Abstract
Cancer-associated fibroblasts (CAFs) play important roles in cancer progression through their complex interactions with cancer cells. The secreted bone morphogenetic protein antagonist, gremlin1 (GREM1) is expressed by the CAFs of basal cell carcinomas (BCCs), and promotes the growth of cancer cells. In this study, we investigated the expression of GREM1 mRNAs in various benign and malignant skin tumors, including various BCC subtypes. Analysis by RNA in situ hybridization (ISH) revealed that fibroblasts in the scar tissue expressed GREM1 and α-smooth muscle actin (α-SMA), whereas resident fibroblasts in the dermis of the normal skin did not express GREM1. Real-time polymerase chain reaction analysis showed significantly higher GREM1 expression in skin cancers and pilomatricomas (PMCs) than in other benign skin tumors. Tissue microarrays analyzed by RNA ISH for GREM1 expression also demonstrated that 23% of BCCs, 42% of squamous cell carcinomas, 20% of melanomas, and 90% of PMCs were positive for GREM1 expression, whereas trichoepitheliomas, eccrine poromas, hidradenomas, and spiradenomas were negative for GREM1 expression. Most BCCs that were GREM1 expression positive were of desmoplastic or mixed subtypes, and GREM1 expression was localized to activated myofibroblasts at the tumoral-stromal interface. Interestingly, most PMCs harbored GREM1-expressing fibroblasts, probably because of the inflammatory responses caused by foreign body reactions to keratin. Additionally, in BCCs, stromal GREM1 expression had a strong correlation with CD10 expression. In conclusion, GREM1 is frequently expressed by myofibroblasts in scars or in the stroma of basal cell carcinomas, suggesting that GREM1 expression can be a marker for activated myofibroblasts in the cancer stroma or in scar tissue.
Introduction
Cancer-associated fibroblasts (CAFs) are essential components of cancer microenvironments, and play critical roles in the cancer progression; they tend to aggregate peritumorally and encircle cancer cells that are invading adjacent normal tissues [1]. CAFs can originate from multiple precursors such as resident fibroblasts, smooth muscle cells, endothelial cells, and bone marrow (BM)-derived mesenchymal stem cells [2, 3]. CAFs secrete a wide spectrum of soluble factors including growth factors, chemokines, and cytokines, and contribute to the growth, migrations, epithelial mesenchymal transitions (EMTs), and metastases of the cancer cells. Thus, molecular markers of CAFs, such as fibroblast activation protein (FAP), C-X-C motif chemokine ligand 12 (CXCL12), and hepatocyte growth factor (HGF) are emerging as selective therapeutic targets in the cancer stroma [4]. Only a few studies on skin cancers have examined the molecular markers in CAFs for developing novel therapeutic strategies [5, 6].
Basal cell carcinomas (BCCs) have a characteristic fibromyxoid stroma with CAFs present around the tumor cells. In 2006, CAFs of human BCCs were first shown to express high levels of gremlin1 (GREM1) expression which promote the proliferation of cultured BCC cells [7]. Moreover, these GREM1-expressing CAFs were also observed in many other carcinomas [7]. GREM1 is a secreted bone morphogenetic protein (BMP) antagonist and is known to be involved in renal development [8, 9] and in the pathogenesis of nephropathy [10, 11]. Intestinal cryptal myofibroblasts and smooth muscle cells also express GREM1 at colon crypts, which contribute in the formation of the intestinal stem cell niche [12]. Interestingly, aberrant epithelial GREM1 expression leads to the development of hereditary mixed polyposis syndrome (HMPS) and traditional serrated adenomas [13]. Some reports on colon cancers have highlighted the stromal expression of GREM1 at the invasion front [14, 15].
BCCs are classified into several subtypes, based on distinct histological features, into nodular, micronodular, desmoplastic, mixed, and superficial subtypes. However, detailed patterns of GREM1 expression in these BCC subtypes remain undefined. In addition, GREM1 expression in the stromal cells of benign skin tumors and of other malignant tumors such as SCCs and MNs is unknown. In particular, trichoepitheliomas (TEs) that often confound a differential diagnosis of BCCs have characteristic peritumoral stromal cells; this raise the question of whether there are any differences in stromal GREM1 expression between BCCs and TEs that can be used as potential diagnostic feature. For the detection of GREM1 expression, RNA in situ hybridization (ISH) has enabled the specific visualization of GREM1 mRNA in human formalin-fixed paraffin-embedded (FFPE) specimens [7, 12, 13]. Therefore, in this study, we have used RNA ISH to investigate the stromal expression of GREM1 in various skin tumors, and compared the expression of GREM1 between benign and malignant tumors, and between the distinct BCC subtypes.
