Abstract
OBJECTIVE: The ROR1 and ROR2 receptor tyrosine kinases have both been implicated in ovarian cancer progression and have been shown to drive migration and invasion. There is an increasing importance of the role of stroma in ovarian cancer metastasis; however, neither ROR1 nor ROR2 expression in tumor or stromal cells has been analyzed in the same clinical cohort. AIM: To determine ROR1 and ROR2 expression in ovarian cancer and surrounding microenvironment and examine associations with clinicopathological characteristics. METHODS: Immunohistochemistry for ROR1 and ROR2 was used to assess receptor expression in a cohort of epithelial ovarian cancer patients (n = 178). Results were analyzed in relation to clinical and histopathological characteristics and survival. Matched patient sample case studies of normal, primary, and metastatic lesions were used to examine ROR expression in relation to ovarian cancer progression. RESULTS: ROR1 and ROR2 are abnormally expressed in malignant ovarian epithelium and stroma. Higher ROR2 tumor expression was found in early-stage, low-grade endometrioid carcinomas. ROR2 stromal expression was highest in the serous subtype. In matched patient case studies, metastatic samples had higher expression of ROR2 in the stroma, and a recurrent sample had the highest expression of ROR2 in both tumor and stroma. CONCLUSION: ROR1 and ROR2 are expressed in tumor-associated stroma in all histological subtypes of ovarian cancer and hold potential as therapeutic targets which may disrupt tumor and stroma interactions.
Introduction
Ovarian cancer (OC) is often diagnosed at an advanced stage with cancer spread to the peritoneal cavity due to the aggressive nature of tumor growth and metastasis. Current treatment options may induce an initial anticancer effect in the patient; however, most cases develop recurrent and resistant cancer, which is difficult to treat. Treatment regimens have yet to change dramatically with advances in genomics as seen in other tumor types, and there is a strong need for useful biomarkers and targeted therapies in this field.
Evidence of altered Wnt signaling is frequently found in human cancers [1]. In many cases, the receptor tyrosine kinases ROR1 and ROR2 are abnormally overexpressed in tumors and associated with stem cell properties, epithelial to mesenchymal transition, and metastasis [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12]. In OC, we have previously shown that both ROR1 and ROR2 are important in cell growth, migration, and invasion [13]. Others have confirmed the role of ROR1 in OC and have found that inhibiting ROR1 using a monoclonal antibody halts the growth of tumor xenografts [14], [15]. Thus, these receptors may be suitable targets for patient therapy.
ROR1 and ROR2 are structurally similar type 1 transmembrane proteins both activated by the Wnt5a ligand. They exhibit similar expression patterns in the developing face and heart but differing patterns in the embryonic brain and limbs. ROR1 and ROR2 are also able to form complexes to modulate synaptogenesis [16]. In cancer, few studies have investigated the functional relationship between ROR1 and ROR2, with most studies indicating separate signaling pathway roles. For example, ROR2 is known to inhibit β-catenin–dependent Wnt signaling and activate independent pathways such as RhoA and JNK [17]. On the other hand, ROR1 has been associated with a range of alternative pathways such as ERK and MET signaling, as it is hypothesized to be a docking protein for other receptor tyrosine kinases [18]. ROR1 has also been associated with caveolae formation in lung cancer [19]. In leukemia, however, it has been shown that the Wnt5a ligand can induce ROR1/ROR2 hetero-oligomerization to promote cell proliferation [20].
To date, there have been no studies investigating the expression of both ROR1 and ROR2 in the same OC clinical cohort. Additionally, there has been no investigation into their significance in ovarian tumor-associated stroma. The role of the tumor microenvironment has become increasingly important, especially for OC as cells metastasize to unique areas such as the omentum and peritoneum [21]. Women with high proportions of stroma in their OC have a worse prognosis [22], with higher levels of cancer-associated fibroblasts shown to be correlated with lymph node metastasis, lymphovascular invasion, and omental metastasis. Additionally, cancer-associated fibroblasts have been shown to induce OC migration in vitro [23]. Recently, high ROR2 expression in both pancreatic cancer cells and stromal cells was found to confer a poor overall prognosis and associated with many malignant clinicopathological features [24]. We aimed to investigate this idea further in a large cohort of epithelial ovarian tumors and correlate tumor and stromal expression of ROR1 and ROR2 with clinical features and survival.
Methods
Ethics
Ethics approval was obtained through UNSW and the Sydney East Area Health Service Human Research Ethics Committee (approval #HC13339). The tissue microarray (TMA) cohort biospecimens and data used in this study were derived from the Gynecological Oncology Biobank at Westmead Hospital, NSW, Australia. Tissue was collected under the project “Molecular Biology of Gynecological Disease,” WSLHD ethics approval number HREC92/10/4.13. Patients were included after informed consent was accepted. Six individual matched sample case studies were collected through the HSA Biobank at the Lowy Cancer Centre, UNSW, in association with the Royal Hospital for Women, Randwick, under ethics approval #HC13339.
Patient Cohort
The TMA cohort consisted of 227 samples from women diagnosed with borderline or invasive epithelial ovarian, fallopian tube, or primary peritoneal carcinoma between 1989 and 2011 and treated at Westmead Hospital, Sydney, Australia. Of these, 210 were invasive carcinomas, including 28 (12.3%) clear cell, 26 (11.5%) endometrioid, 10 (4.4%) mucinous, and 146 (64.3%) serous, and 17 (7.5%) were mucinous borderline tumors. Tumor stage was classified according to the International Federation of Gynecologists and Obstetricians (FIGO) criteria (Table 1). Samples collected post–neoadjuvant chemotherapy (n = 2) were excluded from the analysis. Most women with advanced disease received six cycles of carboplatin/paclitaxel (paclitaxel at 175 mg/m2 intravenously over 3 hours followed by carboplatin over an hour at 6 mg/ml, repeated every 3 weeks) postsurgery, which is the standard treatment regimen (Table 1). Women that did not respond or tolerate this standard of care received other therapies such as cyclophosphamide, or gemcitabine, tamoxifen, or radiotherapy as indicated by “other” (Table 1).
Table 1.
Clinicopathological Characteristics of TMA Cohort
| Serous (n = 146) | Endometrioid (n = 26) | Clear Cell (n = 28) | Mucinous Invasive (n = 10) | Mucinous Borderline (n = 17) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade | ||||||||||
| 1 | 12 | 8% | 12 | 46% | 0 | 0% | 3 | 30% | ||
| 2 | 43 | 29% | 11 | 42% | 14 | 50% | 5 | 50% | ||
| 3 | 90 | 62% | 3 | 12% | 13 | 46% | 2 | 20% | ||
| Grade (WHO) | ||||||||||
| Low | 16 | 11% | ||||||||
| High | 129 | 88% | ||||||||
| Primary site | ||||||||||
| Ovary | 121 | 83% | 25 | 96% | 28 | 100% | 10 | 100% | 17 | 100% |
| Fallopian tube | 8 | 5% | 1 | 4% | 0 | 0% | 0 | 0% | 0 | 0% |
| Peritoneal | 17 | 12% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| FIGO stage | ||||||||||
| I | 4 | 3% | 13 | 50% | 12 | 43% | 6 | 60% | 16 | 94% |
| II | 7 | 5% | 4 | 15% | 4 | 14$ | 0 | 0% | 0 | 0% |
| III | 120 | 82% | 7 | 27% | 11 | 39% | 3 | 30% | 1 | 6% |
| IV | 15 | 10% | 2 | 8% | 0 | 0% | 1 | 10% | 0 | 0% |
| Residual disease | ||||||||||
| Nil | 29 | 20% | 20 | 77% | 20 | 71% | 7 | 70% | 16 | 94% |
| Any | 117 | 80% | 6 | 23% | 8 | 29% | 3 | 30% | 1 | 6% |
| Neoadjuvant therapy | ||||||||||
| No | 145 | 99% | 26 | 100% | 27 | 96% | 10 | 100% | 17 | 100% |
| Yes | 1 | 1% | 0 | 0% | 1 | 4% | 0 | 0% | 0 | 0% |
| Primary treatment | ||||||||||
| Platinum | 20 | 14% | 1 | 4% | 4 | 14% | 0 | 0% | 1 | 6% |
| Platinum/Taxol | 94 | 64% | 11 | 42% | 16 | 57% | 4 | 40% | 0 | 0% |
| Platinum/cyclophosphamide | 14 | 10% | 5 | 19% | 2 | 7% | 0 | 0% | 0 | 0% |
| Platinum/Taxol/other | 16 | 11% | 2 | 8% | 5 | 18% | 1 | 10% | 0 | 0% |
| None | 2 | 1% | 7 | 27% | 1 | 4% | 5 | 50% | 16 | 94% |
The case study cohort (five patients) consisted of one matched normal, primary, and metastatic full-faced slide from each individual, equaling a total of 15 samples. Four patients had serous carcinoma and one patient had endometrioid carcinoma, all moderate- to high-grade stages II-III. All ovarian tumor staging was classified according to FIGO (Table 2). One patient had received neoadjuvant chemotherapy prior to surgery (ID0681), whereas the others had the standard regimen as explained previously. In addition, patient ID0681 relapsed and had resectable disease, and therefore, a matched recurrent sample was collected and analyzed.
