Introduction
Dermatomyositis (DM) is an idiopathic inflammatory myopathy that can affect children and adults and is characterized by muscle inflammation and characteristic cutaneous findings.1 In addition to multiorgan involvement, adult patients with DM may have up to a 30% risk of associated malignancy. Clinically amyopathic DM (CADM) accounts for approximately 20% of all cases and is diagnosed based on the presence of pathognomonic cutaneous involvement.2, 3 Antibody against melanoma differentiation–associated protein 5 (MDA5) was recently found to be specific for CADM associated with rapidly progressive interstitial lung disease (ILD).4 To our knowledge, there are no other cases of MDA5-positive CADM in an HIV-positive patient. However, multiple cases of classic DM have been reported in seropositive patients and are summarized in Table I.5, 6, 7, 8, 9, 10, 11
Table I.
Reported cases of dermatomyositis in HIV-positive individuals
| Study | Country of origin | Age (y) | Sex (F/M) | Type of DM (classic/CADM) | CD4 count (cells/μL) | ANA titer | Other antibodies | Presence of cancer | Treatment |
|---|---|---|---|---|---|---|---|---|---|
| Baguley et al5 | United Kingdom | 22 | M | Classic | 117 | Negative | N/A | None | Prednisolone followed by azathioprine, ART |
| Gresh et al6 | US | 22 | M | Classic | 160 | 1:1,280 | N/A | N/A | Methylprednisolone followed by prednisone, ART |
| Marfatia et al7 | India | 30 | F | Classic | 379 | Negative | N/A | None | Prednisone, ART |
| Carroll and Holmes8 | US | 27 | M | Classic | 135 | Negative | N/A | Not reported | Prednisone, ART |
| Ogoina et al9 | Nigeria | 40 | F | Classic | 383 | N/A | N/A | Not reported | Diclofenac and prednisolone, ART |
| Rajadhyaksha et al10 | India | 50 | F | Classic | 222 | 1:160 | N/A | None | Prednisolone and methotrexate, ART |
| Sharma et al11 | India | 8 | M | Classic | 770 | Negative | N/A | Not reported | Prednisolone followed by low-dose methotrexate, ART |
ANA, Antinuclear antibody; N/A, information not available.
Case report
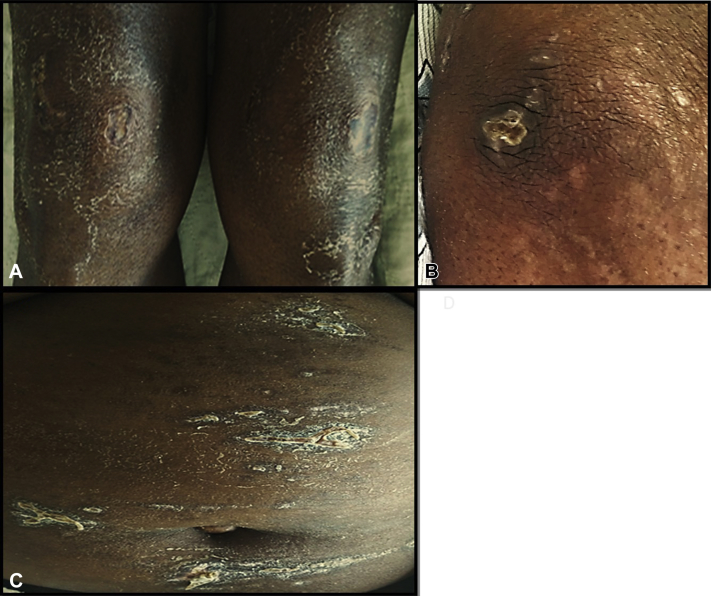
A 52-year-old woman of Haitian origin, known for well-controlled HIV-1 on antiretroviral therapy (ART), presented to the emergency department 30 days before admission with polyarthralgia, dry cough, and pruritic dry, scaly cutaneous lesions over her extensor surfaces as well as erythema of her nail beds. Results of initial laboratory tests were normal except for elevated C-reactive protein (19.1 mg/L), lactate dehydrogenase (537 U/L), and ferritin (553 μg/L). Additionally, a computerized tomography scan of the lungs showed bilateral vague interstitial infiltrates. She was sent home on antibiotics. She presented again 1 month later with progressive dyspnea, arthralgias, hemoptysis and progressive cutaneous lesions. On skin examination, she had dusky violaceous patches with central stellate shallow ulcerations and crusting over elbows, knees, and abdomen (Fig 1). Heliotrope rash was present on the eyelids. The remainder of the cutaneous examination was unremarkable. Physical examination was also remarkable for coarse pulmonary crackles without muscle weakness. Nailfold videocapillaroscopy found alternating areas of capillary dilatation and