Abstract
Background
Transplant renal artery stenosis (TRAS) is a common vascular complication after kidney transplantation and is associated with refractory hypertension, volume overload, and graft injury or loss. This article describes 5-year outcomes of endovascular intervention for TRAS with bare metal and drug eluting stents (DES).
Methods
We investigated, as a prospective cohort study, patient and graft outcomes after the targeted use of DES for vessel diameter less than 5 mm and bare metal stents (BMS) for vessel diameter greater than 5 mm as the primary management for TRAS.
Results
From March 2008 to November 2014, 57 patients were stented for hemodynamically significant TRAS; 29 received DES, 26 received BMS, and 2 patients received both stent types. They were followed up for a mean of 35.1 ± 22.8 months; a subset of these patients who all received DES were followed up for 61.7 ± 17.5 months. Mean serum creatinine declined from 2.87 ± 1.5 mg/dL at the time of intervention to 1.98 ± 0.76 mg/dL (P < 0.001) at one month follow-up and was 1.96 ±0.92 mg/dL (P < 0.001) at 35.1 ± 22.8 months. Mean systolic blood pressure declined from 159.05 ± 19.68 mm Hg at time of intervention to 135.65 ± 15.10 mm Hg (P < 0.001) at most recent visit. Clinically driven restenosis requiring repeat revascularization occurred in 15.7% of patients.
Conclusions
Primary stenting with DES and BMS is both successful in the initial treatment of TRAS and also produced an immediate and long-term reduction in serum creatinine and systolic blood pressure.
Transplant renal artery stenosis (TRAS) is the most frequent vascular complication of renal transplantation1 and a reversible cause of hypertension, volume overload, and graft injury or loss. Its incidence ranges from 6% to 23% depending on screening and diagnostic criteria.1-3 Perhaps, the most accurate estimate comes from a retrospective cohort study where 999 renal transplant recipients were routinely screened for TRAS at the 3-month mark and at times of allograft dysfunction and found to be 13.7%.4 Implicated risk factors include atherosclerosis, Cytomegalovirus infection, multiple renal arteries, delayed graft function (DGF), and most recently, donor-specific antibodies.5-9
Endovascular intervention (EVI) is accepted as the initial therapy for hemodynamically significant TRAS, and composed of percutaneous transluminal angioplasty (PTA) and/or stenting based on individual centers experience. Procedural success rates for PTA are close to 90% with an incidence of restenosis of 15% to 28%.4,5 Combined PTA with stenting and primary stent placement for TRAS shows almost 100% procedural success rates6,10 with minimal complications. Restenosis rates after stenting are superior to PTA alone, but remain as high as 15%.10
Though there is extensive data in coronary vessels, experience with drug eluting stents (DES) in transplant renal arteries is limited. An initial case series in 2008 used DES for in-stent restenotic lesions in the transplant renal arteries in 3 patients.11 In 2011, we reported our experience with DES as the primary treatment of TRAS in 12 patients with transplant renal artery diameter less than 5 mm.12 The use of DES in that population was supported by the high rate of restenosis (40-50%) seen when bare metal stents (BMS) were used in small native renal vessels.13,14 Recently, a similar approach of using DES, BMS and PTA based on clinical parameters was confirmed to have positive short term clinical outcomes.15 Currently, we present our long-term clinical and procedural outcomes on 57 patients with clinically significant TRAS, using DES for vessel diameters of 5 mm or less and BMS for vessel diameters greater than 5 mm. The 12 patients stented in our prior report are included in the current analysis.
MATERIALS AND METHODS
Study Design
This is a single-center, observational, prospective cohort study designed to investigate patient and graft outcomes after the use of DES and BMS as the primary management for TRAS. As previously reported,13 a strategy was implemented in March 2008 at our institution of using primary EVI with DES for vessel diameter of 5 mm or less and BMS for vessel diameter greater than 5 mm to treat TRAS. The study protocol was approved by the institutional review board and informed consent was obtained from all participants. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the 'Declaration of Istanbul on Organ Trafficking and Transplant Tourism'.
Patient Population
From November 2007 to November 2014, a total of 666 transplants were performed at our center. Of these, 453 (68%) were from deceased donors and the rest were from living donors. Among the deceased donors, 220 (48%) were standard criteria donations (SCD), 206 (46%) were expanded criteria donations (ECD) (donor age, > 60 years or > 50 years) with 2 of the following: a history of hypertension, a creatinine greater than 1.4, or death resulting from stroke), and 27 (6%) were from donors after cardiac death. Of the 66 patients transplanted, 577 (86.6%) were primary and the remaining 89 were retransplants. Fifty-seven of these patients were diagnosed with TRAS from March 2008 to November 2014, stented accordingly, and followed up through May 2015.