Table 2.
Clinicopathological Characteristics of Matched Case Study Cohort
| ID0670 | ID0764 | ID0978 | ID1430 | ID0681 | |
|---|---|---|---|---|---|
| Histological subtype | Serous | Serous | Endometrioid | Serous | Serous |
| Grade | 3 | 3 | 2 | 3 | 3 |
| Stage | IIB | IIIA | IIIA | IIIA | IIIB |
| Primary site | Ovary | Ovary | Ovary | Ovary | Ovary |
| Neoadjuvant therapy | No | No | No | No | Yes |
| Normal sample | Fallopian tube fimbria | Fallopian tube fimbria | Ovarian surface epithelium | Ovarian surface epithelium | Fallopian tube fimbria |
Clinical Data Definitions
Progression-free survival was defined as the time between the date of histological diagnosis and the first confirmed sign of disease recurrence or progression based on definitions developed by the Gynecological Cancer Intergroup [25]. In the majority of cases, the date of progression was assigned using CA125 criteria. In cases where CA125 was not a marker of disease or progression preceded an increase in CA125, relapse was based on imaging (appearance of new lesion) or, in a minority of cases, global deterioration in health status attributable to the disease, or death. Overall survival was calculated from the date of histological diagnosis to the date of death and censored at last contact date if the patient was alive.
Univariate analysis associated progression-free survival with stage in serous, mucinous, invasive, and clear cell subtypes and with residual disease in all subtypes (Supplementary Table 6). Overall survival was associated with stage in serous and clear cell subtypes and residual disease in all subtypes (Supplementary Table 7).
Immunohistochemistry
Immunohistochemistry (IHC) was performed by the Histology Facility at the Garvan Institute of Medical Research, Kinghorn Cancer Centre, at Darlinghurst, NSW, Australia. Formalin-fixed, paraffin-embedded specimens were sectioned at 4 μm using a Leica RM2235 microtome (Leica Biosystems, Nussloch, Germany). Sections were placed on a Superfrost plus slide and allowed to incubate for 2 hours in a 60°C oven to allow for maximum adhesion. Antigen retrieval was performed in EDTA-based ER2 buffer, pH 9 for 20 minutes at 100°C. Primary antibody staining was completed using the automated Leica Bond RX IHC machine (Leica Biosystems, Nussloch, Germany). Sections were stained with Anti-ROR1 Ab (Abcam #135669) or Anti-ROR2 Ab (QED Biosciences, #34045) both at a 1:100 dilution in addition to a negative IgG control, and counterstained with hematoxylin.
Thirty-two cores on the TMAs (14.1%) were excluded from analysis because of limited amount of stroma and malignant epithelium present in the sample. ROR1 and ROR2 expression in cancer cells was scored by three blinded pathologists using a 0 to 3 scale, 0 being complete absence of staining and 3 being very strong. Any discrepancies between scores were discussed and agreed by consensus; however, scoring was mostly unanimous among pathologists. Stromal staining was scored by one blinded pathologist using the same 0 to 3 scale. For the matched case studies, stroma and tumor staining was scored by two pathologists independently using the 0 to 3 scale.
Statistical Analysis
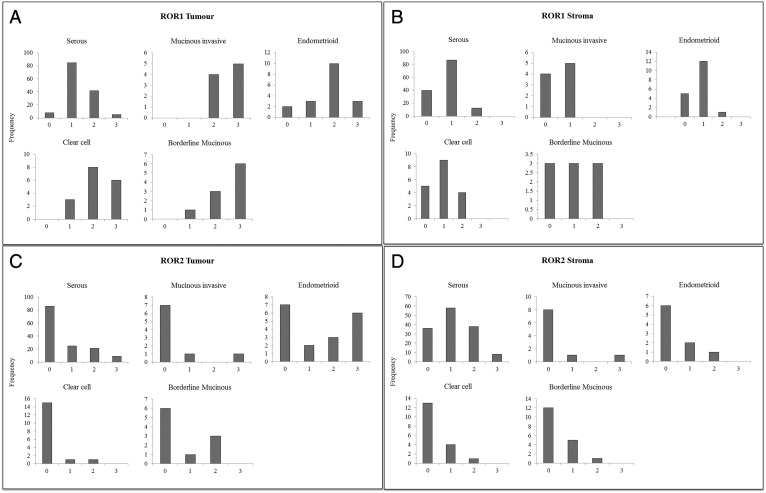
For statistical analyses, ROR staining was dichotomized based on the frequency distribution of scores within each histotype (Supplementary Figure 1). For all histotypes, ROR1 stroma, ROR2 stroma, and ROR2 tumor staining were dichotomized into negative (group 1; score = 0) and positive (group 2; score > 0) groups. ROR1 tumor scores dichotomized at a score of 1 for serous, endometrioid, and clear cell histotypes (group 1, score ≤ 1 and group 2, score > 1) and 2 for mucinous histotypes (group 1, score ≤ 2 and group 2, score > 2). Associations between ROR expression and clinicopathological features were determined using the Fisher exact test. The relationship between ROR1 and ROR2 staining pairs in tumor and stroma was determined using the Wilcoxon matched pairs signed rank test. Differences between progression-free or overall survival were assessed using Cox regression and Kaplan-Meier curves with log-rank test. Univariate and multivariate analysis measured different prognostic factors in patients. Statistical analyses were performed using Stata 10.0 (StataCorp LP, College Station, TX).
Supplementary Figure 1.
Frequency distribution of ROR scores. (A) Frequency distribution of ROR1 scores in tumor cells by subtype (from left to right: serous, mucinous invasive, endometrioid, clear cell, and mucinous borderline). (B) Frequency distribution of ROR1 scores in stroma by subtype (from left to right: serous, mucinous invasive, endometrioid, clear cell, and mucinous borderline). (C) Frequency distribution of ROR2 scores in tumor cells by subtype (from left to right: serous, mucinous invasive, endometrioid, clear cell, and mucinous borderline). (D) Frequency distribution of ROR2 scores in stroma by subtype (from left to right: serous, mucinous invasive, endometrioid, clear cell, and mucinous borderline).
