Abstract
Peripheral nerve injury-caused hyperexcitability and abnormal ectopic discharges in the primary sensory neurons of dorsal root ganglion (DRG) play a key role in neuropathic pain development and maintenance. The two-pore domain background potassium (K2P) channels have been identified as key determinants of the resting membrane potential and neuronal excitability. However, whether K2P channels contribute to neuropathic pain is still elusive. We reported here that K2P1.1, the first identified mammalian K2P channel, was highly expressed in mouse DRG and distributed in small-, medium-, and large-sized DRG neurons. Unilateral lumbar (L) 4 spinal nerve ligation led to a significant and time-dependent reduction of K2P1.1 mRNA and protein in the ipsilateral L4 DRG, but not in the contralateral L4 or ipsilateral L3 DRG. Rescuing this reduction through microinjection of adeno-associated virus-DJ expressing full-length K2P1.1 mRNA into the ipsilateral L4 DRG blocked spinal nerve ligation-induced mechanical, thermal, and cold pain hypersensitivities during the development and maintenance periods. This DRG viral microinjection did not affect acute pain and locomotor function. Our findings suggest that K2P1.1 participates in neuropathic pain development and maintenance and may be a potential target in the management of this disorder.
Keywords: Potassium channels, K2P1.1, TWIK1, dorsal root ganglion, neuropathic pain
Introduction
Peripheral nerve injury-induced neuropathic pain is one of major clinical disorders characterized by spontaneous ongoing or intermittent burning pain, sensory abnormalities (dysesthesia), an increased response to painful stimuli (hyperalgesia), and pain in response to normally innocuous stimuli (allodynia).1 Treatment options of this disorder are limited in part due to our incomplete understanding of the mechanisms that underlie the induction and maintenance of neuropathic pain. One of the primary causes of neuropathic pain is hypersensitivities and abnormal ectopic discharges that arise in the neuromas at the site of peripheral nerve injury and the primary sensory neurons of dorsal root ganglion (DRG).1–4 Exploring how peripheral nerve injury leads to these abnormal neuronal activities in the DRG may provide a potential new avenue for the prevention and treatment of neuropathic pain.
The two-pore domain background potassium (K2P) channel family and the leak currents carried by K2P channels have been identified as key determinants of neuronal activity throughout the nervous system.5 In mammalians, 15 subunits have been identified and were further divided into six subfamilies based on their structural and functional properties including TWIK, TREK, TASK, THIK, TRESK, and TALK.5,6 K2P channels control neuronal excitability by setting the resting membrane potential and input resistance.7,8 Their expression and function could also be modulated by multiple neurotransmitters and modulators.9,10 K2P1.1, also known as TWIK1, was the first identified K2P channel and was found to be opened at rest and therefore is capable of driving the membrane potential towards the equilibrium potential for K+.9,11 K2P1.1 mRNA is highly expressed in mouse DRG and mainly distributed in large and medium DRG neurons.12 Peripheral spared nerve lesion produces a significant downregulation of K2P1.1 mRNA in the injured DRG 1, 2, and 4 weeks after lesion.12 However, whether this downregulation occurs at the development (or the early) period of neuropathic pain is still elusive. More importantly, whether nerve injury-induced DRG K2P1.1 downregulation contributes to the development and maintenance of neuropathic pain is unknown.
In the present study, we first characterized the subpopulation distribution patterns and neurochemical properties of K2P1.1-positive cells. Second, we examined the time-dependent change of K2P1.1 mRNA and protein in the injured mouse DRG after unilateral lumbar (L) 4 spinal nerve ligation (SNL). Finally, we tested whether rescuing K2P1.1 downregulation by overexpressing full-length K2P1.1 gene in the injured DRG affected pain hypersensitivities during the development and maintenance periods of SNL-induced neuropathic pain.
Materials and methods
Animal preparations
Eight-week-old male CD1 mice (Charles River Lab., Wilmington, MA) were used in this experiment. They were housed in a temperature-controlled room with a standard 12-h light-dark cycle and had free access to food and water. Mice were habituated to the testing environment daily for three days before behavioral testing. They were randomly assigned into different experimental or control groups. The group sizes were based on previous experience. All procedures used were approved by the Animal Care and Use Committee at the Rutgers New Jersey Medical School and consistent with the ethical guidelines of the US National Institutes of Health and the International Association for the Study of Pain. All of the experimenters were blind to treatment condition.
DRG microinjection
DRG microinjection was carried out as described previously.9,13–18 Briefly, a dorsal midline incision was made in the lower lumbar region. The left L3/4 articular processes were exposed and then removed with small rongeurs. After the DRG was exposed, the viral solution (1 μl, 2 × 1010) was injected into the left L4 DRG with a glass micropipette connected to a Hamilton syringe. The pipette was removed 10 min after injection. The surgical field was irrigated with sterile saline and the skin incision closed with wound clips.
SNL-induced neuropathic pain model
A mouse neuropathic pain model of unilateral L4 SNL was carried out as described previously.15,16,19 Briefly, animals were anesthetized with 2–3% isoflurane. The left fifth lumbar transverse process was identified and then was separated from its muscle attachments. The underlying fourth spinal nerve was carefully isolated and tightly ligated with a 7-0 silk suture under a surgical microscope. The ligated nerve was then transected just distal to the ligature. The skin and muscles were closed in layers. The surgical procedure for the sham group was identical to that of the SNL group, except that the spinal nerve was not transected or ligated.
Behavioral tests
Mechanical behavioral test was carried out as described previously.15,16 Briefly, mice were placed in a plastic chamber over a metal mesh floor. After 30-min habituation, two calibrated von Frey filaments (0.07 and 0.4 g; Stoelting Co., Wool Dale, IL) were applied to the plantar surface of the hind paw for approximately 1 s, and each stimulation was repeated 10 times to both hind paws. The occurrence of paw withdrawal in each of these 10 trials was expressed as a percent response frequency. This percentage will be used as an indication of the amount of paw withdrawal.