Materials and methods
Patients
A total of 152 FFPE tissues (normal skin, n = 6; scar tissues, n = 10; skin tumors, n = 160) were obtained from the punch or the excisional biopsy specimens at the Jeju National University Hospital, Jeju and at the Seoul National University Hospital, Seoul, Korea, from 2011 to 2015. Skin tumors included basal cell carcinomas (BCCs, n = 81), squamous cell carcinomas (SCCs, n = 17), malignant melanomas (MNs, n = 5), pilomatricomas (PMCs, n = 10), trichoepitheliomas (TEs, n = 16), eccrine poromas (EPs, n = 11), spiradenomas (SPAs, n = 10), and hidradenomas (HDAs, n = 9). Clinicopathological characteristics of patients, including their sex, age, and tumor sizes are summarized in Table 1. All hematoxylin and eosin-stained slides were reviewed independently by two dermatopathologists (B.G.J and C.L.). The BCCs were classified into nodular, micronodular, superficial, desmoplastic, and mixed subtypes. Mixed subtype refers to BCCs containing both nodular and desmoplastic areas. Additionally, surgically excised skin tumors, including 18 BCCs, 9 SCCs, 8 MNs, 8 PMCs, 7 TEs, 9 EPs, 10 HDAs, and 8 SPAs, were collected for real-time polymerase chain reaction (PCR) analyses. All data were analyzed anonymously and this study was approved by the Institutional Review Boards of the Jeju National University Hospital (2016-06-005) and the Seoul National University Hospital (1607-048-774).
Table 1. Clinicopathological characteristics of skin tumors.
| BCC (n = 81) | TE (n = 16) | SCC (n = 17) | MN (n = 5) | PMC (n = 10) | EP (n = 11) | HDA (n = 9) | SPA (n = 10) | |
|---|---|---|---|---|---|---|---|---|
| Sex | ||||||||
| Male (%) | 29 (36) | 3 (19) | 7 (41) | 4 (80) | 2 (20) | 6 (55) | 2 (22) | 5 (50) |
| Female (%) | 52 (64) | 13 (81) | 10 (59) | 1 (20) | 8 (80) | 5 (45) | 7 (78) | 5 (50) |
| Age (years) | ||||||||
| Mean (s.d.) | 75 (13) | 49 (19) | 69 (15) | 64 (15) | 9.8 (4.3) | 56 (20) | 44 (21) | 54 (11) |
| Range | 24–94 | 6–71 | 33–93 | 49–83 | 3–18 | 28–82 | 2–68 | 36–69 |
| Tumor size (cm) | ||||||||
| Mean (s.d.) | 1.0 (0.8) | 1.2 (1.3) | 1.8 (1.4) | 4.3 (2.1) | 1.7 (0.5) | 1.3 (0.5) | 1.5 (0.6) | 1.3 (0.7) |
| Range | 0.2–5.3 | 0.5–4.0 | 0.4–6.0 | 1.8–7.0 | 1.0–2.5 | 0.8–2.0 | 0.8–2.2 | 0.4–2.7 |
Abbreviation: BCC, Basal cell carcinoma; TE, trichoepithelioma; SCC, squamous cell carcinoma; MN, malignant melanoma; PMC, pilomatricoma; EP, eccrine poroma; HDA, hidradenoma, SPA, spiradenoma, s.d., standard deviation
RNA extraction and quantitative real-time PCR
Representative tumor areas were manually dissected from one to four FFPE tissue sections (4-μm thick) from each paraffin block; total RNA from these sections was extracted with an RNeasy FFPE Kit (Qiagen, Valencia, CA, USA) according to the instructions provided by the manufacturer but with a slight modification, as described previously [16]. cDNAs were synthesized from 1–2 μg of RNA using random hexamer primers and the GoScript reverse transcription system (Promega, Madison, Wisconsin, USA). Real-time PCR was performed with a StepOne Plus real-time PCR system (Applied Biosystems, Foster City, CA, USA) using the Premix Ex Taq (Takara Bio, Shiga, Japan) according to the recommendations provided by the manufacturer. The cycling conditions were as follows: initial denaturation for 20 s at 95°C, followed by 40 cycles of 95°C for 1 s and 60°C for 20 s. The following TaqMan gene expression assays were used: (GREM1), and Hs0275899_g1 (GAPDH). GAPDH served as the endogenous control.
Tissue microarray construction
Ten tissue microarrays (TMAs) containing samples from 94 BCCs, 17 SCCs, 5 MNs, 12 PMCs, 18 TEs, 7 EPs, 5 SPAs, 8 HDAs, 6 normal skin tissues, and 10 scar tissues were constructed. Briefly stated, one representative tumor area (4 mm in diameter) was extracted from individual FFPE tumors (donor blocks) and arranged in new recipient paraffin blocks (tissue array block) using a trephine apparatus (SuperBioChips Laboratories, Seoul, Korea).
RNA in situ hybridization
Using previously described protocols, RNA in situ hybridizations (ISH) for GREM1 were performed on 10 TMAs with the RNAscope FFPE assay kit (Advanced Cell Diagnostics, Inc., Hayward, CA, USA) [17]. Briefly, 4-μm TMA sections were baked at 60°C for 1 hour. Next, each of these sections was subjected to protease digestion followed by hybridization with GREM1 probes for 2 hours. An horseradish peroxidase (HRP)-based signal amplification system was hybridized to the probes before color development with 3,3′-diaminobenzidine tetrahydrochloride (DAB). The housekeeping gene, ubiquitin C (UBC), and the bacterial gene, DapB, served as positive and negative controls, respectively. Samples with UBC that were easily visible under a 10 × objective lens were considered adequate, as recommended by the manufacturer. Positive staining was indicated by the presence of brown punctate dots in the nucleus or cytoplasm or both. The expression of GREM1 was quantified according to the recommended scoring guidelines as follows: score 0, no staining or less than one dot per cell; score 1: one to three dots per cell (visible at 20–40 × magnification); score 2, four to 10 dots per cell and no or very few dot clusters (visible at 20–40 ×); score 3, more than10 dots per cell and less than10% positive cells with dot clusters (visible at 20 ×); score 4, more than10 dots per cell and >10% positive cells with dot clusters (visible at 20 ×). When the ISH score of sample was more than 2, the sample was considered to be positive for GREM1.