dropout. Two biopsies specimens were taken (periungual and elbow skin), which showed vacuolar degeneration of the basal cell layer and mild perivascular infiltrate, suggesting dermatomyositis. The creatine kinase, aldolase, and electromyography were normal. Serum ferritin level was increased to 1104 μg/L. Repeat computed tomography scan of the thorax showed rapidly progressive ILD. Wedge lung biopsy confirmed acute fibrinous and organizing pneumonia. Hydroxychloroquine, cyclosporine, prednisone, and intravenous immunoglobulins were started in addition to continuing broad spectrum antibiotics and antifungals until infectious causes were ruled out. Whole-body positron emission tomography scan did not find any suspicious lesions for malignancy. Given the clinicopathologic picture suggestive of amyopathic dermatomyositis with rapidly progressive ILD, an antibody panel was ordered, which found MDA5 positivity (66 median fluorescence units [MFU], immunoblot assay [EUROIMMUN, Luebeck, Germany] [negative, <30; positive, 30–80; strong positive, >80]). Despite treatment, the ILD progressed, and the patient was transferred to the intensive care unit for mechanical ventilation. She subsequently received 3 pulses of intravenous cyclophosphamide, polymyxin B hemoperfusion, plasmapheresis, and, given the lack of clinical response, rituximab (1g intravenous, 2 doses at 2-week interval). After 4 months of supportive care and respiratory assistance, the patient finally improved, ferritin level decreased to 176 μg/L, and she was extubated. She was transferred to a rehabilitation program and is slowly recuperating her respiratory function. She remains on prednisone, 10 mg/d, hydroxychloroquine, 400 mg/d, and cyclosporin 100 mg/d.
Fig 1.
Clinical features on presentation included ulcerated crusted dusky violaceous plaques on the knees (A), elbows (B), and abdomen (C).
Given previous reports suggesting that MDA5 antibodies may be used as an objective marker of disease activity, we have obtained MDA5 titers at baseline (66 MFU), before polymyxin B therapy (32 MFU), after 24 hours (27 MFU), and 48 hours (31 MFU) after polymyxin and upon clinical improvement and discharge from the hospital (42 MFU).12 In our patient, despite marked clinical improvement, MDA5 levels failed to correlate with disease activity.
Discussion
This case highlights several challenges surrounding the diagnosis and management of CADM. Anti-MDA5 positivity was reported to be associated with a specific presentation of CADM featuring painful ulcerations over extensor surfaces and nailfolds, hyperferritinemia, and frequent treatment-resistant, rapidly progressive ILD associated with poor prognosis.3, 13 Interestingly, MDA5 protein functions as an intracellular pathogen sensor involved in the recognition and mounting of immune response to viral RNA.3, 4 Hence, there could be an association between CADM and viral infections perhaps explaining the incidence of ILD. Testing MDA5 antibody may be of clinical utility to predict prognostic outcome and may suggest need for more aggressive therapy; however, further studies are necessary to determine if MDA5 is a useful marker of disease activity, given that it did not correlate with it in our patient.
