Abstract
Objective
Both hypokalemia and hyperkalemia are associated with disease progression in patients with chronic kidney disease (CKD). It is unclear whether similar associations are present in the general population. Our aim was to examine the association of plasma potassium with risk of developing CKD and the role of diuretics in this association in a population-based cohort.
Research design and methods
We studied 5,130 subjects free of CKD at baseline of the Prevention of Renal and Vascular End-Stage Disease (PREVEND) study, a prospective, population-based cohort of Dutch men and women aged 28–75 years. Hypokalemia was defined as plasma potassium <3.5 mmol/L, and hyperkalemia as plasma potassium ≥5.0 mmol/L. Risk of CKD was defined as de novo development of eGFR <60 ml/min/1.73m2 and/or albuminuria >30 mg/24h.
Results
Mean baseline plasma potassium was 4.4±0.3 mmol/L. The prevalences of hypokalemia and hyperkalemia were 0.5% and 3.8%, respectively; 3.0% of the subjects used diuretics. During a median follow-up of 10.3 years (interquartile range: 6.3–11.4 years), 753 subjects developed CKD. The potassium-CKD association was modified by diuretic use (Pinteraction = 0.02). Both hypokalemia without (HR, 7.74, 95% CI, 3.43–17.48) or with diuretic use (HR, 4.32, 95% CI, 1.77–10.51) were associated with an increased CKD risk as compared to plasma potassium 4.0–4.4 mmol/L without diuretic use. Plasma potassium concentrations ≥3.5 mmol/L were associated with an increased CKD risk among subjects using diuretics (Ptrend = 0.01) but not among subjects not using diuretics (Ptrend = 0.74).
Conclusion
In this population-based cohort, hypokalemia was associated with an increased CKD risk, regardless of diuretic use. In the absence of hypokalemia, plasma potassium was not associated with an increased CKD risk, except among subjects using diuretics.
Introduction
Potassium homeostasis is strictly regulated by the kidneys and plasma potassium is normally maintained within narrow limits (typically between 3.5 to 5.0 mmol/L) [1–3]. Such strict regulation of plasma potassium is essential for many physiologic processes, including acid-base homeostasis, systemic blood pressure control, smooth muscle tone, cardiac conduction and rhythm, and membrane potential repolarization [1].
Disturbances in plasma potassium are more common in patients with chronic kidney disease (CKD) compared to the general population. This disturbance typically presents as hypokalemia (<3.5 mmol/L) as a consequence of diuretic administration [4], but CKD patients also have a higher risk of having hyperkalemia (≥5.0 mmol/L) due to impaired kidney function and frequent use of renin angiotensin aldosterone system (RAAS) inhibitors [1]. In subjects with CKD, both hypo- and hyperkalemia are associated with higher risk of all-cause mortality, major cardiovascular events, and hospitalization [5–7]. Also in individuals without impaired kidney function, potassium disturbances are linked to higher rates of mortality [8]. Moreover, in experimental studies, it has been shown that hypokalemia can induce renal injury [9,10]. Prospective cohort studies in CKD patients showed that hypokalemia is associated with increased risk of developing of end-stage renal disease (ESRD) in most [7,11,12], but not all studies [6].
The association of hypokalemia with risk of developing de novo CKD is not well established. A study in Japanese men and women examined this association and found that potassium <4.0 mmol/L was associated with an increased risk of developing CKD [13]. However, this study excluded individuals using medication for hypertension including diuretics–the main risk factor for hypokalemia in the general population [4]–which substantially limits the generalization of the findings. Recently, Chen at al. observed that lower levels of potassium were associated with higher CKD risk among participants of the Atherosclerosis Risk in Communities Study not taking thiazide and loop diuretics [14].
Therefore, the aim of the present study was to prospectively examine the association of plasma potassium with risk of developing CKD in a Dutch large population-based cohort and to investigate the role of diuretic use in this association.
Materials and methods
Study design and population
This study was conducted within the framework of the PREVEND study, which is designed to prospectively investigate the natural course of increased levels of urinary albumin excretion (UAE) and its relation to renal and cardiovascular disease in a large cohort drawn from the general population. Details of this study have been described elsewhere [15]. In brief, from 1997 to 1998, all inhabitants of Groningen, the Netherlands aged 28 to 75 years, were sent a questionnaire and a vial to collect a first morning void urine sample. Pregnant women and subjects with type 1 diabetes mellitus were excluded. Urinary albumin concentration was assessed in 40,856 responders. Subjects with a urinary albumin concentration of ≥10 mg/L (n = 7,768) were invited to participate, of whom 6,000 were enrolled. In addition, a randomly selected group with a urinary albumin concentration of <10 mg/L (n = 3,394) was invited to participate in the cohort, of whom 2,592 were enrolled. These 8,592 individuals form the Prevention of Renal and Vascular End-stage Disease (PREVEND) cohort and completed an extensive examination in 1997 and 1998 (baseline).
For the current study, we excluded subjects with CKD at baseline (n = 1,898) or unknown CKD status (n = 1,191), with missing values of plasma potassium due to missing blood samples or insufficient volume of plasma (n = 358), or invalid concentrations of plasma potassium (n = 8), and with renal disease requiring dialysis (n = 7), leaving 5,130 participants for the analyses.
The PREVEND study has been approved by the medical ethics committee of the University Medical Center Groningen and is performed in accordance with Declaration of Helsinki guidelines. Written informed consent was obtained from all participants.
Data collection
The procedures at each examination in the PREVEND study have been described in detail previously [16]. In brief, each examination included two visits to an outpatient clinic separated by three weeks. Prior to the first visit, all participants completed a self-administered questionnaire regarding demographics, cardiovascular and renal disease history, smoking habits, alcohol consumption and medication use. Information on medication use was combined with information on drug use from the IADB.nl database, containing pharmacy-dispensing data from community pharmacies in the Netherlands [17]. During each examination and during each visit, blood pressure was measured on the right arm, every minute for 10 and 8 minutes, respectively, by an automatic Dinamap XL Model 9300 series device (Johnson-Johnson Medical, Tampa, Florida, USA). The mean of the last two recordings from each of the two visits was used. In the last week before the second visit, subjects collected two consecutive 24-hour specimens after thorough oral and written instruction. Furthermore, fasting serum and plasma blood samples were provided and stored at -80°C.
Potassium measurement and categories
Circulating potassium was determined in plasma with lithium heparin as anticoagulant on a Modular analyzer (Roche Diagnostics, Mannheim, Germany) with an interassay coefficient of variation of 1.4%. Plasma potassium was categorized as <3.5, 3.5–3.9, 4.0–4.4, 4.5–4.9, and ≥5.0 mmol/L. Hypokalemia was defined as a plasma potassium concentration less than 3.5 mmol/L, normokalemia was defined as a potassium concentration between 4.0 and 4.4 mmol/L, and hyperkalemia was defined as a plasma potassium concentration equal to or greater than 5.0 mmol/L.
Ascertainment of chronic kidney disease
The primary outcome of CKD was defined as a combination of reaching an eGFR <60 ml/min per 1.73 m2 and/or an UAE >30 mg/24h de novo. We estimated GFR with the combined creatinine cystatin C-based Chronic Kidney Disease Epidemiology (CKD-EPI) Collaboration equation from 2012, taking into account age, sex, and race [18]. UAE was multiplied by urine volume to obtain a value in mg per 24h. The two 24-hour UAE values of each subject per examination were averaged. Data on both measurements were collected during follow-up every 3 to 5 years.
Measurement of serum creatinine was performed by an isotope dilution mass spectrometry (IDMS) traceable enzymatic method on a Roche Modular analyzer using reagents and calibrators from Roche (Roche Diagnostics, Mannheim, Germany), with intra- and interassay coefficients of variation of 0.9% and 2.9%, respectively. Serum cystatin C concentrations were measured by Gentian Cystatin C Immunoassay (Gentian AS, Moss, Norway) on a Modular analyzer (Roche Diagnostics). Cystatin C was calibrated directly using the standard supplied by the manufacturer (traceable to the International Federation of Clinical Chemistry Working Group for Standardization of Serum Cystatin C) [19]. The intra- and interassay coefficients of variation were <4.1% and <3.3%, respectively. Urinary albumin concentration was measured by nephelometry with a threshold of 2.3 mg/l, and intra- and interassay coefficients of variation of 2.2% and 2.6%, respectively (Dade Behring Diagnostic, Marburg, Germany).
