Abstract
Background
Besides ‘definitive rejection’, the Banff classification includes categories for ‘suspicious for rejection’ phenotypes. The aim of this study was to determine the frequency and phenotypes of rejection episodes in 316 consecutive renal transplants from 2009 to 2014 grouped into patients without/with pretransplant HLA-DSA (ptDSAneg, n = 251; ptDSApos, n = 65).
Methods
All adequate indication (n = 125) and surveillance biopsies (n = 538) performed within the first year posttransplant were classified according to the current Banff criteria.
Results
‘Suspicious for rejection’ phenotypes were 3 times more common than ‘definitive rejection’ phenotypes in biopsies from ptDSAneg patients (35% vs 11%) and equally common in biopsies from ptDSApos patients (25% vs 27%). In both groups, ‘suspicious for rejection’ phenotypes were more frequent in surveillance than in indication biopsies (28% vs 16% in ptDSAneg patients, and 37% vs 29% in ptDSApos patients). ‘Borderline changes: ‘Suspicious' for acute T-cell mediated rejection’ (91%) were the dominant ‘suspicious for rejection’ phenotype in ptDSAneg patients, whereas ‘borderline changes’ (58%) and ‘suspicious for acute/active antibody-mediated rejection’ (42%) were equally frequent in biopsies from ptDSApos patients. Inclusion of ‘suspicious for rejection’ phenotypes increased the 1-year incidence of clinical (ptDSAneg patients: 18% vs 8%, P = 0.0005; ptDSApos patients: 24% vs 18%, P = 0.31) and (sub)clinical rejection (ptDSAneg patients: 59% vs 22%, P < 0.0001; ptDSApos patients: 68% vs 40%, P = 0.004).
Conclusions
‘Suspicious for rejection’ phenotypes are very common in the current era and outnumber the frequency of ‘definitive rejection’ within the first year posttransplant.
As a standardized classification system, the Banff criteria for renal allograft rejection have gained wide acceptance among pathologists and provide a helpful tool in therapeutic decision making.1,2 Since the first conference in 1991, the Banff classification is continuously evolving and incorporating new diagnostic insights and emerging data.2-9 Besides criteria for ‘definitive rejection,’ the current classification also includes criteria for limited forms, coined as ‘borderline changes: ‘Suspicious’ for acute T-cell mediated rejection (TCMR) or briefly ‘borderline changes’ and ‘suspicious for acute/active antibody-mediated rejection (ABMR).’ Although ‘borderline changes’ considered to be ‘suspicious’ for acute TCMR were already anchored in the classification at the first Banff meeting, the term ‘suspicious for acute/active ABMR’ has first been introduced in 2001, in the context of definition of acute and chronic ABMR.9
Using current tacrolimus (Tac)-based immunosuppression the reported 1-year incidence of clinical and subclinical rejection is around 7% to 12% and 3% to 9%, respectively,10-13 suggesting that rejection is well controlled. However, this contrasts with the observation that rejection still accounts for the majority of allograft losses.14-16 One could argue that allograft loss due to rejection mainly results from nonadherence and minimization of immunosuppression beyond the first year posttransplant.15,17 Another possibility is that we miss a substantial proportion of early ongoing rejection processes due to diagnostic and/or interpretation problems, which will culminate in chronic irreversible allograft damage on the long-term.17-20
We hypothesized that current immunosuppressive strategies and improved risk stratification have led to a shift towards more limited forms of rejection phenotypes. Because the clinical significance of ‘suspicious for rejection’ phenotypes is still a matter of debate, the transplant community may report rejection frequencies with or without inclusion of these phenotypes.1,21-25 To the best of our knowledge, a precise analysis of the rejection phenotype distribution in the current era has not been performed so far. Therefore, the aim of this study was to investigate in detail rejection phenotypes observed within the first year posttransplant in an unselected patient population treated with current Tac-based immunosuppression and risk-stratified by the presence/absence of donor-specific HLA antibodies (HLA-DSA).
MATERIALS AND METHODS
Patient Population and Allograft Biopsy Selection
This retrospective single-center study in a Caucasian population was approved by the ethics committee of Northwestern and Central Switzerland (www.eknz.ch).
The study flowchart is illustrated in Figure 1. In total, 372 kidney transplantations performed between January 1, 2009, and December 31, 2014, at the University Hospital Basel were evaluated for study inclusion. Of those, 45 transplantations from ABO-incompatible living donors were excluded due to possible misclassification as a result of almost universal C4d positivity in peritubular capillaries. In addition, we excluded transplantations considered to comprise an immunological risk without detectable pretransplant HLA-DSA (n = 11; mainly husband-to-wife transplantations with shared children). Thus, 316 transplantations were enrolled in the study. These transplantations belonged to 2 distinct risk groups based on the presence/absence of pretransplant HLA-DSA detected by Luminex single HLA-antigen beads (LabScreen SA; One Lambda, Inc., Canoga Park, CA) with mean fluorescence intensity (MFI) greater than 50026-28: (i) patients without pretransplant HLA-DSA (ptDSAneg; n = 251), and (ii) patients with pretransplant HLA-DSA (ptDSApos; n = 65).
FIGURE 1.
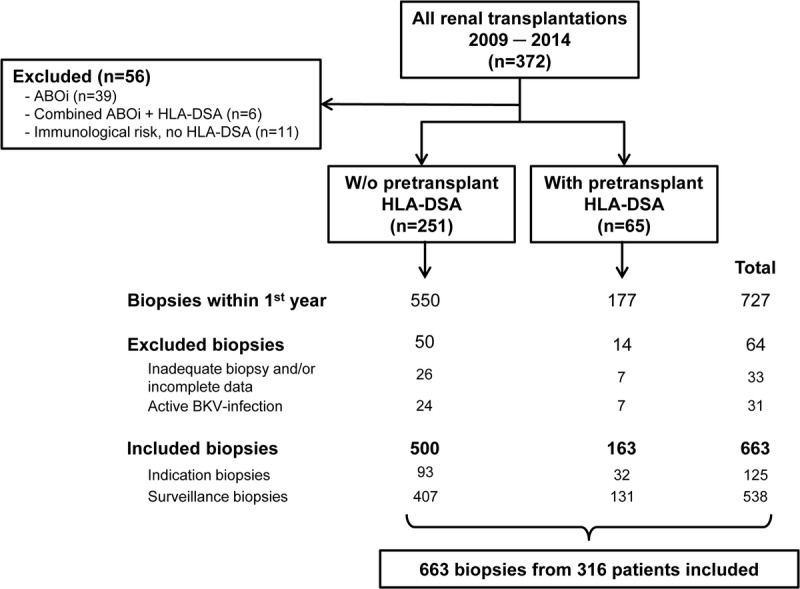
Study flowchart.