Tissue Evaluation, Organ Preservation, and Surgical Techniques
Deceased donor biopsies were evaluated before transplantation. All deceased donor kidneys were preserved with continuous hypothermic pulsatile perfusion. Single renal arteries were connected to the Life Port kidney transporter (Organ Recovery Systems, Inc., Chicago, IL) using a seal ring; multiple renal arteries often required cannulation. The arterial anastomosis was performed using a Carrel patch. In most cases the anastomosis was to the side of the external iliac artery. Rarely, an end-to-end anastomosis was performed.
Immunosuppression Protocol
Induction consisted of a single intravenous dose of alemtuzumab (anti-CD52, Campath-1H) 30 mg, on the day of surgery and rapid steroid withdrawal with intravenous methylprednisolone for 3 days, in sequential doses of 500, 250, and 125 mg. Maintenance immunosuppression consisted of either tacrolimus or cyclosporine with mycophenolate mofetil.
Diagnosis of TRAS
Clinical suspicion for TRAS was based on unexplained worsening allograft function, often associated with volume retention and/or poorly controlled blood pressure requiring incremental doses of antihypertensive medications. Doppler ultrasonography (DU) of the transplanted renal artery was performed as the primary modality to evaluate for stenosis. Hemodynamically significant TRAS was defined as arterial lumen stenosis greater than 50% and/or a peak velocity exceeding 200 cm/s, with the renal to external iliac artery peak velocity ratio greater than 2.
Angiographic Procedures
Angiography of the transplant renal artery was performed by the femoral approach with initial pretreatment of the patient with oral N-acetylcysteine, intravenous hydration with sodium bicarbonate in dextrose solution and utilization of iodixanol as the sole contrast agent. Minimization of contrast load as well as dilution of iodixanol with saline was performed as appropriate during contrast injections. Initial angiography of the iliac artery was followed up by selective transplant renal angiography. Angiographically significant TRAS was defined as a diameter stenosis of 70% or greater or 50% or greater with a lesion gradient greater than 25 mm Hg using a 5 french Berenstein diagnostic catheter across the lesion. If the transplant renal artery diameter was 5 mm or less, the lesion was stented with a DES, whereas if the transplant renal artery diameter was greater than 5 mm, the lesion was stented with a BMS.
All procedures, except for one were performed by a single operator (A.M.), an Interventional Cardiologist. DESs used were the Zotarolimus Eluting Stent (Resolute and Endeavor stents, Medtronic, Minneapolis, MN) and Everolimus Eluting Stent (Xience V, Abott Vascular, Santa Clara, CA). BMSs used were the Palmaz-Genesis Peripheral stent (Cordis, Bridgewater, NJ), Herculink Peripheral Stent (Abott Vascular, Santa Clara, CA), Herculink Elite Renal Stent (Abott Vascula) and the Omnilink Peripheral Stent (Abott Vascular). Postprocedure, patients were initiated on lifelong aspirin, and clopidogrel was prescribed for a minimum of 1 year for DES and 6 weeks for BMS.
Outcome Measures
The primary outcomes were the clinical successes of the procedure measured by renal function and blood pressure control. These parameters were tracked at our transplant clinic, initially one week postprocedure, then monthly and eventually every third month, and as clinically indicated. Each visit included serum creatinine and blood pressure measurement and antihypertensive documentation. The secondary outcome was the procedural success rate, measured by resolution of the stenosis. Endovascular complications, that is, in-stent restenosis and thrombosis and adverse advents, such as contrast nephropathy and major bleeding episodes, while on antiplatelet therapy were also recorded for each cohort.
Statistical Analysis
Means ± SD was used to describe continuous variables and percentages were used to describe categorical variables. Statistical analysis was done using the nonparametric Wilcoxon signed-rank test to compare differences between groups.
RESULTS
Recipient Demographics and Follow-Up
Hemodynamically significant TRAS was confirmed angiographically in 57 (8.5%) of 666 patients during the study period. Of these, 29 patients with vessel diameter ≤5 mm underwent primary EVI with deployment of DES and 26 with vessel diameter >5 mm underwent primary EVI with deployment of BMS. One patient with vessel diameters of 5 mm had initial DES placement at the ostium requiring restenting with BMS due to ostial stent recoil and 1 patient had both a BMS and DES placed for 2 separate lesions. The baseline characteristics of the total recipient cohort and the DES and BMS groups are delineated in Table 1.
TABLE 1.
