Abstract
Elotuzumab is one of the first monoclonal antibodies to be approved for the treatment of multiple myeloma. It is a humanized immunoglobulin G kappa (IgGκ) antibody that targets signaling lymphocytic activation molecule family member 7 (SLAMF7), a surface marker that is highly expressed on normal and malignant plasma cells. This review summarizes the preclinical and clinical data that led to the approval of elotuzumab, along with a discussion on the ongoing and future clinical investigations.
Keywords: multiple myeloma, relapsed, refractory, treatment, elotuzumab, SLAMF7, CS1
Introduction
Multiple myeloma (MM) is a clonal plasma cell malignancy that usually presents with a monoclonal protein in serum or urine, anemia, renal insufficiency and bone disease. Although MM represents ~10% of hematologic malignancies in the US with a median age of 69 years at diagnosis, the annual incidence of MM has been rising steadily over the past decade in the US, with a projected 30,000 new cases in 2016.1 This may be primarily driven by the increment in the proportion of the aging population coupled with an increased awareness about the disease due to the development of novel agents for treatment of MM. In comparison with the 1990s, the survival of MM patients has increased 4-fold, but many patients experience disease relapse and may eventually develop disease that is refractory to all approved agents.1 Therefore, there remains an impetus to understand drug resistance mechanisms and develop novel drug classes and treatment strategies based on disease biology. During relapse, patients may present with different burdens of disease and clinical symptoms that may also be complicated by other co-morbidities (diabetes, heart disease, chronic obstructive pulmonary disease, etc.) – this adds another dimension to the intricacy of their care.
Immunotherapy is a broad terminology applied to several strategies being employed in cancer medicine. These strategies include monoclonal antibodies (mAbs), antibody–drug conjugates and various adoptive cellular therapies (vaccines, natural killer [NK] cells, T cells, dendritic cells, etc.), with the last deemed as the most bona fide “immunotherapy” approach. The mAbs may target the cancer cell, its micro-environment or the immune system (eg, checkpoint inhibition). With the success of mAb therapy in the treatment of solid and hematologic malignancies, several targets are now being explored in MM. In this review, we focus on signaling lymphocytic activation molecule family member 7 (SLAMF7) as a therapeutic target in MM and the relevant preclinical and clinical data of the approved anti-SLAMF7 mAb elotuzumab (Elo).
SLAMF7 – expression on human cells and function
SLAMF7 is a signaling lymphocytic activation molecule F7, previously known as cell surface 1, CS1 (CCND3 subset 1, CD2-like receptor-activating cytotoxic cells [CRACC]). It is a cell surface protein and a member of the signaling lymphocytic activation molecule family, which has been identified on NK cells and is critical for NK cell functions such as adhesion.2 By examining the expressed sequence tag database for CD2-like molecules,3 a novel leukocyte cell surface receptor of the CD2 family called CRACC was identified. CRACC appears to trigger the NK cell-mediated cytotoxicity through a unique SLAM-associated protein-independent extracellular signal-regulated kinase (ERK)-dependent pathway.3 Hsi et al4 confirmed that other normal lymphocyte subsets, such as NK cells, NK-like T cells, CD8+ T cells, activated monocytes and dendritic cells, also express SLAMF7, although at lower levels than normal plasma cells. In 2008, they showed that normal plasma cells and MM cells express high levels of SLAMF7 messenger RNA (mRNA) and protein. This observation eventually led to the development of a panel of murine and humanized mAbs to human SLAMF7 to validate this protein as a potential target for the treatment of MM.4