In total, 727 biopsies were performed in these 316 transplantations within the first year posttransplant. Of those, 33 inadequate biopsies or biopsies with incomplete datasets were excluded. To prevent misclassification of biopsies showing tubulointerstitial inflammation in the context of active polyomavirus BK infection, we further excluded 31 biopsies showing either definitive polyomavirus-associated nephropathy (n = 14) or presumptive/resolving polyomavirus-associated nephropathy (n = 17).29,30
Finally, 663 biopsies (125 indication and 538 surveillance biopsies) were included in the study: 500 from ptDSAneg and 163 from ptDSApos patients. Indication biopsies were performed in cases of inadequate graft function following transplantation, if serum creatinine increased more than 20% from baseline, or in cases of increasing proteinuria or glomerular hematuria. Surveillance biopsies were routinely done at 3 and 6 months posttransplant. ptDSApos patients underwent an additional surveillance biopsy at month 12 posttransplant as well as an optional biopsy on day 7 posttransplant.
Immunosuppression
As reported previously, ptDSAneg patients were mostly treated with basiliximab induction and maintenance immunosuppression with Tac, mycophenolate mofetil (MMF) or mycophenolate sodium (Myf), and prednisone (P). If no clinical or subclinical rejection occurred, the maintenance immunosuppression was reduced to a dual therapy consisting of Tac-MMF/Myf. ptDSApos patients were mostly treated with an induction regimen consisting of antithymocyte globulin (ATG) (either ATG-Fresenius or Thymoglobulin) ± intravenous immunoglobulins (IvIg), and received a maintenance immunosuppression with Tac-MMF/Myf-P, which was continued indefinite.26,28,31
Histopathology
For specimen sampling, ultrasound-guided biopsies using a 16-gauge needle were performed. Samples routinely comprised 2 cores to obtain a sufficient amount of glomeruli and to minimize sampling error. Histological workup followed standard procedures and included light microscopy and immunofluorescence. Electron microscopy was done if necessary for diagnosis on an individual basis. C4d staining was performed by immunofluorescence on frozen sections. Positivity of the peritubular capillaries was graded from 0 to 3. Grades 2 (=focal) and 3 (=diffuse) were classified as C4d positive. All indication and surveillance biopsies were scored according to the Banff criteria3-7 and were mostly evaluated by the same pathologist (H.H.). Noteworthy, as a result of the local policy at our pathology department, the definition of the cg score relied on the Banff 1997 classification.
Assignment as Rejection and Definition of Rejection Phenotypes
The individual parameters required to assign the diagnosis of TCMR (ie, Banff scores i, t, and v) or ABMR (ie, Banff scores g, cg, ptc, v, t, and C4d, as well as presence of HLA-DSA) were analyzed electronically using a script, which calculated the TCMR and ABMR phenotypes following precisely the current Banff classification rules.3,5
For TCMR diagnosis, biopsy results were grouped as (i) no TCMR, (ii) borderline changes, (iii) TCMR IA, (iv) TCMR IB, (v) TCMR IIA, (vi) TCMR IIB, and (vii) TCMR III. For ABMR diagnosis, the categories assigned were (i) no ABMR; (ii) suspicious for acute/active ABMR; (iii) acute/active ABMR; (iv) chronic, active ABMR; and (v) C4d staining without evidence of rejection. Biopsies fulfilling criteria of both TCMR (any type including ‘borderline changes’) and ABMR (any type including ‘suspicious for acute/active ABMR’ and ‘C4d staining without evidence of rejection’) were classified as mixed rejection.
Determination of HLA-DSA at the Time Point of Allograft Biopsies
The presence of HLA-DSA is 1 of 3 features required to diagnose ABMR.3 In all ptDSApos transplantations, we assumed HLA-DSA to persist at the time of the biopsy and did not repeat the analysis. In ptDSAneg transplantations, sera obtained at the time of biopsy were evaluated for circulating de novo HLA-DSA, when biopsies showed minimal features of ABMR such as g > 0 or ptc > 0 or C4d ≥ 2 (≥ focal positivity). All HLA antibody analyses were performed with Luminex SA applying a MFI > 500 cutoff and included the determination of circulating de novo HLA-DSA in all loci (ie, A/B/C/DRB1/DRB3-5/DQ/DP). The mentioned minimal ABMR features were detected in 48 (9.6%) of 500 biopsies or 36 (14.3%) of 251 patients. Unfortunately, we had no available biopsy serum from 5 (10.4%) of 48 biopsies. HLA-antibody testing by Luminex SA revealed de novo HLA-DSA in only 6 (14%) of 43 biopsy sera and 5 (13.9%) of 36 patients, respectively.
Statistical Analyses
Data were analyzed using JMP Version 12 software (SAS institute Inc., Cary, NC). Categorical data are presented as counts and/or percentages and were analyzed by Pearson χ2 test. Continuous data are shown as median and interquartile ranges (IQR) and compared by Wilcoxon rank sum tests. The Kaplan-Meier method was used to generate the time-to-rejection curves and the groups compared using the log-rank test. For all statistical tests, a 2-tailed P value less than 0.05 was considered to indicate statistical significance.
RESULTS
Baseline Characteristics of the Study Population
Table 1 shows the baseline characteristics of the 316 transplantations, stratified by the presence/absence of pretransplant HLA-DSA. The median age of the patients was 56 years. As expected, ptDSApos patients had significantly more sensitizing events compared with ptDSAneg patients (85% vs 42%; P < 0.0001); in particular, they had more previous transplantations (49% vs 10%; P < 0.0001). Among ptDSApos patients, 62% had 1 HLA-DSA, whereas 38% had 2 or more. The median cumulative MFI was 1880 (896-6237).
TABLE 1.