Results
To date, studies indicate that ROR1 and ROR2 are important in many stages of embryogenesis, organizing the structures of the brain, heart, and limbs [26]. It is recognized that ROR1 and ROR2 expression diminishes in adult tissue [27], with many studies indicating increased expression associated with malignant progression [3], [4], [10], [11], [12], [28], [29], [30]. We have previously found ROR2 and its receptor, WNT5A, to be upregulated in OC [13], [31], [32] patient samples and have shown in vitro that both ROR1 and ROR2 are important in OC migration and invasion, indicating a role in OC metastasis [32]. However, it is unknown how the receptors interplay, and therefore, we aimed to analyze the expression of both receptors in the same cohort.
Expression of ROR1 and ROR2 in OC Stroma
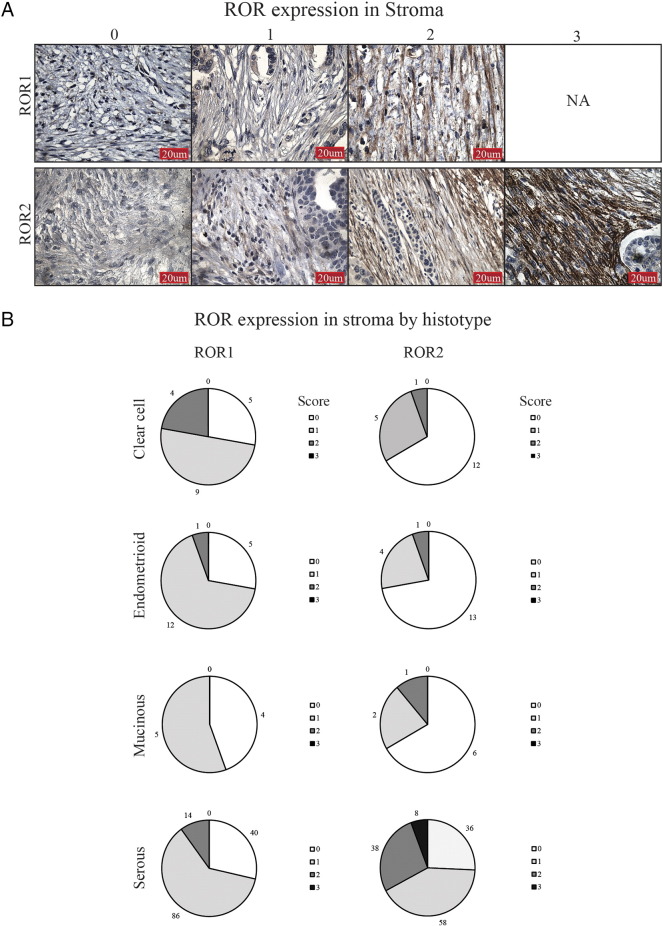
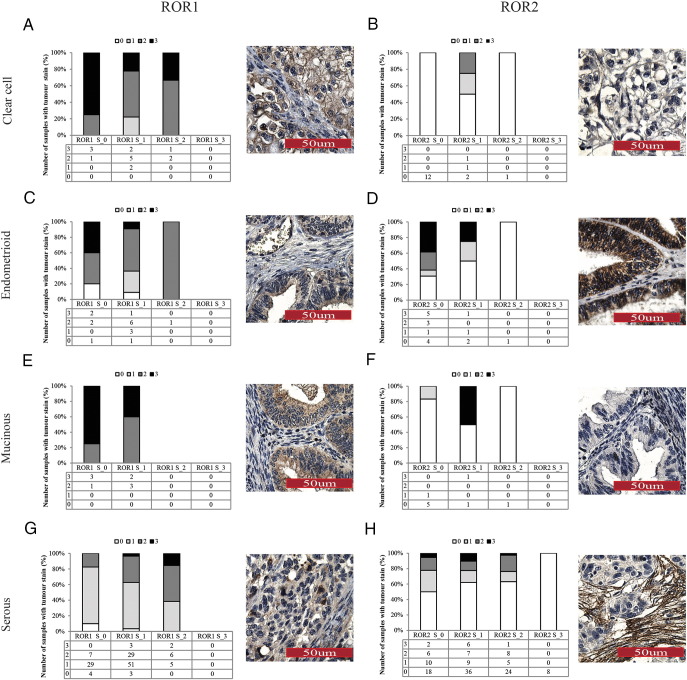
ROR1 and ROR2 stromal staining ranged from low to high intensity across all epithelial ovarian cancer (EOC) subtypes (Figure 1A), and overall, ROR1 stroma was less expressed than ROR2 stroma (Figure 1B). In particular, ROR2 stromal staining was strongest in the serous subtype (P < .0001, Supplementary Table 1); however, this did not associate with a worse prognosis (Supplementary Figure 2). There was no significant relationship found between ROR1 and ROR2 stroma expression.
Figure 1.
ROR expression in OC stroma. (A) Representative images of stromal ROR1 (top panel) and ROR2 (bottom panel) IHC, from a score of 0 to 3. ROR1 staining did not have strong expression in the stroma, and therefore, no representative figure of score 3 is shown (NA). (B) ROR expression in the stroma depicted by histological subtype. Each pie chart represents percentage of samples and ROR score per subtype. Scores of 0 to 3 colored white, light gray, gray, and dark gray, respectively.
Supplementary Figure 2.
HGSOC ROR2 stroma survival analysis. (A) Kaplan-Meier curve representing progression-free survival (PFS) of HGSOC patients with ROR2 weak (group 1, blue) and ROR2 strong (group 2, red) stroma expression. No significant trend was found. (B)Kaplan-Meier curve representing overall survival (OS) of HGSOC patients with ROR2 weak (group 1, blue) and ROR2 strong (group 2, red) stromal expression. No significant trend was found.
Expression of ROR1 and ROR2 in OC Tumor Cells
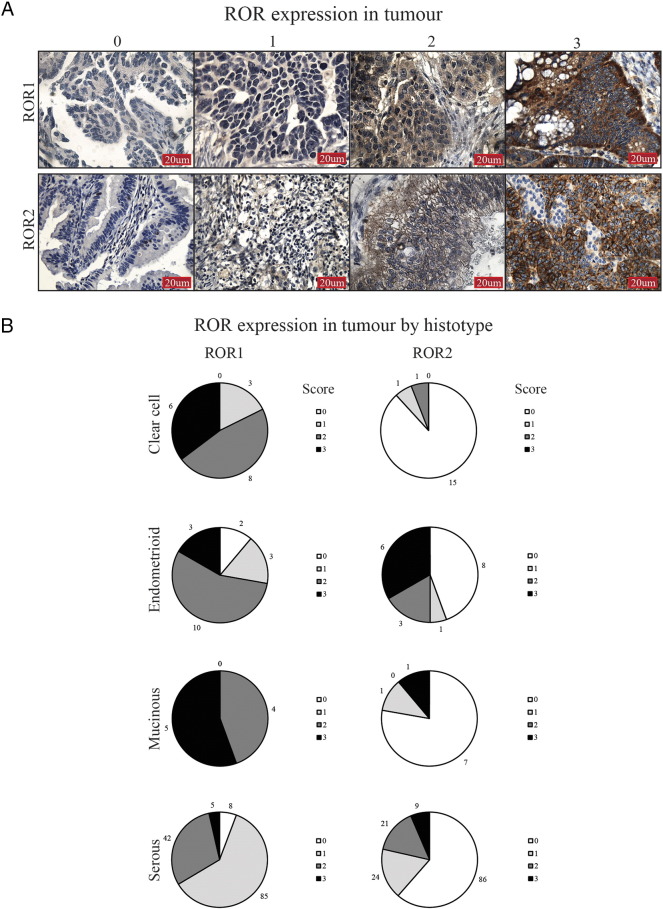
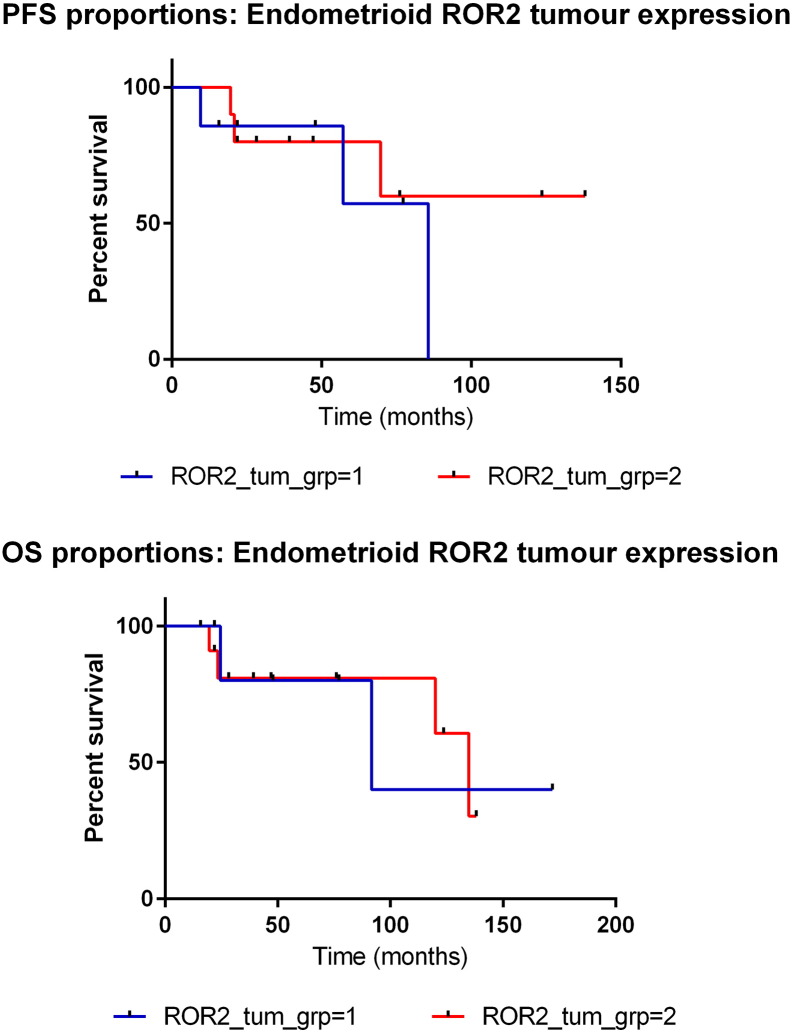
ROR1 and ROR2 had a noticeably different pattern of expression in tumor cells (Figure 2A), and overall, ROR1 tumor positivity was significantly higher than ROR2 (P < .00001, Figure 2B and Supplementary Table 2). Cellular localization of ROR1 and ROR2 was mainly cytoplasmic, with ROR2 exhibiting some membranous patterns in agreement with previous reports [15], [24]. Strong ROR1 staining was most notable in clear cell and mucinous subtypes (Figure 2B). For ROR2, very low tumor expression was noted in the clear cell subtype, alongside strongest expression significant in the endometrioid subtype (P < .016, Figure 2B and Supplementary Table 1). ROR2 expression in endometrioid tumor cells was associated with early stage and low grade (Supplementary Table 3), which was reflected in a trend in better overall survival (Supplementary Figure 3).
Figure 2.
ROR expression in OC tumor. (A) Representative images of tumor ROR1 (top panel) and ROR2 (bottom panel) IHC, from a score of 0 to 3. (B) ROR expression in the tumor depicted by subtype. Each pie chart represents percentage of samples and ROR score per subtype. Scores of 0 to 3 colored white, light gray, gray, and dark gray, respectively.