SLAMF7 – expression on malignant PCs
Both plasma cells and MM cells appear to express high levels of SLAMF7 mRNA and protein, an observation confirmed in animal models, human cell lines and primary patient samples.4,5 This high expression of SLAMF7 on human MM cells appears to be independent of the presence of metaphase cytogenetic abnormalities or molecular subgroup by gene expression profiling.4 It was with this in mind Hsi et al developed a panel of murine and humanized mAbs to human SLAMF7 to validate this protein as a potential target for the treatment of MM. SLAMF7 was analyzed by gene expression profiling and immunohistochemistry of both normal and MM patient samples. Interestingly, it has been observed that the SLAMF7 gene is located on chromosome 1q, amplifications of which are frequent in aggressive myeloma and linked to early myeloma-related death in part due to overexpression of the cell cycle regulator CKS1B.6
Elo – preclinical data
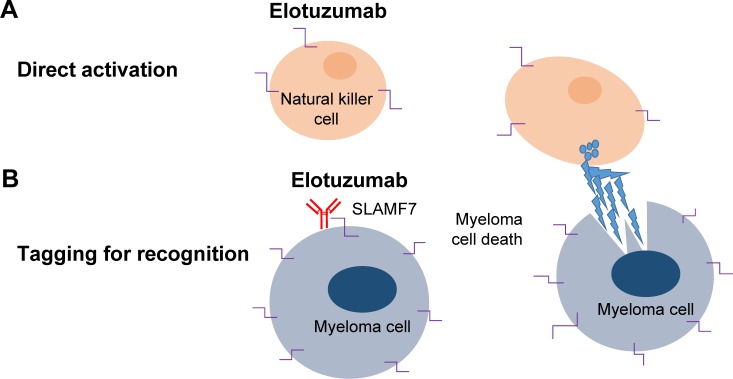
Elo is a humanized IgGκ mAb that targets SLMAF7. It mediates MM cell killing via NK cell activation first demonstrated by Tai et al5 who showed that the novel humanized anti-SLAMF7 mAb HuLuc63 induced antibody-dependent cellular cytotoxicity (ADCC) against human MM cells (Figure 1).5 The same group also found that gene expression of SLAMF7 appears most highly in primary myeloma cells and cell lines and is not detected at significant levels in normal tissues and nonmalignant cells. Elo showed in vivo efficacy in mouse xenograft models of MM by inhibition of MM cell adhesion to bone marrow stromal cells.4,5 Although this activity was limited as a single agent in preclinical studies, immunomodulatory agents such as lenalidomide appeared to enhance the preclinical efficacy of Elo through their potentiation of NK-cell-mediated ADCC and immune function. Furthermore, the combination of Elo with different classes of agents, such as the proteasome inhibitor bortezomib, has been shown to enhance immune lysis of myeloma by enhancing Elo-mediated antibody-dependent cell-mediated cytotoxicity.6 Based on the preclinical rationale mentioned earlier, Elo moved into early phase clinical development.
Figure 1.
Elotuzumab: proposed mechanism of action in myeloma.
Notes: (A) Direct natural killer (NK) cell activation by elotuzumab. (B) Antibody-dependent NK cell-mediated cytotoxicity.
Abbreviation: SLAMF7, signaling lymphocytic activation molecule family member 7.
Elo – clinical data and Phases I, II and III
In the first human Phase I, multicenter, open-label, dose escalation study by Zonder et al,7 the safety of single-agent Elo was studied in relapsed and refractory MM patients. Thirty-five patients with relapsed/refractory MM were enrolled into 6 escalating dose cohorts, with intravenous Elo doses ranging from 0.5 to 20 mg/kg once every 14 days. Trial eligibility included adults aged ≥18 years with a diagnosis of relapsed/refractory MM who had received at least 2 prior MM therapies. No maximum tolerated dose (MTD) was identified up to the maximum planned dose (MPD) of 20 mg/kg. The most common adverse events were primarily infusion related and mild to moderate in severity. To reduce the risk of an infusion-related reaction, the study was amended to include a premedication regimen of methylprednisolone, diphenhydramine and acetaminophen before the first dose of Elo in the 20 mg/kg dosing group. Additional dosing of diphenhydramine and acetaminophen was given on as-needed basis to subsequent cycles. Of the 34 patients treated, 25 completed the initial 8-week treatment. Eight went on to receive another 8 weeks of therapy. Findings revealed that SLAMF7 on bone marrow-derived plasma cells was reliably saturated (≥95%) at the 10 and 20 mg/kg dose levels. Nine patients (26.5%) had stable disease. Results from this study formed the framework for further investigation of Elo in combination with other MM therapies (Table 1).7
Table 1.