Recipient demographics
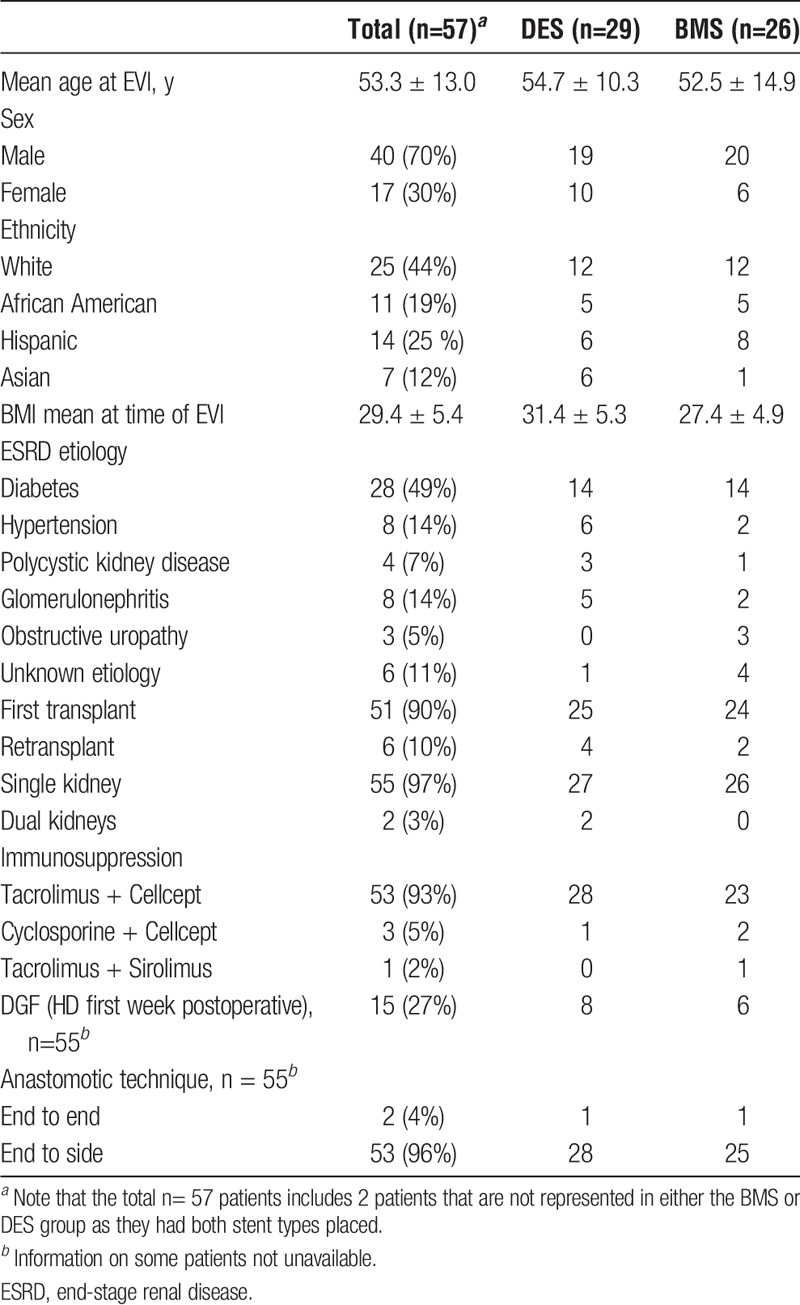
Patients were followed up for a mean of 35.1 ± 22.8 months (range, 2.9-87.8) postprocedure. Those who received DES were followed up for 33.6 ± 24.1 months (range, 2.9-87.8) and patients who received BMS were followed up for 37.4 ± 20.9 months (range, 4.8-78.2). In 2011, we reported on 12 of the patients in the DES group who then had a mean follow up of 16 ± 10.0 months. Apart from 2 lost to follow-up, the remaining 10 patients have now been followed up for 61.7 ± 17.5 months (range, 30.5-87.8) and are included in the 29 patients in the DES group.
Donor Characteristics
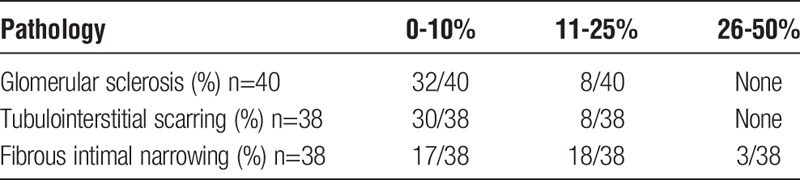
As delineated in Table 2, 44 (77.2%) of 57 kidneys were from deceased donors and of these 26 (45.6%) of 57 were ECD. The donors had a mean age of 53.7 years, about half, 25 (45%) of 55 were women, 31 (56%) of 55 were hypertensive, and 16 (29%) of 55 smoked for more than 20 years. Of note, 19 (33.3%) of 57 kidneys had more than 1 renal artery, and mean cold ischemic time for the deceased donors was 25.3 hours. Warm ischemic time was less than 1 hour in all cases. Pretransplant tissue evaluation (Table 3) indicated that the majority of deceased donor kidneys had less than 10% global glomerular sclerosis and tubulointerstitial scarring (80% and 79%, respectively), and none had greater than 25% of either entity. Significant vascular findings, that is, 26% to 50% fibrous intimal narrowing, were seen in 3 kidneys, with another 18 (47%) having 10% to 25% fibrous intimal narrowing.