Supplementary Figure 3.
Endometrioid ROR2 tumor survival analysis. (A)Kaplan-Meier curve representing PFS of endometrioid patients with ROR2 weak (group 1, blue) and ROR2 strong (group 2, red) tumor expression. Nonsignificant trend shows strong expression may have better prognosis. (B)Kaplan-Meier curve representing OS of endometrioid patients with ROR2 weak (group 1, blue) and ROR2 strong (group 2, red) tumor expression. Nonsignificant trend shows strong expression may have better prognosis.
Association of ROR Staining in the Tumor and Stroma
Overall, expression of ROR1 in the stroma was less than that in the tumor cells (P < .00001, Supplementary Table 4), whereas ROR2 stroma expression was higher than ROR2 tumor cell expression (P < .005, Supplementary Table 5). In clear cell OC, ROR1 expression was strongest in the tumor cells and weakest in stroma (Figure 3A). This pattern was also noticed in endometrioid and mucinous tumors (Figure 3, C and E). However, ROR1 expression in serous OC was similar in both tumor and stroma; for example, when the stroma had low expression, tumor cells exhibited low expression (Figure 3G).
Figure 3.
ROR expression relationship in tumor and stroma. Each panel represents number of tumor ROR expression scores for each stroma score (x-axis, ROR S_0, ROR S_1, ROR S_2 and ROR S_3). Tumor ROR scores are represented by shading and distribution listed underneath. Each graph is paired with a representative image of the overall pattern of expression for that subtype. (A) ROR1 tumor and stroma expression in clear cell OC showed overall low stroma intensity but high tumor intensity. (B) ROR2 expression was overall low in both tumor and stroma in clear cell OC. (C) ROR1 expression in endometrioid OC was overall strong in tumor cells and weak in stroma. (D) ROR2 expression in endometrioid OC was very strong in tumor cells and absent in stroma. (E) ROR1 expression in mucinous OC was high in tumor and low in stroma. (F) ROR2 expression in mucinous OC was overall weak in both tumor and stroma. (G) ROR1 expression in serous tumor increased as stroma expression increased, but overall expression was mid to low. (H) ROR2 expression in serous OC tumor decreased as stroma expression increased. All cases with a stromal score of 3 exhibited absent tumor expression.
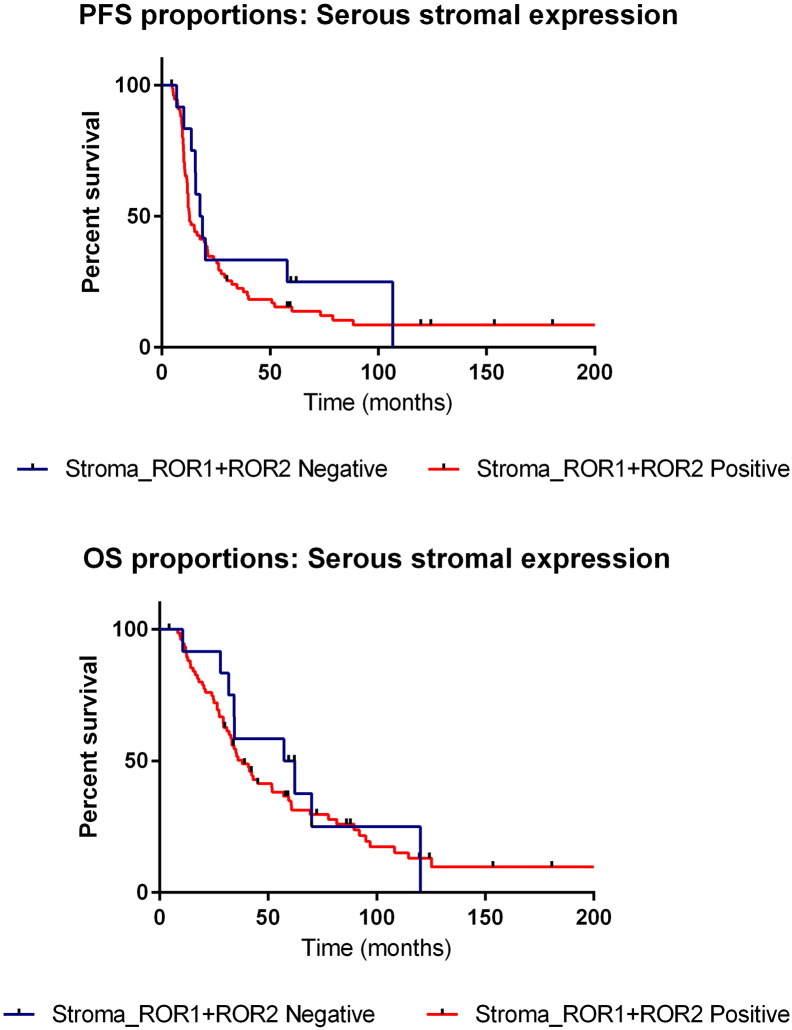
ROR2 expression in clear cell OC was overall low in both tumor and stromal cells (Figure 3B). In the endometrioid subtype, strong ROR2 expression was observed in the tumor cells when the stroma had none (Figure 3D). Mucinous OC had mixed expression of ROR2, with overall low expression in both tumor and stromal components (Figure 3F). Serous OC however exhibited an interesting pattern of expression. When stroma associated with serous OC had low expression of ROR2, approximately 50% of patients had tumor cell positivity. However, when ROR2 stromal expression was strongest, 100% (8 patients) exhibited no ROR2 expression in the tumor cells (Figure 3H). Additionally, when divided into subgroups of negative for both ROR1 and ROR2 stromal expression (23 patients) versus positive ROR1 and ROR2 stromal expression (88 patients), it was found that those with positive staining of both ROR1 and ROR2 in the stroma had a trend in worse overall and progression-free survival (nonsignificant, Figure 4).
