Abstract
INTRODUCTION: High-risk non–muscle-invasive bladder cancer (NMIBC) remains challenging given the high probability of progression. Given that the androgen receptor (AR) has been discussed as a possible factor in the development and progression of bladder cancer, we investigated the predictive value of AR in stage pT1 NMIBC. MATERIALS AND METHODS: We retrospectively analyzed the clinical data and AR mRNA expression in 296 patients with stage pT1 NMIBC who underwent a transurethral resection of the bladder. The mRNA expression of the AR transcript variants 1 (AR1) and 2 (AR2) was measured by reverse transcription quantitative real-time polymerase chain reaction. AR expression was also correlated to KRT5 and KRT20 mRNA expression. RESULTS: Kaplan-Meier analysis indicated that high AR1 mRNA expression ≥35.47 is associated with statistically significant better recurrence-free survival (RFS) (P = .0007), progression-free survival (PFS) (P = .0420), and cancer-specific survival (CSS) (P = .0050). Multivariate Cox regression analysis revealed that high AR1 mRNA expression is an independent prognostic marker for RFS (P = .0029) and CSS (P = .0119). Spearman rank correlation revealed a significant positive association between mRNA expression of AR1 and KRT5 (rs: 0.3171, P < .0001) as well as a negative association with multifocal tumors (rs: 0.1478, P < .0109). No association was noted between AR1 expression and tumor grade, concomitant CIS, gender, tumor size, and KRT20 in patients with stage T1 NMIBC. CONCLUSIONS: AR mRNA expression can predict RFS and CSS in patients with stage T1 NMIBC. Further studies are necessary to refine the relevance of AR mRNA expression compared with immunohistochemically detectable AR expression.
Introduction
Urothelial carcinoma of the bladder (UCB) is the ninth most common cancer worldwide with 430,000 new cases and 165,000 deaths estimated for 2012 [1]. UCB can be divided into non–muscle-invasive (NMIBC) and muscle-invasive bladder cancer (MIBC). NMIBC is typically treated with transurethral resection of the bladder (TURB) and, depending on its risk of progression, the instillation of mitomycin or Bacillus Calmette-Guerin (BCG), whereas radical cystectomy remains the gold standard for MIBC [2], [3]. However, high-risk NMIBC remains challenging given that the probability of progression ranges between 25% and 50% [4]. Patients eligible for early cystectomy are mainly determined by clinicopathological features based upon the scoring system developed by the European Organization for Research and Treatment of Cancer [2]. The research on new molecular markers and therapeutic targets is mandatory with the goal to improve the clinical course of these patients.
The fact that the incidence of UCB is increased by three-fold in men compared with women, even when adjusted for environmental and lifestyle factors [5], and that women have a worse outcome [6], [7] has led to the hypothesis of UCB being an endocrine-related malignancy. Particularly, the role of the androgen receptor (AR) in the tumorigenesis and progression of UCB has been the focus of several studies [8], [9], [10], [11]. For instance, Hsu et al. demonstrated that mice had a lower incidence of UCB and a higher survival rate after exposure to carcinogens when AR was ablated in the bladder [9]. The same study also showed that AR overexpression promoted the chemically induced transformation of normal urothelial cells to carcinoma in the human normal urothelial cell line SV-HUC-1 [9]. The findings made by in vitro and animal experiments are supported by a retrospective analysis of men with primary UCB and concomitant prostate cancer who experienced longer recurrence-free survival (RFS) rates for UCB under androgen deprivation therapy for prostate cancer [12]. However, the association between AR, tumor stage, and tumor grade remains unclear. Although some studies described an association between high AR expression and increased tumor stage and grade as well as a worse outcome [13], [14], other studies reported an association between a decrease of AR expression and tumor progression [15], [16]. Nevertheless, these data suggest that a change in AR expression is associated with the progression of UCB. However, to what extent AR expression can be considered as a possible marker or even a therapeutic target for patients with high-risk NMIBC needs to be determined.
The aim of the present study was to analyze the predictive value of AR expression in patients with stage T1 NMIBC. A reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR)–based assessment was used for the objective quantification of AR mRNA expression from formalin-fixed, paraffin-embedded routine tissues.
Material and Methods
Patient Population
In this study, we retrospectively analyzed 334 patients with stage pT1 NMIBC at initial diagnosis treated with TURB at the Department of Urology of the University of Regensburg between 1989 and 2012. The study was performed under a positive vote (No. 08/108) of the institutional review board of the University of Regensburg. Histopathological parameters of all cases, including grading according to WHO 1973 and WHO 2004 classifications, were assessed by a pathologist specialized in uropathology (A.H.).
