Abstract
Background
Randomized trials show a mortality benefit to adjunctive corticosteroids for human immunodeficiency virus (HIV)-related Pneumocystis jiroveci pneumonia (HIV-PCP). Guidelines for non-HIV PCP (NH-PCP) recommend adjunctive corticosteroids based on expert opinion. We conducted a systematic review and meta-analysis characterizing adjunctive corticosteroids for NH-PCP.
Methods
We searched MEDLINE from 1966 through 2015. Data on clinical outcomes from NH-PCP were extracted with a standardized instrument. Heterogeneity was assessed with the I2 index. Pooled odds ratios and 95% confidence interval were calculated using a fixed effects model.
Results
Our search yielded 5044 abstracts, 277 articles were chosen for full review, and 6 articles described outcomes in moderate to severe NH-PCP. Studies were limited by variable definitions, treatment selection bias, concomitant infections and small sample size. Individual studies reported shorter intensive care unit stay and duration of mechanical ventilation of patients given adjunctive corticosteroids. There was no association between corticosteroids and survival in NH-PCP (odds ratio, 0.66; 95% confidence interval, 0.38-1.15; P = 0.14).
Conclusions
The literature does not support an association between adjunctive corticosteroids and survival from NH-PCP but data are limited and findings should not be considered conclusive. Further research with improved methodology is needed to better understand the role of adjunctive corticosteroids for NH-PCP.
Research during the early acquired immunodeficiency syndrome epidemic fueled major advances in prevention and management of human immunodeficiency virus (HIV)-related Pneumocystis pneumonia (HIV-PCP).1-4 Large scale randomized studies established that adjunctive corticosteroids for treatment of severe HIV-PCP (PaO2 < 70 mm Hg on room air) reduces mortality and adjunctive corticosteroids is standard of care for HIV-PCP.5-7
Non–HIV-related Pneumocystis jirovecii pneumonia (NH-PCP) was previously considered a rare disease. Data are lacking on current burden of NH-PCP, but individual patient risk of NH-PCP in transplant patients is high. NH-PCP now complicates 1% of solid organ transplants and has a cumulative incidence of 0.1% per year for stem cell transplant recipients.8,9
Despite lack of randomized trials, current guidelines recommend adjunctive corticosteroids for NH-PCP.10,11 Retrospective studies of NH-PCP with relatively small sample size have suggested either decreased mortality12-15 or no impact16-19 with increasing the dosage of glucocorticoids as adjunctive treatment of moderate to severe NH-PCP. Older investigations, conducted when utilization of adjunctive glucocorticoid therapy was limited to patients with HIV-PCP, found higher mortality in NH-PCP compared with HIV-PCP.13,20-22 More recent investigations have observed increased use of adjunctive glucocorticoids in NH-PCP, and similar mortality between HIV-PCP and NH-PCP.22-24 With relatively limited available data to support the guideline recommendations for NH-PCP,10,11 the primary objective of this investigation was to systematically review the literature and perform a meta-analysis of available data relating to the use of use of adjunctive glucocorticoids in hospitalized patients with moderate to severe NH-PCP.
MATERIALS AND METHODS
Search Strategy and Study Selection
We performed a literature search of Medline from 1966 to July 2015 and of EMBASE from 1980 to July 2015 to find published articles evaluating adjunctive glucocorticoid therapy for patients with NH-PCP. We limited studies to human subjects and searched for the following terms: (Pneumocysti*[text word] OR PCP[text word] OR “Pneumocystis Infections”[MESH] OR “Pneumocystis jirovecii”[MESH] OR “Pneumonia, Pneumocystis”[MESH] OR “Pneumocystis carinii”[MESH]) AND (treat* [text word] OR adjunct* [text word] OR treatment* [text word] OR steroid*[text word] OR corticosteroid*[text word] OR glucocorticoid*[text word] OR “Glucocorticoids”[MESH] OR “Adrenal Cortex Hormones”[MESH] OR “Steroids”[MESH]) NOT (“animals”[MeSH] NOT “humans”[MeSH]) In addition, we examined the references of all identified articles to look for additional relevant articles. Non-English references were translated by an investigator (J.M.) or a native speaker whenever possible.