This is a case of severe MDA5-positive CADM in an HIV-positive patient. Thanks to highly effective ART therapy, most HIV patients now have increased rate of survival with restoration of immune function. Although autoimmune conditions are usually rare in patients with AIDS because of profound immunosuppression, they can arise in acute HIV infection (normal CD4 count) and after immune reconstitution owing to ART. Molecular mimicry triggered by an infectious pathogen in context of HIV-induced defective cellular immunity and overreactive humoral response may account for this observed surge.14 Zandman et al14 have classified the likelihood and type of autoimmune conditions arising in the setting of HIV according to the infection stage and CD4 count.14 Treatment of autoimmune conditions, and dermatomyositis particularly, in the setting of HIV infection is challenging. Interestingly, hydroxychloroquine was reported to be safe and to even decrease HIV viral load; however, the use of immunosuppressive medications needs to be balanced against the potential increase in viral load and immunosuppression.15 Future research is needed to better understand the pathogenesis and to guide the treatment decisions for autoimmune conditions in seropositive population.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Iaccarino L., Ghirardello A., Bettio S. The clinical features, diagnosis and classification of dermatomyositis. J Autoimmun. 2014;48-49:122–127. doi: 10.1016/j.jaut.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Gerami P., Schope J.M., McDonald L., Walling H.W., Sontheimer R.D. A systematic review of adult-onset clinically amyopathic dermatomyositis (dermatomyositis sine myositis): a missing link within the spectrum of the idiopathic inflammatory myopathies. J Am Acad Dermatol. 2006;54(4):597–613. doi: 10.1016/j.jaad.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 3.Parronchi P., Radice A., Palterer B., Liotta F., Scaletti C. MDA5-positive dermatomyositis: an uncommon entity in Europe with variable clinical presentations. Clin Mol Allergy. 2015;13:22. doi: 10.1186/s12948-015-0031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakashima R., Hosono Y., Mimori T. Clinical significance and new detection system of autoantibodies in myositis with interstitial lung disease. Lupus. 2016;25(8):925–933. doi: 10.1177/0961203316651748. [DOI] [PubMed] [Google Scholar]
- 5.Baguley E., Wolfe C., Hughes G.R.V. Dermatomyositis in HIV infection [letters to the editor] Br J Rheum. 1988;27:493–500. doi: 10.1093/rheumatology/27.6.493-a. [DOI] [PubMed] [Google Scholar]
- 6.Gresh J.P., Aguilar J.L., Espinoza L.R. Human immunodeficiency virus (HIV) infection-associated dermatomyositis [letter] J Rheumatol. 1989;16(10):1397–1398. [PubMed] [Google Scholar]
- 7.Marfatia Y.S., Ghiya R.A., Chaudhary D. Dermatomyositis in a human immunodeficiency virus-infected person. Indian J Dermatol Venereol Leprol. 2008;74(3):241–243. doi: 10.4103/0378-6323.41370. [DOI] [PubMed] [Google Scholar]
- 8.Carroll M., Holmes R. Dermatomyositis and HIV infection: case report and review of the literature. Rheumatol Int. 2011;31(5):673–679. doi: 10.1007/s00296-009-1231-x. [DOI] [PubMed] [Google Scholar]
- 9.Ogoina D., Umar A., Obiako O.R. Dermatomyositis associated with HIV-1 infection in a Nigerian adult female: a case report. African Health Scieneces. 2012;12(1):74–76. [PMC free article] [PubMed] [Google Scholar]
- 10.Rajadhyaksha A., Baheti T., Mehra S., Sonawale A., Jain N. Dermatomyositis: a rare presentation of HIV seroconversion illness. J Clin Rheumatol. 2012;18(6):298–300. doi: 10.1097/RHU.0b013e318268566c. [DOI] [PubMed] [Google Scholar]
- 11.Sharma V.K., Kakkar A., Lodha R., Suri V., Kabra S.K. HIV with juvenile dermatomyositis. Indian J Pediatr. 2014;81(9):926–928. doi: 10.1007/s12098-013-1160-2. [DOI] [PubMed] [Google Scholar]
- 12.Muro Y., Sugiura K., Hoshino K., Akiyama M. Disappearance of anti-MDA-5 autoantibodies in clinically amyopathic DM/interstitial lung disease during disease remission. Rheumatology (Oxford) 2012;51(5):800–804. doi: 10.1093/rheumatology/ker408. [DOI] [PubMed] [Google Scholar]
- 13.Narang N.S., Casciola-Rosen L., Li S., Chung L., Fiorentino D.F. Cutaneous ulceration in dermatomyositis: association with anti-melanoma differentiation-associated gene 5 antibodies and interstitial lung disease. Arthritis Care Res (Hobeken) 2015;67(5):667–672. doi: 10.1002/acr.22498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zandman-Goddard G., Shoenfeld Y. HIV and autoimmunity. Autoimmun rev. 2002;1(6):329–337. doi: 10.1016/s1568-9972(02)00086-1. [DOI] [PubMed] [Google Scholar]
- 15.Roszkiewicz J., Smolewska E. Kaleidoscope of autoimmune diseases in HIV infection. Rheumat Int. 2016;36(11):1481–1491. doi: 10.1007/s00296-016-3555-7. [DOI] [PubMed] [Google Scholar]