TABLE 2.
Donor characteristics
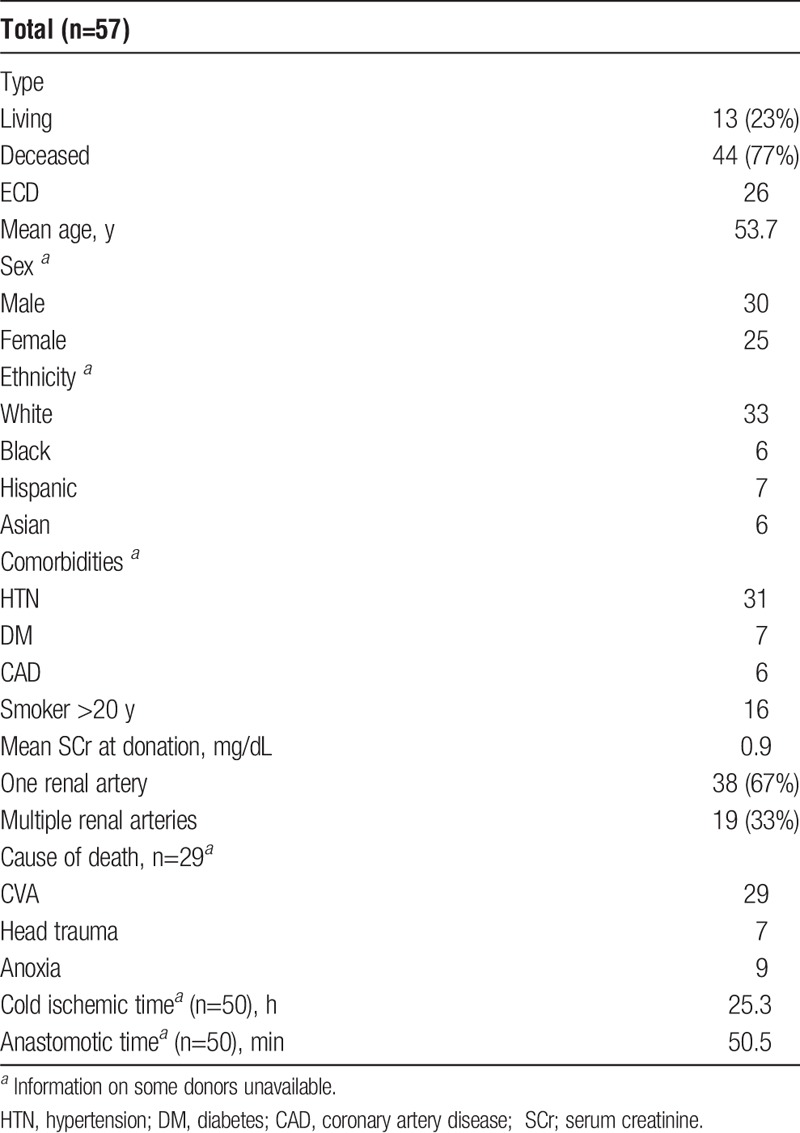
TABLE 3.
Pathologic findings of deceased donor kidney biopsies
Diagnosis of TRAS
On DU, all 57 patients had either a peak systolic velocity greater than 250 cm/s or a V1 to V2 ratio greater than 3:1. TRAS was confirmed by angiogram and occurred at 9.3 ± 18.0 months posttransplant (1.3 to 58.8 months). Those who received DES (vessel diameter, ≤5 mm) were diagnosed at 7.0 ± 9.4 months posttransplant compared with 11.7 ± 24.8 months in those who received BMS (vessel diameter, >5 mm).
Angiographic Findings and Stent Deployment
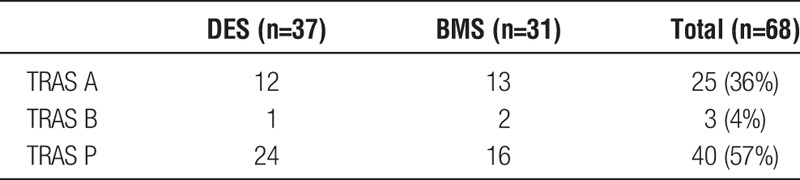
At angiography, 68 discrete stenotic lesions were found in the 57 patients. Of these, 25 (37%) of 68 were anastomotic (TRAS A), 3 (4%) of 68 were kink/bend (TRAS B), and 40 (59%) of 68 were postanastamotic (TRAS P).15 The 68 stented lesions were revascularized using 39 DES and 31 BMS, with some single lesions requiring more than 1 stent. As detailed in Table 4, DES was deployed to 12 (48%) of 25 TRAS A lesions, 1 (33%) of 3 TRAS B lesions and 24 (60%) of 40 TRAS P lesions. The severity of stenosis was 70% or greater in 59 of the lesions. The 9 remaining lesions ranged from 50% to 65% stenosis and had a pressure gradient of above 25 mm Hg. The sole lesion with a 50% stenosis had a pressure gradient of 70 mm Hg. The median DES diameter used was 4.00 mm (range, 2.75-4.0 mm). The median BMS diameter was 5.5 mm (range, 5-7 mm). The median stent length was 12 mm (range, 8-30 mm).