Abstracts from each reference from our electronic search were independently reviewed for relevance by 2 investigators (P.I. and J.M.) using a standardized instrument. Studies were selected for full review if they reported primary data from patients with NH-PCP treated with and without adjunctive corticosteroids. Studies that did not separate data on outcomes between patients treated with and without adjunctive corticosteroids were excluded. Reports of single case experiences were excluded on the basis of publication bias, for example, exceptional circumstances surrounding the diagnosis, underlying conditions, or course of disease. There were no exclusions for patients with different types of immunosuppression or medical comorbidities. The intervention of interest was adjunctive corticosteroid therapy. The comparison groups were patients treated for NH-PCP with and without adjunctive corticosteroids. The outcome of interest was mortality, as defined by the study investigators. The meta-analysis of observational studies in epidemiology criteria were used to conduct this investigation and the article follows PRISMA criteria.25
Data Extraction
Each article underwent independent, blinded, double-data extraction by 2 reviewers (J.A.M., P.I.) using a standardized instrument. Discrepancies in data extraction underwent arbitration by a third reviewer (A.G.) and consensus was obtained by verbal discussion. Data collected from each study included year of study, country, number of patients, method of PCP diagnosis, treatment of PCP, definition of adjunctive corticosteroid use, dose of steroids used, and mortality.
Additional data were collected about the patient cohorts when present, including ethnicity, age, comorbid conditions, malignancy, organ or stem cell transplant, immunological diseases. All-cause mortality and clinical cure rates, as defined by the individual studies, were the primary outcome measures used in this meta-analysis.
Data Analysis and Statistical Methods
Data on NH-PCP outcomes were collected from all articles. Odds ratios for mortality were calculated for each article. Mantel-Haenszel statistical methods were used to calculate the pooled odds ratios, 95% confidence intervals, and the associated P values of each risk factor using a fixed-effects model. We analyzed heterogeneity in publication using the I2 measure of inconsistency and used DerSimonian and Laird random-effects model for I2 > 50% or P < 0.10. We did not use additional weighting criteria for the analysis. To ensure that our results were not biased by the process of combining results from multiple investigations (ie, Simpson’s paradox), we present a forest-plot of data from each individual study.26,27
RESULTS
Our search yielded 5044 references possibly related to NH-PCP. After the abstracts of all references were reviewed, 4767 abstracts were excluded because they were not related to PCP (n = 1575), related to only H-PCP (n = 1270), were basic science or animal models studies (n = 893), related to prophylaxis only (n = 377), a single case report (n = 374), or were only a literature review (n = 278). The full article for 277 references were reviewed.(Figure 1) From the 277 investigations with data on treatment of NH-PCP, 271 references were excluded from further analysis because they did not include outcome data (n = 81), did not have data on adjuvant steroid use (n = 65), untranslatable by the study team (n = 53), reported on HIV only (n = 28), reported single cases only (n = 20), outcomes were unclear (n = 15), reports of PCP colonization without evidence of infection (n = 7), or duplicated reports (n = 2).
FIGURE 1.
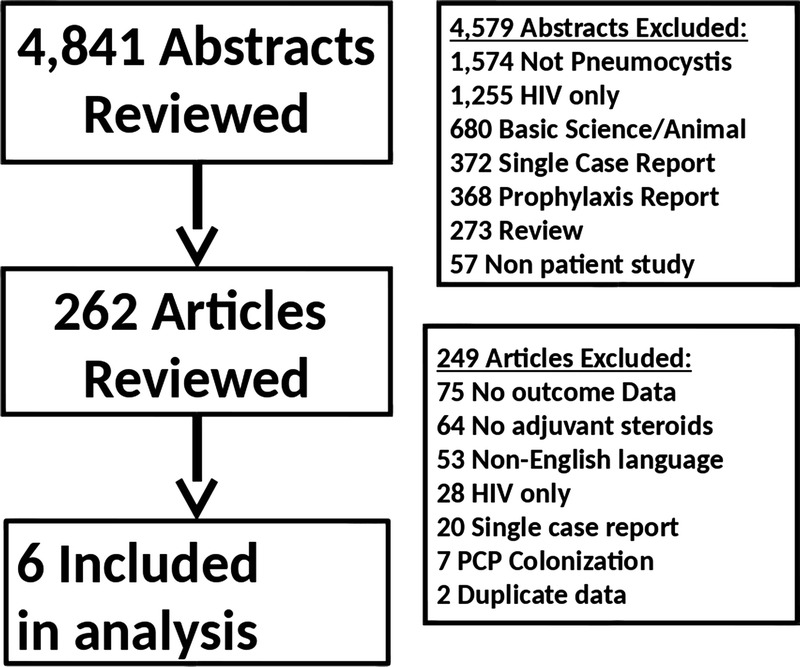
Study selection process and reasons for exclusion of references.