Published elotuzumab clinical trials
| Study | Regimen | Study design (no of participants) | Median prior lines of treatment | Response | PFS | Significant AEs |
|---|---|---|---|---|---|---|
| Lonial et al9 | EloRd | Phase I (n=28) | 3 | ≥PR: 82% ≥VGPR: 29% CR: 4% SD: 11% |
NR | Fatigue: 61% Grade 3/4: neutropenia (36%), thrombocytopenia (21%) |
| Zonder et al7 | Elo | Phase I (n=35) | 4.5 | ≥PR: 0% ≥VGPR: 0% SD: 28.5% |
NR | IRR before institution of infusion prophylaxis: 52% |
| Jakubowiak et al8 | V + Elo | Phase I (n=28) | 2 | ≥PR: 48% ≥VGPR: 7% |
9.5 months | |
| Richardson et al11 | EloRd 10 mg/kg versus EloRd 20 mg/kg | Phase Ib/II (n=36) (n=37) |
≥2 ≥2 |
≥PR: 36% ≥VGPR: 43% CR: 4% |
32.9 months | 78% had grade 3–4 AEs: lymphopenia (21%), neutropenia (19%) |
| Jakubowiak et al10 | EBd versus Bd | Phase II (n=77) (n=75) |
1 1 |
≥PR: 65% VGPR: 30% CR: 4% ≥PR: 63% VGPR: 23% CR: 4% |
9.7 months 1-year OS: 85% 6.9 months 1-year OS: 74% |
IRR in elotuzumab group: 7% (all grade 1/2); most common grade 3–4 AEs were infections (EBd 21%, Bd 13%) and thrombocytopenia (EBd 9%, Bd 17%) |
| Lonial et al12 (ELOQUENT-2) | EloRd 10 mg/kg versus Rd | Phase III (n=321) (n=325) |
2 2 |
≥PR: 79% VGPR: 28% CR: 4% ≥PR: 66% VGPR: 21% CR: 7% |
19.4 months 14.9 months |
Grade 3/4 lymphopenia: 77% Herpes zoster: 4.1 per 100 patient-years IRR: 10% (mostly grade 1/2) Grade 3/4 lymphopenia: 49% Herpes zoster: 2.2 per 100 patient-years |
Abbreviations: PFS, progression-free survival; AE, adverse event; EloRd, elotuzumab, lenalidomide and dexamethasone; Rd, lenalidomide and dexamethasone; PR, partial response; VGPR, very good partial response; CR, complete response; SD, stable disease; EBd, Elo, bortezomib and dexamethasone; Bd, bortezomib and dexamethasone; OS, overall survival; NR, not reported; IRR, infusion related reaction.
In parallel, another Phase I, multicenter, open-label, dose escalation trial was initiated to evaluate the MTD, safety and efficacy of Elo in combination with bortezomib. Patients were required to have received 1–3 prior lines of MM therapy. Bortezomib was given at 1.3 mg/m2 intravenously (IV) on days 1, 4, 8 and 11 of a 21-day cycle along with Elo in 4 dose-escalating doses ranging from 2.5 to 20 mg/kg IV within 30 minutes of bortezomib infusion on days 1 and 11 of each cycle. This study was also amended to include premedication regimens to minimize infusion reactions following a grade 3 infusion reaction of hypersensitivity. Twenty-eight patients were enrolled with 27 evaluable for response based on the European Group for Blood and Marrow Transplantation (EBMT) criteria. Eleven patients had received prior bortezomib therapy and 13 had received prior lenalidomide. Patients were treated with a median of 6 cycles, ranging from 1 to 32. The most frequent grade 3–4 adverse events included lymphopenia (25%), fatigue (14%), thrombocytopenia, neutropenia, hyperglycemia, pneumonia and peripheral neuropathy (11% each).8 The objective response rate (ORR) was 48% and median time-to-progression (TTP) was 9.46 months (Table 1).