Thermal behavioral test was performed as described previously.15,16 In brief, each mouse was placed in a plastic chamber above a glass plate. After 30-min habituation, a radiant heat from Model 336 Analgesic Meter (IITC Life Science, Instruments, Woodland Hills, CA) was applied by aiming a beam of light through a hole in the light box through the glass plate to the middle of the plantar surface of each hind paw. When the animal lifts its paw in response to the heat, the light beam was turned off. The time from the start of light beam to the foot lift was defined as paw withdrawal latency. Five minutes was allowed between stimulations and five measurements were averaged for each side. A cut-off time of 20 s was used to prevent tissue damage.
Cold behavioral test was carried out as previously described.15,16 Each mouse was placed in a Plexiglas chamber on the cold aluminum plate, the temperature of which was set at 0℃ and monitored continuously by a thermometer. The length of time between the placement of the hind paw on the plate and the animal jumping, with or without paw licking and flinching, was defined as the paw withdrawal latency. Ten minutes was allowed between three repeated trials. A cut-off time of 20 s was used to avoid paw tissue damage.
Locomotor function was carried out on day 7 post-SNL or sham surgery. Three reflexes were observed. (1) Placing reflex: The mouse’s hind limbs were hold slightly lower than the fore limbs, and the dorsal surfaces of the hind paws were brought into contact with the edge of a table. Whether the hind paws were placed on the table surface reflexively was recorded. (2) Grasping reflex: The mouse was placed on a wire grid and the experimenter recorded whether the hind paws grasped the wire on contact. And (3) Righting reflex: The mouse was placed on its back on a flat surface and whether it immediately assumed the normal upright position was recorded. Five trials were conducted for each test and scores were derived from counts of normal reflexes for each trial. General behavior, including spontaneous activity, was assessed.
Plasmid construction and virus production
To construct the plasmid expressing full-length K2P1.1 mRNA, total RNA from mouse DRG tissue was extracted by the Trizol method (Invitrogen/ThermoFisher Scientific, Grand Island, NY), treated with DNase I (New England Biolabs, Ipswich, MA), and reverse-transcribed using the gene-specific primer 5’-ATTGGAATGCAAACCCAGAG-3’. Then the full-length sequence of K2P1.1 cDNA was amplified using the following primers (Forward: 5’-ATAGAATTCGCCACCATGCTGCAGTCCCTGG-3’; Reverse: 5’-GTAGGATCCTCAGTGGTCTGCAGAGCCAT-3’). The K2P1.1 cDNA was inserted into the pro-viral plasmid (pAAV-MCS) by the EcoRI and BamHI restriction sites. The cDNA sequence and recombinant clone were verified by using DNA sequencing.
The adeno-associated virus (AAV)-DJ viral particles carrying the cDNA were prepared using AAV-DJ Helper Free Packaging System (Cell Biolabs, Inc., San Diego, CA). Briefly, HEK293-AAV cells were transfected with a pAAV-MCS that expressed K2P1.1 or enhanced green fluorescent protein (EGFP), pAAV-DJ-RC, and pHelper using a PEI Transfection method. Three days later, the transfected cells were collected and suspended in serum-free DMEM. After four freeze-thaw cycles, the AAV supernatant was collected by centrifugation at 10,000 g for 10 min and concentrated using AAV Purification Standard Kit (Cell Biolabs, Inc.).
Quantitative real-time reverse transcription-polymerase chain reaction
Total RNA extraction and quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR) were performed according to our previously published protocols.13–16,18 Briefly, mice were sacrificed under isoflurane anesthesia, and bilateral L4 DRGs was rapidly collected. To obtain enough RNA, L4 DRGs from four mice per time point were pooled. Total RNA was extracted by using the miRNeasy kit with on-column digestion of genomic DNA according to manufacturer’s instructions (QIAGEN, Valencia, CA), treated with an overdose of DNase I (New England Biolabs, Ipswich, MA), and reversed-transcribed with the ThermoScript reverse transcriptase (Invitrogen/ThermoFisher Scientific) and oligo (dT) primers (Invitrogen/ThermoFisher Scientific). Template (4 µl) was amplified in a BIO-RAD CFX96 real-time PCR system (Bio-Rad Laboratories, Hercules, CA) by using the following primers (K2P1.1: 5’-CAACGCCTCGGGAAATTGGA-3’ (forward) and 5’-CCCCAGCGTATGTGGAAGTAG-3’ (reverse). Tuba1a: 5’-GTGCATCTCCATCCATGTTG (forward) and 5’-GTGGGTTCCAGGTCTACGAA-3’ (reverse)). Each sample was run in triplicate in a 20 μl reaction with 250 nM forward and reverse primers, 10 µl of Advanced Universal SYBR Green Supermix (Bio-Rad Laboratories), and 20 ng of cDNA. The PCR amplification consisted of an initial 3 min at 95℃ followed by 40 cycles of 95℃ for 10 s, 60℃ for 30 s, and 72℃ for 30 s. Ratios of ipsilateral side mRNA levels to contralateral side mRNA levels were calculated by using the ΔCt method (2−ΔΔCt) at a threshold of 0.02. All data were normalized to Tuba1a mRNA, which has been demonstrated to be stable even after peripheral nerve injury insult in mice.14–16
Western blot analysis
The detailed protocol for Western blot analysis was described previously.14,18 In brief, mice were sacrificed under isoflurane anesthesia and ipsilateral L4 or L3/4 DRGs were collected and rapidly frozen in liquid nitrogen. The tissues were homogenized in chilled lysis buffer (10 mM Tris, 5 mM MgCl2, 5 mM EGTA, 250 mM sucrose, 1 mM phenylmethylsulfonyl fluoride, 1 mM DTT, and 40 μM leupeptin). After centrifugalized at 4℃ for 15 min at 1000 g, the supernatant was collected. After protein concentration was measured, the samples were heated at 99℃ for 5 min and loaded onto a 4%–20% precast polyacrylamide gel (Bio-Rad Laboratories). The proteins were then electrophoretically transferred onto 0.2 µm pore-size nitrocellulose membranes (Bio-Rad Laboratories). The membranes were blocked with 3% nonfat milk in Tris-buffered saline containing 0.1% Tween-20 for 1 h and then incubated with primary rabbit anti-K2P1.1 (1:1000; Alomone Labs, Jerusalem, Israel) or rabbit anti-GAPDH (1:1000; Santa Cruz Biotechnology, Dallas, TX) at 4℃ overnight under gentle agitation. After being incubated with horseradish peroxidase-conjugated anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA), the blots were developed with chemiluminescent regents (Clarity Western ECL Substrate; Bio-Rad Laboratories). The image signals were visualized by using ChemiDoc XRS+ System (Bio-Rad Laboratories). The intensity of blots was quantified with Image Lab software (Bio-Rad Laboratories). The bands of K2P1.1 at the different time points were normalized to the corresponding bands of GAPDH.