Patient characteristics
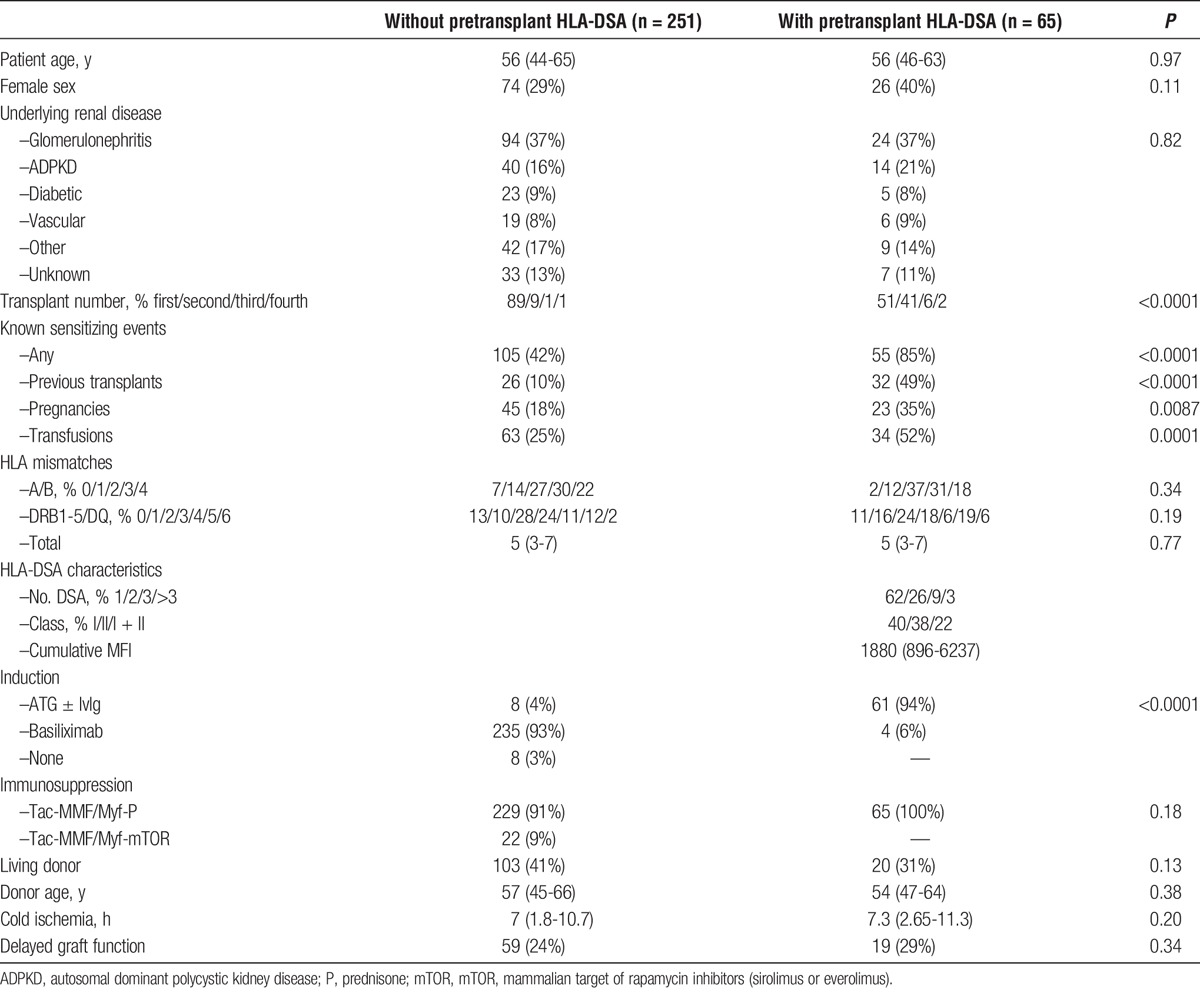
Ninety-four percent of ptDSApos patients received ATG ± IvIg as induction therapy. The remaining 6% were initially treated with basiliximab, but switched to the ATG ± IvIg regimen when HLA-DSA assignment was completed (mostly missing C- and DP-typing). In contrast, the majority (93%) of ptDSAneg patients received basiliximab as an induction therapy. Only 8 (4%) patients in this group were initially treated with ATG, mostly as part of a calcineurin inhibitor sparing protocol in donors with an extended warm or cold ischemia time.
Overview of Rejection Phenotypes
Figure 2 details the rejection phenotype distribution in the 663 investigated allograft biopsies, stratified by the presence/absence of pretransplant HLA-DSA and the reason for allograft biopsy (ie, indication vs surveillance). For this overview analysis, we summarized TCMR IA, IB, IIA, IIB, and III into 1 group called ‘TCMR’. In addition, we summarized acute/active ABMR and chronic, active ABMR into 1 group called ‘acute/chronic active ABMR’. This allows for a better distinction between those rejection phenotypes that fulfill the criteria for ‘definitive rejection’ according to the Banff classification (ie, group ‘TCMR’ and group ‘acute/chronic active ABMR’) and those that are currently regarded as ‘suspicious for rejection’ (ie, ‘borderline changes’ as well as ‘suspicious for acute/active ABMR’ and ‘C4d staining without evidence of rejection’).
FIGURE 2.
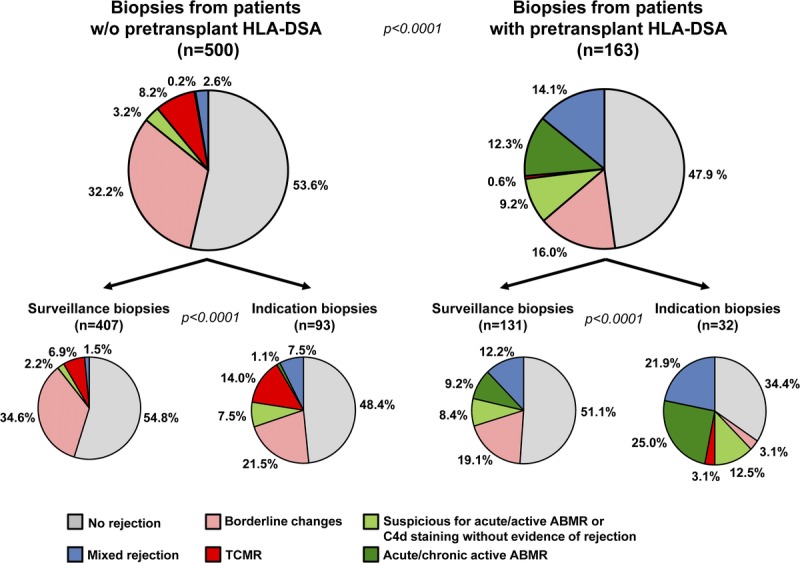
Overview of rejection phenotypes. The distribution of rejection phenotypes is divided into biopsies from ptDSAneg and ptDSApos patients and further subdivided into surveillance and indication biopsies.
Median estimated GFR using the Modification of Diet in Renal Disease equation revealed no significant differences between the 6 rejection phenotypes in both indication (range, 11-24 mL/min per 1.73 m2, P = 0.42) and surveillance biopsies (range, 41-51 mL/min per 1.73 m2, P = 0.31). ‘Suspicious for’ and ‘definitive rejection’ TCMR groups showed very similar allograft function (indication biopsies, 22 and 24 mL/min per 1.73 m2; surveillance biopsies, 50 and 50 mL/min per 1.73 m2, respectively).
The distribution of the 6 rejection phenotype groups was significantly different between ptDSAneg and ptDSApos patients, as well as between indication and surveillance biopsies within these 2 groups (all P < 0.0001) (Figure 2). Biopsies from ptDSApos patients showed a much higher frequency of ABMR and mixed rejection phenotypes than biopsies from ptDSAneg patients (36% vs 6%), whereas the overall rate of any rejection was similar (52% vs 46%). Indication biopsies had generally more ‘definitive rejection’ phenotypes compared to surveillance biopsies.