Assessment of AR mRNA Expression by RT-qPCR
Tumor specimens were assessed by RT-qPCR as previously described [17]. In short, for RNA extraction from formalin-fixed, paraffin-embedded tissue, a single 10-μm curl was processed according to a commercially available bead-based extraction method (Xtract kit; STRATIFYER Molecular Pathology GmbH, Mainz, Germany). RNA was eluted with 100 μl of elution buffer. DNA was digested, and RNA eluates were then stored at −80°C until use.
Primers for the AR transcript variant 1 (AR1; RefSeq NM_000044.3) and transcript variant 2 (AR2; RefSeq NM_001011645.2) were designed. The mRNA expression levels of the genes of interest (GOI), AR1 and AR2, as well as the reference gene (REF) CALM2 were determined by RT-qPCR, which involves the reverse transcription of RNA and subsequent amplification of cDNA executed successively in a one-step reaction. Each patient sample or control was analyzed in triplicate in a Siemens Versant PCR System (Siemens, DE) according to the following protocol: 5 minutes at 50°C and 20 seconds at 95°C followed by 40 cycles of 15 seconds at 95°C and 60 seconds at 60°C.
Forty amplification cycles were applied, and the cycle threshold (CT) values of the two GOI and the REF gene for each sample (S) were estimated as the median of the triplicate measurements. These were then normalized against the mean expression levels of the REF gene using the 40-ΔCT method to ensure that normalized gene expression obtained by the test is proportional to the corresponding mRNA expression levels:
Statistical Methods
The cutoffs for AR1 and AR2 mRNA expression levels were determined by partitioning tests for progression-free survival (PFS) and then tested for the prediction of RFS and cancer-specific survival (CSS). Subsequently, RFS, PFS, and CSS were analyzed using the median mRNA expression of AR as objective cutoffs to validate the prognostic strength of AR mRNA expression. The Spearman product-moment correlation coefficient rs was used as a measure of the strength and direction of the linear relationship between variables in addition to the Wilcoxon/Kruskal-Wallis test, with both resulting in almost identical P values. Statistical analysis, including Kaplan-Meier survival analysis, multivariate Cox regression hazard analysis and partitioning testing, was performed with JMP SAS (SAS Institute, Cary, NC) and Graph Pad Prism software (Version 5.04; Graph Pad Software Inc., La Jolla, CA).
Results
Patient Population
Clinicopathological characteristics of the cohort are summarized in Table 1. Only patients with stage pT1 were included in the study. Any patients with stage pTa or pTx were not considered for inclusion. From this initial cohort of 334 patients, 38 samples were excluded due to low mRNA yields, leaving 296 patients for this study. All of these patients received a Re-TURB within 4 to 6 weeks after initial diagnosis. A total of 168 patients received a postoperative instillation therapy (n = 45 for mitomycin, n = 123 for BCG). The findings of stage ≤T1 were defined as recurrence, while the findings of stage >T1 were defined as progression. Median follow-up was 42 months (interquartile range, 25-72 months). The median mRNA expression levels for AR1 and AR2 were 34.63 and 26.87, respectively.
Table 1.
Patient Characteristics of Patients with T1 NMIBC
| Patient Characteristics | n (%) |
|---|---|
| Total cohort | 296 (100) |
| Male gender | 234 (79.1) |
| Age <75 years | 145 (49.0) |
| Tumor size <3 cm | 140 (47.3) |
| Concomitant CIS | 84 (28.4) |
| Multifocal tumor | 87 (29.4) |
| Mitomycin instillation therapy | 45 (15.2) |
| BCG instillation therapy | 123 (41.6.9) |
| WHO grading 1973 G2 | 95 (32.1) |
| WHO grading 1973 G3 | 201 (67.9) |
Correlation of AR mRNA Expression with Subtype Specific Genes and Clinicopathological Parameters
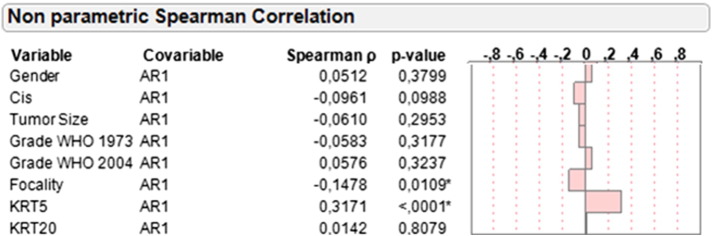
Nonparametric Spearman rank correlation revealed a statistically significant positive association between mRNA expression of AR1 and AR2 (rs: 0.6412, P < .0001). Given that the mRNA expression patterns of AR1 and AR2 were strongly correlated and AR1 expression was significantly increased compared with AR2 expression, subsequent analyses were performed exclusively with AR1. AR1 expression was inversely correlated with tumor multifocality (rs: −0.1478, P = .0109). No significant association was noted between AR1 expression and tumor grade, tumor size, concomitant CIS, and gender (Figure 1).