Assessment of covariates
BMI was calculated as weight (kg) divided by height squared (m2). Smoking status was defined as self-reported never smoker, former smoker, or current smoker (<6, 6–20, or >20 cigarettes/day) and alcohol intake as no/rarely, 1 to 4 drinks/month, 2 to 7 drinks/week, 1 to 3 drinks/day, and 4 or more drinks/day. Parental history of CKD was defined as having a first-degree relative who had a renal disease requiring dialysis for >6 weeks. Educational level was defined as low (primary education or intermediate vocational education), middle (higher secondary education), and high (higher vocational education and university). Use of diuretic medication was defined as use of low-ceiling diuretics including thiazides (ATC code C03A), low-ceiling diuretics excluding thiazides (ATC code C03B), high-ceiling diuretics (ATC code C03C), potassium-sparing agents (ATC code C03D), and diuretics and potassium-sparing agents in combination (ATC code C03E).
Urinary excretions of sodium, potassium, magnesium, and creatinine were determined with a MEGA clinical chemistry analyzer (Merck, Darmstadt, Germany). Sodium, potassium, and magnesium were determined by indirect potentiometry. Glucose was determined from fasting blood samples by using Kodak Ektachem dry chemistry (Eastman Kodak, Rochester, NY). High-sensitivity C-reactive protein was determined by nephelometry (BN II, Dade Behring, Marburg, Germany). Concentrations of 1,25-dihydroxyvitamin D were measured using liquid chromatography tandem mass spectrometry as previously described [20]. An automated two-site immunoassay (Roche, diagnostics, Indianapolis, IN, USA) was used to measure plasma intact parathyroid hormone (PTH). Measurement of blood urea nitrogen (BUN) was performed on a Roche Modular with ultraviolet kinetic assay, which is based on Talke and Schubert’s method and has been optimized for analyzers that permit kinetic measurements.
Type 2 diabetes was defined as a fasting plasma glucose ≥7.0 mmol/L (>126 mg/dL) or the use of glucose-lowering drugs [21]. Hypertension was defined as systolic blood pressure of ≥140 mm Hg, a diastolic blood pressure of ≥90 mm Hg, or both or the use of antihypertensive agents as previously described [22].
Statistical analyses
Baseline characteristics are presented according to categories of plasma potassium. Continuous data are presented as mean with SD or as median with IQR in case of skewed distribution. Categorical data are presented as number with percentage.
Multinomial logistic regression was used to examine the associations of the predefined risk factors with the plasma potassium categories. Plasma potassium of 4.0–4.4 mmol/L was used as reference category. ORs are reported with 95% CIs. Univariable associations with risk of hypo- and hyperkalemia were determined for sex, age, BMI, eGFR, smoking, alcohol, education, race, diabetes, hypertension, use of ACEi, ARBs, beta blockers, thiazide diuretics, loop diuretics, potassium-sparing diuretics, and urinary excretion of potassium, magnesium and albumin. These analyses were also adjusted for eGFR for bivariable ORs (95% CIs).
To study the prospective association between plasma potassium and risk of developing CKD, Cox proportional hazards regression analyses were used to calculate HRs and 95% CIs. We first calculated HRs for the crude model. Second, we additionally adjusted for age, sex, and baseline eGFR. Third, we additionally adjusted for height, weight, urinary potassium excretion, and use of diuretics. Finally, we additionally adjusted for systolic blood pressure, plasma aldosterone, plasma albumin, plasma magnesium [23], hs-CRP, diabetes, smoking, alcohol, education, race, urinary creatinine excretion, plasma chloride, and the BUN/creatinine ratio one at the time. In secondary analyses, CKD incidence was defined by either an eGFR <60 ml/min/1.73 m2 or UAE >30 mg/24h alone. We evaluated potential effect modification by sex, age, hypertension, urinary potassium excretion, baseline eGFR, plasma aldosterone, and diuretic use by fitting models containing both main effects and their cross-product terms.
Several sensitivity analyses were performed to examine the robustness of the associations between plasma potassium and risk of developing CKD. First, we examined the specific role of potassium-wasting diuretics (i.e. loop diuretics and thiazide diuretics) in the association of plasma potassium and risk of developing CKD. Second, we excluded subjects at baseline with an eGFR <66 (instead of <60) ml/min per 1.73 m2 and/or a UAE >25 (instead of >30) mg/24h for a more pronounced decline in kidney function over time in order to reach the primary outcome of CKD. Third, we restricted the analyses of plasma potassium and risk of CKD to subjects who were not using antihypertensive drugs at baseline because of the potential mediating effects of blood pressure in the association between plasma potassium and risk of CKD. Fourth, we excluded subjects from the analyses who developed diabetes during follow-up to eliminate the development of diabetes during follow-up as possible mediator in the association of plasma potassium with risk of developing CKD. Finally, we addressed the oversampling of subjects with higher urinary albumin concentrations by using design-based Cox proportional-hazards regression models that took into account the probability of selection by statistical weighting.
All P-values are two-tailed. A P-value <0.05 was considered statistically significant. All analyses were conducted with the use of the statistical package IBM SPSS (version 22; SPSS, Chicago, IL) and Rstudio (version 0.99.491; Rstudio, Boston, MA).
Results
Baseline characteristics
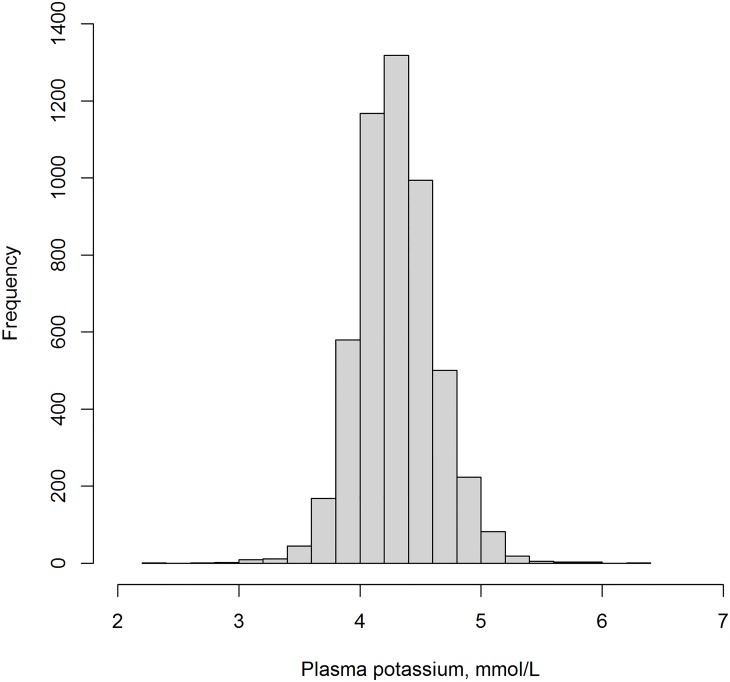
The distribution of plasma potassium is presented in Fig 1. Baseline mean plasma potassium was 4.4±0.3 mmol/L. The prevalence of hypokalemia at baseline was 0.5%. Hyperkalemia was more common with a prevalence of 3.8%. A total of 3.0% of the subjects used diuretics, including thiazides (1.8%), loop diuretics (0.4%), and potassium-sparing diuretics (0.8%).
Fig 1. Distribution of plasma potassium in 5,130 subjects of the Prevention of Renal and Vascular End-Stage Disease (PREVEND) study.
Baseline characteristics according to categories of plasma potassium are shown in Table 1. Subjects with hypokalemia were more likely to be older, to be lower educated, not smoking, consume no alcohol, and to have diabetes compared to subjects with normokalemia. They were also more likely to use beta blockers and diuretics, and had a higher systolic blood pressure and lower plasma chloride concentrations compared to subjects with normokalemia. Furthermore, a slightly lower eGFR and slightly higher UAE were observed in subjects with hypokalemia. Subjects with hyperkalemia were more likely to be male, to smoke, to be White, have a higher urinary potassium excretion, and to not use angiotensin-converting-enzyme inhibitors (ACEi), angiotensin receptor blockers (ARBs), and thiazide diuretics compared to subjects with normokalemia.