In the final analysis, 6 retrospective cohort investigations of patients treated with adjunctive corticosteroids were included.13-18 Among the 6 studies included in the analysis, there were 386 cases of NH-PCP. The 6 studies were conducted in the United States, France, Korea, and Japan. The patient population included solid organ transplantation (n = 111), hematological malignancies (n = 93), nonhematologic malignancies (n = 29), collagen vascular disease (n = 25), rheumatoid arthritis (n = 21), cancer (n = 11), interstitial lung disease (n = 9), connective tissue disease (n = 7), and other inflammatory diseases (n = 50). Diagnosis of NH-PCP was based on immunofluorescent staining, microscopic examination, or polymerase chain reaction of the patients' sputum, bronchoalveolar lavage fluid, or transbronchial biopsy.
The included investigations differed in their definition of adjuvant corticosteroid dose (see Table 1). The investigations defined adjuvant steroid use as; prednisone doses greater than 60 mg, pulse corticosteroids, high dose corticosteroids, adjunctive corticosteroids, prednisone doses of 80 mg or greater, and prednisone doses > 1 mg/kg. Nearly all patients were initially treated with trimethoprim-sulfamethoxazole prior to PCP infection.
TABLE 1.
Papers included in the meta-analysis of the impact of adjunctive corticosteroids on survival from NH-PCP
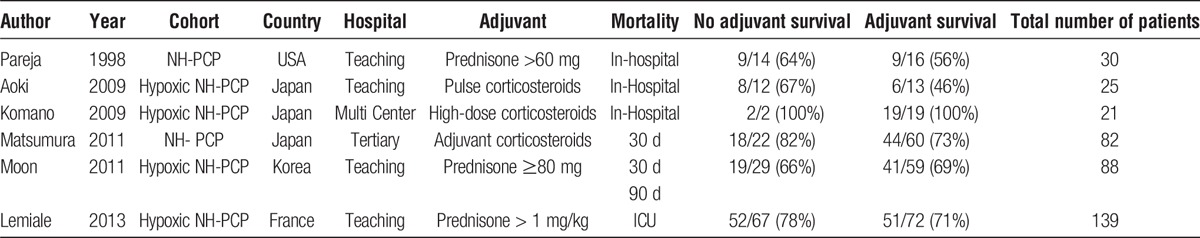
Studies also differed in their definition of mortality. Three investigations used in hospital mortality rates, 1 investigation used 30 day all-cause mortality, 1 used 30 day and 90 day all-cause mortality, and 1 used intensive care unit (ICU) mortality rates. A meta-analysis of the 6 studies showed treatment with adjuvant corticosteroids for NH-PCP did not have an impact on survival (odds ratio, 0.76; 95% confidence interval, 0.47-1.2; P = 0.25) (Figure 2). The most common coinfections described were cytomegalovirus (n = 23) and Aspergillus (n = 9). Patients were administered a wide range of immunosuppression at time of diagnosis. Reports of initial decline in respiratory function in patients not treated with corticosteroids was reported once.16
FIGURE 2.