Figure 1.
Correlation of AR1 mRNA expression with gender, concomitant CIS, tumor size, focality, KRT5, KRT20, and grading according to WHO 1973 and WHO 2004 criteria based on central pathology review.
Moreover, we investigated the correlation of AR1 mRNA expression with the mRNA expression of KRT5 and KRT20, reflecting the basal and luminal subtypes of bladder cancer, respectively [18], [19], [20]. A significant correlation between AR1 and KRT5 was demonstrated (rs: 0.3171, P < .0001). No correlation was noted between the mRNA expression of AR1 and KRT20 (rs: 0.0142, P = .81).
Prognostic Value of AR1 mRNA Expression
Partition testing indicated that AR1 mRNA levels ≥35.47 are associated with improved PFS (5-year survival rate 84% vs 79%; P = .0420). Using this cutoff, Kaplan-Meier analysis revealed that high AR1 mRNA expression (≥35.47) was associated with significantly better RFS (5-year survival rate 75% vs 60%; P = .0007) and CSS (5-year survival rate 94% vs 80%; P = .0050) (Figure 2).
Figure 2.
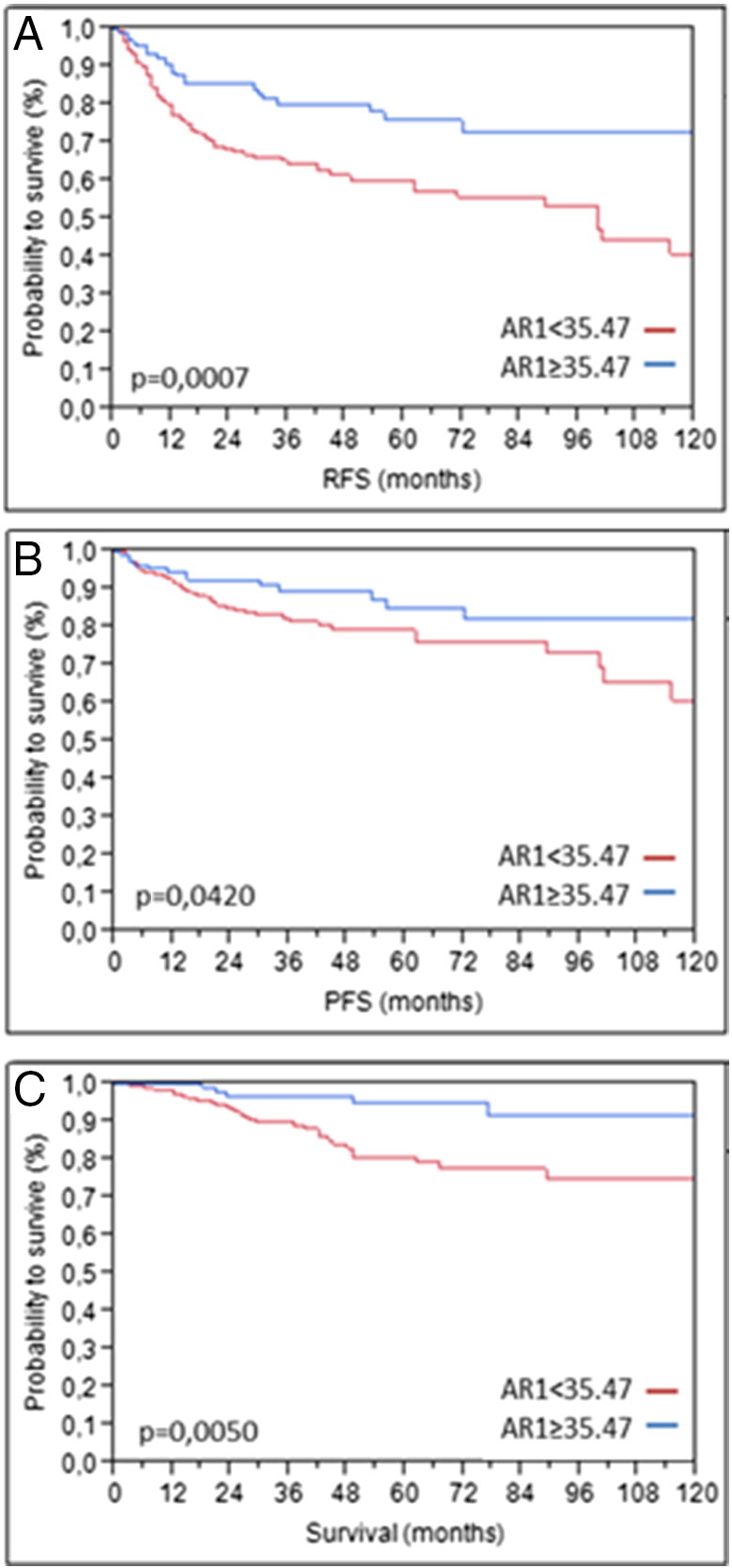
Kaplan-Meier analysis in the total cohort (n = 296) based on AR1 mRNA expression for RFS (A), PFS (B), and CSS (C).