Jakubowiak et al pursued a randomized, Phase II study evaluating the efficacy of Elo combined with bortezomib and dexamethasone. Progression-free survival (PFS) was the primary end point. A total of 150 patients were treated with either EBd (Elo, bortezomib, dexamethasone) or Bd (bort-ezomib, dexamethasone) in a 1:1 ratio, stratified according to prior proteasome inhibitor therapy, presence of at least 2 FcγRIIIa V alleles and number of prior lines of MM therapy (1–3).10 Treatment schema included bortezomib 1.3 mg/m2 IV or subcutaneously administered on days 1, 4, 8 and 11 for cycles 1–8 and then on days 1, 8 and 15 thereafter along with Elo 10 mg/kg IV weekly with the first 2 cycles followed by days 1 and 11 for cycles 3–8 and then on days 1 and 15 thereafter. Treatment was continued until disease progression or intolerable toxicity. Premedication of dexamethasone 20 mg was administered orally on non-Elo-dosing days and as 8 mg orally plus 8 mg IV on Elo-dosing days. Approximately half of the patients included in this study had received prior bortezomib therapy. With the use of premedication, there were no grade 3–4 infusion-related reactions in this study. The most common grade 3–4 adverse events were infections (EBd 21%, Bd 13%) and thrombocytopenia (EBd 9%, Bd 17%). Two on-study deaths occurred in the EBd group versus 6 patients in the Bd group, the primary cause of death being disease in the EBd group. Although the ORR was comparable in both cohorts (EBd 66%, Bd 63%), updated analysis revealed that the response rate of very good partial response (VGPR) or better occurred in 36% of patients with EBd versus 27% of patients with Bd. The study met its primary end point with a 28% reduction in the risk of progression or death with EBd compared to Bd and a median PFS of 9.7 months in the EBd cohort versus 6.9 months in the Bd cohort. In an updated analysis, the 2-year PFS rate was 18% with EBd and 11% with Bd (Table 1). Interestingly, those EBd-treated patients who were homozygous for the high-affinity FcγRIIIa V allele were shown to have a significantly prolonged median PFS of 22.3 months compared with 9.8 months for those homozygous for the low-affinity FcγRIIIa V allele. In contrast, the PFS was 8.2 and 6.9 months for Bd-treated patients homozygous for the high-affinity and low-affinity FcγRIIIa V allele, respectively.10 This interesting observation will warrant further validation in ongoing and future prospective studies.
Following the above-mentioned Phase I study, an open-label, multicenter, dose escalation Phase I study by Lonial et al9 evaluated escalating doses of Elo at 5, 10 and 20 mg/kg, administered intravenously in combination with lenalidomide and dexamethasone for relapsed and refractory MM patients. The primary objective of the study was to identify the MTD of Elo when combined with lenalidomide and dexamethasone. The MTD was defined as the highest dose of Elo at which 1 of 6 patients or fewer experienced a dose-limiting toxicity (DLT) during cycle 1. Elo was administered intravenously on days 1, 8, 15 and 22 for the first 2 treatment cycles and on days 1 and 15 for the remaining treatment cycles. Three patients were enrolled in 5, 10 and 20 mg/kg dose cohorts, beginning at the lowest dose level. If there were no DLTs during cycle 1, the next higher dose cohort could be enrolled. Additional patients who were enrolled were treated with either the MTD or the MPD of 20 mg/kg of Elo. The initial protocol included a treatment plan of 6 months, but with observed durable responses, treatment time was extended to disease progression. The median number of prior therapies was 3. Patients received a median of 10.5 treatment cycles. No DLTs were observed at the maximum proposed dose of 20 mg/kg. It is notable that 89% of patients experienced at least one infusion reaction although most resolved on their own or following treatment with intravenous corticosteroids and premedications (diphenhydramine and ranitidine or equivalent). These reactions were mostly mild to moderate in severity and resolved the same day either with treatment or spontaneously. There were 2 patients who experienced a grade 3 or higher serious infusion reaction leading to treatment discontinuation. It is not clear whether all the patients were given premedication prior to infusion, although the authors make a point in their conclusion that the ongoing Phase II study would better define an improved premedication strategy. The ORR was 82% with 29% achieving at least a VGPR. A total of 11% of patients had stable disease with 7% having progressive disease. The response rate was similar regardless of the number of previous therapies received. However, the activity was most notable in the lenalidomide-naïve patients with a 95% ORR. No first cycle DLT was observed in the dose-escalation phase to the maximum proposed dose of 20 mg/kg. At a median of 16.4 months follow-up, the median TTP was not reached for those patients in the 20 mg/kg cohort (Table 1).9
Based on the encouraging safety and efficacy signal seen with Elo in combination with lenalidomide and dexamethasone in the above-noted Phase I study,9 a follow-up Phase II extension was implemented to further assess the efficacy of the combination and better characterize the optimal dose.11 Inclusion criteria included adults aged >18 years with relapsed/refractory MM who were treated with 1–3 prior lines of therapy. Seventy-three patients were assessed for eligibility into the Phase II portion, and all were enrolled to either Elo 10 mg/kg or Elo 20 mg/kg with Len/Dex. Treatment was administered in 28-day cycles given until disease progression or unacceptable toxicity. To minimize infusion reactions seen in the earlier studies, a premedication regimen was utilized that included dexamethasone given as a split dose (28 mg given orally 3–24 hours before Elo infusion and 8 mg given IV 45 minutes before infusion), as well as an H1 blocker, an H2 blocker and acetaminophen all given 30–90 minutes prior to infusion. A total of 44 patients (split evenly between the 2 cohorts) had previous bortezomib exposure and 45 patients had previously been treated with thalidomide. Patients received a median of 17 cycles with a range of 1–51 cycles. Grade 3–4 treatment-emergent adverse events were noted in 73% of patients, with lymphopenia (21%) and neutropenia (19%) being the most common. Eighty-four percent achieved an objective response (92% with the 10 mg/kg dosing and 72% with the 20 mg/kg dosing). Forty-two percent of patients enrolled achieved at least a VGPR by International Uniform Response criteria. The median PFS was an impressive 32.9 months. Most pertinent in this Phase Ib/II study was that the lower 10 mg/kg dosing had a higher ORR over the 20 mg/kg dosing validating later Phase III study dosing (Table 1).