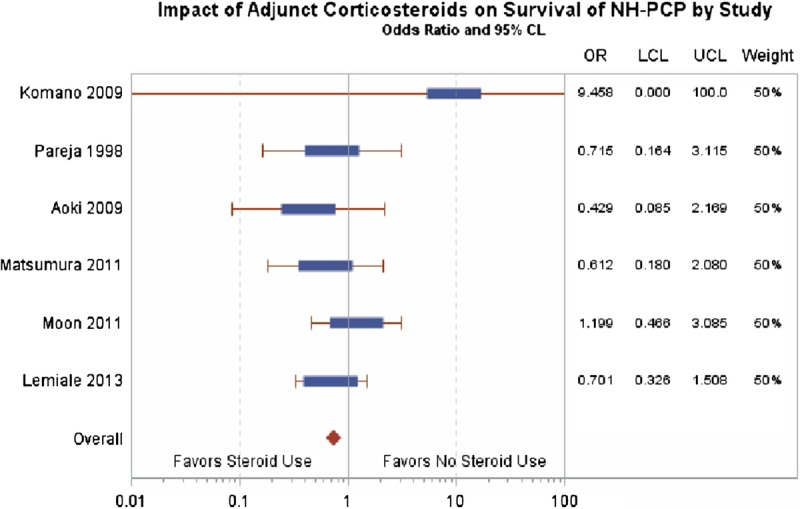
Impact of adjunctive corticosteroids on survival of NH-PCP by study.
DISCUSSION
Current guidelines recommend adjunctive corticosteroids for cases of severe NH-PCP.10,11 However, our systematic review found little data to support the role of adjunctive corticosteroids in the treatment of NH-PCP. We found no clinical trials data and only few articles that provided comparative data. Based on our analysis of 6 articles reporting on over 350 cases of NH-PCP, we found no association between adjunctive corticosteroids and survival in NH-PCP and very scant information on potential risks.
The effect of adjuvant corticosteroids on the duration of mechanical ventilation or ICU stay could not be assessed because only 1 of 6 articles included such intermediate outcomes data. Pareja et al13 found the ICU stay was shorter for patients treated with high-dose steroids (≥60 mg/d; 8.5 ± 7 days) compared with patients treated with low dose steroids or on a steroid taper (≤30 mg/d; 15.8 ± 8 days) (P = 0.025). The duration of mechanical ventilation was also significantly reduced in the increased high - dose steroid group (6.3 ± 6 days) compared with the low dose/taper steroid group (18 ± 21 days) (P = 0.047). Although these data suggest potential benefit to the use of adjuvant corticosteroids, these results should be confirmed with additional studies.
We would also highlight that there was relatively little safety data reported in these investigations. Moon et al compared NH-PCP patients treated with and without adjunctive corticosteroids and found no significant differences in the rates of concomitant bacterial infection, viral infections, or respiratory failure. None of the articles described incidence of hyperglycemia, psychiatric complications, gastrointestinal bleeding episodes, or other known complications of corticosteroids. Future investigations should carefully assess the safety of adjunctive corticosteroids on NH-PCP. Moreover, future investigations should carefully consider the question of how to approach patients on high-dose corticosteroids at the time of NH-PCP diagnosis versus those on no or very low corticosteroid treatment.
Our findings should not be used to drive treatment recommendations, nor challenge existing guidelines, but we would highlight that the literature is limited to small, single center, retrospective cohort studies with significant heterogeneity in study design. Despite the large number of cases, there were few investigations included in our analysis, and all were retrospective in nature. Moreover, there was evidence for treatment selection bias in these investigations that was not adjusted in the original analysis and could not be adjusted for in our meta-analysis. We also caution that differences in definitions of mortality may further have introduced bias.
Overall, the impact of adjunctive corticosteroids on NH-PCP remains unclear and requires further research. The currently published literature does not support a mortality benefit for adjunctive corticosteroids in NH-PCP and the benefit in terms of ICU stay or mechanical ventilation was only seen in 1 study. Notably, safety information on high-dose corticosteroids in NH-PCP is largely lacking. Due to the inability to enroll an adequate number of subjects in a clinical trial, a standard prospective randomized clinical trial of NH-PCP is unlikely. Until such a trial is performed, we strongly believe that future research should make substantial changes in methodologies to provide more valuable insight. For example, a mortality endpoint, particularly a late mortality endpoint, may not be optimal in determining outcomes from NH-PCP as these patients have high risk for non–infection-related deaths. Alternative outcome endpoints, including duration of ventilation, length of ICU stay, and the reporting on safety data would be valuable. Furthermore, Cox proportional hazards modeling, propensity score analysis, or instrumental variable analyses are needed to better understand outcomes from observations studies.28-30 Until additional research using improved methodologies are published, clinicians should carefully consider the risk and benefits of adjunctive corticosteroids for NH-PCP.10,11
Footnotes
Published online 15 February, 2017.