To exclude a potential influence of instillation therapies on our results, we conducted a subgroup analysis based on postoperative instillation therapy. In the subgroup without instillation therapy (n = 128), high AR expression was significantly associated with improved RFS (P = .0241) and exhibited a strong tendency toward prolonged CSS (P = .0521). On the other hand, the subgroup that received a postoperative instillation therapy (n = 168) exhibited significantly improved RFS (P = .0070), PFS (P = .0230), and CSS (P = .0119) when high AR expression was present (Figure 3).
Figure 3.
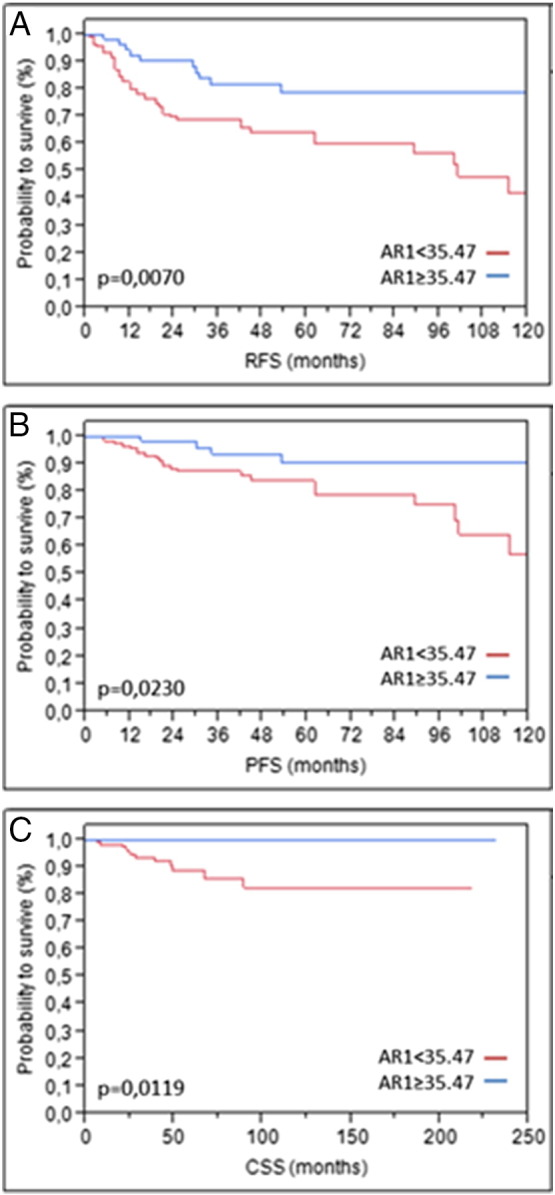
Kaplan-Meier analysis in the subcohort of pT1 patients who received instillation therapy (n = 168) based on AR1 mRNA expression for RFS (A), PFS (B), and CSS (C).
While there was no association between grading and AR mRNA expression according to nonparametric Spearman rank correlation, grade 3 NMIBC based on the WHO classification of 1973 was also associated with significantly worse PFS (P = .0421) and almost statistically significant worse CSS (P = .0585) (Supplemental Figure 1).
Supplemental Figure 1.
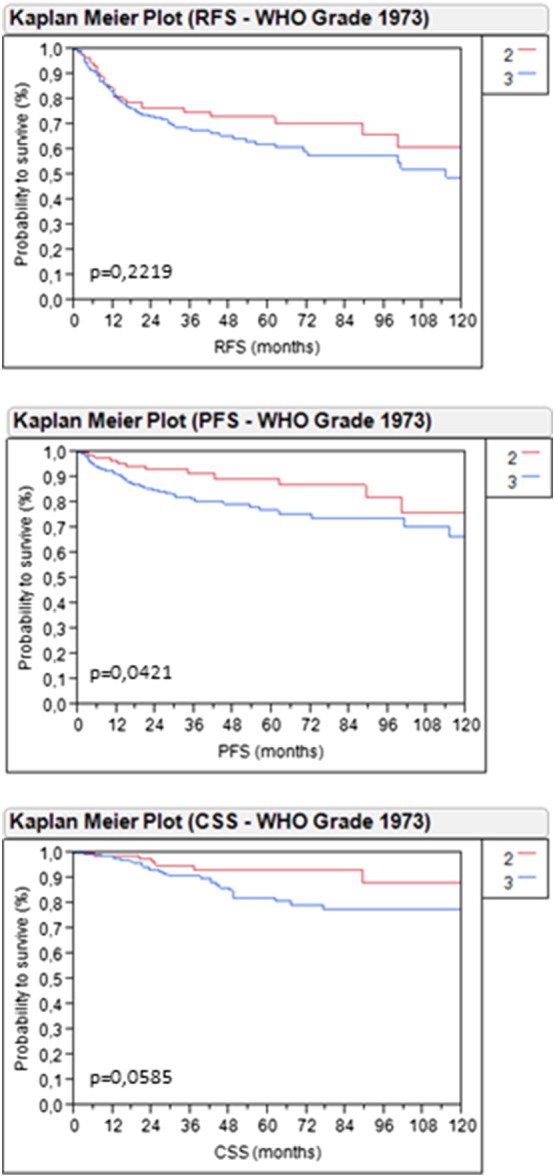
Kaplan-Meier analysis in the total cohort (n = 296) based on grading according to the WHO classification of 1973 for RFS (A), PFS (B), and CSS (C).
