Abstract
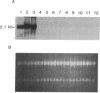
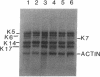
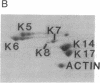
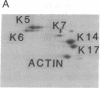
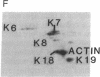
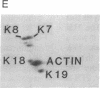
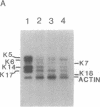
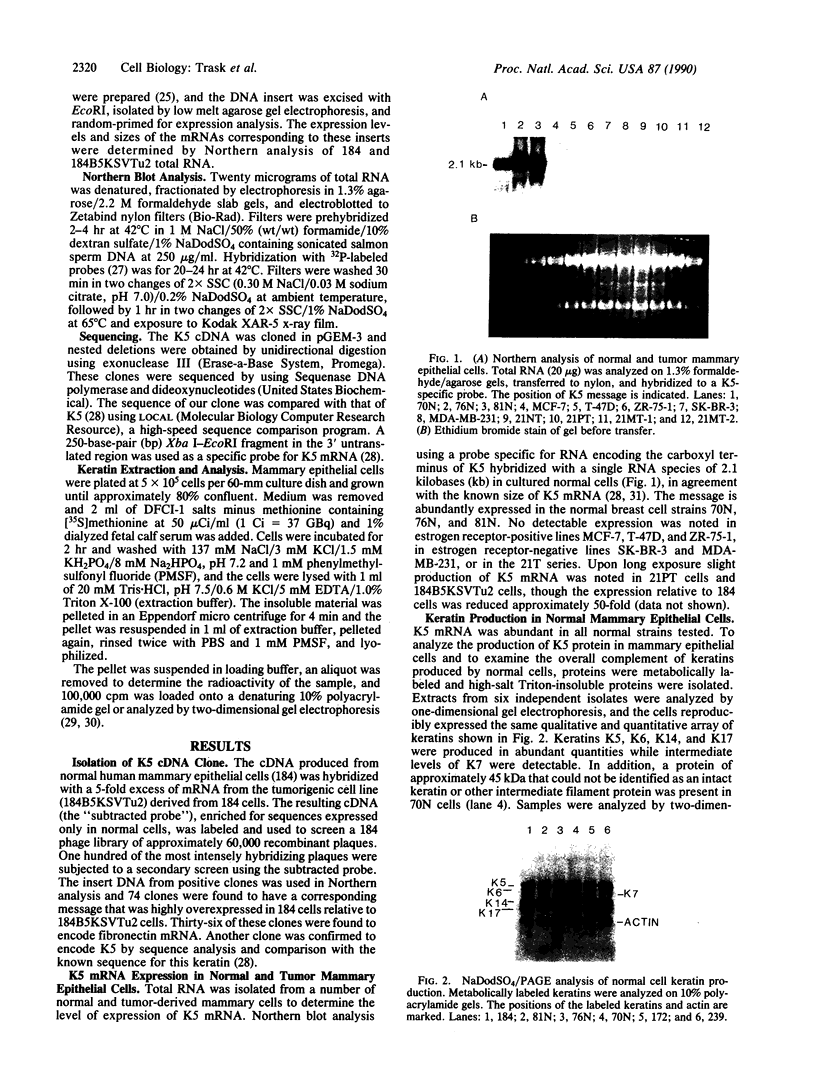
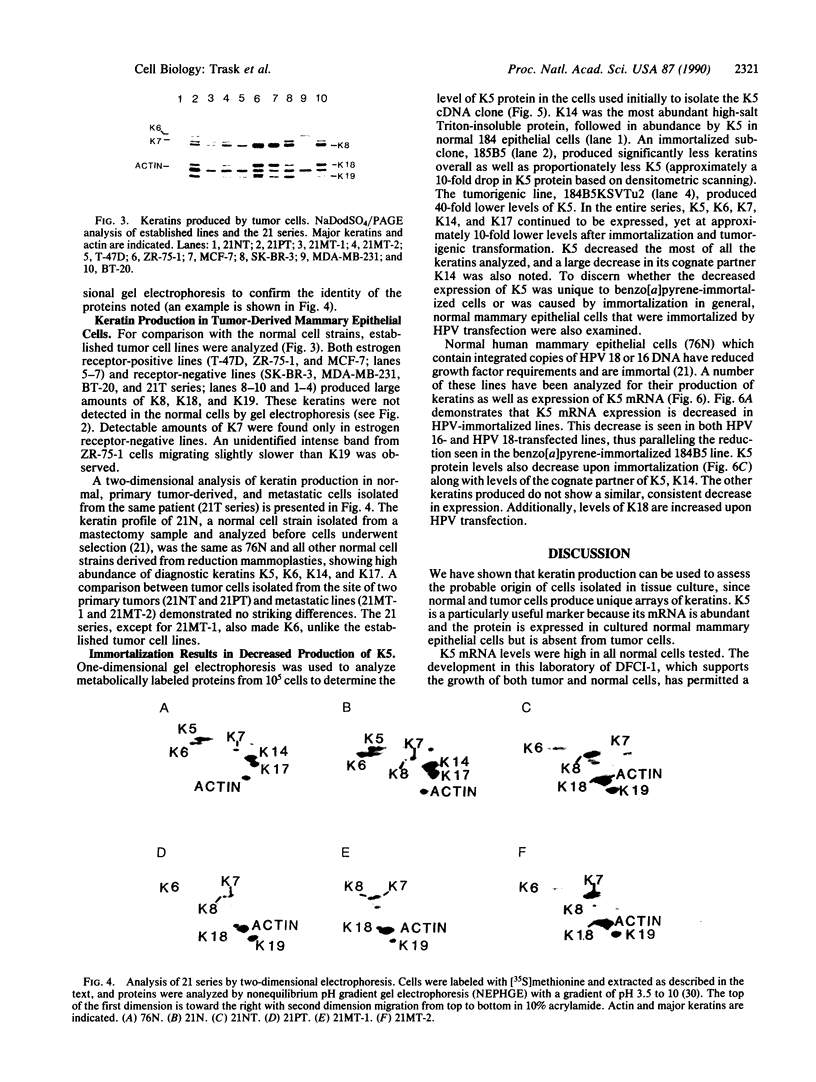
Keratin 5 (K5) mRNA and protein are shown to be expressed in normal mammary epithelial cells in culture and are absent from tumor-derived cell lines. To extend these findings, the full complements of keratins in normal, immortalized, and tumor cells were compared. It is shown here that normal cells produce keratins K5, K6, K7, K14, and K17, whereas tumor cells produce mainly keratins K8, K18, and K19. In immortalized cells, which are preneoplastic or partially transformed, the levels of K5 mRNA and protein are lower than in normal cells, whereas the amount of K18 is increased. Thus, K5 is an important marker in the tumorigenic process, distinguishing normal from tumor cells, and decreased K5 expression correlates with tumorigenic progression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Band V., Sager R. Distinctive traits of normal and tumor-derived human mammary epithelial cells expressed in a medium that supports long-term growth of both cell types. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1249–1253. doi: 10.1073/pnas.86.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band V., Zajchowski D., Kulesa V., Sager R. Human papilloma virus DNAs immortalize normal human mammary epithelial cells and reduce their growth factor requirements. Proc Natl Acad Sci U S A. 1990 Jan;87(1):463–467. doi: 10.1073/pnas.87.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band V., Zajchowski D., Stenman G., Morton C. C., Kulesa V., Connolly J., Sager R. A newly established metastatic breast tumor cell line with integrated amplified copies of ERBB2 and double minute chromosomes. Genes Chromosomes Cancer. 1989 Sep;1(1):48–58. doi: 10.1002/gcc.2870010109. [DOI] [PubMed] [Google Scholar]
- Blobel G. A., Moll R., Franke W. W., Vogt-Moykopf I. Cytokeratins in normal lung and lung carcinomas. I. Adenocarcinomas, squamous cell carcinomas and cultured cell lines. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45(4):407–429. doi: 10.1007/BF02889883. [DOI] [PubMed] [Google Scholar]