Most strikingly, ‘suspicious for rejection’ phenotypes were 3 times more common than ‘definitive rejection’ phenotypes in ptDSAneg patients (35% vs 11%) and equally common in ptDSApos patients (25% vs 27%). In both patient groups, ‘suspicious for rejection’ phenotypes were more frequent in surveillance than in indication biopsies (37% vs 29% in ptDSAneg and 28% vs 16% in ptDSApos patients, respectively). The dominant ‘suspicious for rejection’ phenotype in ptDSAneg patients were ‘borderline changes’, accounting for 91% of the ‘suspicious for rejection’ phenotypes. In ptDSApos patients, ‘borderline changes’ accounted for 58% and 42% were of the ‘suspicious for acute/active ABMR’ or ‘C4d staining without evidence of rejection’ phenotype.
Mixed rejection phenotypes accounted for 14% of biopsies from ptDSApos patients, whereas these phenotypes were only observed in 3% of biopsies from ptDSAneg patients. In both groups, mixed rejection phenotypes were more frequent in indication than in surveillance biopsies (ptDSAneg, 8% vs 2%; ptDSApos, 22% vs 12%).
Grid View of Individual Banff Lesion Scores Used for the Diagnosis of TCMR and ABMR
To analyze the distribution of the individual Banff lesion scores in more detail, we created grids consisting of those lesion scores, which contribute to the diagnosis of TCMR (i, t, and v) and ABMR (g, ptc, v), respectively. For ABMR, the parameter “C4d” was incorporated by the shape of the individual data points. The grids are separately shown for ptDSAneg patients (Figure 3A) and ptDSApos patients (Figure 3B).
FIGURE 3.
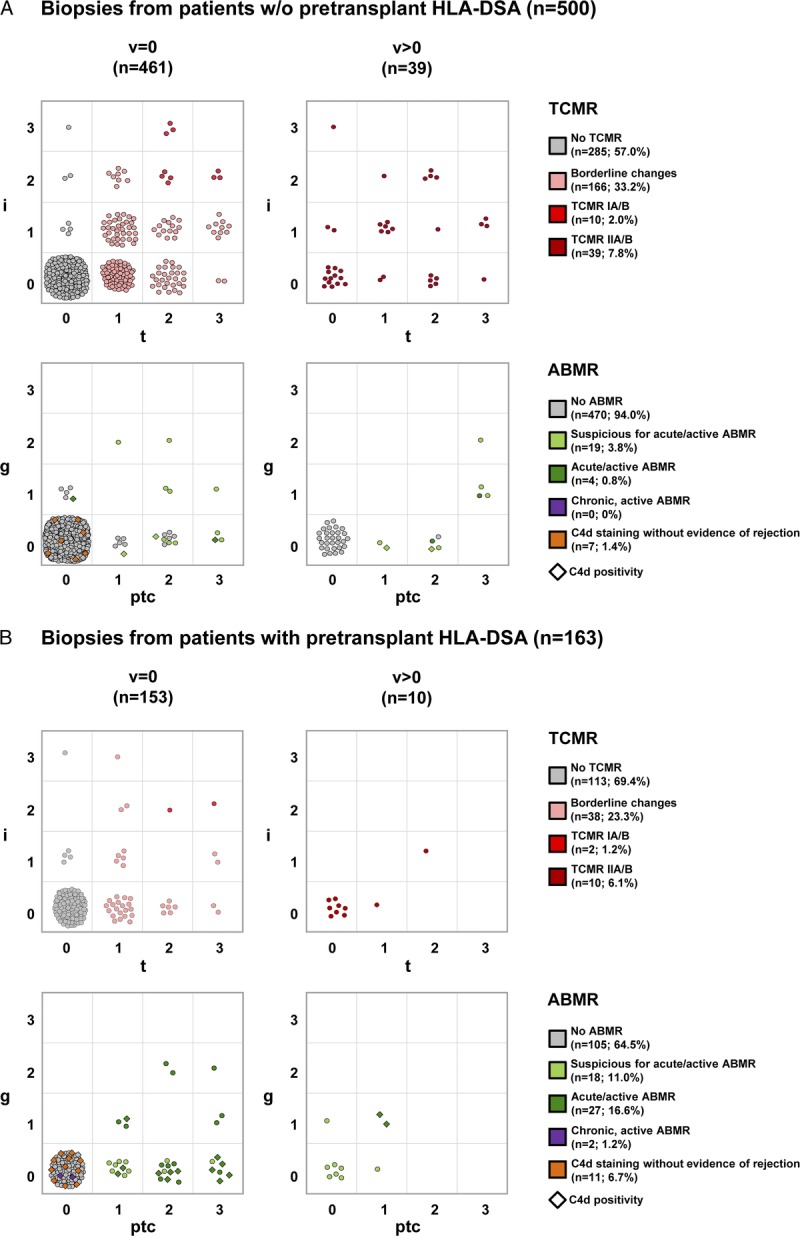
Grid view of individual Banff lesion scores used for the diagnosis of TCMR and ABMR. A, Biopsies from ptDSAneg patients; (B) biopsies from ptDSApos patients. For TCMR, the grid view includes the i- and t-scores, stratified by the v-score. For ABMR, the grid view includes the g- and ptc-scores, stratified by the v-score. C4d staining results are incorporated in the shape of the individual data points (◊, C4d positivity) in the ABMR section. The assigned diagnostic category of TCMR and ABMR derived from the individual Banff lesion scores including the parameter HLA-DSA are marked with different colors and are given in the figure legend as count and percentage.
In biopsies from ptDSAneg patients, any lesion leading to classification as TCMR was found in 215 (43%) of 500 biopsies. Of those 215 TCMR classifications, 77% were assigned as ‘borderline changes’, 5% as TCMR IA/B, and 18% as TCMR IIA/B. No TCMR III rejection was observed. Within the ‘borderline changes’ category, the most frequent i-t combinations were i0t1 (n = 68; 41%), i1t1 (n = 36; 22%), and i0t2 (n = 28; 17%). Among the TCMR IIA/B lesions, one third (33%) showed only a positive v-score without tubulointerstitial inflammation. Only 30 (6%) of 500 biopsies demonstrated any lesions leading to classification as ABMR. Of those 30 ABMR classifications, 64% were assigned as ‘suspicious for acute/active ABMR’, 23% as ‘C4d staining without evidence of rejection’, and only 13% as acute/active ABMR. The reason for the rare assignment as acute/active ABMR was mainly missing detection of circulating HLA-DSA. Interestingly, 15 (71%) of 21 biopsies classified as ‘suspicious for acute/active ABMR’ were C4d negative. In these biopsies lacking C4d positivity, the extent of microvascular inflammation (g + ptc ≥ 2) was sufficient to fulfill the feature of evidence of recent antibody interaction with vascular endothelium according to the Banff ABMR criteria. Therefore, moderate microvascular inflammation (g + ptc ≥ 2) alone resulted in classification as ‘suspicious for acute/active ABMR’ in those biopsies.