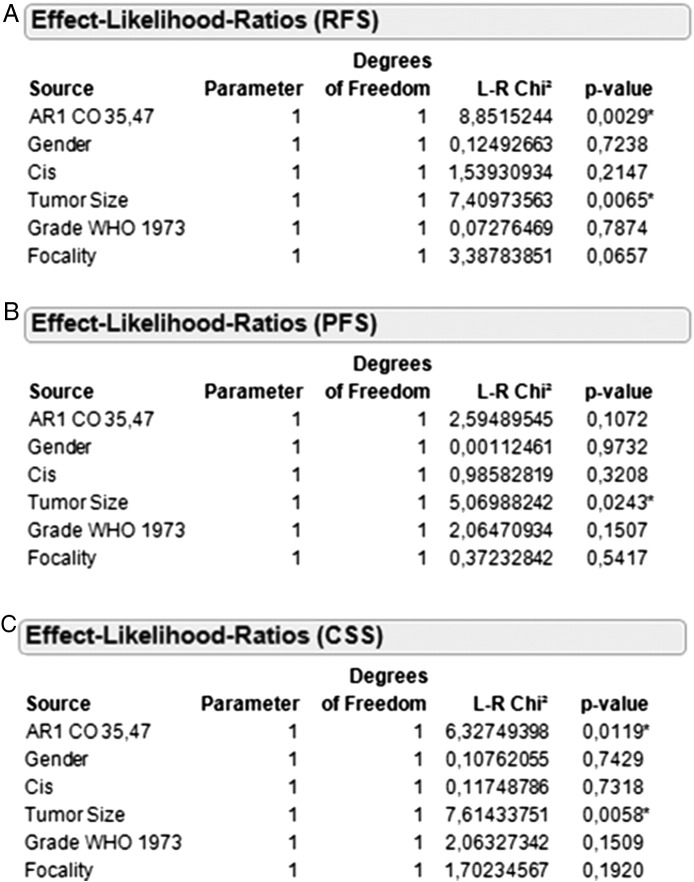
Multivariate Cox regression analysis adjusted for grading, gender, concomitant CIS, tumor size, and tumor focality revealed that high AR1 mRNA expression (≥35.47) is an independent prognostic marker for RFS (L-R χ2: 8.85, P = .0029) and CSS (L-R χ2: 6.33, P = .0119) (Figure 4). From the clinicopathological parameters, only tumor size was a statistically significant independent predictor for RFS (L-R χ2: 7.41, P = .0065), PFS (L-R χ2: 5.07, P = .0243), and CSS (L-R χ2: 7.61, P = .0058).
Figure 4.
Cox regression analysis of AR1 mRNA expression ≥35.47 in the total cohort for RFS (A), PFS (B), and CSS (C).
Discussion
Treatment options for UCB have minimally evolved in the last two decades [21]. However, recent genetic analyses revealed important new insight into the pathophysiology of UCB. Several independent studies revealed distinct molecular subtypes of UCB, reminiscent of the basal and luminal subtypes found in breast cancer [19], [20], [22]. These subtypes bear different clinicopathological features and survival outcomes given that basal UCBs are intrinsically more aggressive but chemosensitive, whereas luminal UCBs are less aggressive but chemoresistant [19], [20], [23]. Moreover, it was shown that genes and genomic regions are either mutated or altered frequently in UCB, which could be relevant for future targeted therapies [22]. These and other molecular findings are capable of revolutionizing the diagnostic and therapeutic approach toward UCB in a manner similar to that in breast cancer [24]. The recent approval of the PD-1/PD-L1 inhibitor atezolizumab by the FDA as a second-line treatment against metastatic UCB is a first step in this direction [25].