Immunohistochemistry
After being deeply anesthetized with isoflurane, the mice were perfused through the left ventricle with 20 mL of 0.01 M phosphate-buffered saline (PBS, pH 7.4) followed by 100 mL of 4% paraformaldehyde in 0.01 M phosphate buffer (pH 7.4). Bilateral L4 DRGs were removed, post-fixed in the same fixative at 4℃ overnight, and dehydrated in 30% sucrose in 0.01 M PBS overnight at 4℃. The DRG was sectioned at the thickness of 20 µm.
For the single labeling, every fourth section was collected (at least 3–4 sections/DRG). Single-label immunofluorescence histochemistry was carried out as described previously.20,21 After being blocked for 1 h at 37℃ in PBS containing 10% goat serum and 0.3% TritonX-100, the sections were incubated alone with primary rabbit anti-K2P1.1 (1:1000, Alomone Labs) over two nights at 4℃. The sections were then washed with PBS and incubated with goat anti-rabbit IgG conjugated with Cy2 (1:300, Jackson ImmunoResearch Laboratories) for 2 h at room temperature. Control experiments included substitution of normal rabbit serum for the primary antiserum and omission of the primary antiserum. Finally, the sections were rinsed in 0.01 M PBS and mounted onto gelatin-coated glass slides. Cover slips were applied with a mixture of 50% glycerin and 2.5% triethylene diamine in 0.01 M PBS. All immunofluorescence-labeled images were examined under a Nikon TE2000E fluorescence microscope (Nikon Co., Japan) and randomly captured with a CCD spot camera (1–3 fields/section). All labeled and unlabeled neurons with nuclei were counted. Cell profiles were outlined, and cell area was calculated by using the imaging software Image-Pro Plus (Media Cybernetics, Silver Spring, MD).
For the double labeling, two sets of sections (at least 2–3 sections/set) were collected from each tissue by grouping every sixth serial section. Double-label immunofluorescence histochemistry was carried out as described previously.20,21 The sections were incubated overnight at 4℃ with primary rabbit anti-K2P1.1 (1:1000, Alomone Labs) plus one of following primary antibody: chicken anti-βIII-tubulin (1:300, Millipore), mouse anti-Glutamine synthetase (1:500, Millipore), mouse anti-NF200 (1:1000, Sigma), biotinylated IB4 (1:300, Sigma), and mouse anti-CGRP (1:500, Abcam). The sections were then incubated for 2 h at room temperature with a mixture of goat anti-rabbit IgG conjugated with Cy2 (1:200, Jackson) and donkey anti-chicken IgG conjugated with Cy3 (1:200, Jackson) or goat anti-mouse IgG conjugated with Cy3 (1:200, Jackson) or avidin labeled with Texas Red (1:200, Sigma). Control experiments as described above were performed in parallel.
Statistical analysis
All of the results are given as means ± SEM. The data were statistically analyzed with two-tailed, paired Student’s t-test and a one-way or two-way analysis of variance (ANOVA). When ANOVA showed a significant difference, pairwise comparisons between means were tested by the post hoc Turkey method (SigmaPlot 12.5, San Jose, CA). Significance was set at P < 0.05.