Figure 4.
Survival proportions of ROR stroma expression. (A): Kaplan-Meier curve representing PFS of patients (serous subtype only) with both ROR1 and ROR2 stroma positivity (red) and ROR1 and ROR2 stromal absence (blue). (B) Kaplan Meier curve representing OS of patients (serous subtype only) with both ROR1 and ROR2 stroma positivity (red) and ROR1 and ROR2 stromal absence (blue).
ROR Expression in Metastasis and Recurrence
To investigate the role of ROR1 and ROR2 in both tumor and stromal cells in OC metastasis, five case studies with matched normal, primary tumor, and metastatic lesions were obtained from the HSA Biobank at UNSW Australia. These five case studies were chosen due to their widespread, high-grade disease and availability of matched normal samples. Normal samples were taken from the uninvolved fallopian tube or ovary and were confirmed benign by pathologists.
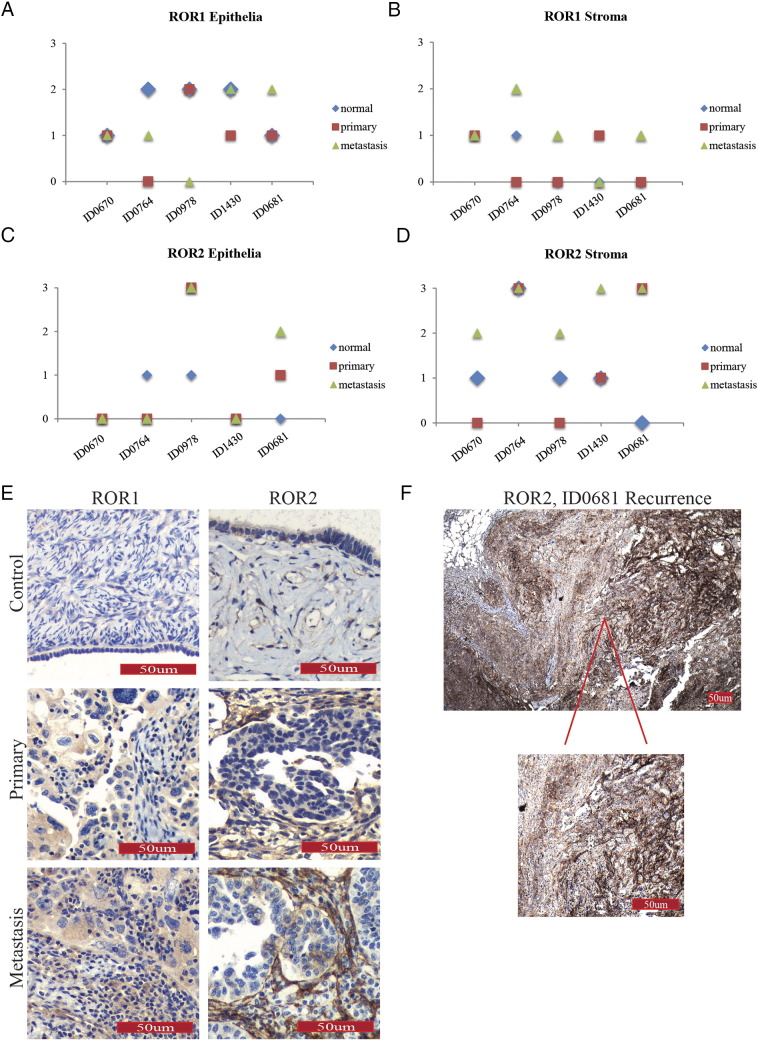
Overall, ROR1 expression was mid to highly expressed in tumor cells but expressed at low levels in the stroma, as seen with the larger Westmead cohort (Figure 5, A and B). We noted that a number of patients had ROR2 expression in tumor cells that increased in metastatic sites (Figure 5C); however, all patients had increased/highest levels of ROR2 stroma expression in their metastatic sites with scores of either 2 or 3 (Figure 5D). Examples of patterns of staining are indicated in Figure 5E. Interestingly, patient ID0764 had very low expression (scores 0-1) of ROR2 in epithelial/tumor cells yet the highest expression (3) in stromal cells across all normal, primary, and metastatic sites (Figure 5D). This patient did not have any remarkable clinicopathological features in comparison to the rest of the cohort.
Figure 5.
Tracking ROR expression in individual case studies. (A) ROR1 expression in epithelial/tumor cells in individual cases as indicated by colored dots (blue = normal, red = primary, green = metastatic samples). Y-axis represents ROR expression score, and x-axis represents individual patient. (B) ROR1 expression in stromal cells in individual cases as indicated by colored dots (blue = normal, red = primary, green = metastatic samples). Y-axis represents ROR expression score, and x-axis represents individual patient. (C): ROR2 expression in epithelial/tumor cells in individual cases as indicated by colored dots (blue = normal, red = primary, green = metastatic samples). Y-axis represents ROR expression score, and x-axis represents individual patient. (D) ROR2 expression in stromal cells in individual cases as indicated by indicated by colored dots (blue = normal, red = primary, green = metastatic samples). Y-axis represents ROR expression score, and x-axis represents individual patient. (E) Representative images of ROR1 (left panel) and ROR2 (right panel) in case studies, with noticeable ROR2 expression in stromal components in primary and metastatic samples. (F) Representative image of case ID0681 recurrence showing diffuse and strong ROR2 expression in tumor and stroma.
Interestingly, patient ID0681, who had neoadjuvant chemotherapy prior to surgery, had an increase in ROR1 and ROR2 expression in both primary cancer stroma and tumor cells in comparison to her benign control. This woman had resectable disease at recurrence, and biopsies from her right diaphragmatic mass noted serous adenocarcinoma with some focal clear cell features. This biopsy was stained for ROR1 and ROR2, and interestingly, expression of both receptors had increased. In the primary and metastatic tumor samples, ROR2 expression was seen focally; that is, clusters of cells and stroma stained strongly, and other areas did not. It is interesting to note that the relapsed biopsy was diffusely and extremely strong for ROR2 (Figure 5F).