The discovery of molecular subtypes of UCB has also renewed the interest in the possible involvement of hormones and hormonal receptors in the development of UCB given that parameters such as ESR2 and ERBB2 expression have been used to define the luminal subtype of UCB [22]. Recently, we demonstrated that the mRNA expression of ESR1, ERBB2, and MKI67 is significantly associated with stage and grade in NMIBC [17].
AR as a possible factor in the development and progression of UCB has already been the focus of several studies. Although an association seems likely, the true prognostic significance of AR in UCB remains unclear mainly due to conflicting results [13], [14], [15], [16], [26]. For instance, Mashadi et al. showed increased AR expression in UCB compared with normal urothelium. Moreover, patients with positive AR expression had a poorer prognosis and a higher rate of metastases compared with their AR-negative counterparts given that AR expression positively correlated with tumor stage and grade in this study [14]. Zheng et al. found an association between AR positivity and progression of UCB as well [13]. In the study by Mir et al., only 9.0% of NMIBCs expressed the AR compared to 15.1% of MIBCs [27]. Miyamoto et al. demonstrated an increased AR expression in metastatic UCB compared with primary tumors. However, unlike the previously cited studies, the loss of AR expression was associated with high-grade and invasive tumors in this study [26]. Tuygun et al. also found that high AR expression was associated with low-grade tumors and NMIBC [16]. In all of the mentioned studies, AR expression in tumor specimens was assessed by immunohistochemistry. However, immunohistochemistry is prone to high interobserver variability and different results, depending on the applied antibody [27], [28]. Furthermore, immunohistochemistry was previously shown to detect only 5.7% of nuclear AR expression and 26.3% of combined nuclear and cytoplasmic AR expression, thereby masking prognostically meaningful results. [29]. This finding might contribute to the inconclusive results of the previous mentioned studies.
To avoid these technical limitations of immunohistochemistry in the assessment of AR protein expression in UCB, we measured AR mRNA expression using RT-qPCR. In the present study, we present a significant association between high AR expression and better survival in stage T1 UCB. We identified no association between AR expression and gender, which is akin to previous findings [14], [16], [27].
In addition, by stratifying AR expression with the molecular subtypes of stage T1 NMIBC, we identified a significant association between high AR1 mRNA expression and the basal subtype reflected by KRT5 mRNA expression, whereas no association was noted between AR1 and KRT20, reflecting the luminal subtype. High expression of KRT5 and AR1 is associated with a better outcome in our study of stage T1 NMIBC. However, it remains unclear whether this positive effect can be attributed to either of the two markers or if another unknown associated factor is responsible for this result.
Although our results present a clear diagnostic advantage for patients with stage T1 NMIBC when subject to an additional evaluation of AR mRNA expression, the therapeutic significance for stage T1 NMIBC remains unclear. Although previous results in mouse models suggest that functional AR signaling is involved in the initiation of UCB [7], our data implicate a better outcome upon elevated AR expression in NMIBC. However, available data from other studies suggest an improved course of disease for patients with UCB who received antiandrogen therapy for prostate cancer [12]. Moreover, another preclinical study described the negative effect of androgens on the expression of interleukin IL6, which is necessary for the adherence of BCG to urothelial carcinoma and thus the effectiveness of BCG in the treatment of NMIBC. Antiandrogens reverse this effect. Therefore, BCG therapy might improve when administered together with antiandrogens [30]. Regarding the potential use of antiandrogens in UCB, an analogy might be drawn to breast cancer, where the estrogen receptor–positive luminal subtypes are associated with better survival, yet antihormonal therapy is regarded standard of care for the luminal breast cancer subtypes [31].
Additionally, it has to be considered that AR expression, and thus the role of AR, might change during the progression of UCB, as indicated by the previously cited studies. Further research is necessary to clarify the therapeutic relevance of AR expression and antiandrogen therapies in NMIBC and MIBC.
Some limitations to our study should be noted. The first limitation is the retrospective nature of this study. Moreover, no immunohistochemistry was used to determine AR protein expression in this study, which is probably the most readily available form of AR detection.
In conclusion, we demonstrated that the measurement of AR mRNA expression predicts the outcome of patients with stage T1 NMIBC. This information might help to better stratify patients with this challenging tumor entity beyond the clinicopathological methods currently available. Further studies are necessary to investigate the possible therapeutic impact of these findings as well as the relevance of AR expression in advanced states of urothelial cancer.
The following are the supplementary data related to this article.
Conflict of Interest
R.M.W. is a founder of STRATIFYER Molecular Pathology GmbH.