Results
Distribution of K2P1.1-positive immunoreactivity in DRG
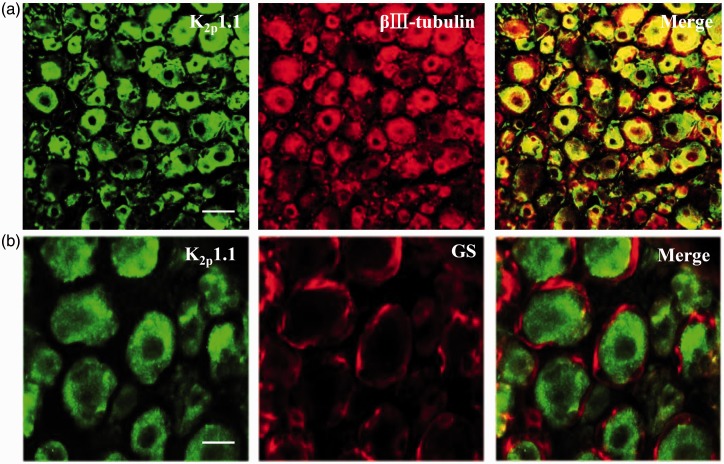
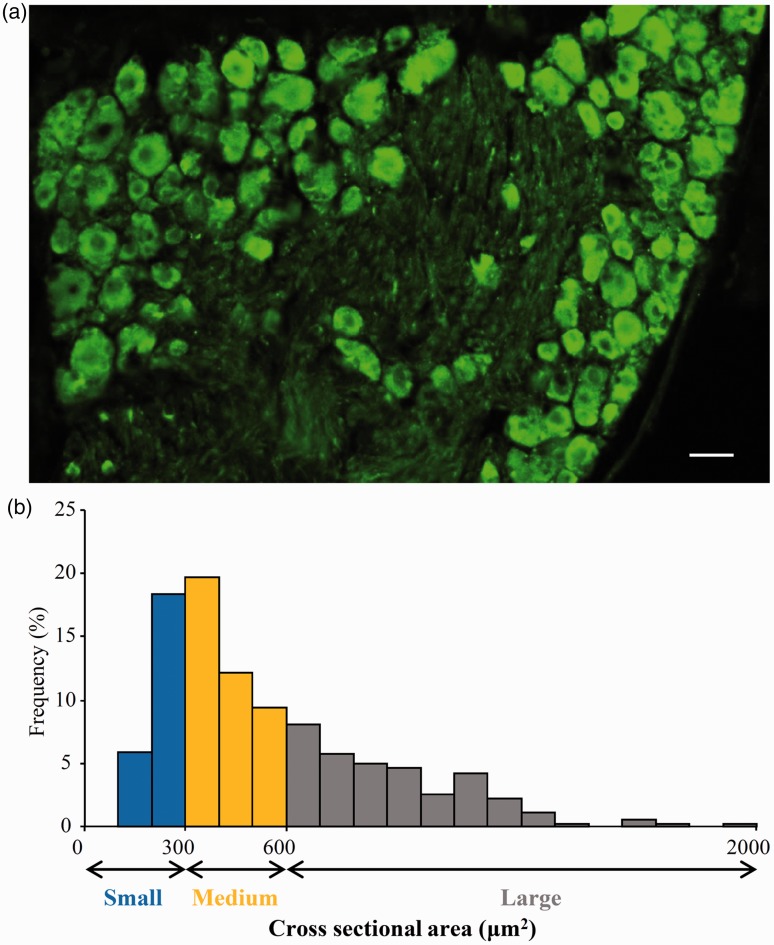
We first examined the distribution pattern of K2P1.1 in the mouse DRG. Using double labeling for K2P1.1 and βIII-tubulin (a specific neuronal marker) or glutamine synthetase (GS, a marker for satellite glial cells), we found that K2P1.1 co-expressed with βIII-tubulin (Figure 1(a)), but not with GS (Figure 1(b)), indicating that K2P1.1 is expressed exclusively in the neurons of DRG. Approximately 89.4% (1184/1324) of DRG neurons were positive for K2P1.1 (Figure 2(a)). A cross-sectional area analysis of neuronal somata found that approximately 24.3% of K2P1.1-labeled neurons are small (<300 µm2 in area), 41.2% are medium (300–600 µm2 in area), and 34.5% are large (>600 µm2 in area) (Figure 2(b)). To further confirm the subpopulation distribution of K2P1.1 in DRG, we also did the double labeling for K2P1.1 and distinct DRG neuronal markers. Approximately 45.8% (258/563) of K2P1.1-labeled neurons are positive for neurofilament-200 (NF200, a marker for medium/large diameter neurons and myelinated A-fibers) (Figure 3(a)), 31.9% (158/495) for calcitonin gene-related peptide (CGRP, a marker for small/medium peptidergic neurons) (Figure 3(b)), and 18.9% (92/488) for isolectin B4 (IB4, a marker for small non-peptidergic neurons) (Figure 3(c)).
Figure 1.
K2P1.1 is expressed exclusively in dorsal root ganglion (DRG) neurons of naive mice. (a). K2P1.1 is co-expressed with βIII-tubulin in the DRG neurons. (b) K2P1.1 is undetected in GS-labeled DRG satellite glial cells. Scale bar: 40 µm in (a); 20 µm in (b). GS: glutamine synthetase.
Figure 2.
K2P1.1 is distributed in the small-, medium-, and large-sized DRG neurons of naive mice. (a) A representative image from immunohistochemical staining showing the distribution of K2P1.1-positive neurons. Scale bar: 50 µm. (b) Histogram showing the distribution of K2P1.1-positive somata.
Figure 3.
Co-location of K2P1.1 with NF200, CGRP, and IB4 in DRG neurons. Double immunohistochemical staining shows that approximately 45.8% of K2P1.1-labeled neurons are positive for NF200 (a), 31.9% for CGRP (b), and 18.9% for IB4 (c). Scale bar: 40 µm.
Downregulation of K2P1.1 in the injured DRG after SNL
We further examined whether peripheral nerve injury changed the expression of DRG K2P1.1. SNL, but not sham surgery, time-dependently decreased the levels of K2P1.1 mRNA and protein in the L4 DRG on the ipsilateral side (Figure 4(a) and (b)). The amount of K2P1.1 protein in the ipsilateral L4 DRG was reduced by 53.8% on day 3 (P < 0.05), 58.3% on day 7 (P < 0.05), and 52.8% on day 14 (P < 0.05) post-SNL compared to sham surgery mice at the corresponding time point (Figure 4(a)). No significant changes in the levels of K2P1.1 protein were seen in either contralateral L4 DRG or ipsilateral intact L3 DRG (Figure 4(c)). Consistently, the ratios of ipsilateral side to contralateral side of K2P1.1 mRNA levels were decreased by 85.8% on day 3 (P < 0.05), 84.0% on day 7 (P < 0.05), and 79.2% on day 14 (P < 0.05) post-SNL compared to naive mice (Figure 4(b)). We also quantified the number of K2P1.1-positive neurons in the contralateral and ipsilateral L4 DRGs on day 7 post-SNL or sham surgery. SNL, but not sham surgery, reduced the number of K2P1.1-positive neurons by 56.6% in the ipsilateral L4 DRG compared to that in the corresponding contralateral L4 DRG (P < 0.05) (Figure 4(d)). To further verify the effect of SNL on the expression of K2P1.1 in the injured DRG neurons, we examined K2P1.1 protein level in the ipsilateral L4 DRG on day 7 after sciatic nerve axotomy or sham surgery. Like after SNL, the level of K2P1.1 protein in the ipsilateral L4 DRG post-axotomy was reduced by 44.7% compared to that post-sham surgery (Figure 4(e)).