P.I. received support from the Western University of Health Sciences Research Committee. J.M. received support from the NIH/NCRR/NCATS UCLA CTSI grant KL2TR000122 and the Perkins Foundation. ALG received support from the NIH/NHLBI grant K23HL102220.
The authors declare no conflicts of interest.
P.I., A.L.G., J.A.M. participated in conception, hypothesis, data review and generation, article development, and revision. S.J.E. participated in statistical analysis and article revision. H.W. and I.M. participated in data review and article revision.
REFERENCES
- 1.Gagnon S, Boota AM, Fischl MA, et al. Corticosteroids as adjunctive therapy for severe Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. A double-blind, placebo-controlled trial. N Engl J Med. 1990;323:1444–1450. [DOI] [PubMed] [Google Scholar]
- 2.Bozzette SA, Sattler FR, Chiu J, et al. A controlled trial of early adjunctive treatment with corticosteroids for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. California Collaborative Treatment Group. N Engl J Med. 1990;323:1451–1457. [DOI] [PubMed] [Google Scholar]
- 3.Montaner JS, Lawson LM, Levitt N, et al. Corticosteroids prevent early deterioration in patients with moderately severe Pneumocystis carinii pneumonia and the acquired immunodeficiency syndrome (AIDS). Ann Intern Med. 1990;113:14–20. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan JE, Hanson D, Dworkin MS, et al. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;30(Suppl 1):S5–S14. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan JE, Masur H, Holmes KK. Guidelines for preventing opportunistic infections among HIV-infected persons—2002. Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. MMWR Recomm Rep. 2002;51:1–52. [PubMed] [Google Scholar]
- 6.Huang L, Morris A, Limper AH, et al. An Official ATS Workshop Summary: recent advances and future directions in pneumocystis pneumonia (PCP). Proc Am Thorac Soc. 2006;3:655–664. [DOI] [PubMed] [Google Scholar]
- 7.Briel M, Bucher HC, Boscacci R, et al. Adjunctive corticosteroids for Pneumocystis jiroveci pneumonia in patients with HIV-infection. Cochrane Database Syst Rev. 2006;3:CD006150. [DOI] [PubMed] [Google Scholar]
- 8.Kontoyiannis DP, Marr KA, Park BJ, et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin Infect Dis. 2010;50:1091–1100. [DOI] [PubMed] [Google Scholar]
- 9.Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. 2010;50:1101–1111. [DOI] [PubMed] [Google Scholar]
- 10.Martin SI, Fishman JA, AST Infectious Diseases Community of Practice. Pneumocystis pneumonia in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):272–279. [DOI] [PubMed] [Google Scholar]
- 11.McKinnell JA, Cannella AP, Injean P, et al. Adjunctive glucocorticoid therapy for non-HIV-related Pneumocystis carinii pneumonia (NH-PCP). Am J Transplant. 2014;14:982–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burke BA, Good RA. Pneumocystis carinii infection. Medicine (Baltimore). 1973;52:23–51. [DOI] [PubMed] [Google Scholar]
- 13.Pareja JG, Garland R, Koziel H. Use of adjunctive corticosteroids in severe adult non-HIV Pneumocystis carinii pneumonia. Chest. 1998;113:1215–1224. [DOI] [PubMed] [Google Scholar]
- 14.Matsumura Y, Shindo Y, Iinuma Y, et al. Clinical characteristics of Pneumocystis pneumonia in non-HIV patients and prognostic factors including microbiological genotypes. BMC Infect Dis. 2011;11:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aoki Y, Iwamoto M, Kamata Y, et al. Prognostic indicators related to death in patients with Pneumocystis pneumonia associated with collagen vascular diseases. Rheumatol Int. 2009;29:1327–1330. [DOI] [PubMed] [Google Scholar]