Acknowledgements
This study was funded by the German Cancer Aid (Deutsche Krebshilfe, grant number 110541). D.S. is supported by a Ferdinand Eisenberger grant of the German Society of Urology (Deutsche Gesellschaft für Urologie, grant ID SiD1/FE-16).
The authors thank Stefanie Herlein and Silke Claas for excellent technical support.
Contributor Information
Danijel Sikic, Email: danijel.sikic@uk-erlangen.de.
Johannes Breyer, Email: johannes.breyer@ukr.de.
Arndt Hartmann, Email: arndt.hartmann@uk-erlangen.de.
Maximilian Burger, Email: maximilian.burger@ukr.de.
Philipp Erben, Email: philipp.erben@medma.uni-heidelberg.de.
Stefan Denzinger, Email: stefan.denzinger@ukr.de.
Markus Eckstein, Email: markus.eckstein@uk-erlangen.de.
Robert Stöhr, Email: robert.stoehr@uk-erlangen.de.
Sven Wach, Email: sven.wach@uk-erlangen.de.
Bernd Wullich, Email: bernd.wullich@uk-erlangen.de.
Bastian Keck, Email: bastian.keck@uk-erlangen.de.
Ralph M. Wirtz, Email: ralph.wirtz@STRATIFYER.de.
Wolfgang Otto, Email: wolfgang.otto@ukr.de.
References
- 1.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2016;71:96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Babjuk M, Bohle A, Burger M, Capoun O, Cohen D, Comperat EM, Hernandez V, Kaasinen E, Palou J, Roupret M. EAU guidelines on non–muscle-invasive urothelial carcinoma of the bladder: update 2016. Eur Urol. 2016;71:447–461. doi: 10.1016/j.eururo.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 3.Novotny V, Froehner M, Ollig J, Koch R, Zastrow S, Wirth MP. Impact of adjuvant intravesical Bacillus Calmette-Guerin treatment on patients with high-grade T1 bladder cancer. Urol Int. 2016;96:136–141. doi: 10.1159/000443705. [DOI] [PubMed] [Google Scholar]
- 4.Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, Palou J, Vicente-Rodriguez J. Multivariate analysis of the prognostic factors of primary superficial bladder cancer. J urol. 2000;163:73–78. doi: 10.1016/s0022-5347(05)67975-x. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 6.Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer. 2009;115:68–74. doi: 10.1002/cncr.23986. [DOI] [PubMed] [Google Scholar]
- 7.Burge F, Kockelbergh R. Closing the gender gap: can we improve bladder cancer survival in women? — A systematic review of diagnosis, treatment and outcomes. Urol Int. 2016;97:373–379. doi: 10.1159/000449256. [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto H, Yang Z, Chen YT, Ishiguro H, Uemura H, Kubota Y, Nagashima Y, Chang YJ, Hu YC, Tsai MY. Promotion of bladder cancer development and progression by androgen receptor signals. J Natl Cancer Inst. 2007;99:558–568. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- 9.Hsu JW, Hsu I, Xu D, Miyamoto H, Liang L, Wu XR, Shyr CR, Chang C. Decreased tumorigenesis and mortality from bladder cancer in mice lacking urothelial androgen receptor. Am J Pathol. 2013;182:1811–1820. doi: 10.1016/j.ajpath.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imada S, Akaza H, Ami Y, Koiso K, Ideyama Y, Takenaka T. Promoting effects and mechanisms of action of androgen in bladder carcinogenesis in male rats. Eur Urol. 1997;31:360–364. doi: 10.1159/000474484. [DOI] [PubMed] [Google Scholar]
- 11.Lin C, Yin Y, Stemler K, Humphrey P, Kibel AS, Mysorekar IU, Ma L. Constitutive beta-catenin activation induces male-specific tumorigenesis in the bladder urothelium. Cancer Res. 2013;73:5914–5925. doi: 10.1158/0008-5472.CAN-12-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izumi K, Taguri M, Miyamoto H, Hara Y, Kishida T, Chiba K, Murai T, Hirai K, Suzuki K, Fujinami K. Androgen deprivation therapy prevents bladder cancer recurrence. Oncotarget. 2014;5:12665–12674. doi: 10.18632/oncotarget.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Y, Izumi K, Yao JL, Miyamoto H. Dihydrotestosterone upregulates the expression of epidermal growth factor receptor and ERBB2 in androgen receptor-positive bladder cancer cells. Endocr Relat Cancer. 2011;18:451–464. doi: 10.1530/ERC-11-0010. [DOI] [PubMed] [Google Scholar]
- 14.Mashhadi R, Pourmand G, Kosari F, Mehrsai A, Salem S, Pourmand MR, Alatab S, Khonsari M, Heydari F, Beladi L. Role of steroid hormone receptors in formation and progression of bladder carcinoma: a case-control study. Urol J. 2014;11:1968–1973. [PubMed] [Google Scholar]