Figure 4.
Peripheral nerve injury leads to the decreases in the levels of K2P1.1 mRNA and protein in the injured DRG. (a) K2P1.1 protein expression in the ipsilateral L4 DRG after SNL or sham surgery. Representative Western blots (left panels) and a summary of densitometric analysis (right graphs). n = 8 mice/time point. *P < 0.05 vs. the corresponding control group (Sham), one-way ANOVA followed by post hoc Tukey test. (b) K2P1.1 mRNA expression in the ipsilateral and contralateral L4 DRGs after SNL. n = 8 mice/time point. *P < 0.05 vs. the corresponding control group (0 day), one-way ANOVA followed by post hoc Tukey test. (c) K2P1.1 protein expression in the ipsilateral L3 DRG after SNL. Representative Western blots (Top panels) and a summary of densitometric analysis (bottom graphs). n = 8 mice/time point. One-way ANOVA followed by post hoc Tukey test. (d) Number of K2P1.1-labeled neurons in the ipsilateral and contralateral L4 DRGs on day 7 after SNL or sham surgery. Representative immunohistochemical staining (left panels) and a summary of the analysis on the number of K2P1.1-labeled neurons (right graphs). n = 5 mice/group. *P < 0.05 vs. the corresponding sham group by two-tailed paired t-test. Scale bar: 60 µm. (e) K2P1.1 protein expression in the ipsilateral L3/4 DRGs on day 7 after axotomy or sham surgery. Representative Western blots (top panels) and a summary of densitometric analysis (bottom graphs). n = 8 mice/group. *P < 0.05 vs. the corresponding control group (Sham) by two-tailed paired t-test. SNL = spinal nerve ligation.
Rescuing DRG K2P1.1 reduction blocked neuropathic pain development
Is the reduced K2P1.1 in the injured DRG involved in neuropathic genesis? To address this question, we examined the effect of overexpressing K2P1.1 in the injured DRG through microinjection of AAV-DJ expressing full-length K2P1.1 mRNA (AAV-K2P1.1) into the ipsilateral L4 DRG to rescue SNL-induced downregulation of K2P1.1. AAV-DJ expressing EGFP (AAV-EGFP) was used as a control. Like AAV5 reported previously,13–16,18 AAV-DJ requires three to four weeks to become expressed and its expression lasts for at least six weeks (Figure 5). EGFP-labeled AAV-DJ was limited to the ipsilateral L4 DRG neurons and their fibers and terminals four weeks after microinjection (Figure 5(e), (g), and (h)). Approximately 66.2% of L4 DRG neurons were labeled by EGFP (Figure 5(e)). EGFP fluorescence was detected in many nerve fibers and terminals innervating the dorsal horn ipsilateral to the microinjection (Figure 5(g) and (h)). No cell bodies of spinal cord neurons were labeled. Consistent with previous reports,13–16,18 the injected DRG, stained with hematoxylin and eosin, retained its structural integrity and contained no visible leukocytes (data not shown).
Figure 5.
EGFP-labeled AAV-DJ is limited in L4 DRG and its fibers and terminals on the ipsilateral side after AAV-EGFP injection into the unilateral L4 DRG. (a–f). Time course of EGFP-labeled AAV-DJ expression in the ipsilateral L4 DRG after viral injection. (g). EGFP-labeled AAV-DJ expression in the ipsilateral L4 spinal cord on day 28 after viral injection. (h). High magnification of the outlined region from g. Scale bars: 50 µm in a–f; 100 µm in g; 25 µm in h.
To examine the effect of AAV-K2P1.1 microinjection into the injured DRG on SNL-induced development of pain hypersensitivity, we carried out the SNL model or sham surgery four weeks after viral microinjection. As expected, the level of K2P1.1 protein was significantly decreased in the ipsilateral L4 DRG on day 7 post-SNL from the SNL plus PBS or AAV-EGFP group compared to the naive group (Figure 6(a)). This decrease was completely rescued in the SNL plus AAV-K2P1.1 group (Figure 6(a)). In the sham plus AAV-K2P1.1 group, the amount of K2P1.1 protein in the injected DRG markedly increased by 2.27-fold compared to the naive group (Figure 6(a)). Consistent with previous studies,13,15,18,22–24 SNL led to mechanical, thermal, and cold pain hypersensitivities on the ipsilateral side from day 3 to 7 after SNL in the PBS- or AAV-EGFP-injected group. The paw withdrawal frequencies in response to mechanical stimuli applied to the ipsilateral hind paw were significantly increased as compared with preinjury baseline values, a behavioral indication of mechanical pain hypersensitivity (Figure 6(b) and (c)). The paw withdrawal latencies of the ipsilateral hind paw in response to thermal and cold were markedly less than those at baseline, an indication of thermal and cold pain hypersensitivities, respectively (Figure 6(d) and (e)). Microinjection of AAV-K2P1.1 did not alter basal paw withdrawal responses to mechanical, thermal, or cold stimuli on the ipsilateral hid paw of sham group (Figure 6(b) to (e)), but microinjection of AAV-K2P1.1 abolished SNL-induced pain hypersensitivities (Figure 6(b) to (e)). Compared with the baseline level, there were no significant increases in paw withdrawal frequencies in response to mechanical stimuli and no marked decreases in paw withdrawal latency in response to thermal or cold stimulation on the ipsilateral side from the AAV-K2P1.1 plus SNL group (Figure 6(b) to (e)). As expected, microinjection of neither PBS nor virus altered basal paw withdrawal responses on the contralateral side (Figure 6(f) to (h)).