Most recently, ELOQUENT-2, an open-label, multi-center Phase III study of lenalidomide and dexamethasone (Rd) versus Elo, lenalidomide and dexamethasone (EloRd) was conducted by Lonial et al.12 This study included adults >18 years old who were diagnosed with relapsed and/or refractory MM and had received 1–3 prior lines of therapy. A total of 646 patients were randomly assigned to either EloRd (321 patients) or Rd (325 patients). The median number of previous lines of therapy was 2 in both groups. Exposure to prior bortezomib and thalidomide therapy was similar between the 2 arms; 5 patients in the EloRd group and 6 patients in the Rd group received prior lenalidomide therapy. The median duration of treatment in the EloRd group was 17 and 12 months in the Rd group. The most common reason for therapy discontinuation was disease progression, 65% in the EloRd group and 79% in the Rd group. The most common grade 3–4 adverse events in the EloRd group were lymphopenia (77%) and neutropenia (34%) compared to 49% and 44%, respectively, for those in the Rd group. The ORR in the EloRd group was 79% versus 66% in the Rd group. Twenty-eight percent in the EloRd group versus 21% in the RD group achieved a VGPR. Interestingly, the complete response (CR) in the EloRd group was 4% versus 7% in the Rd group, which could have been due to the fact that the therapeutic mAb, detectable on serum protein electrophoresis and immunofixation assays, may have led to underestimation of the CR rate in the EloRd group. This phenomenon has also been observed in patients receiving another IgGκ mAb such as daratumumab.
There was an improvement in PFS favoring the patients who received EloRd. Specifically, there was a relative reduction of 30% in the risk of disease progression or death compared to Rd alone. Median follow-up was 24.5 months with PFS at 1 year being 68% versus 57% favoring the EloRd group.12 Although not yet mature, preliminary overall survival data published thus far have recorded 210 deaths (30%) in the EloRd group versus 116 (37%) in the Rd group, representing 49% of the 427 deaths that are prespecified for final analysis. The fluorescence in situ hybridization (FISH) analyses were performed in a central laboratory upon study enrollment, which makes this trial unique compared to other Phase III trials in similar setting. It is notable that 32% of patients included in this study had the del(17p) variant (17p deletion), a poor prognostic biomarker in MM. The authors define del(17p) positivity without a cutoff noting that if any cell in the analyzed sample was positive for the mutation, the patient was considered del(17p) positive. The benefit of PFS in the Elo group was consistent across this key subgroup along with other typically poor outcome subgroups including patients aged ≥65 years, patients with resistance to most recent therapy, with an international staging system (ISS) stage III disease at diagnosis, previous exposure to bortezomib or immunomodulatory drugs, and those patients with previous stem cell transplantation or with a creatinine clearance of <60 mL per minute.
Based on these data reviewed, in November 2015, Elo in combination with lenalidomide and dexamethasone was approved by the FDA for use in MM patients who have received 1–3 prior therapies.
Future directions
While the ELOQUENT-2 data are provocative, many patients are treated with lenalidomide-based therapy until disease progression as part of first-line therapy. Thus, many patients will be lenalidomide, immunomodulatory drug (IMiD) class refractory at the time of first relapse and may not be the ideal candidates for EloRd. Therefore, data perhaps need to be generated in the clinically relevant patient populations. There are several clinical trials examining the role of Elo combinations in the upfront treatment setting, maintenance setting and the relapsed/refractory setting (Table 2).13 The most exciting of these combinations may be with other mAbs, especially the checkpoint inhibitor combination trials.