In biopsies from ptDSApos patients, we found very similar results regarding TCMR frequency and TCMR phenotype distribution as compared with ptDSAneg patients. By contrast, ABMR phenotypes were much more frequent in biopsies from ptDSApos patients (36% vs 6%). Acute/active ABMR was the most common phenotype (47%), followed by ‘suspicious for acute/active ABMR’ (31%), ‘C4d staining without evidence of rejection’ (19%), and chronic, active ABMR (3%). Notably, thrombotic microangiopathy contributing to ABMR diagnosis according to the Banff classification was found in only 1 biopsy.
Details of Mixed Rejection Phenotypes
As mentioned in the Materials and Methods, biopsies fulfilling any diagnosis of TCMR (including ‘borderline changes’) and ABMR (including ‘suspicious for acute/active ABMR’) were classified as mixed rejection. The composition of these mixed phenotypes consisting of TCMR and ABMR phenotypes is shown in Figure 4.
FIGURE 4.
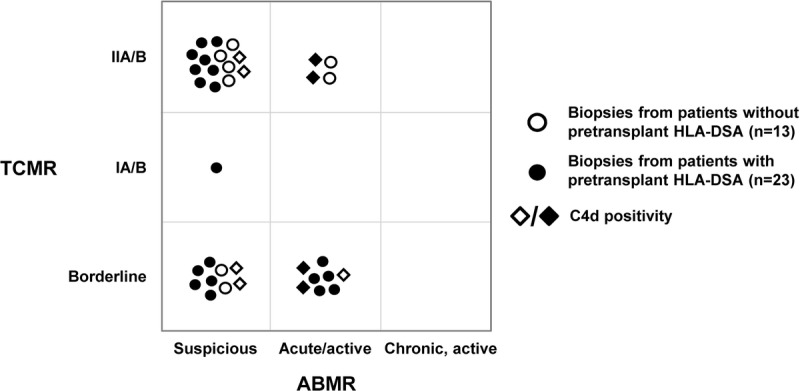
Overview of mixed rejection phenotypes. The composition of mixed rejection phenotypes consisting of the assigned diagnostic categories of both TCMR and ABMR is shown for biopsies from ptDSAneg (loops) and ptDSApos (spots) patients. C4d staining results are incorporated in the shape of the individual data points (◊, C4d positivity).
Overall, mixed rejection phenotypes were 5 times less frequent in biopsies from ptDSAneg patients (13/500; 3%) compared with biopsies from ptDSApos patients (23/163; 14%). In both groups, ‘borderline changes’ and/or ‘suspicious for acute/active ABMR’ phenotypes were observed in 11 (85%) of 13 biopsies and 21 (91%) of 23 biopsies, respectively. Four and 5 different TCMR/ABMR phenotype combinations were noticed in the 2 groups. The most frequent combination in both groups was TCMR IIA/B together with ‘suspicious for acute/active ABMR’ (46% and 35%, respectively).
One-Year Incidence of Clinical and (Sub)Clinical Rejection
Figure 5 illustrates how the inclusion/exclusion of ‘suspicious for rejection’ phenotypes (ie, ‘borderline changes’, ‘suspicious for acute/active ABMR’, and ‘C4d staining without evidence of rejection’) affects the 1-year incidence of clinical and (sub)clinical rejection. In ptDSAneg patients, the 1-year incidence of clinical rejection significantly dropped from 18% to 8% when ‘suspicious for rejection’ phenotypes were excluded (P = 0.0005). Similarly, the 1-year incidence of (sub)clinical rejection was significantly lower (59% vs 22%; P < 0.0001). In ptDSApos patients, the 1-year incidence of clinical rejection dropped from 24% to 18% (P = 0.31) and for (sub)clinical rejection from 68% to 40% (P = 0.004) when ‘suspicious for rejection’ phenotypes were excluded (P = 0.31).
FIGURE 5.
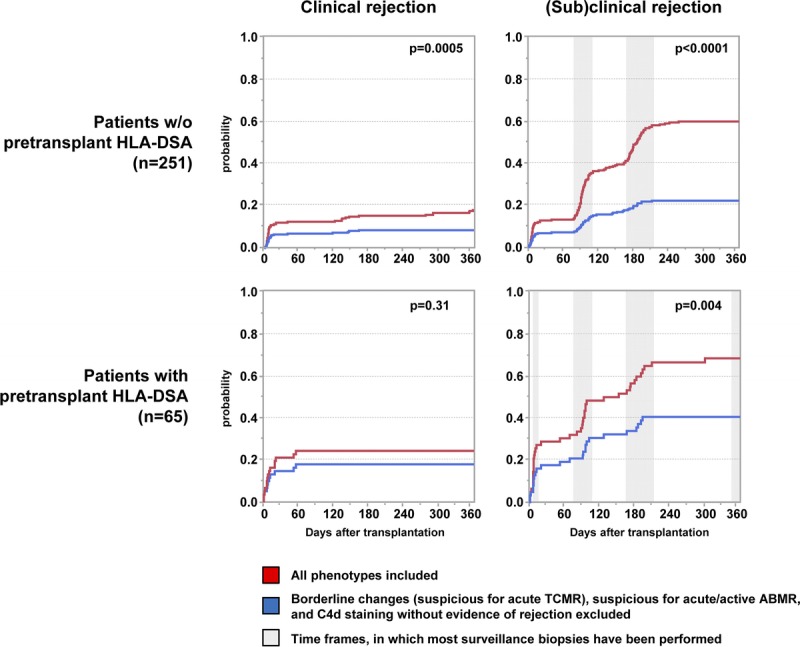
One-year incidence of clinical and (sub)clinical rejection, divided into ptDSAneg and ptDSApos patients. The grey-shaded areas in the (sub)clinical rejection boxes approximate the windows, in which most surveillance biopsies have been performed.
DISCUSSION
The principal finding of this study is that ‘borderline changes’ considered to be ‘suspicious’ for acute TCMR and ‘suspicious for acute/active ABMR’ phenotypes are very common in the current era of immunosuppression and risk stratification and outnumber the frequency of ‘definitive rejection’ phenotypes. This observation was evident in ptDSAneg as well as ptDSApos patients and significantly influenced the 1-year incidence of both clinical and (sub)clinical rejection. We relate this considerable shift towards these limited forms of rejection to an improved risk stratification and more potent maintenance immunosuppression, attenuating lymphocytic infiltration into the graft.