Immunohistochemistry
Immunohistochemistry was performed on 4μm thick TMA sections using a BOND-MAX automated immunostainer and a Bond Polymer Refine Detection kit (Leica Microsystems, Wetzlar, Germany), according to the instructions provided by the manufacturer. The primary antibodies used were anti-BCL2 (1:50; Dako, Carpenteria, CA, USA), anti-CD34 (1:300; Immunotech, Marseille, France), anti-CD10 (1:200; Novocatra, Newcastle, UK), and anti-α-smooth muscle actin (α-SMA) (1:1000; Neomarkers, Lab Vision Corporation, Fremont, CA, USA). Staining for BCL2 was considered as positive when over 10% of tumor cell nuclei were strongly positively stained for BCL2. Staining for CD34, CD10, and α-SMA were considered as positive when over 10% of stromal fibroblasts showed positive cytoplasmic staining.
Statistical analysis
Statistical analyses were performed with Prism version 5.0 (GraphPad Software, Inc., San Diego, CA, USA). Between-group comparisons of real-time PCR data were tested using Student’s t-test. The associations between GREM1 positivity and the immunohistochemical markers were tested by Pearson chi-square test. A P-value < 0.05 was considered statistically significant.
Results
RNA in situ hybridization for GREM1 in normal skin and scar tissue
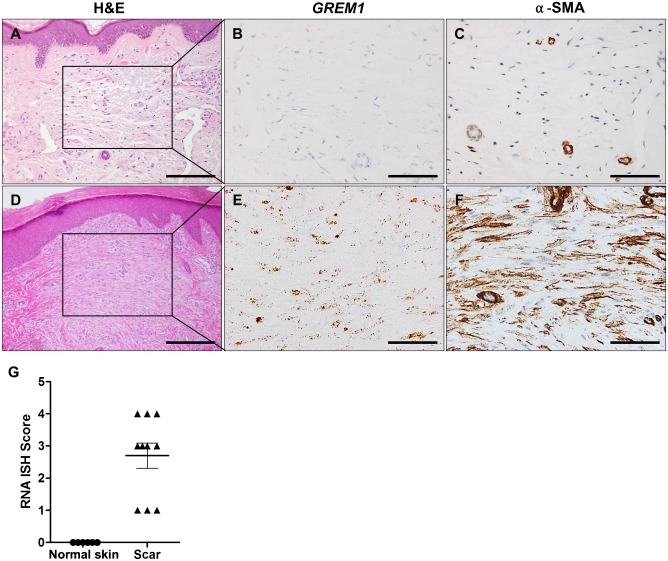
Firstly, we validated the use of RNA ISH for detection of GREM1 mRNAs in human FFPE specimens containing normal colon tissues, in which GREM1-expressing cells are known to be present. As previously shown, results of RNA ISH clearly demonstrated that the smooth muscle cells in the muscularis mucosa expressed GREM1 (S1 Fig) [12]. Secondly, we examined GREM1 expression in normal skin and scar tissues. Formation of scar is a non-tumorous condition in which fibroblasts proliferate and become activated. As expected, RNA ISH revealed that GREM1 transcripts were not found in the resident dermal fibroblasts. On the other hand, a number of fibroblasts that had proliferated in the scar tissues highly expressed GREM1 as well as α-SMA, demonstrating that GREM1-expressing myofibroblasts may also appear under physiological conditions such as wound healing (Fig 1).
Fig 1. GREM1 expression in normal skin and scar tissue.
RNA in situ hybridization (ISH) for GREM1 and immunohistochemical analysis for α-SMA was performed on the normal skin (n = 6) and scar tissues (n = 10). (A–C) Dermal fibroblasts of normal skin were negative for GREM1 or α-smooth muscle actin (α-SMA). (D–F) Scar tissue fibroblasts were positive for both GREM1 and α-SMA. (G) RNA ISH scores for GREM1 in normal skin and scar tissues. Scale bar: 40 μm (A and D), 20 μm (B, C, E, and F).