TABLE 4.
TRAS type and stent(s) deployed
Clinical Outcomes
In both cohorts, posttransplant baseline creatinine was 1.82 ± 0.72 mg/dL which increased to 2.87 ± 1.5 mg/dL at time of EVI (P <0.001). One month postprocedure serum creatinine was 1.98 ± 0.76 mg/dL (P <0.001), and at 35.1 ± 22.8 months, follow-up was 1.96 ± 0.92 mg/dL (P < 0.001) compared with preprocedure value (Figure 1). Their systolic blood pressure at the time of intervention was 159.1 ± 19.7 mm Hg, which was increased from their posttransplant baseline of 132.5 ± 8.3 mm Hg (P < 0.001). One month post-EVI systolic blood pressure was 134.5 ± 15.6 mm Hg (P < 0.001) and at 35.1 ± 22.8 months was 135.7 ± 15.1 mm Hg (P<0.001), compared with preprocedure value. Similarly, diastolic blood pressure was 79.3 ± 11.3 mm Hg at time of intervention and was 71.0 ± 10.6 mm Hg (P<0.001) at last follow-up (Figure 2). All but 3 patients are requiring the same or lesser amounts of blood pressure medications.
FIGURE 1.
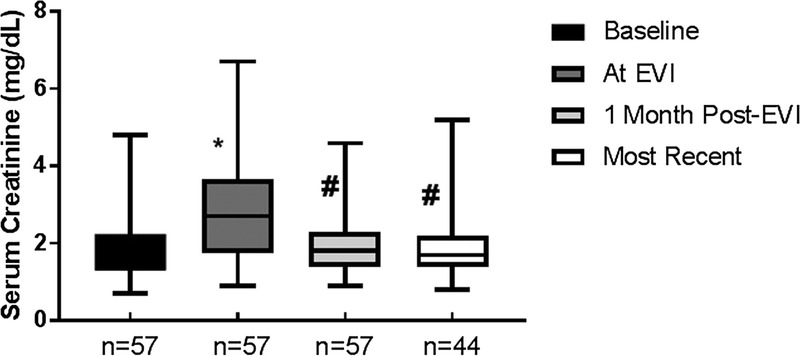
Trends in serum creatinine pre and post-EVI. The mean serum creatinine (Cr) at the indicated time intervals is shown. * Significantly different from baseline (b/l) creatinine. # Significantly different from creatinine at time of EVI.
FIGURE 2.
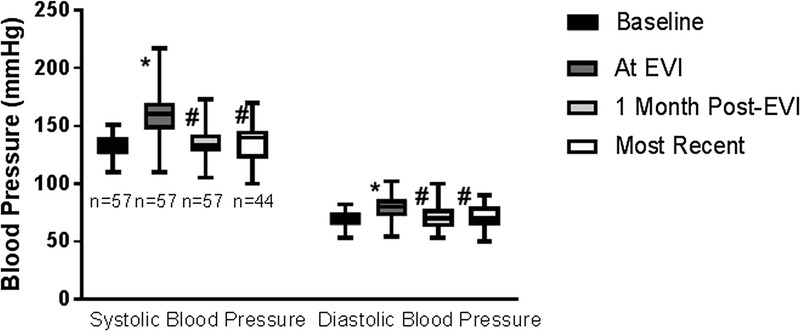
Trends in systolic blood pressure and diastolic blood pressure pre and post-EVI. *Significantly different from baseline blood pressure. # Significantly different from blood pressure at time of EVI.
The subset of the 12 patients reported on in 2011, stented with DES, have now been followed up for 61.7 ± 17.5 months. One has returned to hemodialysis, and 2 were lost to follow up. The remaining 9 patients had a serum creatinine of 3.1 ± 1.3 mg/dL at the time of TRAS diagnosis,12 and most recently at 61.7 ± 17.5 months is 2.5 ± 1.4 mg/dL. The timeline of their renal function is further depicted in Figure 3. Their systolic blood pressure was 156 ± 15.0 mm Hg before EVI12 and was 131.2 ± 14.2 mm Hg on latest measurement.
FIGURE 3.