Discussion
This study is the first to analyze the expression of both ROR1 and ROR2 in the same clinical cohort, the first to analyze their expression in OC tumor-associated stroma, and the first to follow their progression in OC metastasis and recurrence. We found that ROR2 tumor expression was strongest in the endometrioid subtype and was significantly associated with early stage and low grade. We noted very low expression of ROR1 and ROR2 in clear cell OC, a unique subtype of OC which has a poor prognosis and different histological phenotype. However, we found that, in the serous subtype, ROR2 expression in stroma was significantly overexpressed. When ROR2 expression in serous stroma was strongest, 100% of patients had no expression in tumor cells. Our case studies supported our large cohort findings, indicating a role of ROR2 in stromal signaling but also suggesting importance in metastasis and recurrence, more so than ROR1.
The importance of stroma in OC is well recognized. OC has a different anatomical progression in contrast to other solid tumors, and therefore, the interactions between stroma and cancer may behave likewise. For example, cancer cells disseminate throughout the peritoneal cavity and implant in areas such as the omentum [33]. Recently, one study aimed to investigate the molecular changes in OC stroma compared with normal matched tissue. Microarray profiling found significant differences between normal and cancer-associated stroma, as well as two distinct subgroups of cancer-associated stroma: one with similar receptor/ligand expression to the cancer and one different [34]. They indicated that these might be more permissive or resistant to cancer growth and chemoresistance; however, there has been no follow-up on their profiling data to date. Other preliminary studies have correlated poor survival with increase in stromal compartments in OC [22]. Limited in vitro studies have provided some evidence for the role of ovarian stroma in progressive disease mainly through cancer-associated fibroblasts (CAFs). The presence of CAFs is significantly higher in OC than borderline or benign lesions and was associated with advanced-stage disease and lymph node metastasis [23]. Furthermore, it was found that isolated OC CAFs induced cancer cell invasion and migration [23]. In this study, we provided evidence of ROR upregulation in OC stroma, with a trend in poor survival when both receptors were present.
Endometrioid OC accounts for approximately 20% of EOCs and exhibit a histological appearance similar to the well-differentiated gland-like structures seen in the endometrium [35]. Endometrioid OC can arise from malignant transformation of atypical endometriosis [35]; however, the genetic signature of endometriosis-related OC and endometriosis-unrelated OC is quite different, and therefore, they may need to be treated clinically as different diseases [36]. Overall, endometrioid OCs display different genetic mutations to other EOCs, such as KRAS, PTEN, and β-catenin (CCNTB1) [37]. Additionally, endometrioid OCs have high expression of nuclear β-catenin [38]. Patients with an endometrioid OC often have a better prognosis than other EOC subtypes [39]. ROR2 signaling is known to inhibit the canonical Wnt pathway, which involved the translocation of β-catenin to the nucleus to activate transcription factors and downstream targets involved in proliferation such as MYC, AXIN2, and Cyclin D1 [40]. It has been shown that tumors with β-catenin mutations show β-catenin expression in the nucleus, indicating an activation of signaling pathways [41]. Therefore, if ROR2 is overexpressed, this would be inhibiting aberrant β-catenin regulation and having an antitumor effect. Our findings support those found by Tothill et al. [42], who classified subtypes of OC by molecular gene signatures. The C6 expression subtype, consisting of predominantly low-grade and early-stage endometrioid tumors and consistent with deregulation of Wnt signaling, was associated with good survival [42]. Additionally, their profiling data found ROR2 to be upregulated in that subtype. Therefore, the presence of ROR2 could be used as a positive prognostic indicator for patients with endometrioid OC. This indicates a specific role of ROR2 in endometrioid OC and shows its fluidity of roles in different cancer types. This finding additionally indicates the importance of classifying OC histological subtypes and separation as individual diseases.
Clear cell ovarian carcinoma account for approximately 5% of EOCs globally and exhibits a unique hobnail cell shape lining tubules and cysts and is also thought to be associated with endometriosis; however, this progression is not well understood. Clear cell tumors have low proliferation rates and high frequency of PIK3CA mutations which often coexist with loss of ARID1A which is thought to be an early-stage event in the tumorigenesis of clear cell carcinoma [43], [44]. The survival rate of clear cell carcinoma is one of the worst in OC, and it exhibits a poor response to chemotherapy with recurrence. We found high staining of ROR1 in tumor cells of clear cell subtypes and lowest expression of ROR2. This may indicate a role of ROR1 over ROR2 in this subtype. Ovarian clear cell carcinoma is relatively rare, and clear cell ovarian cases make up approximately 12% of this cohort, with only 28 samples. It would be important to evaluate the role of ROR1 in clear cell OC using a large cohort and appropriate cell line models. Currently, ROR1 monoclonal antibodies are in phase I clinical trials for leukemia [45]. These studies could be extended to women with clear cell OC.
We showed that serous OC exhibited strong stromal expression of ROR2. Serous OC is the most common subtype, contributing to approximately 80% of OC deaths. High-grade serous OC (HGSOC) has a papillary-like architecture with high–nuclear grade cells in sheets often with slit-like structures. Even though it was recently confirmed that many HGSOCs actually arise in the fallopian tube from early serous tubal intraepithelial carcinoma lesions found in the fallopian tube [46], [47], [48], a recent study indicated that a proportion of these lesions are actually metastatic from the ovary [49]. Thus, diagnosis of HGSOC primary site is often challenging. Although Wnt activation in HGSOC stroma through ROR2 did not have any prognostic significance, it may be involved in metastasis and recurrence as shown through our case study investigation. Recently, Wang et al (2016) found ROR2 upregulation in pancreatic stroma, which was significantly associated with metastasis and stage [24]. Even though we found no association with clinicopathological features, we did note high expression of ROR2 in OC-associated stroma which warrants further investigation.
Our case study cohort, although small, demonstrated ROR expression in matched normal, primary, and metastatic samples from individual patients. Even though serous OC is most commonly diagnosed at stage IIIC, due to selection of cases with uninvolved tissue yet some metastatic sites, our case studies ranged from stage II to IIIB. Women with widespread OC undergo debulking surgery followed by chemotherapy, and when it recurs, there is usually no role for surgery unless the disease is focal and contained within an area that is resectable. In this cohort, we obtained one recurrent sample which interestingly showed the strongest expression of ROR2 seen in both tumor and stroma. It would be important to continue this study of the role of ROR2 in recurrent OC as it indicates a potential role for Wnt targeted therapy.
It is important to note the specificity of antibodies for use in IHC studies. Recently, we analyzed three commercial antibodies used for ROR2 IHC due to contradicting results found on expression patterns in colorectal cancer [28], [50], [51] and found that only one appropriately measured expression of ROR2 [52]. In this study, we used this validated ROR2 antibody. However, as investigations into ROR1 signaling are more recent, there are few validated ROR1 antibodies for IHC analysis. Our study used a ROR1 antibody previously used to investigate OC [15]. Balakrishnan et al [53] have developed a ROR1-specific monoclonal antibody targeting the carboxy terminus of ROR1. They tested this antibody in cohorts of OC subtypes which showed a range of expression. These subtype cohorts were limited in size; however, it would be interesting to test this antibody on our large TMA cohort for comparison to our IHC analysis.
Conclusion
We have investigated for the first time the relationship between ROR1 and ROR2 in OC tumor and stroma. Through a large TMA cohort and individual case studies, we indicated a protective role of ROR2 in the endometrioid subtypes, inhibiting increased β-catenin signaling, yet an oncogenic role in the stroma of the serous subtype. We also showed a unique importance of ROR1 in clear cell OC. Our results are interesting in understanding OC subtypes and disease progression and warrant further investigation through appropriate in vitro and in vivo models to elucidate novel therapeutic potential.
The following are the supplementary data related to this article.
Supplementary tables
Acknowledgements
We would like to acknowledge Alice Boulghourjan and Anaiis Zaratzian from the Garvan Medical Research Centre, Sydney, Australia, for providing the IHC services. We thank all coauthors and contributors for their feedback on this study.