In our study, every third biopsy investigated showed ‘borderline changes’, Among the biopsies showing any TCMR lesion, ‘borderline changes’ accounted for 77% and 76% among ptDSAneg and ptDSApos patients, respectively. The majority of these phenotypes classified as ‘borderline changes’ contained only mild tubulitis ± interstitial inflammation in less than 25% of the parenchyma (i-t score i0t1 and i1t1). Notably, TCMR type IA/IB lesions were rare events and accounted for only 5% or less.
The distinction between ‘definitive’ and ‘borderline changes’ relies on arbitrary cutoffs. Noteworthy, the pathology of rejection encompasses a continuum ranging from a focal finding with only very few involved tubules with mild tubulitis to a diffuse process containing multiple tubules with moderate to severe tubulitis. Based on the findings of our study, the question arises whether the current category of ‘borderline changes: ‘Suspicious’ for acute TCMR’ accurately reflects the biological process of rejection. The discussion related to this issue faces several challenges. First, as recently reported by Becker et al,1 the definition of ‘borderline changes’ is not homogenously used among pathologists. As demonstrated by the results of their survey, two thirds of the nephropathologists as well as the majority of the most influential manuscripts applied the Banff 1997 definition instead of the revised classification of 2005. To highlight this problem, use of the Banff 1997 definition (minimal i-score i1 needed to diagnose ‘borderline changes’) would have reduced the frequency of ‘borderline changes’ in ptDSAneg patients from 33% to 20%. Therefore, a widely accepted and uniformly used definition of ‘borderline changes’ is needed. Second, the definition of TCMR should ideally be based on pertinent clinical outcomes. Although several studies indicate that subclinical inflammation precedes chronic injury and is associated with deteriorating graft function,12,25,32-35 there are no data on whether treatment of ‘borderline changes’ reduces chronic lesions in the allograft and improves long-term allograft survival. As histopathology provides a static assessment of the extent of allograft inflammation/injury, additional parameters reflecting the composition, activity and dynamics of infiltrating cells, for example by molecular phenotyping or urinary chemokines, might be helpful to define the threshold for ‘definitive TCMR’ beyond the currently used i- and t-scores.15,19,24,36-40
Although TCMR Type IA/B lesions were rare in our study, Type IIA/B rejections, defined by a positive v-score, were the most frequent phenotypes leading to classification as TCMR. TCMR Type IIA/B lesions were observed in 7.8% and 6.1% of biopsies from ptDSAneg and ptDSApos patients, respectively. The observed frequency is consistent with the study of Salazar et al41 investigating 703 indication biopsies. This finding allows for 2 possible explanations. First, it might be possible that current immunosuppression is able to prevent severe tubulointerstitial inflammation, but has only limited capacity to control vascular lesions. Indeed, this could explain why one-third of biopsies classified as TCMR IIA/B showed no tubulointerstitial inflammation. Another possible explanation is related to the overlapping scoring of vascular lesions within the current Banff classification, as arteritis can occur in the context of both TCMR and ABMR.3,42-44 Vascular lesions may lead to classification as TCMR IIA/B rejections according to Banff, but may indicate ABMR. Indeed, among the mixed rejection phenotypes in our study, TCMR IIA/B together with ‘suspicious for acute/active ABMR’ was the most frequent combination in both groups (46% and 35%, respectively). These phenotypes might in fact mainly mirror antibody-mediated processes.43
There have been considerable changes in the classification of ABMR since 2001, although the basic components for diagnosis of acute/active and chronic, active ABMR remained the same: Morphological evidence of tissue injury, evidence of antibody interaction with vascular endothelium and serologic evidence of DSA.3,5-7,9 Among ptDSAneg patients whose biopsies were classified as ‘suspicious for acute/active ABMR’, the lacking feature to diagnose ‘definitive ABMR’ was the serologic evidence of DSA. Although we evaluated all biopsies showing minimal features of ABMR (ie, g > 0 or ptc > 0 or C4d ≥ focal positive) for circulating de novo HLA-DSA by the most sensitive Luminex SA assays, they were only found in 13.9% of those biopsies. This corresponds to an estimated de novo HLA-DSA frequency of 2% within the first year posttransplant, which is completely in line with Wiebe et al.17 We cannot explain this low association of de novo HLA-DSA with the observed histological ABMR features. Potential reasons are (i) these cases of ABMR are mainly caused by non-HLA-DSA,45-47 (ii) circulating de novo HLA-DSA were not detected due to absorption in the graft, and (iii) glomerulitis and/or peritubular capillaritis were not indicative of ABMR.
Based on the low frequency of de novo HLA-DSA within the first year after transplantation, diagnosis of ABMR in ptDSAneg patients mainly relied on histologic features as well as on complement C4d deposition. If present, C4d positivity is considered to be very specific for ABMR48,49 and has been associated with an adverse outcome.50 In our study, 71% and 48% of biopsies, classified as ‘suspicious for acute/active ABMR’ and acute/active ABMR, were C4d negative, but showed at least moderate microvascular inflammation. This is an interesting finding. However, it is important to keep in mind that C4d deposition in the graft is a dynamic process and requires target molecules in close proximity as well as a sufficient amount of DSA.44,51,52
Overall, the frequency of ABMR in ptDSApos patients was 5 times higher as compared with ptDSAneg patients despite augmented induction and maintenance therapy, which is consistent with a recent meta-analysis.53 Acute/active ABMR was diagnosed in the majority of cases (47%), followed by ‘suspicious for acute/active ABMR’ phenotypes. In our opinion, this finding truly reflects the real life situation. However, we have to admit that we did not repeat the circulating HLA antibody analysis at the time of biopsy posttransplant in these patients. This has likely led to an overestimation of ABMR and ‘suspicious for acute/active ABMR’ phenotypes.
Our study has several strengths. To the best of our knowledge, this is the first study investigating the Banff-defined rejection phenotype frequency in such detail in an unselected patient population. Second, as we routinely perform surveillance biopsies, we were able to calculate the incidence of both clinical and (sub)clinical rejection within the first year posttransplant. Third, the use of 16-gauge needles for biopsies and immunofluorescence for C4d detection reduces sampling error and increases sensitivity for accurate diagnosis of rejection.54 Fourth, the assignment of the Banff diagnosis was performed by a computer-based calculation allowing us to strictly follow the Banff classification rules. Fifth, the vast majority of biopsies were evaluated by the same pathologist minimizing interobserver variability.