Figure 6.
Rescuing K2P1.1 expression in the injured DRG blocked SNL-induced pain hypersensitivities during the development period. SNL or sham surgery was carried out four weeks after microinjection of PBS, AAV-K2P1.1, or AAV-EGFP into the ipsilateral L4 DRG. (a) K2P1.1 protein expression in the ipsilateral L4 DRG on day 7 after SNL or sham surgery from the treated groups as indicated. Representative Western blots (left panels) and a summary of densitometric analysis (right graphs). n = 8 mice/group. *P < 0.05 vs. naive mice. #P < 0.05 vs. the PBS plus SNL group, one-way ANOVA followed by post hoc Tukey test. (b)–(h) Paw withdrawal responses to 0.07 g von Frey filament (b), 0.4 g von Frey filament (c), thermal stimulation (d), and cold stimulation (e) on the ipsilateral side and paw withdrawal responses to 0.07 g von Frey filament (f), 0.4 g von Frey filament (g), and thermal stimulation (h) on the contralateral side from the treated groups as indicated. n = 8 mice/group. *P < 0.05 vs. the PBS plus SNL group at the corresponding time points, by two-way ANOVA followed by post hoc Tukey test.
Rescuing DRG K2P1.1 reduction attenuated neuropathic pain maintenance
We also investigated the role of DRG K2P1.1 in the maintenance of neuropathic pain. We subjected mice to SNL two weeks after DRG viral microinjection. Mechanical, thermal, and cold pain hypersensitivities were completely developed in both the AAV-EGFP- and AAV-K2P1.1-injected groups on day 7 post-SNL (Figure 7(a) to (d)). These pain hypersensitivities were significantly attenuated on days 14, 21, and 28 after SNL in the AAV-K2P1.1-injected group, but not in the AAV-EGFP-injected group (Figure 7(a) to (d)). Microinjection of neither AAV-K2P1.1 nor AAV-EGFP altered paw withdrawal frequency or latency on the contralateral side (Figure 7(e) to (g)).
Figure 7.
Rescuing K2P1.1 expression in the injured DRG blocked SNL-induced pain hypersensitivities during the maintenance period. SNL or sham surgery was carried out two weeks after microinjection of AAV-K2P1.1 or AAV-EGFP into the ipsilateral L4 DRG. (a)–(g) Paw withdrawal responses to 0.07 g von Frey filament (a), 0.4 g von Frey filament (b), thermal stimulation (c), and cold stimulation (d) on the ipsilateral side and paw withdrawal responses to 0.07 g von Frey filament (e), 0.4 g von Frey filament (f), and thermal stimulation (g) on the contralateral side from the treated groups as indicated. n = 6 mice/group. *P < 0.05 vs. the AAV-EGFP plus SNL group at the corresponding time points by two-way ANOVA followed by post hoc Tukey test.
Overexpressing K2P1.1 in the DRG did not affect locomotor functions
We finally determined whether overexpressing K2P1.1 in the injured DRG influenced locomotor function of experimental animals. As shown in Table 1, microinjection of AAV-K2P1.1, AAV-EGFP, and PBS did not produce any effects on locomotor functions including placing, grasping, and righting reflexes among the treated groups. Convulsions and hypermobility were not observed in any of the injected animals. In addition, we did not observe any significant difference in general behaviors, including the gait and spontaneous activity, between the PBS- and virus-injected groups.
Table 1.
Mean (±SEM) changes in locomotor test.
| Groups | Placing | Grasping | Righting |
|---|---|---|---|
| PBS + SNL | 5 (0) | 5 (0) | 5 (0) |
| EGFP + SNL | 5 (0) | 5 (0) | 5 (0) |
| K2P1.1 + SNL | 5 (0) | 5 (0) | 5 (0) |
| K2P1.1 + sham | 5 (0) | 5 (0) | 5 (0) |
N = 8 mice/group, five trials. PBS: phosphate-buffered saline; SNL: spinal nerve ligation; EGFP: enhanced green fluorescent protein.
Discussion
Four major findings arose from the present study. First, K2P1.1 was highly expressed in the DRG and distributed in small-, medium-, and large-sized DRG neurons. Second, K2P1.1 channel was significantly downregulated in the injured DRG during both the development and maintenance periods of SNL-induced pain hypersensitivities. Third, rescuing this downregulation by overexpressing K2P1.1 mRNA in the injured DRG blocked the development and maintenance of SNL-induced neuropathic pain. Finally, overexpressing DRG K2P1.1 did not affect acute pain and locomotor functions. These findings suggest that the DRG K2P1.1 channel may be a novel potential target for preventing and treating neuropathic pain.
K2P1.1 mRNA is abundant in peripheral and central nerve systems of rodents and human beings.25,26 In mouse forebrain, K2P1.1 was highly expressed in astrocyte and contributed to its passive conductance.27 In contrast, in mouse and rat DRG, the data from present study and the previous work12 showed that K2P1.1 was expressed exclusively in the neurons, but not in the satellite glial cells. These findings indicate that the cell-type expression pattern of K2P1.1 may have tissue-related specificity. We further observed that, in addition to medium- and large-sized DRG neurons reported previously,12 K2P1.1 channel was also distributed in small-sized DRG neurons. The discrepancy in DRG subpopulation distribution pattern between the present study and previous work12 is unknown, but may be related to the differences in the use of primary antibodies.