Footnotes
Funding: This study was supported by the Ovarian Cancer Research Foundation (to C.F.). The Gynaecological Oncology Biobank at Westmead was funded by NHMRC Enabling Grants ID310670 and ID628903 and Cancer Institute NSW Grants 12/RIG/1-17 and 15/RIG/1-16. A.d.F. is funded by the University of Sydney Cancer Research Fund and the Cancer Institute NSW through the Sydney-West Translational Cancer Research Centre.
References
- 1.Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. 2012;4(5):a008052. doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan H, He Q, Gong G, Wang Y, Li J, Wang J, Zhu D, Wu X. miR-382 inhibits migration and invasion by targeting ROR1 through regulating EMT in ovarian cancer. Int J Oncol. 2016;48(1):181–190. doi: 10.3892/ijo.2015.3241. [DOI] [PubMed] [Google Scholar]
- 3.Zhang S, Chen L, Cui B, Chuang HY, Yu J, Wang-Rodriguez J, Tang L, Chen G, Basak GW, Kipps TJ. ROR1 is expressed in human breast cancer and associated with enhanced tumor-cell growth. PLoS One. 2012;7(3):e31127. doi: 10.1371/journal.pone.0031127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chien HP, Ueng SH, Chen SC, Chang YS, Lin YC, Lo YF, Chang HK, Chuang WY, Huang YT, Cheung YC. Expression of ROR1 has prognostic significance in triple negative breast cancer. Virchows Arch. 2016;468(5):589–595. doi: 10.1007/s00428-016-1911-3. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez NB, Lorenzo D, Picco ME, Barbero G, Dergan-Dylon LS, Marks MP, Garcia-Rivello H, Gimenez L, Labovsky V, Grumolato L. ROR1 contributes to melanoma cell growth and migration by regulating N-cadherin expression via the PI3K/Akt pathway. Mol Carcinog. 2015;55:1772–1785. doi: 10.1002/mc.22426. [DOI] [PubMed] [Google Scholar]
- 6.Jung EH, Lee HN, Han GY, Kim MJ, Kim CW. Targeting ROR1 inhibits the self-renewal and invasive ability of glioblastoma stem cells. Cell Biochem Funct. 2016;34(3):149–157. doi: 10.1002/cbf.3172. [DOI] [PubMed] [Google Scholar]
- 7.Potratz J, Tillmanns A, Berning P, Korsching E, Schaefer C, Lechtape B, Schleithoff C, Unland R, Schafer KL, Muller-Tidow C. Receptor tyrosine kinase gene expression profiles of Ewing sarcomas reveal ROR1 as a potential therapeutic target in metastatic disease. Mol Oncol. 2016;10(5):677–692. doi: 10.1016/j.molonc.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J, Chen L, Cui B, Widhopf GF, II, Shen Z, Wu R, Zhang L, Zhang S, Briggs SP, Kipps TJ. Wnt5a induces ROR1/ROR2 heterooligomerization to enhance leukemia chemotaxis and proliferation. J Clin Invest. 2015;126:585–598. doi: 10.1172/JCI83535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren D, Minami Y, Nishita M. Critical role of Wnt5a-Ror2 signaling in motility and invasiveness of carcinoma cells following Snail-mediated epithelial-mesenchymal transition. Genes Cells. 2011;16(3):304–315. doi: 10.1111/j.1365-2443.2011.01487.x. [DOI] [PubMed] [Google Scholar]
- 10.Henry C, Quadir A, Hawkins NJ, Jary E, Llamosas E, Kumar D, Daniels B, Ward RL, Ford CE. Expression of the novel Wnt receptor ROR2 is increased in breast cancer and may regulate both beta-catenin dependent and independent Wnt signalling. J Cancer Res Clin Oncol. 2015;141(2):243–254. doi: 10.1007/s00432-014-1824-y. [DOI] [PubMed] [Google Scholar]
- 11.Lu C, Wang X, Zhu H, Feng J, Ni S, Huang J. Over-expression of ROR2 and Wnt5a cooperatively correlates with unfavorable prognosis in patients with non–small cell lung cancer. Oncotarget. 2015;6(28):24912–24921. doi: 10.18632/oncotarget.4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun B, Ye X, Lin L, Shen M, Jiang T. Up-regulation of ROR2 is associated with unfavorable prognosis and tumor progression in cervical cancer. Int J Clin Exp Pathol. 2015;8(1):856–861. [PMC free article] [PubMed] [Google Scholar]
- 13.Henry C, Llamosas E, Knipprath-Meszaros A, Schoetzau A, Obermann E, Fuenfschilling M, Caduff R, Fink D, Hacker N, Ward R. Targeting the ROR1 and ROR2 receptors in epithelial ovarian cancer inhibits cell migration and invasion. Oncotarget. 2015;6(37):40310–40326. doi: 10.18632/oncotarget.5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, Cui B, Lai H, Liu G, Ghia EM, Widhopf GF, Zhang Z, Wu CC, Chen L, Wu R. Ovarian cancer stem cells express ROR1, which can be targeted for anti-cancer-stem-cell therapy. Proc Natl Acad Sci U S A. 2014;111(48):17266–17271. doi: 10.1073/pnas.1419599111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Qiu J, Ye C, Yang D, Gao L, Su Y, Tang X, Xu N, Zhang D, Xiong L. ROR1 expression correlated with poor clinical outcome in human ovarian cancer. Sci Rep. 2014;4:5811. doi: 10.1038/srep05811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paganoni S, Bernstein J, Ferreira A. Ror1-Ror2 complexes modulate synapse formation in hippocampal neurons. Neuroscience. 2010;165(4):1261–1274. doi: 10.1016/j.neuroscience.2009.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford CE, Qian Ma SS, Quadir A, Ward RL. The dual role of the novel Wnt receptor tyrosine kinase, ROR2, in human carcinogenesis. Int J Cancer. 2013;133(4):779–787. doi: 10.1002/ijc.27984. [DOI] [PubMed] [Google Scholar]
- 18.Gentile A, Lazzari L, Benvenuti S, Trusolino L, Comoglio PM. The ROR1 pseudokinase diversifies signaling outputs in MET-addicted cancer cells. Int J Cancer. 2014;135(10):2305–2316. doi: 10.1002/ijc.28879. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi T, Lu C, Ida L, Yanagisawa K, Usukura J, Cheng J, Hotta N, Shimada Y, Isomura H, Suzuki M. ROR1 sustains caveolae and survival signalling as a scaffold of cavin-1 and caveolin-1. Nat Commun. 2016;7:10060. doi: 10.1038/ncomms10060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu J, Chen L, Cui B, Widhopf GF, Shen Z, Wu R, Zhang L, Zhang S, Briggs SP, Kipps TJ. Wnt5a induces ROR1/ROR2 heterooligomerization to enhance leukemia chemotaxis and proliferation. J Clin Invest. 2016;126(2):585–598. doi: 10.1172/JCI83535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo Z, Wang Q, Lau WB, Lau B, Xu L, Zhao L, Yang H, Feng M, Xuan Y, Yang Y. Tumor microenvironment: the culprit for ovarian cancer metastasis? Cancer Lett. 2016;377(2):174–182. doi: 10.1016/j.canlet.2016.04.038. [DOI] [PubMed] [Google Scholar]
- 22.Labiche A, Heutte N, Herlin P, Chasle J, Gauduchon P, Elie N. Stromal compartment as a survival prognostic factor in advanced ovarian carcinoma. Int J Gynecol Cancer. 2010;20(1):28–33. doi: 10.1111/IGC.0b013e3181bda1cb. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Tang H, Cai J, Zhang T, Guo J, Feng D, Wang Z. Ovarian cancer–associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett. 2011;303(1):47–55. doi: 10.1016/j.canlet.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Huang J, Fan X, Wang X, Lu Y, Zhu H, Wang W, Zhang S, Wang Z. High ROR2 expression in tumor cells and stroma is correlated with poor prognosis in pancreatic ductal adenocarcinoma. Sci Rep. 2015;5:12991. doi: 10.1038/srep12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rustin GJS, Vergote I, Eisenhauer E, Pujade-Lauraine E, Quinn M, Thigpen T, du Bois A, Kristensen G, Jakobsen A, Sagae S. Definitions for response and progression in ovarian cancer clinical trials incorporating RECIST 1.1 and CA 125 agreed by the Gynecological Cancer Intergroup (GCIG) Int J Gynecol Cancer. 2011;21(2):419–423. doi: 10.1097/IGC.0b013e3182070f17. [DOI] [PubMed] [Google Scholar]