Table 1. Baseline characteristics of the 5,130 participants of the Prevention of Renal and Vascular End-Stage Disease (PREVEND) study.
| Plasma potassium, mmol/L | P-value | |||||
|---|---|---|---|---|---|---|
| 2.3–3.4 | 3.5–3.9 | 4.0–4.4 | 4.5–4.9 | 5.0–6.3 | ||
| Subjects, % | 24 (0.5) | 404 (7.9) | 2,873 (56.0) | 1,636 (31.9) | 193 (3.8) | <0.001 |
| Women, % | 20 (83.3) | 279 (69.1) | 1588 (55.3) | 790 (48.3) | 90 (46.6) | <0.001 |
| Age, y | 53.4 ± 12.5 | 48.6 ± 12.5 | 48.1 ± 11.7 | 48.4 ± 11.7 | 48.6 ± 11.9 | 0.20 |
| Height, cm | 166 ± 9 | 171 ± 9 | 173 ± 9 | 174 ± 10 | 174 ± 9 | <0.001 |
| Weight, kg | 73 ± 15 | 75 ± 14 | 77 ± 13 | 77 ± 13 | 77 ± 14 | 0.01 |
| BMI, kg/m2 | 26.4 ± 4.5 | 25.7 ± 4.3 | 25.7 ± 3.9 | 25.6 ± 3.9 | 25.4 ± 3.8 | 0.53 |
| Current smoker, % | 6 (25.0) | 74 (18.3) | 826 (28.8) | 627 (38.3) | 68 (35.2) | <0.001 |
| No alcohol consumption, % | 12 (50.0) | 114 (28.2) | 681 (23.7) | 361 (22.1) | 34 (17.6) | <0.001 |
| White, % | 20 (83.3) | 371 (92.5) | 2,736 (95.9) | 1,575 (97.0) | 189 (97.9) | <0.001 |
| Education, high, % | 2 (8.3) | 126 (31.2) | 965 (33.6) | 520 (31.8) | 64 (33.2) | 0.07 |
| Diabetes Mellitus, % | 4 (16.7) | 10 (2.5) | 35 (1.2) | 34 (2.1) | 3 (1.6) | <0.001 |
| Family history of CKD, % | 0 (0) | 3 (0.7) | 41 (1.4) | 22 (1.3) | 5 (2.6) | 0.45 |
| Systolic blood pressure, mm Hg | 134 ± 19 | 127 ± 20 | 125 ± 18 | 125 ± 18 | 125 ± 18 | 0.03 |
| Diastolic blood pressure, mm Hg | 77 ± 10 | 73 ± 10 | 72 ± 9 | 73 ± 9 | 72 ± 8 | 0.15 |
| Cardiovascular disease, % | 1 (4.2) | 11 (2.7) | 85 (3.0) | 64 (3.9) | 9 (4.7) | 0.34 |
| Medication: | ||||||
| ACEi*, % | 1 (5.0) | 18 (5.0) | 78 (3.2) | 37 (2.7) | 4 (2.6) | 0.23 |
| ARB*, % | 0 (0) | 2 (0.6) | 9 (0.4) | 8 (0.6) | 1 (0.6) | 0.89 |
| Beta blockers*, % | 5 (25.0) | 29 (8.1) | 135 (5.5) | 81 (5.8) | 11 (7.1) | 0.002 |
| Thiazide diuretics*, % | 6 (30.0) | 35 (9.7) | 43 (1.8) | 8 (0.6) | 1 (0.6) | <0.001 |
| Loop diuretics*, % | 1 (5.0) | 6 (1.7) | 8 (0.3) | 7 (0.5) | 1 (0.6) | 0.001 |
| Potassium-sparing diuretics*, % | 0 (0) | 17 (4.7) | 17 (0.7) | 4 (0.3) | 4 (2.6) | <0.001 |
| Proton pump inhibitors*, % | 0 (0) | 12 (3.0) | 63 (2.3) | 51 (3.2) | 7 (3.7) | 0.28 |
| Lipid lowering drugs, % | 1 (4.2) | 28 (6.9) | 136 (4.7) | 101 (6.2) | 11 (5.7) | 0.17 |
| Glucose lowering drugs, % | 0 (0) | 5 (1.2) | 20 (0.7) | 26 (1.6) | 1 (0.5) | 0.06 |
| Plasma potassium, mmol/L | 3.2 ± 0.3 | 3.8 ± 0.1 | 4.2 ± 0.1 | 4.6 ± 0.1 | 5.1 ± 0.2 | <0.001 |
| Plasma magnesium, mmol/L | 0.80 ± 0.07 | 0.80 ± 0.06 | 0.81 ± 0.06 | 0.82 ± 0.05 | 0.83 ± 0.05 | 0.30 |
| Plasma albumin, g/L | 44.8 ± 2.9 | 45.3 ± 2.9 | 45.9 ± 2.6 | 45.9 ± 2.6 | 46.6 ± 2.5 | <0.001 |
| Plasma sodium, mmol/L | 141 ± 4 | 141 ± 3 | 142 ± 2 | 142 ± 2 | 142 ± 3 | 0.18 |
| Plasma chloride, mmol/L | 101 ± 5 | 105 ± 8 | 106 ± 2 | 106 ± 2 | 106 ± 2 | <0.001 |
| Plasma aldosterone†, ng/dL | 13.1 ± 8.0 | 14.2 ± 7.8 | 13.3 ± 6.9 | 12.9 ± 6.6 | 12.4 ± 5.1 | 0.12 |
| Plasma PTH‡, pmol/L | 5.6 ± 2.1 | 4.0 ± 1.4 | 3.8 ± 1.4 | 3.8 ± 1.5 | 3.8 ± 1.7 | <0.001 |
| Plasma 1,25 dihydroxyvitamin D§, pg/mL | 57.4 ± 23.8 | 58.1 ± 20.0 | 55.8 ± 18.2 | 55.3 ± 17.7 | 55.2 ± 17.8 | 0.10 |
| Plasma phosphorus, mg/dL | 3.15 ± 0.55 | 3.10 ± 0.50 | 3.11 ± 0.48 | 3.16 ± 0.49 | 3.22 ± 0.51 | 0.001 |
| Plasma glucose, mmol/L | 5.4 ± 2.9 | 4.8 ± 1.3 | 4.7 ± 0.8 | 4.8 ± 0.9 | 4.7 ± 0.7 | 0.89 |
| hs-CRP¶, mg/L | 3.3 ± 3.4 | 2.8 ± 5.5 | 2.2 ± 4.0 | 2.2 ± 3.2 | 2.0 ± 2.7 | 0.01 |
| BUN, mg/dL | 13.8 ± 4.9 | 13.8 ± 3.6 | 14.4 ± 3.3 | 14.8 ± 3.5 | 14.9 ± 3.4 | <0.001 |
| Serum creatinine, mg/dL | 0.7 (0.6–0.80) | 0.7 (0.6–0.8) | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) | <0.001 |
| BUN/creatinine ratio | 17.8 (13.6–22.0) | 17.9 (15.1–21.6) | 18.0 (15.3–21.3) | 17.7 (15.1–21.3) | 17.6 (14.8–21.4) | 0.82 |
| eGFR, ml/min/1.73 m2 | 91 ± 21 | 99 ± 15 | 98 ± 15 | 96 ± 15 | 95 ± 15 | 0.06 |
| Urinary excretion of: | ||||||
| Potassium, mmol/24h | 60.3 (41.3–70.3) | 62.9 (48.8–80.6) | 70.3 (56.9–83.9) | 70.6 (58.2–85.3) | 72.6 (60.2–88.8) | <0.001 |
| Sodium, mmol/24h | 98 (80–148) | 125 (98–148) | 135 (104–169) | 136 (107–167) | 131 (106–169) | <0.001 |
| Magnesium, mmol/24h | 2.54 (1.31–3.80) | 3.58 (2.64–4.43) | 3.76 (2.89–4.72) | 3.88 (2.99–4.85) | 3.78 (3.04–5.06) | <0.001 |
| Urea, mmol/24h | 288 (183–388) | 324 (259–389) | 343 (284–413) | 348 (284–419) | 337 (277–423) | <0.001 |
| Creatinine, mmol/24 | 9.2 (7.3–11.7) | 10.8 (9.2–13.1) | 11.7 (9.5–14.3) | 11.9 (9.8–14.7) | 11.4 (9.5–14.4) | <0.001 |
| Albumin, mg/24h | 8.3 (7.3–21.3) | 8.2 (6.1–11.4) | 7.8 (5.8–11.0) | 7.6 (5.7–11.1) | 8.0 (5.8–11.6) | 0.02 |
Continuous variables are reported as mean ± SD or median (interquartile range) and categorical variables are reported as n (%).
* Data available in 4,378 subjects.
† Data available in 5,008 subjects.
§ Data available in 4,971 subjects.
‡ Data available in 4,967 subjects.
¶ Data available in 4,959 subjects.
Abbreviations: ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blockers; BMI, body mass index; BUN, blood urea nitrogen; hs-CRP, high-sensitivity C-reactive protein; PTH, parathyroid hormone.
Both eGFR and UAE were regularly measured at baseline and at several follow-up screening waves, of which the first took place at a median follow-up of 4.1 years (IQR, 4.0–4.3 years), the second at 6.3 years (IQR, 6.0–6.7 years), the third at 9.2 years (IQR, 8.8–9.5 years), and the fourth at 11.5 years (IQR, 11.2–12.4 years).