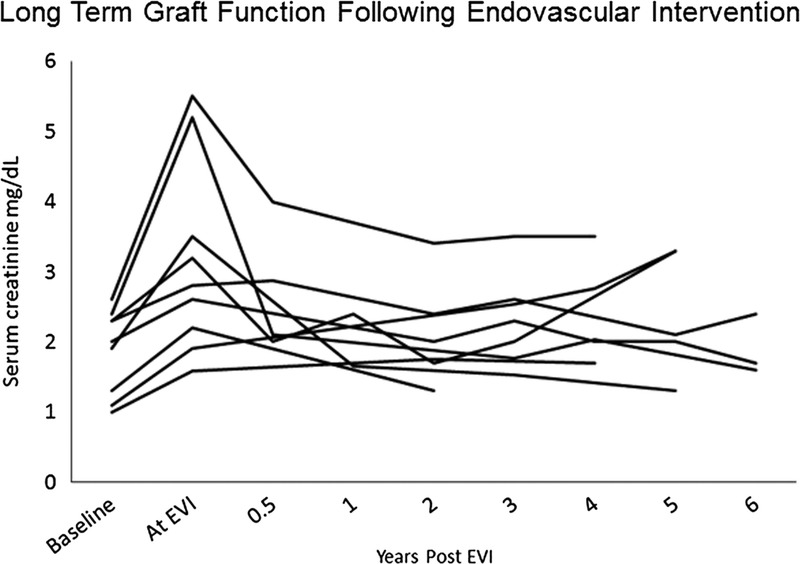
Long term trends in serum creatinine for each of the 9 patients reported in a previous analysis13 that underwent EVI with a DES.
Overall, 11 (19.2%) of 57 patients (6 in the DES and 5 in BMS groups and 1 who had both stent types) progressed to end-stage renal disease and resumed dialysis at a mean of 24.5 ± 17.1 months posttransplant and 19 ± 16.2 months post EVI. Of these patients, 1 had recurrent focal segmental glomerulosclerosis, 6 had refractory acute rejection, and in 4 the cause was multifactorial. In all these patients TRAS was excluded on DU. Two patients moved out of state after their EVI and were lost to follow up. These 2 patients along with the 11 that returned to dialysis are not included in analysis of renal function and blood pressure control beyond one month post EVI.
Procedural Outcomes
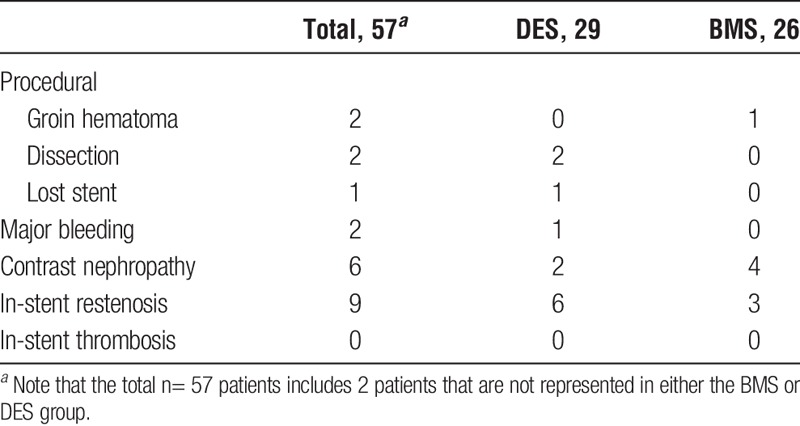
The secondary outcome, primary procedural success was 100% at 30 days. Clinically driven restenosis confirmed angiographically occurred in 9 patients (15.7%), 6 with DES, and 3 with BMS. Median time to restenosis in DES was 8 months (range, 1-70 months), and BMS was 5.5 months (range, 1-6 months).
Adverse events and complications are detailed in Table 5. Five periprocedural complications were encountered, including 1 stent stripping and loss into a distal profunda femoral artery branch with no adverse outcome, 2 distal edge dissections that required further stenting and 2 groin hematoma, all without functional impairment of the graft. Two patients experienced a major bleeding episode while on clopidogrel, 1 being a subdural hematoma after a fall which was treated with Burr Hole drainage and left the patient without neurological deficits, and the second a large groin hematoma after catheterization. In all 6 (10.5%) of 57 patients developed contrast nephropathy defined by a 20% rise in creatinine within 48 hours of procedure, and all experienced full renal recovery. There were no instances of in-stent thrombosis.
TABLE 5.
Adverse events and complications
DISCUSSION
The findings presented indicate that primary EVI with DES, and BMS was successful in the initial treatment of TRAS and produced an immediate and long term reduction in serum creatinine and systolic blood pressure. This was achieved with few adverse events. The suggestion that primary stenting is at least comparable to PTA in safety and efficacy, in the treatment of TRAS, has been reported previously,6,10 but additionally, we propose that the targeted use of BMS and DES in large and small transplant renal arteries, may offer a reasonable approach to decrease the need for repeat revascularizations.