- Brawer M. K., Peehl D. M., Stamey T. A., Bostwick D. G. Keratin immunoreactivity in the benign and neoplastic human prostate. Cancer Res. 1985 Aug;45(8):3663–3667. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Connell N. D., Rheinwald J. G. Regulation of the cytoskeleton in mesothelial cells: reversible loss of keratin and increase in vimentin during rapid growth in culture. Cell. 1983 Aug;34(1):245–253. doi: 10.1016/0092-8674(83)90155-1. [DOI] [PubMed] [Google Scholar]
- Cooper D., Schermer A., Sun T. T. Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications, and limitations. Lab Invest. 1985 Mar;52(3):243–256. [PubMed] [Google Scholar]
- Dairkee S. H., Puett L., Hackett A. J. Expression of basal and luminal epithelium-specific keratins in normal, benign, and malignant breast tissue. J Natl Cancer Inst. 1988 Jul 6;80(9):691–695. doi: 10.1093/jnci/80.9.691. [DOI] [PubMed] [Google Scholar]
- Eckert R. L., Green H. Cloning of cDNAs specifying vitamin A-responsive human keratins. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4321–4325. doi: 10.1073/pnas.81.14.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Regulation of terminal differentiation of cultured human keratinocytes by vitamin A. Cell. 1981 Sep;25(3):617–625. doi: 10.1016/0092-8674(81)90169-0. [DOI] [PubMed] [Google Scholar]
- Kim K. H., Stellmach V., Javors J., Fuchs E. Regulation of human mesothelial cell differentiation: opposing roles of retinoids and epidermal growth factor in the expression of intermediate filament proteins. J Cell Biol. 1987 Dec;105(6 Pt 2):3039–3051. doi: 10.1083/jcb.105.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krepler R., Denk H., Weirich E., Schmid E., Franke W. W. Keratin-like proteins in normal and neoplastic cells of human and rat mammary gland as revealed by immunofluorescence microscopy. Differentiation. 1981;20(3):242–252. doi: 10.1111/j.1432-0436.1981.tb01179.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lersch R., Fuchs E. Sequence and expression of a type II keratin, K5, in human epidermal cells. Mol Cell Biol. 1988 Jan;8(1):486–493. doi: 10.1128/mcb.8.1.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lersch R., Stellmach V., Stocks C., Giudice G., Fuchs E. Isolation, sequence, and expression of a human keratin K5 gene: transcriptional regulation of keratins and insights into pairwise control. Mol Cell Biol. 1989 Sep;9(9):3685–3697. doi: 10.1128/mcb.9.9.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg K., Rheinwald J. G. Suprabasal 40 kd keratin (K19) expression as an immunohistologic marker of premalignancy in oral epithelium. Am J Pathol. 1989 Jan;134(1):89–98. [PMC free article] [PubMed] [Google Scholar]