Total 15 members of the K2P channel family have been identified.5,6 The expression of several members of this family has been found to be downregulated in the DRG under pathological pain conditions. DRG expression of TASK1, TASK2, TASK3, and TRESK mRNAs was decreased in the first four days after Complete Freund’s Adjuvant-induced inflammation and their decrease was associated with inflammation-evoked spontaneous pain behavior.28 The expression of TRESK transcript, but not TEEK1, TREK2, TASK1, and TRAAK transcripts, in the injured DRG was diminished three weeks after axotomizing sciatic nerve.29 Injured DRG displayed a significant reduction in the level of K2P1.1 mRNA one, two, and four weeks following spared nerve injury and in the level of TASK3 mRNA only one week following spared nerve injury.12 Any knowledge of the changes in the expression of DRG K2P channels in the development of neuropathic pain (i.e., within one week after peripheral nerve injury) is still lacking. The present study demonstrated a marked downregulation of K2p1.1 mRNA and protein in the injured DRG 3, 7, and 14 days after SNL. Given that SNL-induced pain hypersensitivities appears on day 3, reaches a peak on day 7, and lasts for at least 14 days post-SNL,18,30 our finding strongly suggests that SNL-induced downregulation of K2P1.1 expression in the injured DRG is associated with the development and maintenance of SNL-induced neuropathic pain. How SNL led to DRG K2P1.1 downregulation is still unclear, but this downregulation might be caused by epigenetic modifications (e.g., DNA methylation and histone modification) and/or the decrease in RNA stability. Our recent work showed that the expression of one DNA methyltransferase, DNMT3a, was increased in the injured DRG neurons and that this increase was essential for the silencing of DRG K2P1.1 under chemotherapy-induced neuropathic pain conditions (data not shown). Whether DNMT3a contributes to SNL-induced DRG K2P1.1 downregulation remains to be determined.
We provided what we believe to be the first evidence that K2P1.1 downregulation in the injured DRG contributes to neuropathic pain development and maintenance. Extensive studies have revealed that K2P channels are responsible for the regulation of resting membrane potential and cellular excitability.7,8 Under physiological conditions, K2P generates hyperpolarizing leak currents that stabilize cells below firing threshold.7,8 As K2P1.1 exhibits an inwardly rectifying profile in heterologous expression system,11 K2P1.1 is thought to be important in setting the resting membrane potential of cells. Interestingly, we found that overexpressing K2P1.1 in the DRG neurons did not affect acute pain. This no effect may be attributed to the fact that DRG neuronal excitability is dependent on the expression and function of sodium channels, calcium channels, and potassium channels. Our recent work demonstrated that DRG K2P1.1 knockdown in naive mice did not alter action potential threshold although this knockdown depolarized resting membrane potential (data not shown). Thus, without any changes in the expression and function of other channels under normal conditions, the hyperdepolarization of the resting membrane potential produced alone by DRG overexpressing K2P1.1 might not be enough to affect action potential as well as acute pain induced by acute noxious stimulation.
However, SNL-induced DRG K2P 1.1 downregulation observed in the present study is likely involved in the formation of abnormal ectopic discharges in the injured DRG and consequent initiation and expression of SNL-induced pain hypersensitivities. Our recent work revealed that DRG K2P1.1 knockdown depolarized resting membrane potential, decreased current threshold for the activation in the action potentials, and increased the number of action potentials in small, medium, and large DRG neurons and led to neuropathic pain-like symptoms (data not shown). It is very likely that SNL-induced K2P1.1 downregulation in the DRG neurons renders those neurons more prone to hyperexcitability. The increase in excitability of small, medium, and large DRG neurons may drive the release of the neurotransmitters and/or neuromodulators from their primary afferent terminals and lead to spinal central sensitization.1,3,31,32 The latter contributes to the development and maintenance of neuropathic pain.1,3,31,32 This conclusion is strongly supported by our behavioral observations that rescuing K2P1.1 downregulation in the injured DRG through microinjection of AAV-K2P1.1 into the ipsilateral L4 DRG attenuated the development and maintenance of SNL-induced pain hypersensitivities. Therefore, nerve injury-induced DRG K2P1.1 downregulation likely participates in the mechanisms underlying neuropathic pain induction and maintenance.
In summary, we have identified the expression and distribution of K2P1.1 in DRG neurons and revealed its time-dependent downregulation in the injured DRG following peripheral nerve injury. Given that restoring the downregulation of K2P1.1 in the injured DRG neurons alleviated pain hypersensitivities in both the development and maintenance of SNL-induced neuropathic pain, without altering acute pain and motor function, K2P1.1 may be a target for the management of this disorder. Nevertheless, it is worth noting that K2P1.1 is also expressed in other body tissues. Targeting K2P1.1 may have potential off-target effects that remains to be further investigated.
Acknowledgments
The authors thank Dr. Maha Abdellatif for kindly providing pAAV-DJ-RC, pAAV-MCS, pHelper vector, and HEK293-AAV cell line.
Author Contributions
Y-XT and TY conceived the project and supervised all experiments. QM, JY, SW, and Y-XT assisted with experimental design. QM, JY, MX, and SW carried out animal surgery and molecular, biochemical, immunostaining, and behavioral experiments. QM, JY, SW, MX, AB, TY, and Y-XT analyzed the data. QM and Y-XT drafted the manuscript. All authors read and discussed the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH Grants (DA033390, NS094664, NS094224, and HL117684).