Risk factors for hypokalemia and hyperkalemia
Univariable ORs and 95% CIs according to categories of plasma potassium are shown in S1 Table. Bivariable ORs (95% CIs) in which associations were adjusted for the potential influence of eGFR are shown in Table 2. Female sex, alcohol consumption, diabetes, hypertension, use of beta blockers, use of thiazide and loop diuretics, and UAE were associated with higher risk of hypokalemia, whereas high educational level, and higher urinary potassium and magnesium excretion were associated with lower risk of hypokalemia, after accounting for eGFR. Female sex and alcohol consumption were associated with a lower risk of hyperkalemia. Use of potassium-sparing diuretics, urinary excretions of potassium and magnesium were associated with higher risk of hyperkalemia, independent of eGFR.
Table 2. Bivariable adjusted odds ratios (95% confidence intervals) combined with eGFR for risk factors of hypokalemia and hyperkalemia in 5,130 participants of the Prevention of Renal and Vascular End-Stage Disease (PREVEND) study.
| Plasma potassium, mmol/L | |||||
|---|---|---|---|---|---|
| 2.3–3.4 | 3.5–3.9 | 4.0–4.4 | 4.5–4.9 | 5.0–6.3 | |
| Sex, female vs male | 4.00 (1.36–11.72)* | 1.81 (1.45–2.26)*** | 1.00 (ref) | 0.75 (0.67–0.85)*** | 0.70 (0.53–0.94)* |
| Age, y | 1.02 (0.98–1.07) | 1.01 (1.00–1.03)* | 1.00 (ref) | 0.99 (0.99–1.00)* | 0.99 (0.97–1.00) |
| BMI, kg/m2 | 1.02 (0.92–1.13) | 1.00 (0.98–1.03) | 1.00 (ref) | 0.99 (0.97–1.00) | 0.97 (0.93–1.01) |
| Current smoker, yes vs no | 0.82 (0.33–2.08) | 0.56 (0.43–0.73)*** | 1.00 (ref) | 1.53 (1.35–1.74)*** | 1.34 (0.99–1.82) |
| Alcohol consumption, yes vs no | 2.96 (1.32–6.67)** | 1.29 (1.02–1.62)* | 1.00 (ref) | 0.89 (0.77–1.03) | 0.66 (0.45–0.96)* |
| Education, high, yes vs no | 0.18 (0.04–0.77)* | 0.90 (0.72–1.12) | 1.00 (ref) | 0.92 (0.81–1.05) | 0.98 (0.72–1.34) |
| White, yes vs no | 0.18 (0.02–1.40) | 0.66 (0.29–1.51) | 1.00 (ref) | 2.68 (1.11–6.45)* | - |
| Type 2 Diabetes, yes vs no | 13.88 (4.44–43.35)*** | 2.13 (1.04–4.34)* | 1.00 (ref) | 1.63 (1.01–2.63)* | 1.18 (0.36–3.87) |
| Hypertension, yes vs no | 6.74 (2.54–17.92)*** | 1.62 (1.29–2.05)*** | 1.00 (ref) | 0.81 (0.70–0.94)** | 0.75 (0.52–1.07) |
| ACEi, yes vs no | 1.20 (0.16–9.28) | 1.71 (1.01–2.90) | 1.00 (ref) | 0.76 (0.51–1.13) | 0.69 (0.25–1.92) |
| ARB, yes vs no | - | 1.64 (0.35–7.66) | 1.00 (ref) | 1.40 (0.54–3.65) | 1.45 (0.18–11.61) |
| Beta blockers, yes vs no | 4.61 (1.59–13.39)** | 1.61 (1.06–2.47)* | 1.00 (ref) | 0.97 (0.73–1.29) | 1.13 (0.59–2.16) |
| Thiazide diuretics, yes vs no | 20.12 (7.05–57.40)*** | 6.65 (4.16–10.63)*** | 1.00 (ref) | 0.29 (0.14–0.63)** | 0.31 (0.04–2.26) |
| Loop diuretics, yes vs no | 10.94 (1.25–95.73)* | 5.71 (1.96–16.66)** | 1.00 (ref) | 1.36 (0.49–3.77) | 1.60 (0.20–12.95) |
| Potassium-sparing diuretics, yes vs no | - | 8.00 (4.01–15.96)*** | 1.00 (ref) | 0.37 (0.12–1.09) | 3.14 (1.04–9.55)* |
| Urinary potassium excretion, mmol/24h | 0.96 (0.94–0.98)** | 0.99 (0.98–0.99)*** | 1.00 (ref) | 1.00 (1.00–1.01)* | 1.01 (1.00–1.01)* |
| Urinary magnesium excretion, mmol/24h | 0.55 (0.41–0.74)*** | 0.89 (0.83–0.95)** | 1.00 (ref) | 1.05 (1.01–1.09)* | 1.14 (1.04–1.25)** |
| Urinary albumin excretion, mg/24h† | 3.00 (1.39–6.48)** | 1.15 (0.92–1.43) | 1.00 (ref) | 0.97 (0.85–1.10) | 1.14 (0.84–1.54) |
ORs (95% CIs) are calculated with multinomial regression analyses.
* P<0.05,
**P<0.01,
***P<0.001.
† Natural log(ln)-transformed.
Abbreviations: ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blockers; BMI, body mass index; eGFR, estimated glomerular filtration rate.
Risk of developing CKD
During a median follow-up of 10.3 years (IQR: 6.3–11.4 years), 753 subjects developed CKD. In the crude model, subjects with hypokalemia had a higher risk of developing CKD compared to subjects with normokalemia (HR, 5.31; 95% CI, 2.99–9.24; Table 3). After adjustment of age, sex, eGFR, height, weight, urinary potassium excretion, and use of diuretics, this association remained significant (HR, 3.65; 95% CI, 1.94–6.85). Further adjustment for systolic blood pressure, plasma aldosterone, plasma albumin, plasma magnesium, hs-CRP, type 2 diabetes, smoking, alcohol, education, race, urinary creatinine excretion, plasma chloride, or the BUN/creatinine ratio did not materially alter the association. Secondary analyses in which CKD was defined as either eGFR <60 ml/min/1.73 m2 or UAE >30 mg/24h alone, rendered essentially similar results (S2 Table).
Table 3. Association of plasma potassium with risk of developing chronic kidney disease defined as eGFRcreatinine-cystatin C <60 ml/min/1.73m2 or urinary albumin excretion >30 mg/24h in 5,130 participants of the Prevention of Renal and Vascular End-Stage Disease (PREVEND) study.
| Plasma potassium, mmol/L | |||||
|---|---|---|---|---|---|
| 2.3–3.4 | 3.5–3.9 | 4.0–4.4 | 4.5–4.9 | 5.0–6.3 | |
| Person-years | 149 | 3541 | 25,767 | 14,434 | 1,644 |
| Number of events | 12 | 63 | 401 | 249 | 28 |
| Rates* | 805 | 178 | 156 | 173 | 170 |
| Crude model | 5.31 (2.99–9.24) | 1.14 (0.88–1.49) | 1.00 (ref) | 1.11 (0.95–1.30) | 1.09 (0.75–1.61) |
| Multivariable model 1† | 4.33 (2.43–7.72) | 1.18 (0.91–1.55) | 1.00 (ref) | 1.02 (0.87–1.20) | 1.00 (0.69–1.47) |
| Multivariable model 2‡ | 3.65 (1.94–6.85) | 0.83–0.61–1.14) | 1.00 (ref) | 1.07 (0.90–1.26) | 1.00 (0.66–1.53) |
| + Systolic blood pressure | 3.70 (1.97–6.93) | 0.81 (0.60–1.11) | 1.00 (ref) | 1.08 (0.91–1.28) | 1.04 (0.68–1.59) |
| + Aldosterone | 3.11 (1.60–6.04) | 0.88 (0.64–1.21) | 1.00 (ref) | 1.04 (0.86–1.24) | 1.08 (0.70–1.69) |
| + Plasma albumin | 3.65 (1.94–6.86) | 0.83 (0.61–1.14) | 1.00 (ref) | 1.07 (0.90–1.27) | 1.00 (0.65–1.52) |
| + Plasma magnesium | 3.36 (1.78–6.33) | 0.82 (0.60–1.12) | 1.00 (ref) | 1.05 (0.89–1.25) | 1.01 (0.66–1.55) |
| + Hs-CRP | 3.94 (2.09–7.41) | 0.86 (0.63–1.18) | 1.00 (ref) | 1.09 (0.92–1.30) | 1.03 (0.67–1.58) |
| + Type 2 diabetes | 3.27 (1.75–6.11) | 0.83 (0.61–1.14) | 1.00 (ref) | 1.05 (0.89–1.24) | 1.02 (0.67–1.56) |
| + Smoking | 3.65 (1.94–6.86) | 0.84 (0.62–1.15) | 1.00 (ref) | 1.06 (0.89–1.26) | 1.00 (0.65–1.52) |
| + Alcohol consumption | 3.40 (1.81–6.41) | 0.83 (0.60–1.13) | 1.00 (ref) | 1.07 (0.90–1.27) | 1.01 (0.66–1.54) |
| + Education | 3.61 (1.92–6.78) | 0.83 (0.61–1.13) | 1.00 (ref) | 1.07 (0.90–1.26) | 1.00 (0.66–1.53) |
| + Race | 3.70 (1.97–6.95) | 0.83 (0.61–1.14) | 1.00 (ref) | 1.07 (0.90–1.27) | 1.01 (0.66–1.55) |
| + Urinary creatinine excretion | 3.70 (1.97–6.95) | 0.82 (0.60–1.12) | 1.00 (ref) | 1.07 (0.90–1.26) | 1.01 (0.66–1.54) |
| + Plasma chloride | 3.50 (1.83–6.71) | 0.83 (0.61–1.13) | 1.00 (ref) | 1.07 (0.90–1.27) | 1.00 (0.66–1.53) |
| + BUN/creatinine ratio | 3.66 (1.95–6.88) | 0.84 (0.61–1.14) | 1.00 (ref) | 1.06 (0.90–1.26) | 1.00 (0.66–1.53) |
Hazard ratios (HR) and 95% confidence intervals were derived from Cox proportional hazards regression models.