The sustained and significant improvement in graft function and blood pressure control was observed despite the occurrence of various posttransplant complications such as recurrent glomerulonephritis, acute rejection and calcineurin inhibitor toxicity. Additionally, although pretransplant biopsies of the deceased donor kidneys had minimal global sclerosis and interstitial fibrosis, almost 50% did show 10% 25% or greater intimal changes and 8% had greater than 25% fibrous intimal narrowing. The nature of the donor vessels is not surprising considering that 56% of the donors were hypertensive and 29% were more than 20 years smokers.
DGF and multiple renal arteries have both been implicated as risk factors in the development of TRAS late after transplantation.7,9 A considerable portion of our cohort (26.3%) had DGF (dialysis in first week after transplant), higher than our center's overall DGF rate of 17%,16 which is close to the national reported incidence of DGF, approximately 15% for SCD and approximately 35% for ECD.17 The higher proportion in our TRAS patients is explained by their mean cold ischemic time longer than 25 hours and 47% of the donor kidneys being ECD. In addition, 33.3% of our cohort received kidneys with more than 1 renal artery. The use of grafts with multiple renal arteries varies but was found to be 20% in 1 report.17 The higher rates of multiple renal arteries and DGF observed in our TRAS cohort concur with previous reports citing their association.
The use of DES in coronary arteries has dramatically reduced restenosis rates, particularly in small vessels, where it was first observed that a small lumen diameter is a strong predictor for in-stent restenosis.18 There is no long-term data regarding restenosis rates after using DES in these high-risk vessels, but recently, the tailored use of BMS and DES based on transplant renal artery size was shown to have relatively low rates of restenosis over the first year after intervention.15 This is in contrast to previous reports that found 1 year restenosis rates as high as 40% when these small renal arteries (<5 mm) are stented with BMS.13,14
In addition to lumen diameter, our patient population has many established risk factors for in-stent restenosis, namely, chronic kidney disease (CKD), diabetes, and hypertension.18,19 In patients with CKD, percutaneous coronary intervention with BMS carries an increased risk of restenosis and cardiac events when compared to those with normal renal function.20 In contrast, when DES were used in CKD patients, there was no difference in rates of restenosis when compared to non-CKD patients,19 suggesting that DES may be preferable in this group.
A potential obstacle to the use of DES is the longer duration of antiplatelet therapy required, which carries an increased risk of bleeding. This was not encountered in our experience. All but 1 patient with a DES remained on clopidigrel for 1 year or longer (1 patient stopped early due to cost). The 2 bleeding events observed, a postprocedural groin hematoma that required 3 units of red blood cells and a subdural hematoma after a fall might have been exacerbated by clopidogrel but both occurred after inciting events.
We recognize the limitations of this study; participants were not randomized, various types of DES and BMS were used, and it occurred at a single center. We also acknowledge that although the overall incidence of TRAS at our center was 8.5% during the study period, comparable to other reports,1-3 it may be an underestimation because we do not routinely screen. Additionally, we used vessel diameter alone as a guide to therapy and did not factor in stenosis location. A similar tailored approach to TRAS management recently reported that in TRAS-P lesions, the use of DES as compared to BMS was a predictor of future patency.15 In the same report, lesions less than 5 mm demonstrated a trend toward improved patency when stented with DES.15 Pertinent strengths of our study include all procedures being performed by a single interventional cardiologist and long duration of follow-up.
In summary, primary stenting with both DES and BMS was demonstrated to be a safe and effective therapy for TRAS. The cohort stented with DES has demonstrated long-term stability in their renal function and blood pressure control, adding to the sparse data available regarding the long-term use of DES in the management of TRAS. Furthermore, directed deployment of DES and BMS based on the artery size has the potential to offer fewer incidences of in-stent restenosis and subsequent procedures. Based on our experience, it is reasonable to conclude that primary stenting for TRAS is the approach of choice, and future randomized studies are needed to determine which vessels are most amendable to DES as compared with BMS.
ACKNOWLEDGMENTS
The expert secretarial assistance of Sherryl Krulik is gratefully acknowledged. The authors thank Carrie Lindower for her assistance with data retrieval.
Footnotes
Published online 17 January, 2017.
Funding: Dialysis Clinics Incorporated.
The authors declare no conflicts of interest.
C.C.E. participated in the acquisition of data, analysis and interpretation of data, drafting of the article, and critical revision. M.M. participated in the acquisition of data, analysis and interpretation of data, and drafting of the article. F.D. participated in the study conception and design, analysis and interpretation of data, and critical revision. H.S. participated in the study conception and design, analysis and interpretation of data, and critical revision. M.T.A. participated in the acquisition of data, analysis and interpretation of data, drafting of the article, critical revision. A.M. participated in the study conception and design, acquisition of data, and critical revision. E.P.N. participated in the study conception and design, analysis and interpretation of data, drafting of the article, and critical revision.