Real-time PCR analysis for GREM1 in various skin tumors
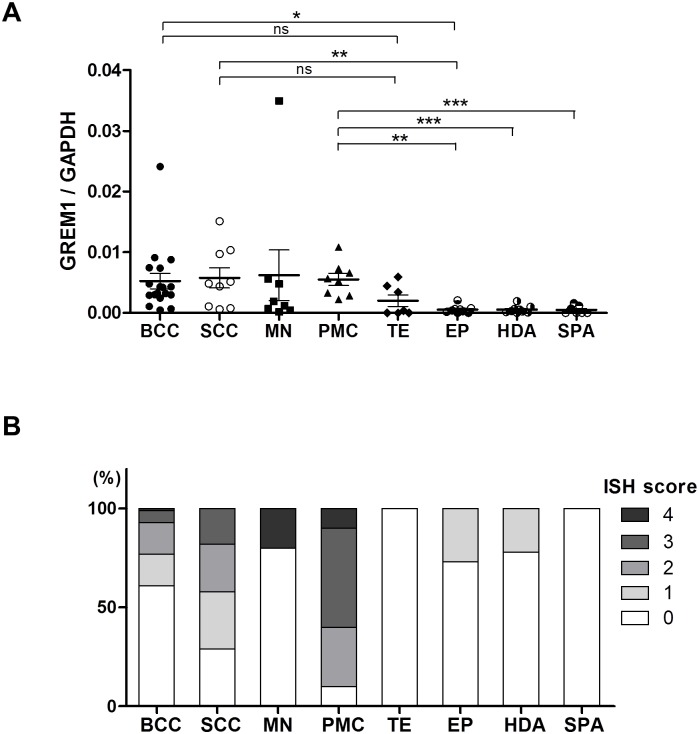
Next, we assessed the transcription levels of GREM1 in various benign and malignant skin tumors. The following FFPE samples were collected: BCCs (n = 18), SCCs (n = 9), MNs (n = 8), PMCs (n = 8), TEs (n = 7), EPs (n = 9), HDAs (n = 9), and SPAs (n = 7). Real-time PCR analysis showed that GREM1 expression was higher in malignant tumors, such as BCCs (P < 0.05) and SCCs (P < 0.01) than in benign tumors, such as EPs, HDAs, and SPAs (Fig 2A). Although GREM1 expression in melanomas was not significantly higher than in other tumors, one case had the highest level of GREM1 among the tumors examined in this analysis. Notably, compared to other benign tumors, PMCs expressed remarkably higher levels of GREM1. In contrast, compared to other tumors, TEs showed no significant differences in GREM1 expression.
Fig 2. mRNA levels of GREM1 in various skin tumors.
(A) Real-time PCR analysis was performed to examine the expression of GREM1 in a variety of skin tumors, including basal cell carcinomas (BCCs, n = 18), squamous cell carcinomas (SCCs, n = 9), melanomas (MNs, n = 8), pilomatricomas (PMCs, n = 8), trichoepitheliomas (TEs, n = 7), eccrine poromas (EPs, n = 9), hidradenomas (HADs, n = 9), and spiradenomas (SPAs, n = 7). *P < 0.05; **P < 0.00; 5***P < 0.001; ns, not significant. (B) The proportion of RNA in situ hybridization (ISH) scores for GREM1 in skin tumors. Analysis of GREM1 expression by RNA ISH was performed on tissue microarrays, including BCCs (n = 81), SCCs (n = 17), MNs (n = 5), PMCs (n = 10), TEs (n = 16), EPs (n = 11), HDAs (n = 9), and SPAs (n = 10). RNA ISH for GREM1 was scored from 0 to 4, reflecting the intensity of GREM1 expression.
Stromal GREM1 expression in benign and malignant skin tumors
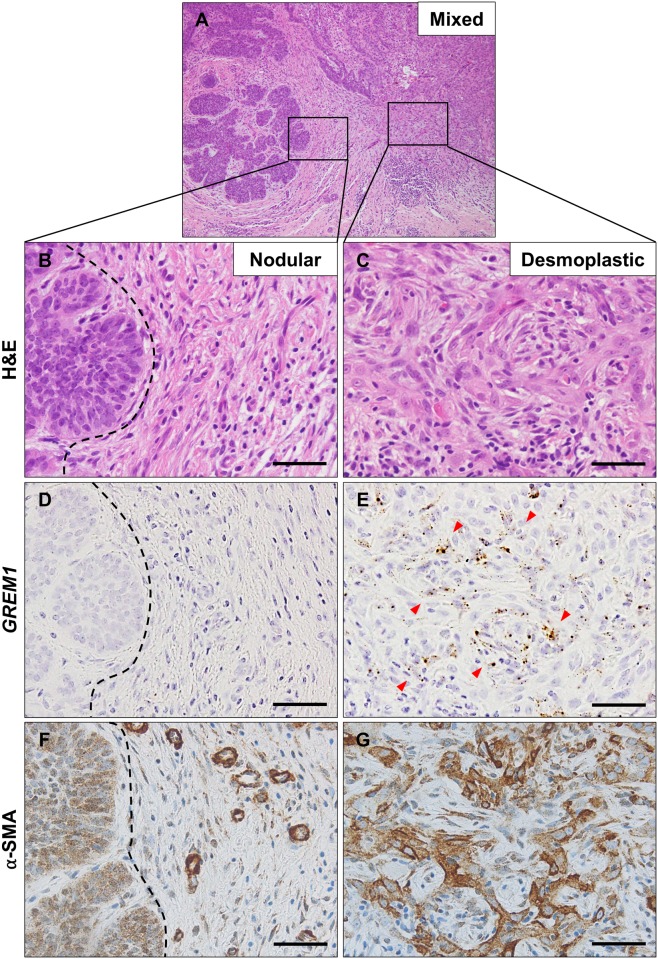
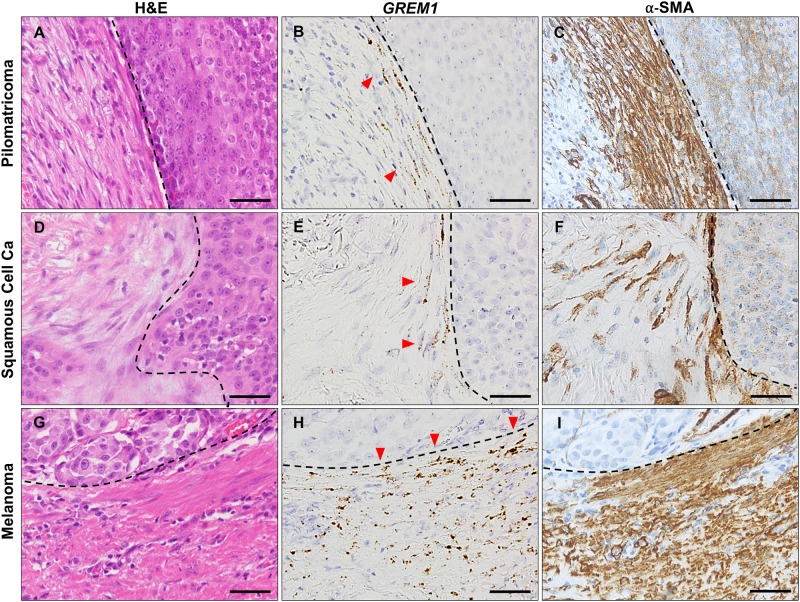
To specifically identify GREM1-expressing cells, we performed RNA ISH on tissue microarrays containing various samples of skin tumors. The results of RNA ISH are shown in Table 2 and Fig 2B. None of the epithelial components of skin tumors included in this study showed GREM1 mRNA expression; GREM1 mRNA localized to the stromal fibroblasts only. Of the malignant tumors, GREM1 positivity (score >2) was observed in 19 cases (23%) of BCCs, in 7 cases (42%) of SCCs, and in one case (20%) of MN. Among benign skin