Some limitations apply to our study. This is a single-center study and the observed rejection phenotypes and frequencies are related to the used immunosuppressive regimens. For ptDSAneg patients, we use a steroid withdrawal concept, which might lead to a higher rejection frequency compared with protocols using indefinite triple therapy with 5 to 7.5 mg prednisone per day. As the study is restricted to biopsies within the first year posttransplant, we are not able to make statements regarding the frequency of ‘suspicious for rejection’ phenotypes on the long-term. Furthermore, because we did not apply the Banff 2013 definition for the cg score, the frequency of chronic, active ABMR within the first year posttransplant might be higher than reported (ie, 2/663 biopsies [0.3%]). Although our aim was to describe the detailed frequency of rejection phenotypes, correlation of our findings with hard end points would have been of interest. However, the restricted follow-up time (median, 3.9 years) and the low number of graft losses (6%) do not allow for this correlation in a meaningful way.
In conclusion, the results of our study pinpoint how recent advances in both immunosuppressive regimens and risk stratification have dramatically changed rejection phenotypes to more limited or so-called ‘suspicious for rejection’ forms. Inclusion and exclusion of these phenotypes critically impacts the reported rejection frequency. Our findings emphasize the need to keep the discussion on definition of rejection open and to continuously question and potentially adapt current classification systems.42 Further research is required to investigate the clinical significance of these emerging and presumably low-grade rejection lesions.
ACKNOWLEDGMENTS
The authors thank the members of the HLA laboratory for performance of the additional Luminex analyses.
Footnotes
Published online 9 February, 2017.
The authors declare no funding or conflicts of interest.
H.H., S.S. contributed equally as senior authors.
C.W. designed and performed the research, analyzed the data and wrote the article. P.A., P.H.-M., A.G., G.H., F.B., J.S., and M.D. performed the research and wrote the article. T.M. and M.M. performed histopathology and wrote the article. H.H. designed the research, performed histopathology, analyzed the data and wrote the article. S.S. designed the research, analyzed the data and wrote the article.
REFERENCES
- 1.Becker JU, Chang A, Nickeleit V, et al. Banff borderline changes suspicious for acute T-cell mediated rejection: where do we stand? Am J Transplant. 2016;16:2654–2660. [DOI] [PubMed] [Google Scholar]
- 2.Mengel M, Sis B, Halloran PF. SWOT analysis of Banff: strengths, weaknesses, opportunities and threats of the international Banff consensus process and classification system for renal allograft pathology. Am J Transplant. 2007;7:2221–2226. [DOI] [PubMed] [Google Scholar]
- 3.Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14:272–283. [DOI] [PubMed] [Google Scholar]
- 4.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. [DOI] [PubMed] [Google Scholar]
- 5.Sis B, Mengel M, Haas M, et al. Banff '09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10(3):464–471. [DOI] [PubMed] [Google Scholar]
- 6.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–760. [DOI] [PubMed] [Google Scholar]
- 7.Solez K, Colvin RB, Racusen LC, et al. Banff '05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy ('CAN'). Am J Transplant. 2007;7:518–526. [DOI] [PubMed] [Google Scholar]
- 8.Solez K, Racusen LC. The Banff classification revisited. Kidney Int. 2013;83:201–206. [DOI] [PubMed] [Google Scholar]
- 9.Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria—an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708–714. [DOI] [PubMed] [Google Scholar]
- 10.Gloor JM, Cohen AJ, Lager DJ, et al. Subclinical rejection in tacrolimus-treated renal transplant recipients. Transplantation. 2002;73:1965–1968. [DOI] [PubMed] [Google Scholar]
- 11.Rush D, Arlen D, Boucher A, et al. Lack of benefit of early protocol biopsies in renal transplant patients receiving TAC and MMF: a randomized study. Am J Transplant. 2007;7:2538–2545. [DOI] [PubMed] [Google Scholar]
- 12.Nankivell BJ, Borrows RJ, Fung CL, et al. Natural history, risk factors, and impact of subclinical rejection in kidney transplantation. Transplantation. 2004;78:242–249. [DOI] [PubMed] [Google Scholar]
- 13.Ekberg H, Tedesco-Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562–2575. [DOI] [PubMed] [Google Scholar]
- 14.El-Zoghby ZM, Stegall MD, Lager DJ, et al. Identifying specific causes of kidney allograft loss. Am J Transplant. 2009;9:527–535. [DOI] [PubMed] [Google Scholar]
- 15.Sellares J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012;12:388–399. [DOI] [PubMed] [Google Scholar]
- 16.Wehmeier C, Georgalis A, Hirt-Minkowski P, et al. 2222 kidney transplantations at the University Hospital Basel: a story of success and new challenges. Swiss Med Wkly. 2016;146:w14317. [DOI] [PubMed] [Google Scholar]
- 17.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Rates and determinants of progression to graft failure in kidney allograft recipients with de novo donor-specific antibody. Am J Transplant. 2015;15:2921–2930. [DOI] [PubMed] [Google Scholar]
- 18.Cosio FG, Grande JP, Wadei H, et al. Predicting subsequent decline in kidney allograft function from early surveillance biopsies. Am J Transplant. 2005;5:2464–2472. [DOI] [PubMed] [Google Scholar]
- 19.Modena BD, Kurian SM, Gaber LW, et al. Gene expression in biopsies of acute rejection and interstitial fibrosis/tubular atrophy reveals highly shared mechanisms that correlate with worse long-term outcomes. Am J Transplant. 2016;16:1982–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreso F, Carrera M, Goma M, et al. Early subclinical rejection as a risk factor for late chronic humoral rejection. Transplantation. 2012;93:41–46. [DOI] [PubMed] [Google Scholar]
- 21.Beimler J, Zeier M. Borderline rejection after renal transplantation—to treat or not to treat. Clin Transplant. 2009;23(Suppl 21):19–25. [DOI] [PubMed] [Google Scholar]
- 22.de Freitas DG, Sellarés J, Mengel M, et al. The nature of biopsies with “borderline rejection” and prospects for eliminating this category. Am J Transplant. 2012;12:191–201. [DOI] [PubMed] [Google Scholar]
- 23.Loupy A, Vernerey D, Tinel C, et al. Subclinical rejection phenotypes at 1 year post-transplant and outcome of kidney allografts. J Am Soc Nephrol. 2015;26:1721–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Németh D, Ovens J, Opelz G, et al. Does borderline kidney allograft rejection always require treatment? Transplantation. 2010;90:427–432. [DOI] [PubMed] [Google Scholar]