REFERENCES
- 1.Srivastava A, Kumar J, Sharma S, et al. Vascular complication in live related renal transplant: an experience of 1945 cases. Indian J Urol. 2013;29:42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bessede T, Droupy S, Hammoudi Y, et al. Surgical prevention and management of vascular complications of kidney transplantation. Transpl Int. 2012;25:994–1001. [DOI] [PubMed] [Google Scholar]
- 3.Bruno S, Remuzzi G, Ruggenenti P. Transplant renal artery stenosis. J Am Soc Nephrol. 2004;15:134–141. [DOI] [PubMed] [Google Scholar]
- 4.Willicombe M, Sandhu B, Brookes P, et al. Postanastomotic transplant renal artery stenosis: association with de novo class II donor-specific antibodies. Am J Transplant. 2014;14:133–143. [DOI] [PubMed] [Google Scholar]
- 5.Audard V, Matignon M, Hemery F, et al. Risk factors and long-term outcome of transplant renal artery stenosis in adult recipients after treatment by percutaneous transluminal angioplasty. Am J Transplant. 2006;6:95–99. [DOI] [PubMed] [Google Scholar]
- 6.Su CH, Lian JD, Chang HR, et al. Long-term outcomes of patients treated with primary stenting for transplant renal artery stenosis: a 10-year case cohort study. World J Surg. 2012;36:222–228. [DOI] [PubMed] [Google Scholar]
- 7.Kamali K, Abbasi MA, Ani A, et al. Renal transplantation in allografts with multiple versus single renal arteries. Saudi J Kidney Dis Transpl. 2012;23:246–250. [PubMed] [Google Scholar]
- 8.Benedetti E, Troppmann C, Gillingham K, et al. Short- and long-term outcomes of kidney transplants with multiple renal arteries. Ann Surg. 1995;221:406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamali K, Abbasi MA, Behzadi AH, et al. Incidence and risk factors of transplant renal artery stenosis in living unrelated donor renal transplantation. J Ren Care. 2010;36:149–152. [DOI] [PubMed] [Google Scholar]
- 10.Marini M, Fernandez-Rivera C, Cao I, et al. Treatment of transplant renal artery stenosis by percutaneous transluminal angioplasty and/or stenting: study in 63 patients in a single institution. Transplant Proc. 2011;43:2205–2207. [DOI] [PubMed] [Google Scholar]
- 11.Douis H, Shabir S, Lipkin G, et al. Drug-eluting stent insertion in the treatment of in-stent renal artery restenosis in three renal transplant recipients. J Vasc Interv Radiol. 2008;19:1757–1760. [DOI] [PubMed] [Google Scholar]
- 12.Abate MT, Kaur J, Suh H, et al. The use of drug-eluting stents in the management of transplant renal artery stenosis. Am J Transplant. 2011;11:2235–2241. [DOI] [PubMed] [Google Scholar]
- 13.Shammas NW, Kapalis MJ, Dippel EJ, et al. Clinical and angiographic predictors of restenosis following renal artery stenting. J Invasive Cardiol. 2004;16:10–13. [PubMed] [Google Scholar]
- 14.Biederman DM, Fischman AM, Titano JJ, et al. Tailoring the endovascular management of transplant renal artery stenosis. Am J Transplant. 2015;15:1039–1049. [DOI] [PubMed] [Google Scholar]
- 15.Lederman RJ, Mendelsohn FO, Santos R, et al. Primary renal artery stenting: characteristics and outcomes after 363 procedures. Am Heart J. 2001;142:314–323. [DOI] [PubMed] [Google Scholar]
- 16.Scientific Registry of Transplant Recipients.
- 17.Saidi RF, Elias N, Kawai T, et al. Outcome of kidney transplantation using expanded criteria donors and donation after cardiac death kidneys: realities and costs. Am J Transplant. 2007;7:2769–2774. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann R, Mintz GS. Coronary in-stent restenosis—predictors, treatment and prevention. Eur Heart J. 2000;21:1739–1749. [DOI] [PubMed] [Google Scholar]
- 19.Osório Gomes V, Blaya P, Lasevitch R, et al. Impact of chronic kidney disease on the efficacy of drug-eluting stents: long-term follow-up study. Arq Bras Cardiol. 2011;96:346–351. [DOI] [PubMed] [Google Scholar]
- 20.Wanitschek M, Pfisterer M, Hvelplund A, et al. Long-term benefits and risks of drug-eluting compared to bare-metal stents in patients with versus without chronic kidney disease. Int J Cardiol. 2013;168:2381–2388. [DOI] [PubMed] [Google Scholar]