tumors, only PMCs (9 cases, 90%) showed GREM1 positivity. No GREM1 positivity was observed in cases of TEs, EPs, HDAs, and SPAs. In BCCs, GREM1 positivity was higher in the desmoplastic (43%) and mixed subtypes (39%) than in nodular (13%), micronodular (0%), and superficial subtypes (0%) (Table 3). In the cases of mixed BCC subtypes, only the myofibroblasts in the desmoplastic area expressed GREM1 (Fig 3). In contrast, fibroblasts around nodular areas expressed neither GREM1 nor α-SMA. Likewise, GREM1-expressing fibroblasts in PMCs, SCCs and MNs were observed in areas where activated myofibroblasts were present (Fig 4). In particular, fibroblasts immediately adjacent to the tumoral-stromal interface had high GREM1 expression. Based on these findings, we suggest that GREM1 expression is induced in myofibroblasts in the stroma, upon activation by invasive cancer cells or inflammation.
Table 2. RNA ISH scores for GREM1 in various skin tumors.
| RNA ISH score | BCC (%) | SCC (%) | MN (%) | PMC (%) | TE (%) | EP (%) | HDA (%) | SPA (%) | |
|---|---|---|---|---|---|---|---|---|---|
| GREM1 | 0 | 50 (62) | 5 (29) | 4 (80) | 1 (10) | 16 (100) | 8 (73) | 7 (78) | 10 (100) |
| 1 | 12 (15) | 5 (29) | 0 (0) | 0 (0) | 0 (0) | 3 (27) | 2 (22) | 0 (0) | |
| 2 | 13 (16) | 4 (24) | 0 (0) | 3 (30) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| 3 | 5 (6) | 3 (18) | 0 (0) | 5 (50) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| 4 | 1 (1) | 0 (0) | 1 (20) | 1 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Total | 81 (100) | 17 (0) | 5 (100) | 10 (100) | 16 (100) | 11 (100) | 9 (100) | 10 (100) | |
Abbreviation: BCC, basal cell carcinoma; SCC, squamous cell carcinoma; MN, malignant melanoma; PMC, pilomatricoma; TE, trichoepithelioma; EP, eccrine poroma; HAD, hidradenoma; SPA, spiradenoma
Table 3. RNA ISH scores for GREM1 in basal cell carcinomas subtypes.
| RNA ISH score | Nodular (%) | Micronodular (%) | Desmoplastic (%) | Mixed* (%) | Superficial (%) | |
|---|---|---|---|---|---|---|
| GREM1 | 0 | 22 (73) | 7 (100) | 5 (36) | 9 (39) | 5 (83) |
| 1 | 4 (13) | 0 (0) | 4 (21) | 5 (22) | 1 (17) | |
| 2 | 4 (13) | 0 (0) | 4 (29) | 5 (22) | 0 (0) | |
| 3 | 0 (6) | 0 (0) | 1 (7) | 4 (17) | 0 (0) | |
| 4 | 0 (1) | 0 (0) | 1 (7) | 0 (0) | 0 (0) | |
| Total | 30 (100) | 7 (100) | 14 (100) | 23 (0) | 6 (100) | |
* Mixed type refers to basal cell carcinomas showing both nodular and desmoplastic features.