* Number of events divided by time at risk standardized per 10,000 person-years.
† Multivariable model 1 is adjusted for age, sex, and eGFR.
‡ Multivariable model 2 is additionally adjusted for height, weight, urinary potassium excretion, and use of diuretics.
Abbreviations: BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; hs-CRP, high-sensitivity C-reactive protein.
We found neither an association of hyperkalemia with risk of developing CKD in the crude model (HR, 1.09; 95% CI, 0.75–1.61; Table 3), nor after multivariable adjustment for age, sex, eGFR, height, weight, urinary potassium excretion, and use of diuretics (HR, 1.00; 95% CI, 0.66–1.53).
Effect modification by diuretic use
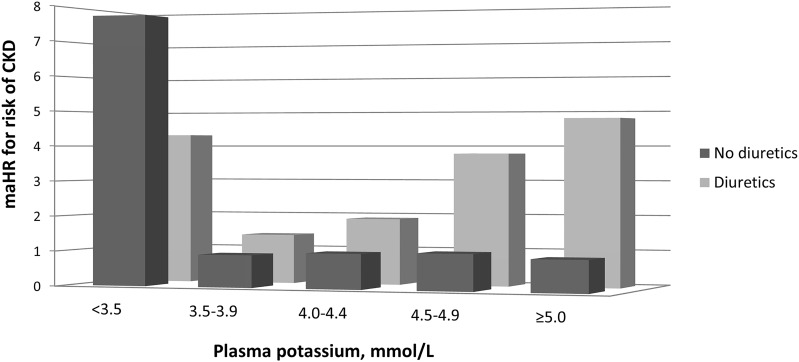
The association of plasma potassium with risk of developing CKD was modified by use of diuretics (Pinteraction = 0.02; Fig 2). Hypokalemia was associated with a higher risk of developing CKD in both subjects using diuretics (HR, 4.32; 95% CI, 1.77–10.51) and not using diuretics (HR, 7.74; 95% CI, 3.43–17.48), compared to subjects with normokalemia and not using diuretics (S3 Table). Moreover, in the absence of hypokalemia, plasma potassium was associated with an increased risk of CKD in subjects using diuretics (Ptrend = 0.01) but not in subjects not using diuretics (Ptrend = 0.74). We found no further evidence for effect modification by sex, age, hypertension status, urinary potassium excretion, baseline eGFR, and plasma aldosterone (Pinteraction>0.1).
Fig 2. Association of plasma potassium with risk of developing chronic kidney disease stratified by use of diuretics in 5,130 participants of the Prevention of Renal and Vascular End-Stage Disease (PREVEND) study.
Hazard ratios were calculated with Cox proportional hazards regression models and were adjusted for age, sex, height, weight, eGFR, and urinary potassium excretion. The category plasma potassium 4.0–4.4 mmol/L and no diuretics was set as reference. Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; maHR, multivariable-adjusted hazard ratio.
Sensitivity analyses for risk of CKD
When stratifying the analyses by use of potassium-wasting diuretics, hypokalemia was associated with a higher risk of developing CKD in both subjects using potassium-wasting diuretics (HR, 4.24; 95% CI, 1.74–10.32) and not using potassium-wasting diuretics (HR, 7.58; 95% CI, 3.36–17.10), compared to subjects with normokalemia not using potassium-wasting diuretics. Since there were no subjects using potassium-sparing diuretics among the hypokalemic subjects, we could not examine the specific role of potassium-sparing diuretics in the association of hypokalemia with risk of CKD. Second, results were essentially the same when we excluded subjects at baseline with an eGFR <66 (instead of <60) ml/min/1.73m2 and/or a UAE <25 (instead of <30) mg/24h (N = 4,923, n = 539) for a more pronounced decline in kidney function during follow-up before reaching the CKD endpoint (HR for hypokalemia compared to normokalemia, 3.62; 95% CI, 1.80–7.30). Third, when we restricted the analyses to subjects who were not using antihypertensive drugs at baseline (N = 4,507, n = 438) results for risk of CKD did not materially change (HR for hypokalemia compared to normokalemia, 6.30; 95% CI, 1.55–25.65). Fourth, we excluded subjects from the analysis who developed diabetes during follow-up (N = 423). During a median follow-up of 10.4 years (IQR: 6.4–11.4 years), 541 subjects developed CKD. The results of this sensitivity analysis did not differ materially from that of the primary analyses, with hypokalemia associated with a higher risk of developing CKD in both subjects using diuretics (HR, 3.81; 95% CI, 1.41–10.30) and not using diuretics (HR, 8.43; 95% CI, 3.11–22.88), compared to subjects with normokalemia and not using diuretics. Finally, in weighted analyses that accounted for the sampling design of the study, results were slightly attenuated (HR for hypokalemia compared to normokalemia, 2.98; 95% CI, 0.79–11.31). However, when stratified for use of diuretics, hypokalemic subjects not using diuretics had a significant increased risk of developing CKD (HR, 8.72; 95% CI, 3.66–20.81, compared to subjects with normokalemia not using diuretics).
Discussion
In this prospective population-based cohort study, hypokalemia was associated with a higher risk of developing CKD, regardless of diuretic use. The association between plasma potassium and risk of CKD was modified by use of diuretics in such a way that non-hypokalemic potassium concentrations were associated with an increased CKD risk among subjects using diuretics but not among subjects not using diuretics. The associations remained after adjustment of confounders, CKD risk factors, and potential mediators of the association, such as systolic blood pressure, plasma magnesium, plasma albumin, hs-CRP, aldosterone, plasma chloride, and the BUN/creatinine ratio.
An observational study in healthy Japanese subjects not using diuretics observed that subjects with lower plasma potassium concentrations had an increased risk of developing CKD (potassium <4.0 mmol/L; HR, 2.65; 95% CI, 2.04–3.44) [13]. These results are in line with a recent study of Chen et al., who investigated the association of potassium concentrations and risk of CKD in the Atherosclerosis Risk of Community Study, a general population cohort from the United States [14]. They observed that hypokalemia was associated with adverse kidney outcomes among participants not taking potassium-wasting drugs, i.e. loop diuretics, thiazide diuretics, and thiazide-like diuretics (potassium <3.5 mmol/24h; HR 1.65; 95 CI, 1.16–2.35). In our present study, we observed that hypokalemia was associated with a higher risk of developing CKD, regardless of diuretic use. Moreover, in contrast to Chen et al., among subjects using diuretics, non-hypokalemic plasma potassium concentrations were positively associated with risk of CKD. The latter finding might reflect confounding by indication or an adverse effect of diuretics.
The prevalence of hypokalemia at baseline in the Prevention of Renal and Vascular End-stage Disease (PREVEND) study (0.5%) was lower compared to a previous reported prevalence of hypokalemia (also defined as potassium <3.5 mmol/L) in the third National Health and Nutrition Examination Survey (NHANES III) study (3.1%), a general population cohort in the US [24]. The prevalence of hyperkalemia (defined as ≥5.0 mmol/L) was higher in the PREVEND study (3.8%) compared to the NHANES III study (0.7%). These difference in prevalences of hypo- and hyperkalemia might be explained by the lower number of Black subjects included in our cohort (0.9%) compared to the NHANES III (10.4%) [25], since Black people are known to have lower potassium concentrations compared to White people [26].