- Miettinen M., Franssila K., Lehto V. P., Paasivuo R., Virtanen I. Expression of intermediate filament proteins in thyroid gland and thyroid tumors. Lab Invest. 1984 Mar;50(3):262–270. [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Moll R., Krepler R., Franke W. W. Complex cytokeratin polypeptide patterns observed in certain human carcinomas. Differentiation. 1983;23(3):256–269. doi: 10.1111/j.1432-0436.1982.tb01291.x. [DOI] [PubMed] [Google Scholar]
- Nagle R. B., Böcker W., Davis J. R., Heid H. W., Kaufmann M., Lucas D. O., Jarasch E. D. Characterization of breast carcinomas by two monoclonal antibodies distinguishing myoepithelial from luminal epithelial cells. J Histochem Cytochem. 1986 Jul;34(7):869–881. doi: 10.1177/34.7.2423579. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Schlegel R., Banks-Schlegel S., McLeod J. A., Pinkus G. S. Immunoperoxidase localization of keratin in human neoplasms: a preliminary survey. Am J Pathol. 1980 Oct;101(1):41–49. [PMC free article] [PubMed] [Google Scholar]
- Stampfer M. R., Bartley J. C. Induction of transformation and continuous cell lines from normal human mammary epithelial cells after exposure to benzo[a]pyrene. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2394–2398. doi: 10.1073/pnas.82.8.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Stampfer M., Bartek J., Lewis A., Boshell M., Lane E. B., Leigh I. M. Keratin expression in human mammary epithelial cells cultured from normal and malignant tissue: relation to in vivo phenotypes and influence of medium. J Cell Sci. 1989 Nov;94(Pt 3):403–413. doi: 10.1242/jcs.94.3.403. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Eichner R., Sun T. T. Monoclonal antibody analysis of keratin expression in epidermal diseases: a 48- and 56-kdalton keratin as molecular markers for hyperproliferative keratinocytes. J Cell Biol. 1984 Apr;98(4):1397–1406. doi: 10.1083/jcb.98.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H., Schweizer J. Carcinoma-specific keratin polypeptide patterns in keratinizing epithelia of rodents : independence of species- and tissue-specific variations. Carcinogenesis. 1981;2(7):613–621. doi: 10.1093/carcin/2.7.613. [DOI] [PubMed] [Google Scholar]
- Wu Y. J., Parker L. M., Binder N. E., Beckett M. A., Sinard J. H., Griffiths C. T., Rheinwald J. G. The mesothelial keratins: a new family of cytoskeletal proteins identified in cultured mesothelial cells and nonkeratinizing epithelia. Cell. 1982 Dec;31(3 Pt 2):693–703. doi: 10.1016/0092-8674(82)90324-5. [DOI] [PubMed] [Google Scholar]
- Wu Y. J., Rheinwald J. G. A new small (40 kd) keratin filament protein made by some cultured human squamous cell carcinomas. Cell. 1981 Sep;25(3):627–635. doi: 10.1016/0092-8674(81)90170-7. [DOI] [PubMed] [Google Scholar]