References
- 1.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron 2006; 52: 77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung JM, Chung K. Importance of hyperexcitability of DRG neurons in neuropathic pain. Pain Pract 2002; 2: 87–97. [DOI] [PubMed] [Google Scholar]
- 3.Devor M. Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp Brain Res 2009; 196: 115–128. [DOI] [PubMed] [Google Scholar]
- 4.Wang W, Gu J, Li YQ, et al. Are voltage-gated sodium channels on the dorsal root ganglion involved in the development of neuropathic pain? Mol Pain 2011; 7: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kindler CH, Yost CS. Two-pore domain potassium channels: new sites of local anesthetic action and toxicity. Reg Anesth Pain Med 2005; 30: 260–274. [DOI] [PubMed] [Google Scholar]
- 6.Lotshaw DP. Biophysical, pharmacological, and functional characteristics of cloned and native mammalian two-pore domain K+ channels. Cell Biochem Biophys 2007; 47: 209–256. [DOI] [PubMed] [Google Scholar]
- 7.Berg AP, Bayliss DA. Striatal cholinergic interneurons express a receptor-insensitive homomeric TASK-3-like background K+ current. J Neurophysiol 2007; 97: 1546–1552. [DOI] [PubMed] [Google Scholar]
- 8.Taverna S, Tkatch T, Metz AE, et al. Differential expression of TASK channels between horizontal interneurons and pyramidal cells of rat hippocampus. J Neurosci 2005; 25: 9162–9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol 2000; 279: F793–F801. [DOI] [PubMed] [Google Scholar]
- 10.Talley EM, Lei Q, Sirois JE, et al. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron 2000; 25: 399–410. [DOI] [PubMed] [Google Scholar]
- 11.Lesage F, Guillemare E, Fink M, et al. A pH-sensitive yeast outward rectifier K+ channel with two pore domains and novel gating properties. J Biol Chem 1996; 271: 4183–4187. [DOI] [PubMed] [Google Scholar]
- 12.Pollema-Mays SL, Centeno MV, Ashford CJ, et al. Expression of background potassium channels in rat DRG is cell-specific and down-regulated in a neuropathic pain model. Mol Cell Neurosci 2013; 57: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan L, Guan X, Wang W, et al. Impaired neuropathic pain and preserved acute pain in rats overexpressing voltage-gated potassium channel subunit Kv1.2 in primary afferent neurons. Mol Pain 2014; 10: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Gu X, Sun L, et al. Dorsal root ganglion myeloid zinc finger protein 1 contributes to neuropathic pain after peripheral nerve trauma. Pain 2015; 156: 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang L, Gu X, Zhao JY, et al. G9a participates in nerve injury-induced Kcna2 downregulation in primary sensory neurons. Sci Rep 2016; 6: 37704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang L, Zhao JY, Gu X, et al. G9a inhibits CREB-triggered expression of mu opioid receptor in primary sensory neurons following peripheral nerve injury. Mol Pain. Epub ahead of print 7 December 2016. DOI: 10.1177/1744806916682242. [DOI] [PMC free article] [PubMed]
- 17.Zhang J, Liang L, Miao X, et al. Contribution of the suppressor of variegation 3-9 homolog 1 in dorsal root ganglia and spinal cord dorsal horn to nerve injury-induced nociceptive hypersensitivity. Anesthesiology 2016; 125: 765–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao X, Tang Z, Zhang H, et al. A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat Neurosci 2013; 16: 1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rigaud M, Gemes G, Barabas ME, et al. Species and strain differences in rodent sciatic nerve anatomy: Implications for studies of neuropathic pain. Pain 2008; 136: 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang L, Fan L, Tao B, et al. Protein kinase B/Akt is required for complete Freund’s adjuvant-induced upregulation of Nav1.7 and Nav1.8 in primary sensory neurons. J Pain 2013; 14: 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu JT, Zhou X, Zhao X, et al. Opioid receptor-triggered spinal mTORC1 activation contributes to morphine tolerance and hyperalgesia. J Clin Invest 2014; 124: 592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atianjoh FE, Yaster M, Zhao X, et al. Spinal cord protein interacting with C kinase 1 is required for the maintenance of complete Freund’s adjuvant-induced inflammatory pain but not for incision-induced post-operative pain. Pain 2010; 151: 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liaw WJ, Zhu XG, Yaster M, et al. Distinct expression of synaptic NR2A and NR2B in the central nervous system and impaired morphine tolerance and physical dependence in mice deficient in postsynaptic density-93 protein. Mol Pain 2008; 4: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Petralia RS, Takamiya K, et al. Preserved acute pain and impaired neuropathic pain in mice lacking protein interacting with C kinase 1. Mol Pain 2011; 7: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Talley EM, Solorzano G, Lei Q, et al. Cns distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci 2001; 21: 7491–7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu S, Marie LB, Miao X, et al. Dorsal root ganglion transcriptome analysis following peripheral nerve injury in mice. Mol Pain 2016; 12: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou M, Xu G, Xie M, et al. TWIK-1 and TREK-1 are potassium channels contributing significantly to astrocyte passive conductance in rat hippocampal slices. J Neurosci 2009; 29: 8551–8564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsh B, Acosta C, Djouhri L, et al. Leak K(+) channel mRNAs in dorsal root ganglia: relation to inflammation and spontaneous pain behaviour. Mol Cell Neurosci 2012; 49: 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tulleuda A, Cokic B, Callejo G, et al. TRESK channel contribution to nociceptive sensory neurons excitability: modulation by nerve injury. Mol Pain 2011; 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao YX, Rumbaugh G, Wang GD, et al. Impaired NMDA receptor-mediated postsynaptic function and blunted NMDA receptor-dependent persistent pain in mice lacking postsynaptic density-93 protein. J Neurosci 2003; 23: 6703–6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009; 10: 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weissner W, Winterson BJ, Stuart-Tilley A, et al. Time course of substance P expression in dorsal root ganglia following complete spinal nerve transection. J Comp Neurol 2006; 497: 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]