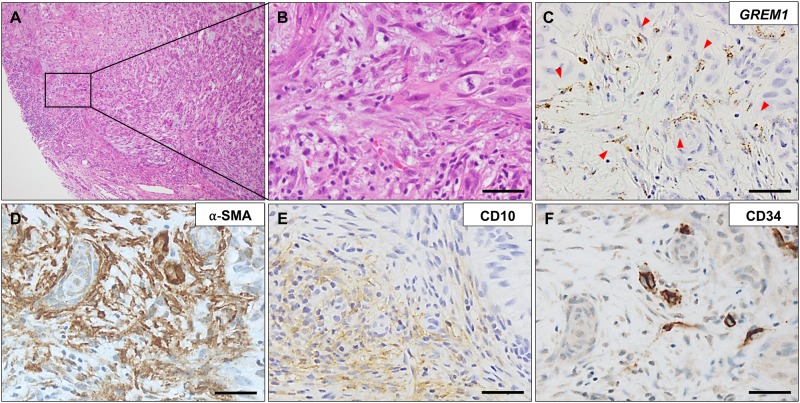
Fig 3. GREM1 expression in mixed type basal cell carcinoma.
A representative hematoxylin and eosin staining of a basal cell carcinoma (A) having both nodular (B) and desmoplastic (C) features. Fibroblasts around the nodular area (indicated by black dotted line) were negative for GREM1 (D) and α-smooth muscle actin (F), whereas those around the desmoplastic area were positive for both GREM1 (indicated by red arrow heads) (E) and α-SMA (G). RNA in situ hybridization for GREM1 and immunohistochemical analysis for α-SMA. Scale bar: 25 μm.
Fig 4. GREM1 expression in the myofibroblasts at the tumoral-stromal interface.
GREM1-positive myofibroblasts (indicated by red arrows) were frequently concentrated at the tumor-stromal interface (indicated by dotted line) in pilomatricoma (A–C), squamous cell carcinoma (D–F), and melanoma (G–I). RNA in situ hybridization for GREM1 and immunohistochemical analysis for α-smooth muscle actin. Scale bar: 25 μm.
Associations between GREM1 and the immunohistochemical markers in basal cell carcinomas
To enable the differential diagnosis of BCCs from TEs, several immunohistochemical markers have been studied, for example BCL2, CD10, and CD34, of which CD10 and CD34 are known to be expressed by peritumoral stromal cells [18]. To examine whether the stromal expression of GREM1 has any correlation with the presence of these diagnostic markers, we performed immunohistochemical analysis for CD10, CD34, and BCL2. Expression of GREM1 showed a strong association with stromal CD10 expression (Table 4). On the other hand, there was no association between expression of stromal GREM1 and that of epithelial CD10. Expression of stromal CD34 or epithelial BCL2 did not have any correlation with GREM1 expression. A representative case is shown in Fig 5.
Table 4. Associations between GREM1 and immunohistochemical markers in basal cell carcinomas.
| GREM1 | P-value# | ||
|---|---|---|---|
| Negative (%) | Positive (%) | ||
| BCL2 (tumor) | |||
| Negative | 20 (69) | 9 (31) | 0.314 |
| Positive | 38 (79) | 10 (21) | |
| CD34 (stroma) | |||
| Negative | 35 (71) | 14 (29) | 0.179 |
| Positive | 27 (84) | 5 (16) | |
| CD10 (tumor) | |||
| Negative | 27 (75) | 9 (25) | 0.812 |
| Positive | 34 (77) | 10 (23) | |
| CD10 (stroma) | |||
| Negative | 49 (88) | 7 (12) | 0.000 |
| Positive | 12 (50) | 12 (50) | |
| α-SMA (stroma) | |||
| Negative | 45 (94) | 3 (6) | 0.000 |
| Positive | 16 (50) | 16 (50) | |
# Pearson Chi-Square test
Fig 5. Association of GREM1 expression with expression of stromal CD10 and CD34 in basal cell carcinoma.
A representative picture of basal cell carcinoma with accompanying GREM1-positive myofibroblasts (indicated by red arrow heads) (A–D), in which stromal CD10 expression (E) was positive but that of CD34 (F) was negative. RNA in situ hybridization for GREM1 and immunohistochemical analysis for α-smooth muscle actin, CD10, and CD34. Scale bar: 25 μm.
Discussion
In this study, we demonstrated that GREM1 is frequently expressed by CAFs in the tumoral-stromal interface of invasive skin cancers, whereas the resident fibroblasts in normal skin do not express GREM1. Notably, we observed the appearance of GREM1-positive fibroblasts in scar tissue, showing that GREM1 expression is not confined to cancer-associated stromal cells, but can also be a phenotypic marker for activated fibroblasts under physiological conditions. Cancers have been demonstrated to activate the latent wound-healing program in an exaggerated and prolonged manner [19]. Therefore, both CAFs and myofibroblasts in scar tissue may express GREM1 possibly because of the activation of a shared molecular pathway. Considering that recently GREM1 has been identified as a marker of mesenchymal stem cells in the bone marrow that can generate bone, cartilage, and the periepithelial intestinal mesenchymal sheath [20], it is also possible to hypothesize that GREM1-expressing myofibroblasts in the scar tissue and the cancer stroma originate from GREM1-positive mesenchymal stem cells in the bone marrow upon tissue destruction either by cancer invasion or inflammation.