Our data regarding the determinants of potassium abnormalities mainly confirmed previous observations. For example, previous studies also identified sex as a risk factor for hypo- and hyperkalemia, where women had higher risk of hypokalemia and men had higher risk of hyperkalemia [7,11,24,27,28], possibly because of differences in body composition. Diuretic use is also known in the literature as a risk factor for hypokalemia [4,7,11,29], due to increased renal potassium loss. Renal insufficiency is reported to be the most common cause of hyperkalemia [30]. In the present study, these risk factors were all associated with hyperkalemia independent of eGFR. No associations of hypokalemia and hyperkalemia with age were observed in our study, while other studies argue that increasing age is an important risk factor for both conditions, especially hyperkalemia [27,28,31].
Several possible mechanisms by which hypokalemia could induce kidney injury are proposed in literature. We investigated some of these possible underlying mechanisms in the present study. First, despite the strict regulation of plasma potassium concentrations between narrow limits and the subsequent limited effect of potassium intake on plasma potassium concentrations [32–37], in some cases hypokalemia can be a reflection of insufficient potassium intake–and thereby also lower intake of fiber, vitamins and antioxidants–or poor nutritional status, which are both associated with risk of developing CKD via oxidative stress, hypertension, or inflammation [38–41]. Despite that subjects with hypokalemia had a significantly lower potassium excretion in the present study, when additionally adjusting for urinary potassium excretion, results remained similar for the association of hypokalemia with risk of CKD. Second, high plasma potassium is associated with reduced activity of the renin-angiotensin-aldosterone system, which has been shown to slow progression of renal disease [42], whereas hypokalemia was reported to stimulate renin and angiotensin II despite direct suppression of aldosterone synthesis leading to salt-sensitive hypertension, intrarenal vasoconstriction and ischemia [10,43–45]. Hypokalemic subjects in the present study had a higher blood pressure and were more likely to use antihypertensive medication, however, results for risk of CKD were essentially the same when additionally adjusting for systolic blood pressure or aldosterone. Third, experimental studies determined that induced hypokalemia led to declines in insulin release in response to hyperglycemia and to a decrease in pancreatic β-cell sensitivity to hyperglycemia with a reduction in insulin release [46–48]. Although subjects with hypokalemia were more likely to have diabetes, additional adjustment for diabetes or excluding subjects who developed diabetes during follow-up rendered similar results as the primary analyses. Finally, low potassium concentrations could have induced diabetes insipidus or could have been accompanied with metabolic alkalosis. However, additional adjustment for the BUN/creatinine ratio or serum chloride levels did not alter the association of hypokalemia with risk of CKD. Taken together, these results suggest that other mechanisms are responsible for the observed association between hypokalemia and risk of CKD. Other mechanisms proposed in literature involves hypokalemia-induced tubulointerstitial injury by ammoniagenesis which has been observed in experimental animal models [9,49]. In line with these studies, our results show that hypokalemia is associated with increased UAE, which can be considered a proxy of tubulointerstitial damage [50,51]. However, we do not have data on urinary ammonia excretion, so we could not further investigate this mechanism. Another mechanism proposed includes the renoprotective effects of higher plasma potassium by upregulating renal kinins, such as kallikrein [52]. Experimental studies showed that an increase in plasma potassium concentration reduces cultured vascular smooth muscle cell proliferation [53], inhibits free radical formation from endothelial cells and macrophages [54], and inhibits platelet aggregation and arterial thrombosis [55,56]. However, also no data on plasma kallikrein were available in the PREVEND study, so we could not investigate this possible mechanism.
The absence of an association between hyperkalemia and risk of developing CKD in the present study is consistent with the findings of Chen et al. and Fukui et al. [13,14]. Both studies did not find associations of high plasma potassium concentrations with risk of developing CKD in subjects free of CKD at baseline and not taking (potassium-sparing) diuretics. Interestingly, Chen and colleagues also observed that higher potassium concentrations were associated with CKD in participants taking potassium-wasting diuretics[14]. Furthermore, studies in CKD patients did not observe associations of hyperkalemia with risk of ESRD [6,12], expect for one study [11]. Moreover, It is important to realize that due to the observational design of our study, and that of Chen et al. and Fukui et al., conclusions about no causal relationship between hyperkalemia and risk of CKD cannot be drawn.
We observed in our study that both the use of diuretics and beta blockers were associated with hypokalemia in cross-sectional analyses, which raises several interesting insights. Diuretics are often used in patients with heart and/or renal disease with volume overload and are associated with potassium loss via the urine. Further, experimental studies as well as clinical and observational studies have suggested a correlation between diuretic use and progression of renal injury [57]. Thiazides have been proposed to associate with renal injury via the induction of hypokalemia, metabolic abnormalities, and volume depletion and to lead to additional focal glomerular damage not observed in states of equivalent hypokalemia in the absence of diuretics [58].
Some limitations of this study should be mentioned. First, as with any observational study, there may be unmeasured or residual confounding despite the substantial number of potentially confounding factors for which we adjusted. Second, due to the small number of subjects with hypo- and hyperkalemia, we could not calculate ORs (95% CIs) for use of thiazide and potassium-sparing diuretics and race for all potassium categories, or reach significance due to the large 95% CIs. However, despite the small number of subjects in some of the potassium categories, we observed a significant association of hypokalemia with increased risk of CKD. Unfortunately, the underlying cause of hypokalemia could not be identified. Third, our results may not be readily generalizable to different demographic populations because >95% of the individuals of the PREVEND study are white. Fourth, our results may not be readily generalizable to different demographic populations because >95% of the individuals of the PREVEND study are White. Blacks, for instance, typically show a lower urinary excretion rate of potassium than do whites [59,60] due to a higher loss of potassium via other routes, a slower rate of disposal as a result of slower skeletal muscle uptake of potassium, or genetic differences in renal potassium handling [60].
Finally, plasma potassium was measured only at baseline. However, when the intra-individual variability of variables is taken into account, this results in strengthening of associations [61]. Therefore, our use of a single plasma potassium measurement at baseline rather than several or multiple ones, will likely provide an underestimation of the true effect.
A strength of this study is the use of the combined CKD end point consisting of a creatinine-cystatin C-based eGFR and two consecutive 24-hour UAE at each screening to ascertain CKD events. Other strengths of this study were the prospective design, the relatively large sample size, and the availability of detailed data on potential confounders.
In conclusion, in this Dutch prospective population-based study, hypokalemia was associated with an increased risk of developing CKD, regardless of diuretic use. Furthermore, the association between plasma potassium and risk of CKD was modified by use of diuretics, in such a way that in the absence of hypokalemia, plasma potassium was associated with an increased risk of CKD in subjects using diuretics, but not in subjects not using diuretics. Traditionally, the concern for nephrologists is hyperkalemia. However, these data show that hypokalemia might be a risk factor for developing CKD among subjects with normal kidney function. Further research is needed to identify the role of the underlying cause of hypokalemia in this association, and to investigate whether the higher risk of developing CKD could be diminished when regulating plasma potassium levels strictly.
Supporting information
Odds ratios (95% confidence intervals) are calculated with multinomial regression analyses. * P<0.05, ** P<0.01, *** P<0.001. † Natural log(ln)-transformed. Abbreviations: ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blockers; BMI, body mass index; PREVEND, Prevention of Renal and Vascular End-Stage Disease.
(DOCX)
Hazard ratios and 95% confidence intervals were derived from Cox proportional hazards regression models. * Number of events divided by time at risk standardized per 10,000 person-years. † Multivariable model 1 is adjusted for age, sex, and eGFR. ‡ Multivariable model 2 is additionally adjusted for height, weight, urinary potassium excretion, and use of diuretics. Abbreviations: eGFR, estimated glomerular filtration rate; hs-CRP, high-sensitivity C-reactive protein; PREVEND, Prevention of Renal and Vascular End-Stage Disease; UAE, urinary albumin excretion.
(DOCX)
Hazard ratios and 95% confidence intervals were derived from Cox proportional hazards regression models. * Number of events divided by time at risk standardized per 10,000 person-years. † Multivariable model 1 is adjusted for age, sex, eGFR, height, weight, and urinary potassium excretion. Abbreviations: eGFR, estimated glomerular filtration rate; PREVEND, Prevention of Renal and Vascular End-Stage Disease.
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The Prevention of Renal and Vascular End-Stage Disease (PREVEND) study has been made possible by grants from the Dutch Kidney Foundation. The funder of the work had no role in the conception, execution, or analysis of the research and had no role in drafting the manuscript.