Table 2.
Ongoing clinical trials of elotuzumab combinations in high-risk smoldering MM, newly diagnosed MM and RRMM
| Trial | Phase | Patient population | Primary end point | Study arm(s) |
|---|---|---|---|---|
| Phase II trial of combination of Elo, lenalidomide and dexamethasone in high-risk smoldering MM (NCT02279394) | Phase II | High-risk MM | PFS | Elotuzumab: 10 mg/kg IV; D1, 8, 15, 22, C1–2; 10 mg/kg IV; D1, 15, C3–8 Lenalidomide: 25 mg oral; D1–21, C1–24 Dexamethasone: 40 mg oral; D1, 8, 15, 22, C1–2; 40 mg oral; D1, 8, 15, C3–8 |
| A Phase II study of Elo with pomalidomide, bortezomib and dexamethasone in relapsed and refractory MM (NCT02718833) | Phase II | RRMM | ORR, treatment-related adverse events | 28-Day cycle: For C1–2, Elo IV is given weekly. For C3–8, Elo IV is given every other week. For C9+, Elo IV is given on D1, pomalidomide PO on D1–21, bortezomib SC on D1, 8, 15, dexamethasone PO and IV combination |
| A Phase 1 study of Elo in combination with ASCT and lenalidomide maintenance for MM (NCT02655458) | Phase Ib | MM patients who are receiving or have completed induction chemo and achieved at least a PR; eligible for ASCT for consolidation | Incidence of completion of treatment, safety and tolerability | Experimental: ASCT, Elo, lenalidomide Maximum number of cycles is 12. Elo IV 20 mg/kg on D1 of each cycle. Lenalidomide dosing starts with C4 at 10 mg PO on D1–21. Dexamethasone 8 mg IV on the day of Elo infusion |
| A randomized Phase I/II study of optimal induction therapy of bortezomib, dexamethasone and lenalidomide with or without Elo in treating patients with newly diagnosed high-risk MM (NCT01668719) | Phase I/II | Newly diagnosed high-riska MM | PFS | Arm 1: Induction: bortezomib SC or IV on D1, 4, 8, 11; lenalidomide PO QD on D1–14; dexamethasone PO or IV on D1, 2, 4, 5, 8, 9, 11, 12. Q21-day cycles ×8C. Maintenance: bortezomib SC or IV on D1, 8, 15; lenalidomide PO QD on D1–21; dexamethasone PO on D1, 8, 15. Q28-day cycles Arm 2: Induction: Arm 1+ elo IV on D1, 8, 15 of C1 and C2 and on D1, 11 of C3–8. Q21-day cycles ×8C Maintenance: Arm 1+ elo IV on D1, 15. Q28-day cycles |
| CheckMate 602 An open-label, randomized Phase III trial of combinations of nivolumab, Elo, pomalidomide and dexamethasone in RRMM (NCT02726581) |
Phase III (CheckMate 602) |
RRMM patients who have received ≥2 lines of prior therapy that must have included an IMiD and a proteasome inhibitor |
ORR PFS |
Arm A: (Investigational arm) nivolumab, pomalidomide, dexamethasone Arm B: (Control arm) pomalidomide and dexamethasone Arm C: (Exploratory arm) nivolumab, elotuzumab, pomalidomide and dexamethasone |
| An open-label, single arm, Phase IIa study of bortezomib, lenalidomide, dexamethasone and Elo in newly diagnosed MM (NCT02375555) | Phase II | Newly diagnosed MM | ORR | Experimental: elotuzumab, lenalidomide, bortezomib, dexamethasone (E-RVD). Induction cycles (1–8) are 21-day cycles. Stem cell mobilization performed for all subjects at the end of cycle 4. Subjects may elect to stop at C4 and proceed to ASCT. Those who do not wish to proceed will receive 8 cycles of induction therapy. Maintenance schedule (28 days) will start after 8 cycles of induction regimen for patients not proceeding with ASCT or after recovery from SCT for patients proceeding with it. Specific maintenance regimen determined by risk category |
| ELOQUENT-3 An open-label, randomized Phase II trial of pomalidomide/dexamethasone with or without Elo in RRMM (NCT02654132) |