Previously, Sneddon et al. have reported that most BCCs (80%, 12 of 15 cases) express GREM1 [7]. In contrast, our results showed that only 28% (or up to 38% when including cases with score of 1) of BCCs (23 of 81 cases) were positive for GREM1. This discrepancy in the overall expression of GREM1 can be explained in part by the differences between the two studies, vis-à-vis the use of RNA ISH and the criteria for GREM1 positivity. In addition, our results suggest that the BCC subtypes included in the study can be important determinants of GREM1 expression because desmoplastic or mixed BCC subtypes exhibited greater GREM1-positivity. Thus, if a study includes a larger number of desmoplastic or mixed subtypes, the GREM1 positivity is expected to be higher. Histologically, desmoplastic and mixed subtypes are characterized by cancer cell invasion into the surrounding tissues, leading to stronger stromal reactions than those seen with other subtypes, and these subtypes have larger numbers of activated myofibroblasts around the cancer cells. On the other hand, CAFs in micronodular and superficial subtypes are relatively smaller in number, and are not in an activated state. These findings seem to indicate that stromal GREM1 expression in BCCs largely depends on the extent of invasiveness of cancer cells and the damage sustained by adjacent stromal tissue.
As mentioned earlier, stromal GREM1 expression has been demonstrated not only in BCCs but also in many invasive carcinomas, such as carcinomas of the esophagus, pancreas, colon, lung, breast, and mesotheliomas [7, 21]. This suggests that GREM1-expressing fibroblasts are essential components of the cancer microenvironment. Additionally, we found that other skin cancers such as SCCs and MNs harbor GREM1-positive CAFs. It is well known that CAFs secret many soluble factors, and promote tumor growth and invasion through tumoral-stromal interactions [22, 23]. Some in vitro studies have shown that GREM1 promotes proliferation and EMT in cancer cells [7, 14]; therefore, GREM1 expression is likely to be one of the properties of CAFs that promote the cancer progression. However, it may be entirely possible that GREM1 expression in CAFs is merely a marker of activated fibroblasts seen in scar tissues rather than an active promoter of cancer progression. Further investigations are required to address the exact roles of GREM1 secreted by CAFs in the cancer microenvironment.
The strong GREM1 expression seen in PMC stromal cells was an unexpected finding. PMCs are basaloid tumors with follicular differentiation, and are biologically benign, unless they undergo malignant transformation to pilomatrix carcinomas. A distinct histological feature exhibited by PMCs is that they often co-occur with active inflammation around tumor nodules; this inflammation arises because of foreign body reaction to keratin materials produced by the abrupt keratinization of the tumor cells. This inflammatory response may recruit the fibroblasts into the tumor, and induce GREM1 expression in these fibroblasts. Therefore, although the tumor alone may not have invasive properties, if it could cause inflammation resulting in tissue damage and stromal reaction, this may result in recruiting the GREM1-expressing myofibroblasts in the tumor stroma.
Occasionally, it is challenging to differentiate BCCs from other basaloid tumors, particularly TEs. Many studies have explored the ability of immunohistochemical markers, such as BCL2, CD10, CD34, D2-40, CK15, and CK20, to help in the diagnosis of BCCs [18, 24]. We evaluated the possibility of the practical usage of stromal GREM1 expression as a diagnostic marker; however, the low rate of stromal GREM1 positivity (28%) in BCCs indicated that it cannot be used as a biomarker, even though all TEs were negative for GREM1 expression. Because CD10 and CD34 are expressed by peritumoral stromal cells as well as tumor cells, we examined whether GREM1 has any associations with expression of stromal CD10 and CD34, and found a strong correlation of GREM1 expression with CD10 expression. However, CD10 expression did not seem to adequately co-localize with GREM1 expression well (Fig 5). It is possible to speculate that expressions of both GREM1 and CD10 are simultaneously induced by a factor in their microenvironment; however, they are not expressed in the same fibroblasts.
In summary, we demonstrated that the GREM1- positive myofibroblasts appear in scar tissue and in invasive skin cancers, including BCCs, SCCs, and MNs. Expression of GREM1 tends to be accentuated in the tumoral-stromal interface. In BCCs, GREM1 expression was observed mostly in the desmoplastic and mixed subtypes that induce strong stromal reactions, and appeared to be closely associated with the expression of α-SMA and CD10. Among benign skin tumors, only PMCs showed high levels of GREM1 expression, probably due to the underlying inflammation. These findings suggest that stromal GREM1 expression can be a marker for activated myofibroblasts in the cancer stroma or in scar tissue.