References
- 1.Gumz ML, Rabinowitz L, Wingo CS. An Integrated View of Potassium Homeostasis. N Engl J Med 2015. July 2;373(1):60–72. 10.1056/NEJMra1313341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanton BA. Renal potassium transport: morphological and functional adaptations. Am J Physiol 1989. November;257(5 Pt 2):R989–97. [DOI] [PubMed] [Google Scholar]
- 3.Gonick HC, Kleeman CR, Rubini ME, Maxwell MH. Functional impairment in chronic renal disease. 3. Studies of potassium excretion. Am J Med Sci 1971. May;261(5):281–290. [DOI] [PubMed] [Google Scholar]
- 4.Knochel JP. Diuretic-induced hypokalemia. Am J Med 1984. November 5;77(5A):18–27. [DOI] [PubMed] [Google Scholar]
- 5.Luo J, Brunelli SM, Jensen DE, Yang A. Association between Serum Potassium and Outcomes in Patients with Reduced Kidney Function. Clin J Am Soc Nephrol 2016. January 7;11(1):90–100. 10.2215/CJN.01730215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakhoul GN, Huang H, Arrigain S, Jolly SE, Schold JD, Nally JV Jr, et al. Serum Potassium, End-Stage Renal Disease and Mortality in Chronic Kidney Disease. Am J Nephrol 2015;41(6):456–463. 10.1159/000437151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korgaonkar S, Tilea A, Gillespie BW, Kiser M, Eisele G, Finkelstein F, et al. Serum potassium and outcomes in CKD: insights from the RRI-CKD cohort study. Clin J Am Soc Nephrol 2010. May;5(5):762–769. 10.2215/CJN.05850809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goyal A, Spertus JA, Gosch K, Venkitachalam L, Jones PG, Van den Berghe G, et al. Serum potassium levels and mortality in acute myocardial infarction. JAMA 2012. January 11;307(2):157–164. 10.1001/jama.2011.1967 [DOI] [PubMed] [Google Scholar]
- 9.Tolins JP, Hostetter MK, Hostetter TH. Hypokalemic nephropathy in the rat. Role of ammonia in chronic tubular injury. J Clin Invest 1987. May;79(5):1447–1458. 10.1172/JCI112973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suga SI, Phillips MI, Ray PE, Raleigh JA, Vio CP, Kim YG, et al. Hypokalemia induces renal injury and alterations in vasoactive mediators that favor salt sensitivity. Am J Physiol Renal Physiol 2001. October;281(4):F620–9. [DOI] [PubMed] [Google Scholar]
- 11.Wang HH, Hung CC, Hwang DY, Kuo MC, Chiu YW, Chang JM, et al. Hypokalemia, its contributing factors and renal outcomes in patients with chronic kidney disease. PLoS One 2013. July 2;8(7):e67140 10.1371/journal.pone.0067140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes J, Kalantar-Zadeh K, Lu JL, Turban S, Anderson JE, Kovesdy CP. Association of hypo- and hyperkalemia with disease progression and mortality in males with chronic kidney disease: the role of race. Nephron Clin Pract 2012;120(1):c8–16. 10.1159/000329511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukui M, Tanaka M, Toda H, Asano M, Yamazaki M, Hasegawa G, et al. Low serum potassium concentration is a predictor of chronic kidney disease. Int J Clin Pract 2014. June;68(6):700–704. 10.1111/ijcp.12367 [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Chang AR, McAdams DeMarco MA, Inker LA, Matsushita K, Ballew SH, et al. Serum Potassium, Mortality, and Kidney Outcomes in the Atherosclerosis Risk in Communities Study. Mayo Clin Proc 2016. October;91(10):1403–1412. 10.1016/j.mayocp.2016.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillege HL, Janssen WM, Bak AA, Diercks GF, Grobbee DE, Crijns HJ, et al. Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med 2001. June;249(6):519–526. [DOI] [PubMed] [Google Scholar]
- 16.Halbesma N, Brantsma AH, Bakker SJ, Jansen DF, Stolk RP, De Zeeuw D, et al. Gender differences in predictors of the decline of renal function in the general population. Kidney Int 2008. August;74(4):505–512. 10.1038/ki.2008.200 [DOI] [PubMed] [Google Scholar]
- 17.Visser ST, Schuiling-Veninga CC, Bos JH, de Jong-van den Berg LT, Postma MJ. The population-based prescription database IADB.nl: its development, usefulness in outcomes research and challenges. Expert Rev Pharmacoecon Outcomes Res 2013. June;13(3):285–292. 10.1586/erp.13.20 [DOI] [PubMed] [Google Scholar]
- 18.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012. July 5;367(1):20–29. 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grubb A, Blirup-Jensen S, Lindstrom V, Schmidt C, Althaus H, Zegers I, et al. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med 2010. November;48(11):1619–1621. 10.1515/CCLM.2010.318 [DOI] [PubMed] [Google Scholar]
- 20.van Ballegooijen AJ, Gansevoort RT, Lambers-Heerspink HJ, de Zeeuw D, Visser M, Brouwer IA, et al. Plasma 1,25-Dihydroxyvitamin D and the Risk of Developing Hypertension: The Prevention of Renal and Vascular End-Stage Disease Study. Hypertension 2015. September;66(3):563–570. 10.1161/HYPERTENSIONAHA.115.05837 [DOI] [PubMed] [Google Scholar]
- 21.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997. July;20(7):1183–1197. [DOI] [PubMed] [Google Scholar]
- 22.Kieneker LM, Gansevoort RT, Mukamal KJ, de Boer RA, Navis G, Bakker SJ, et al. Urinary Potassium Excretion and Risk of Developing Hypertension: The Prevention of Renal and Vascular End-Stage Disease Study. Hypertension 2014. July 21;64:769–776. 10.1161/HYPERTENSIONAHA.114.03750 [DOI] [PubMed] [Google Scholar]
- 23.Joosten MM, Gansevoort RT, Bakker SJ, PREVEND Study Group. Low plasma magnesium and risk of developing chronic kidney disease: results from the PREVEND Study. Kidney Int 2015. June;87(6):1262–1263. [DOI] [PubMed] [Google Scholar]
- 24.Wysowski DK, Kornegay C, Nourjah P, Trontell A. Sex and age differences in serum potassium in the United States. Clin Chem 2003. January;49(1):190–192. [DOI] [PubMed] [Google Scholar]
- 25.Foley RN, Wang C, Ishani A, Collins AJ. NHANES III: influence of race on GFR thresholds and detection of metabolic abnormalities. J Am Soc Nephrol 2007. September;18(9):2575–2582. 10.1681/ASN.2006121411 [DOI] [PubMed] [Google Scholar]
- 26.Chatterjee R, Yeh HC, Shafi T, Anderson C, Pankow JS, Miller ER, et al. Serum potassium and the racial disparity in diabetes risk: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 2011. May;93(5):1087–1091. 10.3945/ajcn.110.007286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hawkins RC. Gender and age as risk factors for hypokalemia and hyperkalemia in a multiethnic Asian population. Clin Chim Acta 2003. May;331(1–2):171–172. [DOI] [PubMed] [Google Scholar]
- 28.Kleinfeld M, Borra S, Gavani S, Corcoran A. Hypokalemia: are elderly females more vulnerable? J Natl Med Assoc 1993. November;85(11):861–864. [PMC free article] [PubMed] [Google Scholar]
- 29.Gennari FJ. Hypokalemia. N Engl J Med 1998. August 13;339(7):451–458. 10.1056/NEJM199808133390707 [DOI] [PubMed] [Google Scholar]
- 30.Hollander-Rodriguez JC, Calvert JF Jr. Hyperkalemia. Am Fam Physician 2006. January 15;73(2):283–290. [PubMed] [Google Scholar]
- 31.Flynn MA, Nolph GB, Baker AS, Martin WM, Krause G. Total body potassium in aging humans: a longitudinal study. Am J Clin Nutr 1989. October;50(4):713–717. [DOI] [PubMed] [Google Scholar]
- 32.Barden AE, Vandongen R, Beilin LJ, Margetts B, Rogers P. Potassium supplementation does not lower blood pressure in normotensive women. J Hypertens 1986. June;4(3):339–343. [DOI] [PubMed] [Google Scholar]
- 33.Fotherby MD, Potter JF. Potassium supplementation reduces clinic and ambulatory blood pressure in elderly hypertensive patients. J Hypertens 1992. November;10(11):1403–1408. [DOI] [PubMed] [Google Scholar]
- 34.Grobbee DE, Hofman A, Roelandt JT, Boomsma F, Schalekamp MA, Valkenburg HA. Sodium restriction and potassium supplementation in young people with mildly elevated blood pressure. J Hypertens 1987. February;5(1):115–119. [DOI] [PubMed] [Google Scholar]