Phase II | RRMM patients who have received ≥2 prior lines of therapy that must have included 2 consecutive cycles of lenalidomide and a proteasome inhibitor alone or in combination | PFS | Experimental arm: elotuzumab IV 10 mg/kg (C1 and C2 weekly on D1, 8, 15, 22), Elo IV 20 mg/kg (C3 and beyond on D1) + pomalidomide PO 4 mg QD on D1–21+ dexamethasone weekly on D1, 8, 15, 22 Control arm: pomalidomide PO 4 mg QD on D1–21+ dexamethasone weekly on D1, 8, 15, 22 |
| Phase II study of combination of Elo with lenalidomide as maintenance post ASCT in patients with MM (NCT02420860) | Phase II | MM patients who have undergone ASCT, within 18 months of initiation of induction therapy. Studied in the maintenance setting following ASCT | PFS | Experimental arm: Elo + lenalidomide Elo 10 mg/kg IV on D1, 8, 15, 21 for the first 2 cycles, then on D1, 15 only during cycles 3–6, then on D1 each cycle after that. Then for cycles beyond 6, once per cycle at 20 mg/kg on D1 of each cycle. Dexamethasone PO and IV. Lenalidomide 10 mg/day; after 3 cycles, dose may be increased to 15 mg/day at MD discretion and tolerability. Cycle length is 28 days |
| A randomized Phase III trial on the effect of Elo in VRD induction/consolidation and lenalidomide maintenance in newly diagnosed myeloma (NCT0249522) | Phase III (GMMG-HD6) |
Newly diagnosed MM | PFS | Active comparator (A1): Induction therapy with VRD 21 days per cycle, intensification (mobilization and ASCT), consolidation therapy with 2 cycles VRD, 21 days per cycle. Maintenance therapy: 26 cycles (28 days) with lenalidomide (dexamethasone on D1, 15 in C1–6 and on D1 in C7–26) Experimental (A2): Induction therapy with 4 cycles VRD 21 days per cycle, intensification (mobilization and ASCT), consolidation therapy with 2 cycles VRD + elotuzumab 21 days per cycle. Maintenance therapy: 26 cycles (28 days) with lenalidomide + elotuzumab (dexamethasone on D1, 15 in C1–6 and on D1 in C7–26) Experimental (B1): Induction therapy with 4 cycles VRD + elotuzumab, 21 days per cycle, intensification (mobilization and ASCT), consolidation therapy with 2 cycles VRD 21 days per cycle. Maintenance therapy: 26 cycles (28 days) with lenalidomide (dexamethasone on D1, 15 in C1–6 and on D1 in C7–26) Experimental (B2): Induction therapy with 4 cycles VRD + elotuzumab, 21 days per cycle, intensification (mobilization and ASCT), consolidation therapy with 2 cycles VRD + elotuzumab, 21 days per cycle. Maintenance therapy: 26 cycles (28 days) with lenalidomide + elotuzumab (dexamethasone on D1, 15 in C1–6 and on D1 in C7–26) |
| A Phase II, randomized, open-label trial of lenalidomide/dexamethasone with or without Elo in subjects with previously untreated MM in Japan (NCT02272803) | Phase II | Newly diagnosed MM | ORR | Arm A: lenalidomide PO 25 mg QD on D1–21+ dexamethasone PO 28 mg and IV 8 mg once daily on D1, 8, 15, 22 (C1–2); D1, 15 (C3–18); D1 (C19 and beyond), PO 40 mg QD on D8, 22 (C3–18); D8, 15, 22 (C19 and beyond) + Elo IV 10 mg/kg, weekly on D1, 8, 15, 22 (C1, 2); D1, 15 (C3–18), Elo IV 20 mg/kg, D1 (C19 and beyond). Repeat every 28 days until subject meets criteria for discontinuation of study drug Arm B: lenalidomide PO 25 mg QD on D1–21+ dexamethasone PO 40 mg weekly on D1, 8, 15, 22. Repeat every 28 days until subject meets criteria for discontinuation of study drug |
| A Phase II single-arm study of Elo in combination with pomalidomide and low-dose dexamethasone (EPd) in patients with MM relapsed or refractory to prior treatment with lenalidomide (NCT02612779) | Phase II | RRMM patients who have received ≥1 but no more than 2 prior lines of therapy, including ≥2 cycles of lenalidomide | PFS | Elo + pomalidomide + low-dose dexamethasone (EPd) |
Note:
High-risk MM is defined by GEP70, t(14;16), t(14;20), del(17p) by FISH or cytogenetics, primary plasma cell leukemia, serum LDH ≥2 times of institutional upper limit of normal value.