- 16.Moon SM, Kim T, Sung H, et al. Outcomes of moderate-to-severe Pneumocystis pneumonia treated with adjunctive steroid in non-HIV-infected patients. Antimicrob Agents Chemother. 2011;55:4613–4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komano Y, Harigai M, Koike R, et al. Pneumocystis jiroveci pneumonia in patients with rheumatoid arthritis treated with infliximab: a retrospective review and case-control study of 21 patients. Arthritis Rheum. 2009;61:305–312. [DOI] [PubMed] [Google Scholar]
- 18.Lemiale V, Debrumetz A, Delannoy A, et al. Adjunctive steroid in HIV-negative patients with severe Pneumocystis pneumonia. Respir Res. 2013;14:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zahar JR, Robin M, Azoulay E, et al. Pneumocystis carinii pneumonia in critically ill patients with malignancy: a descriptive study. Clin Infect Dis. 2002;35:929–934. [DOI] [PubMed] [Google Scholar]
- 20.Limper AH, Offord KP, Smith TF, et al. Pneumocystis carinii pneumonia. Differences in lung parasite number and inflammation in patients with and without AIDS. Am Rev Respir Dis. 1989;140:1204–1209. [DOI] [PubMed] [Google Scholar]
- 21.Kovacs JA, Hiemenz JW, Macher AM, et al. Pneumocystis carinii pneumonia: a comparison between patients with the acquired immunodeficiency syndrome and patients with other immunodeficiencies. Ann Intern Med. 1984;100:663–671. [DOI] [PubMed] [Google Scholar]
- 22.Mansharamani NG, Garland R, Delaney D, et al. Management and outcome patterns for adult Pneumocystis carinii pneumonia, 1985 to 1995: comparison of HIV-associated cases to other immunocompromised states. Chest. 2000;118:704–711. [DOI] [PubMed] [Google Scholar]
- 23.Nüesch R, Bellini C, Zimmerli W. Pneumocystis carinii pneumonia in human immunodeficiency virus (HIV)-positive and HIV-negative immunocompromised patients. Clin Infect Dis. 1999;29:1519–1523. [DOI] [PubMed] [Google Scholar]
- 24.McKinnell JA, Cannella AP, Kunz DF, et al. Pneumocystis pneumonia in hospitalized patients: a detailed examination of symptoms, management, and outcomes in human immunodeficiency virus (HIV)-infected and HIV-uninfected persons. Transpl Infect Dis. 2012;14:510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 26.Bickel PJ, Hammel EA, O'Connell JW. Sex bias in graduate admissions: data from Berkeley. Science. 1975;187:398–404. [DOI] [PubMed] [Google Scholar]
- 27.Pearl J. Causality: models, reasoning, and inference. Cambridge, U.K.; New York: Cambridge University Press; 2000; xvi, 384 p. p. [Google Scholar]
- 28.Berger ML, Mamdani M, Atkins D, et al. Good research practices for comparative effectiveness research: defining, reporting and interpreting nonrandomized studies of treatment effects using secondary data sources: the ISPOR Good Research Practices for Retrospective Database Analysis Task Force Report–Part I. Value Health. 2009;12:1044–1052. [DOI] [PubMed] [Google Scholar]
- 29.Johnson ML, Crown W, Martin BC, et al. Good research practices for comparative effectiveness research: analytic methods to improve causal inference from nonrandomized studies of treatment effects using secondary data sources: the ISPOR Good Research Practices for Retrospective Database Analysis Task Force Report—Part III. Value Health. 2009;12:1062–1073. [DOI] [PubMed] [Google Scholar]
- 30.Cox E, Martin BC, Van Staa T, et al. Good research practices for comparative effectiveness research: approaches to mitigate bias and confounding in the design of nonrandomized studies of treatment effects using secondary data sources: the International Society for Pharmacoeconomics and Outcomes Research Good Research Practices for Retrospective Database Analysis Task Force Report–Part II. Value Health. 2009;12:1053–1061. [DOI] [PubMed] [Google Scholar]