- 25.Thierry A, Thervet E, Vuiblet V, et al. Long-term impact of subclinical inflammation diagnosed by protocol biopsy one year after renal transplantation. Am J Transplant. 2011;11:2153–2161. [DOI] [PubMed] [Google Scholar]
- 26.Amico P, Hirt-Minkowski P, Hoenger G, et al. Risk stratification by the virtual crossmatch: a prospective study in 233 renal transplantations. Transpl Int. 2011;24:560–569. [DOI] [PubMed] [Google Scholar]
- 27.Amico P, Hoenger G, Mayr M, et al. Clinical relevance of pretransplant donor-specific HLA antibodies detected by single-antigen flow-beads. Transplantation. 2009;87:1681–1688. [DOI] [PubMed] [Google Scholar]
- 28.Baechler K, Amico P, Hoenger G, et al. Efficacy of induction therapy with ATG and intravenous immunoglobulins in patients with low-level donor-specific HLA-antibodies. Am J Transplant. 2010;10:1254–1262. [DOI] [PubMed] [Google Scholar]
- 29.Menter T, Mayr M, Schaub S, et al. Pathology of resolving polyomavirus-associated nephropathy. Am J Transplant. 2013;13:1474–1483. [DOI] [PubMed] [Google Scholar]
- 30.Schaub S, Hirsch HH, Dickenmann M, et al. Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy. Am J Transplant. 2010;10:2615–2623. [DOI] [PubMed] [Google Scholar]
- 31.Bielmann D, Hoenger G, Lutz D, et al. Pretransplant risk assessment in renal allograft recipients using virtual crossmatching. Am J Transplant. 2007;7:626–632. [DOI] [PubMed] [Google Scholar]
- 32.Min SI, Park YS, Ahn S, et al. Chronic allograft injury by subclinical borderline change: evidence from serial protocol biopsies in kidney transplantation. J Korean Surg Soc. 2012;83:343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rush D, Nickerson P, Gough J, et al. Beneficial effects of treatment of early subclinical rejection: a randomized study. J Am Soc Nephrol. 1998;9:2129–2134. [DOI] [PubMed] [Google Scholar]
- 34.Wu K, Budde K, Lu H, et al. The severity of acute cellular rejection defined by Banff classification is associated with kidney allograft outcomes. Transplantation. 2014;97:1146–1154. [DOI] [PubMed] [Google Scholar]
- 35.Moreso F, Ibernon M, Gomà M, et al. Subclinical rejection associated with chronic allograft nephropathy in protocol biopsies as a risk factor for late graft loss. Am J Transplant. 2006;6:747–752. [DOI] [PubMed] [Google Scholar]
- 36.Grimm PC, McKenna R, Nickerson P, et al. Clinical rejection is distinguished from subclinical rejection by increased infiltration by a population of activated macrophages. J Am Soc Nephrol. 1999;10:1582–1589. [DOI] [PubMed] [Google Scholar]
- 37.Hirt-Minkowski P, Amico P, Ho J, et al. Detection of clinical and subclinical tubulo-interstitial inflammation by the urinary CXCL10 chemokine in a real-life setting. Am J Transplant. 2012;12:1811–1823. [DOI] [PubMed] [Google Scholar]
- 38.Reeve J, Chang J, Salazar ID, et al. Using molecular phenotyping to guide improvements in the histologic diagnosis of T cell-mediated rejection. Am J Transplant. 2016;16:1183–1192. [DOI] [PubMed] [Google Scholar]
- 39.Schaier M, Seissler N, Becker LE, et al. The extent of HLA-DR expression on HLA-DR(+) Tregs allows the identification of patients with clinically relevant borderline rejection. Transpl Int. 2013;26:290–299. [DOI] [PubMed] [Google Scholar]
- 40.Schaub S, Nickerson P, Rush D, et al. Urinary CXCL9 and CXCL10 levels correlate with the extent of subclinical tubulitis. Am J Transplant. 2009;9:1347–1353. [DOI] [PubMed] [Google Scholar]
- 41.Salazar ID, Merino Lopez M, Chang J, et al. Reassessing the significance of intimal arteritis in kidney transplant biopsy specimens. J Am Soc Nephrol. 2015;26:3190–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haas M. The Revised (2013) Banff classification for antibody-mediated rejection of renal allografts: update, difficulties, and future considerations. Am J Transplant. 2016;16:1352–1357. [DOI] [PubMed] [Google Scholar]
- 43.Lefaucheur C, Loupy A, Vernerey D, et al. Antibody-mediated vascular rejection of kidney allografts: a population-based study. Lancet. 2013;381:313–319. [DOI] [PubMed] [Google Scholar]
- 44.Nickeleit V, Zeiler M, Gudat F, et al. Detection of the complement degradation product C4d in renal allografts: diagnostic and therapeutic implications. J Am Soc Nephrol. 2002;13:242–251. [DOI] [PubMed] [Google Scholar]
- 45.Amico P, Höenger G, Bielmann D, et al. Incidence and prediction of early antibody-mediated rejection due to non-human leukocyte antigen-antibodies. Transplantation. 2008;85:1557–1563. [DOI] [PubMed] [Google Scholar]
- 46.Cardinal H, Dieudé M, Brassard N, et al. Antiperlecan antibodies are novel accelerators of immune-mediated vascular injury. Am J Transplant. 2013;13:861–874. [DOI] [PubMed] [Google Scholar]
- 47.Dragun D, Müller DN, Bräsen JH, et al. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med. 2005;352:558–569. [DOI] [PubMed] [Google Scholar]
- 48.Boehmig GA, Exner M, Habicht A, et al. Capillary C4d deposition in kidney allografts: a specific marker of alloantibody-dependent graft injury. J Am Soc Nephrol. 2002;13:1091–1099. [DOI] [PubMed] [Google Scholar]
- 49.Collins AB, Schneeberger EE, Pascual MA, et al. Complement activation in acute humoral renal allograft rejection: diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol. 1999;10:2208–2214. [DOI] [PubMed] [Google Scholar]
- 50.Kikic Z, Kainz A, Kozakowski N, et al. Capillary C4d and kidney allograft outcome in relation to morphologic lesions suggestive of antibody-mediated rejection. Clin J Am Soc Nephrol. 2015;10:1435–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoenger G, Kraehenbuhl N, Dimeloe S, et al. Inter-individual differences in HLA expression can impact the CDC crossmatch. Tissue Antigens. 2015;85:260–266. [DOI] [PubMed] [Google Scholar]
- 52.Loupy A, Hill GS, Suberbielle C, et al. Significance of C4d Banff scores in early protocol biopsies of kidney transplant recipients with preformed donor-specific antibodies (DSA). Am J Transplant. 2011;11:56–65. [DOI] [PubMed] [Google Scholar]
- 53.Mohan S, Palanisamy A, Tsapepas D, et al. Donor-specific antibodies adversely affect kidney allograft outcomes. J Am Soc Nephrol. 2012;23:2061–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Serres SA, Noel R, Cote I, et al. 2013 Banff criteria for chronic active antibody-mediated rejection: assessment in a real-life setting. Am J Transplant. 2016;16:1516–1525. [DOI] [PubMed] [Google Scholar]