- 35.MacGregor GA, Smith SJ, Markandu ND, Banks RA, Sagnella GA. Moderate potassium supplementation in essential hypertension. Lancet 1982. September 11;2(8298):567–570. [DOI] [PubMed] [Google Scholar]
- 36.Riphagen IJ, Gijsbers L, van Gastel MD, Kema IP, Gansevoort RT, Navis G, et al. Effects of potassium supplementation on markers of osmoregulation and volume regulation: results of a fully controlled dietary intervention study. J Hypertens 2016. February;34(2):215–220. 10.1097/HJH.0000000000000786 [DOI] [PubMed] [Google Scholar]
- 37.He FJ, Marciniak M, Carney C, Markandu ND, Anand V, Fraser WD, et al. Effects of potassium chloride and potassium bicarbonate on endothelial function, cardiovascular risk factors, and bone turnover in mild hypertensives. Hypertension 2010. March;55(3):681–688. 10.1161/HYPERTENSIONAHA.109.147488 [DOI] [PubMed] [Google Scholar]
- 38.Kieneker LM, Bakker SJ, de Boer RA, Navis GJ, Gansevoort RT, Joosten MM. Low potassium excretion but not high sodium excretion is associated with increased risk of developing chronic kidney disease. Kidney Int 2016. August 26. [DOI] [PubMed] [Google Scholar]
- 39.Erlinger TP, Tarver-Carr ME, Powe NR, Appel LJ, Coresh J, Eberhardt MS, et al. Leukocytosis, hypoalbuminemia, and the risk for chronic kidney disease in US adults. Am J Kidney Dis 2003. August;42(2):256–263. [DOI] [PubMed] [Google Scholar]
- 40.Lampe JW. Health effects of vegetables and fruit: assessing mechanisms of action in human experimental studies. Am J Clin Nutr 1999. September;70(3 Suppl):475S–490S. [DOI] [PubMed] [Google Scholar]
- 41.Jiang R, Jacobs DR Jr, Mayer-Davis E, Szklo M, Herrington D, Jenny NS, et al. Nut and seed consumption and inflammatory markers in the multi-ethnic study of atherosclerosis. Am J Epidemiol 2006. February 1;163(3):222–231. 10.1093/aje/kwj033 [DOI] [PubMed] [Google Scholar]
- 42.Ruster C, Wolf G. Renin-angiotensin-aldosterone system and progression of renal disease. J Am Soc Nephrol 2006. November;17(11):2985–2991. 10.1681/ASN.2006040356 [DOI] [PubMed] [Google Scholar]
- 43.Ray PE, Suga S, Liu XH, Huang X, Johnson RJ. Chronic potassium depletion induces renal injury, salt sensitivity, and hypertension in young rats. Kidney Int 2001. May;59(5):1850–1858. 10.1046/j.1523-1755.2001.0590051850.x [DOI] [PubMed] [Google Scholar]
- 44.Adrogue HJ, Madias NE. Sodium and potassium in the pathogenesis of hypertension. N Engl J Med 2007. May 10;356(19):1966–1978. 10.1056/NEJMra064486 [DOI] [PubMed] [Google Scholar]
- 45.Suga S, Mazzali M, Ray PE, Kang DH, Johnson RJ. Angiotensin II type 1 receptor blockade ameliorates tubulointerstitial injury induced by chronic potassium deficiency. Kidney Int 2002. March;61(3):951–958. 10.1046/j.1523-1755.2002.00208.x [DOI] [PubMed] [Google Scholar]
- 46.Rowe JW, Tobin JD, Rosa RM, Andres R. Effect of experimental potassium deficiency on glucose and insulin metabolism. Metabolism 1980. June;29(6):498–502. [DOI] [PubMed] [Google Scholar]
- 47.Helderman JH, Elahi D, Andersen DK, Raizes GS, Tobin JD, Shocken D, et al. Prevention of the glucose intolerance of thiazide diuretics by maintenance of body potassium. Diabetes 1983. February;32(2):106–111. [DOI] [PubMed] [Google Scholar]
- 48.Gorden P. Glucose intolerance with hypokalemia. Failure of short-term potassium depletion in normal subjects to reproduce the glucose and insulin abnormalities of clinical hypokalemia. Diabetes 1973. July;22(7):544–551. [DOI] [PubMed] [Google Scholar]
- 49.Nath KA, Hostetter MK, Hostetter TH. Increased ammoniagenesis as a determinant of progressive renal injury. Am J Kidney Dis 1991. June;17(6):654–657. [DOI] [PubMed] [Google Scholar]
- 50.Eddy AA, McCulloch L, Liu E, Adams J. A relationship between proteinuria and acute tubulointerstitial disease in rats with experimental nephrotic syndrome. Am J Pathol 1991. May;138(5):1111–1123. [PMC free article] [PubMed] [Google Scholar]
- 51.Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, et al. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med 1995. November 15;123(10):754–762. [DOI] [PubMed] [Google Scholar]
- 52.Ardiles L, Cardenas A, Burgos ME, Droguett A, Ehrenfeld P, Carpio D, et al. Antihypertensive and renoprotective effect of the kinin pathway activated by potassium in a model of salt sensitivity following overload proteinuria. Am J Physiol Renal Physiol 2013. June 15;304(12):F1399–410. 10.1152/ajprenal.00604.2012 [DOI] [PubMed] [Google Scholar]
- 53.McCabe RD, Young DB. Potassium inhibits cultured vascular smooth muscle cell proliferation. Am J Hypertens 1994. April;7(4 Pt 1):346–350. [DOI] [PubMed] [Google Scholar]
- 54.McCabe RD, Bakarich MA, Srivastava K, Young DB. Potassium inhibits free radical formation. Hypertension 1994. July;24(1):77–82. [DOI] [PubMed] [Google Scholar]
- 55.Lin H, Young DB. Interaction between plasma potassium and epinephrine in coronary thrombosis in dogs. Circulation 1994. January;89(1):331–338. [DOI] [PubMed] [Google Scholar]
- 56.Young DB, Lin H, McCabe RD. Potassium's cardiovascular protective mechanisms. Am J Physiol 1995. April;268(4 Pt 2):R825–37. [DOI] [PubMed] [Google Scholar]
- 57.Reungjui S, Pratipanawatr T, Johnson RJ, Nakagawa T. Do thiazides worsen metabolic syndrome and renal disease? The pivotal roles for hyperuricemia and hypokalemia. Curr Opin Nephrol Hypertens 2008. September;17(5):470–476. 10.1097/MNH.0b013e328305b9a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reungjui S, Hu H, Mu W, Roncal CA, Croker BP, Patel JM, et al. Thiazide-induced subtle renal injury not observed in states of equivalent hypokalemia. Kidney Int 2007. December;72(12):1483–1492. 10.1038/sj.ki.5002564 [DOI] [PubMed] [Google Scholar]
- 59.Pratt JH, Jones JJ, Miller JZ, Wagner MA, Fineberg NS. Racial differences in aldosterone excretion and plasma aldosterone concentrations in children. N Engl J Med 1989. October 26;321(17):1152–1157. 10.1056/NEJM198910263211703 [DOI] [PubMed] [Google Scholar]
- 60.Turban S, Miller ER 3rd, Ange B, Appel LJ. Racial differences in urinary potassium excretion. J Am Soc Nephrol 2008. July;19(7):1396–1402. 10.1681/ASN.2007101142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koenig W, Sund M, Frohlich M, Lowel H, Hutchinson WL, Pepys MB. Refinement of the association of serum C-reactive protein concentration and coronary heart disease risk by correction for within-subject variation over time: the MONICA Augsburg studies, 1984 and 1987. Am J Epidemiol 2003. August 15;158(4):357–364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Odds ratios (95% confidence intervals) are calculated with multinomial regression analyses. * P<0.05, ** P<0.01, *** P<0.001. † Natural log(ln)-transformed. Abbreviations: ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blockers; BMI, body mass index; PREVEND, Prevention of Renal and Vascular End-Stage Disease.
(DOCX)
Hazard ratios and 95% confidence intervals were derived from Cox proportional hazards regression models. * Number of events divided by time at risk standardized per 10,000 person-years. † Multivariable model 1 is adjusted for age, sex, and eGFR. ‡ Multivariable model 2 is additionally adjusted for height, weight, urinary potassium excretion, and use of diuretics. Abbreviations: eGFR, estimated glomerular filtration rate; hs-CRP, high-sensitivity C-reactive protein; PREVEND, Prevention of Renal and Vascular End-Stage Disease; UAE, urinary albumin excretion.
(DOCX)
Hazard ratios and 95% confidence intervals were derived from Cox proportional hazards regression models. * Number of events divided by time at risk standardized per 10,000 person-years. † Multivariable model 1 is adjusted for age, sex, eGFR, height, weight, and urinary potassium excretion. Abbreviations: eGFR, estimated glomerular filtration rate; PREVEND, Prevention of Renal and Vascular End-Stage Disease.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.