Supporting information
(TIF)
Acknowledgments
We are grateful to Hyun Ju Park, Seung Hee Jung, and Hye Jung Lee (SuperBioChips Laboratories, Seoul, Korea) who provided technical support.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a research grant from Jeju National University Hospital development fund in 2015. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Karagiannis GS, Poutahidis T, Erdman SE, Kirsch R, Riddell RH, Diamandis EP. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Molecular Cancer Research. 2012;10(11):1403–18. 10.1158/1541-7786.MCR-12-0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer cell. 2011;19(2):257–72. 10.1016/j.ccr.2011.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125(23):5591–6. [DOI] [PubMed] [Google Scholar]
- 4.Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer cell. 2005;7(6):513–20. 10.1016/j.ccr.2005.05.024 [DOI] [PubMed] [Google Scholar]
- 5.Huber MA, Kraut N, Schweifer N, Dolznig H, Peter RU, Schubert RD, et al. Expression of stromal cell markers in distinct compartments of human skin cancers. Journal of cutaneous pathology. 2006;33(2):145–55. 10.1111/j.0303-6987.2006.00446.x [DOI] [PubMed] [Google Scholar]
- 6.Kan S, Konishi E, Arita T, Ikemoto C, Takenaka H, Yanagisawa A, et al. Podoplanin expression in cancer-associated fibroblasts predicts aggressive behavior in melanoma. Journal of cutaneous pathology. 2014;41(7):561–7. 10.1111/cup.12322 [DOI] [PubMed] [Google Scholar]
- 7.Sneddon JB, Zhen HH, Montgomery K, van de Rijn M, Tward AD, West R, et al. Bone morphogenetic protein antagonist gremlin 1 is widely expressed by cancer-associated stromal cells and can promote tumor cell proliferation. Proceedings of the national academy of sciences. 2006;103(40):14842–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyazaki Y, Oshima K, Fogo A, Ichikawa I. Evidence that bone morphogenetic protein 4 has multiple biological functions during kidney and urinary tract development. Kidney international. 2003;63(3):835–44. 10.1046/j.1523-1755.2003.00834.x [DOI] [PubMed] [Google Scholar]
- 9.Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. The Journal of clinical investigation. 2000;105(7):863–73. 10.1172/JCI8256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michos O, Panman L, Vintersten K, Beier K, Zeller R, Zuniga A. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development. 2004;131(14):3401–10. 10.1242/dev.01251 [DOI] [PubMed] [Google Scholar]
- 11.Khokha MK, Hsu D, Brunet LJ, Dionne MS, Harland RM. Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nature genetics. 2003;34(3):303–7. 10.1038/ng1178 [DOI] [PubMed] [Google Scholar]
- 12.Kosinski C, Li VS, Chan AS, Zhang J, Ho C, Tsui WY, et al. Gene expression patterns of human colon tops and basal crypts and BMP antagonists as intestinal stem cell niche factors. Proceedings of the National Academy of Sciences. 2007;104(39):15418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis H, Irshad S, Bansal M, Rafferty H, Boitsova T, Bardella C, et al. Aberrant epithelial GREM1 expression initiates colonic tumorigenesis from cells outside the stem cell niche. Nature medicine. 2015;21(1):62–70. 10.1038/nm.3750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karagiannis GS, Musrap N, Saraon P, Treacy A, Schaeffer DF, Kirsch R, et al. Bone morphogenetic protein antagonist gremlin-1 regulates colon cancer progression. Biological chemistry. 2015;396(2):163–83. 10.1515/hsz-2014-0221 [DOI] [PubMed] [Google Scholar]
- 15.Karagiannis GS, Treacy A, Messenger D, Grin A, Kirsch R, Riddell RH, et al. Expression patterns of bone morphogenetic protein antagonists in colorectal cancer desmoplastic invasion fronts. Molecular oncology. 2014;8(7):1240–52. 10.1016/j.molonc.2014.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang BG, Lee BL, Kim WH. Distribution of LGR5+ cells and associated implications during the early stage of gastric tumorigenesis. PLoS One. 2013;8(12):e82390 10.1371/journal.pone.0082390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang BG, Kim HS, Kim KJ, Rhee YY, Kim WH, Kang GH. Distribution of intestinal stem cell markers in colorectal precancerous lesions. Histopathology. 2015. [DOI] [PubMed] [Google Scholar]
- 18.Tebcherani AJ, de Andrade HF, Sotto MN. Diagnostic utility of immunohistochemistry in distinguishing trichoepithelioma and basal cell carcinoma: evaluation using tissue microarray samples. Modern Pathology. 2012;25(10):1345–53. 10.1038/modpathol.2012.96 [DOI] [PubMed] [Google Scholar]
- 19.Schäfer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nature reviews Molecular cell biology. 2008;9(8):628–38. 10.1038/nrm2455 [DOI] [PubMed] [Google Scholar]
- 20.Worthley DL, Churchill M, Compton JT, Tailor Y, Rao M, Si Y, et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160(1):269–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D-J, Zhi X-Y, Zhang S-C, Jiang M, Liu P, Han X-P, et al. The bone morphogenetic protein antagonist Gremlin is overexpressed in human malignant mesothelioma. Oncology reports. 2012;27(1):58 10.3892/or.2011.1463 [DOI] [PubMed] [Google Scholar]
- 22.Rupp C, Scherzer M, Rudisch A, Unger C, Haslinger C, Schweifer N, et al. IGFBP7, a novel tumor stroma marker, with growth-promoting effects in colon cancer through a paracrine tumor–stroma interaction. Oncogene. 2015;34(7):815–25. 10.1038/onc.2014.18 [DOI] [PubMed] [Google Scholar]
- 23.Torres S, Bartolomé RA, Mendes M, Barderas R, Fernandez-Aceñero MJ, Peláez-García A, et al. Proteome profiling of cancer-associated fibroblasts identifies novel proinflammatory signatures and prognostic markers for colorectal cancer. Clinical cancer research. 2013;19(21):6006–19. 10.1158/1078-0432.CCR-13-1130 [DOI] [PubMed] [Google Scholar]
- 24.Sellheyer K, Nelson P. Follicular stem cell marker PHLDA1 (TDAG51) is superior to cytokeratin-20 in differentiating between trichoepithelioma and basal cell carcinoma in small biopsy specimens. Journal of cutaneous pathology. 2011;38(7):542–50. 10.1111/j.1600-0560.2011.01693.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.