- 15.Boorjian S, Ugras S, Mongan NP, Gudas LJ, You X, Tickoo SK, Scherr DS. Androgen receptor expression is inversely correlated with pathologic tumor stage in bladder cancer. Urology. 2004;64:383–388. doi: 10.1016/j.urology.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Tuygun C, Kankaya D, Imamoglu A, Sertcelik A, Zengin K, Oktay M, Sertcelik N. Sex-specific hormone receptors in urothelial carcinomas of the human urinary bladder: a comparative analysis of clinicopathological features and survival outcomes according to receptor expression. Urol Oncol. 2011;29:43–51. doi: 10.1016/j.urolonc.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 17.Breyer J, Wirtz RM, Laible M, Schlombs K, Erben P, Kriegmair MC, Stoehr R, Eidt S, Denzinger S, Burger M. ESR1, ERBB2, and Ki67 mRNA expression predicts stage and grade of non–muscle-invasive bladder carcinoma (NMIBC) Virchows Arch. 2016;469:547–552. doi: 10.1007/s00428-016-2002-1. [DOI] [PubMed] [Google Scholar]
- 18.Dadhania V, Zhang M, Zhang L, Bondaruk J, Majewski T, Siefker-Radtke A, Guo CC, Dinney C, Cogdell DE, Zhang S. Meta-analysis of the luminal and basal subtypes of bladder cancer and the identification of signature immunohistochemical markers for clinical use. EBioMedicine. 2016;12:105–117. doi: 10.1016/j.ebiom.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, Roth B, Cheng T, Tran M, Lee I-L. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25:152–165. doi: 10.1016/j.ccr.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damrauer JS, Hoadley KA, Chism DD, Fan C, Tiganelli CJ, Wobker SE, Yeh JJ, Milowsky MI, Iyer G, Parker JS. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A. 2014;111:3110–3115. doi: 10.1073/pnas.1318376111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carballido EM, Rosenberg JE. Optimal treatment for metastatic bladder cancer. Curr Oncol Rep. 2014;16:404. doi: 10.1007/s11912-014-0404-2. [DOI] [PubMed] [Google Scholar]
- 22.Network CGAR Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi W, Czerniak B, Ochoa A, Su X, Siefker-Radtke A, Dinney C, McConkey DJ. Intrinsic basal and luminal subtypes of muscle-invasive bladder cancer. Nat Rev Urol. 2014;11:400–410. doi: 10.1038/nrurol.2014.129. [DOI] [PubMed] [Google Scholar]
- 24.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ, Panel m Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH, Balmanoukian A, Loriot Y. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyamoto H, Yao JL, Chaux A, Zheng Y, Hsu I, Izumi K, Chang C, Messing EM, Netto GJ, Yeh S. Expression of androgen and oestrogen receptors and its prognostic significance in urothelial neoplasm of the urinary bladder. BJU Int. 2012;109:1716–1726. doi: 10.1111/j.1464-410X.2011.10706.x. [DOI] [PubMed] [Google Scholar]
- 27.Mir C, Shariat SF, van der Kwast TH, Ashfaq R, Lotan Y, Evans A, Skeldon S, Hanna S, Vajpeyi R, Kuk C. Loss of androgen receptor expression is not associated with pathological stage, grade, gender or outcome in bladder cancer: a large multi-institutional study. BJU Int. 2011;108:24–30. doi: 10.1111/j.1464-410X.2010.09834.x. [DOI] [PubMed] [Google Scholar]
- 28.Otto W, Denzinger S, Fritsche HM, Burger M, Rossler W, Bertz S, May M, Hartmann A, Hofstadter F, Wieland WF. Introduction and first clinical application of a simplified immunohistochemical validation system confirms prognostic impact of KI-67 and CK20 for stage T1 urothelial bladder carcinoma: single-center analysis of eight biomarkers in a series of three hundred six patients. Clin Genitour Cancer. 2013;11:537–544. doi: 10.1016/j.clgc.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Hartmann A, Bertz S, Keck B, Dyrskjot L, Orntoft T, Wullich B, Hake R, Eidt S, Wirtz R. Proceedings: AACR 103rd Annual Meeting. 2012. Abstract 3654: prognostic role of androgen receptor in bladder cancer. [Google Scholar]
- 30.Chen F, Langenstroer P, Zhang G, Iwamoto Y, See W. Androgen dependent regulation of Bacillus Calmette-Guerin induced interleukin-6 expression in human transitional carcinoma cell lines. J Urol. 2003;170:2009–2013. doi: 10.1097/01.ju.0000092238.15685.10. [DOI] [PubMed] [Google Scholar]
- 31.Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, Zackrisson S, Cardoso F, Committee EG. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl. 5):v8–30. [Google Scholar]