Abbreviations: MM, multiple myeloma; RRMM, relapsed/refractory MM; PFS, progression-free survival; IV, intravenously; D, days; C, cycles; ORR, overall response rate; SC, subcutaneously; ASCT, autologous stem cell transplantation; PR, partial response; Elo, elotuzumab; IMiD, immuno modulatory drug; VRD, velcade, revlimid, dexamethasone; FISH, fluorescence in situ hybridization; LDH, lactate dehydrogenase; PO, by mouth; QD, every day.
Footnotes
Disclosure
SZU has consulted for Amgen, Celgene, Takeda, Novartis, Janssen and Sanofi; received speaker fees for Celgene, Millennium Takeda and Onyx and received research funding from Amgen, Array Biopharma, Celgene, Janssen Oncology, Onyx, Pharmacyclics, Sanofi and Takeda. The other authors report no conflicts of interest in this work.
References
- 1. seer.cancer.gov [homepage on the Internet] [Accessed November 4, 2016]. Available from: http://seer.cancer.gov/
- 2.Lonial S. Monoclonal antibodies for the treatment of myeloma: targeting SLAMF7 and CD38. Cancer J. 2016;22(1):3–6. doi: 10.1097/PPO.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 3.Bouchon A, Cella M, Grierson HL, Cohen JI, Colonna M. Cutting edge: activation of NK cell-mediated cytotoxicity by a SAP-independent receptor of the CD2 family. J Immunol. 2001;167(10):5517–5521. doi: 10.4049/jimmunol.167.10.5517. [DOI] [PubMed] [Google Scholar]
- 4.Hsi ED, Steinle R, Balasa B, et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin Cancer Res. 2008;14(9):2775–2784. doi: 10.1158/1078-0432.CCR-07-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tai Y-T, Dillon M, Song W, et al. Anti-CS1 humanized monoclonal antibody HuLuc63 inhibits myeloma cell adhesion and induces antibody-dependent cellular cytotoxicity in the bone marrow milieu. Blood. 2008;112(4):1329–1337. doi: 10.1182/blood-2007-08-107292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Rhee F, Szmania SM, Dillon M, et al. Combinatorial efficacy of anti-CS1 monoclonal antibody elotuzumab (HuLuc63) and bortezomib against multiple myeloma. Mol Cancer Ther. 2009;8(9):2616–2624. doi: 10.1158/1535-7163.MCT-09-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zonder JA, Mohrbacher AF, Singhal S, et al. A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood. 2012;120(3):552–559. doi: 10.1182/blood-2011-06-360552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakubowiak AJ, Benson DM, Bensinger W, et al. Phase I trial of anti-CS1 monoclonal antibody elotuzumab in combination with bortezomib in the treatment of relapsed/refractory multiple myeloma. J Clin Oncol. 2012;30(16):1960–1965. doi: 10.1200/JCO.2011.37.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lonial S, Vij R, Harousseau J-L, et al. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J Clin Oncol. 2012;30(16):1953–1959. doi: 10.1200/JCO.2011.37.2649. [DOI] [PubMed] [Google Scholar]
- 10.Jakubowiak A, Offidani M, Pégourie B, et al. Randomized phase 2 study: elotuzumab plus bortezomib/dexamethasone vs bortezomib/dexamethasone for relapsed/refractory MM. Blood. 2016;127(23):2833–2840. doi: 10.1182/blood-2016-01-694604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson PG, Jagannath S, Moreau P, et al. 1703 Study Investigators Elotuzumab in combination with lenalidomide and dexamethasone in patients with relapsed multiple myeloma: final phase 2 results from the randomised, open-label, phase 1b-2 dose-escalation study. Lancet Haematol. 2015;2(12):e516–e527. doi: 10.1016/S2352-3026(15)00197-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lonial S, Dimopoulos M, Palumbo A, et al. ELOQUENT-2 Investigators Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373(7):621–631. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 13.Weisel K. Spotlight on elotuzumab in the treatment of multiple myeloma: the evidence to date. Onco Targets Ther. 2016;9:6037–6048. doi: 10.2147/OTT.S94531. [DOI] [PMC free article] [PubMed] [Google Scholar]