- 26.Matsuda T, Nomi M, Ikeya M, Kani S, Oishi I, Terashima T, Takada S, Minami Y. Expression of the receptor tyrosine kinase genes, Ror1 and Ror2, during mouse development. Mech Dev. 2001;105(1–2):153–156. doi: 10.1016/s0925-4773(01)00383-5. [DOI] [PubMed] [Google Scholar]
- 27.Al-Shawi R, Ashton SV, Underwood C, Simons JP. Expression of the Ror1 and Ror2 receptor tyrosine kinase genes during mouse development. Dev Genes Evol. 2001;211(4):161–171. doi: 10.1007/s004270100140. [DOI] [PubMed] [Google Scholar]
- 28.Mei H, Lian S, Zhang S, Wang W, Mao Q, Wang H. High expression of ROR2 in cancer cell correlates with unfavorable prognosis in colorectal cancer. Biochem Biophys Res Commun. 2014;453(4):703–709. doi: 10.1016/j.bbrc.2014.09.141. [DOI] [PubMed] [Google Scholar]
- 29.Rabbani H, Ostadkarampour M, Danesh Manesh AH, Basiri A, Jeddi-Tehrani M, Forouzesh F. Expression of ROR1 in patients with renal cancer—a potential diagnostic marker. Iran Biomed J. 2010;14(3):77–82. [PMC free article] [PubMed] [Google Scholar]
- 30.Rasmussen NR, Wright TM, Brooks SA, Hacker KE, Debebe Z, Sendor AB, Walker MP, Major MB, Green J, Wahl GM. Receptor tyrosine kinase-like orphan receptor 2 (Ror2) expression creates a poised state of Wnt signaling in renal cancer. J Biol Chem. 2013;288(36):26301–26310. doi: 10.1074/jbc.M113.466086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ford CE, Punnia-Moorthy G, Henry CE, Llamosas E, Nixdorf S, Olivier J, Caduff R, Ward RL, Heinzelmann-Schwarz V. The non-canonical Wnt ligand, Wnt5a, is upregulated and associated with epithelial to mesenchymal transition in epithelial ovarian cancer. Gynecol Oncol. 2014;134(2):338–345. doi: 10.1016/j.ygyno.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Henry CE, Llamosas E, Djordjevic A, Hacker NF, Ford CE. Migration and invasion is inhibited by silencing ROR1 and ROR2 in chemoresistant ovarian cancer. Oncogenesis. 2016;5(5):e226. doi: 10.1038/oncsis.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177(3):1053–1064. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lili LN, Matyunina LV, Walker LD, Benigno BB, McDonald JF. Molecular profiling predicts the existence of two functionally distinct classes of ovarian cancer stroma. Biomed Res Int. 2013;2013:846387. doi: 10.1155/2013/846387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terada T. Endometrioid adenocarcinoma of the ovary arising in atypical endometriosis. Int J Clin Exp Pathol. 2012;5(9):924–927. [PMC free article] [PubMed] [Google Scholar]
- 36.Banz C, Ungethuem U, Kuban R-J, Diedrich K, Lengyel E, Hornung D. The molecular signature of endometriosis-associated endometrioid ovarian cancer differs significantly from endometriosis-independent endometrioid ovarian cancer. Fertil Steril. 2010;94(4):1212–1217. doi: 10.1016/j.fertnstert.2009.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McConechy MK, Ding J, Senz J, Yang W, Melnyk N, Tone AA, Prentice LM, Wiegand KC, McAlpine JN, Shah SP. Ovarian and endometrial endometrioid carcinomas have distinct CTNNB1 and PTEN mutation profiles. Mod Pathol. 2014;27(1):128–134. doi: 10.1038/modpathol.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schlosshauer PW, Ellenson LH, Soslow RA. Beta-catenin and E-cadherin expression patterns in high-grade endometrial carcinoma are associated with histological subtype. Mod Pathol. 2002;15(10):1032–1037. doi: 10.1097/01.MP.0000028573.34289.04. [DOI] [PubMed] [Google Scholar]
- 39.Makar AP, Baekelandt M, Tropé CO, Kristensen GB. The prognostic significance of residual disease, FIGO substage, tumor histology, and grade in patients with FIGO stage III ovarian cancer. Gynecol Oncol. 1995;56(2):175–180. doi: 10.1006/gyno.1995.1027. [DOI] [PubMed] [Google Scholar]
- 40.Yuan Y, Niu CC, Deng G, Li ZQ, Pan J, Zhao C, Yang ZL, Si WK. The Wnt5a/Ror2 noncanonical signaling pathway inhibits canonical Wnt signaling in K562 cells. Int J Mol Med. 2011;27(1):63–69. doi: 10.3892/ijmm.2010.560. [DOI] [PubMed] [Google Scholar]
- 41.Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through β-catenin. Science. 2002;296(5573):1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- 42.Tothill RW, Tinker AV, George J, Brown R, Fox SB, Lade S, Johnson DS, Trivett MK, Etemadmoghadam D, Locandro B. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14(16):5198–5208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- 43.Chan JK, Teoh D, Hu JM, Shin JY, Osann K, Kapp DS. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol. 2008;109(3):370–376. doi: 10.1016/j.ygyno.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto S, Tsuda H, Takano M, Tamai S, Matsubara O. Loss of ARID1A protein expression occurs as an early event in ovarian clear-cell carcinoma development and frequently coexists with PIK3CA mutations. Mod Pathol. 2012;25(4):615–624. doi: 10.1038/modpathol.2011.189. [DOI] [PubMed] [Google Scholar]
- 45.Choi MY, Widhopf GF, Castro J, Li H, Kidwell RL, Zhang SC, Juarez T, Gorak S, Rassenti LZ, Messer K. Cirmtuzumab (UC-961), a first-in-class anti-ROR1 monoclonal antibody: planned interim analysis of initial phase 1 cohorts. Blood. 2015;126(23):1736. [Google Scholar]
- 46.Kim J, Coffey DM, Creighton CJ, Yu Z, Hawkins SM, Matzuk MM. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc Natl Acad Sci. 2012;109(10):3921–3926. doi: 10.1073/pnas.1117135109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karst AM, Levanon K, Drapkin R. Modeling high-grade serous ovarian carcinogenesis from the fallopian tube. Proc Natl Acad Sci U S A. 2011;108(18):7547–7552. doi: 10.1073/pnas.1017300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carlson JW, Miron A, Jarboe EA, Parast MM, Hirsch MS, Lee Y, Muto MG, Kindelberger D, Crum CP. Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J Clin Oncol. 2008;26(25):4160–4165. doi: 10.1200/JCO.2008.16.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eckert MA, Pan S, Hernandez KM, Loth RM, Andrade J, Volchenboum SL, Faber P, Montag A, Lastra R, Peter ME. Genomics of ovarian cancer progression reveals diverse metastatic trajectories including intraepithelial metastasis to the fallopian tube. Cancer Discov. 2016;6:1342–1351. doi: 10.1158/2159-8290.CD-16-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lara E, Calvanese V, Huidobro C, Fernández AF, Moncada-Pazos Á, Obaya ÁJ, Aguilera O, González-Sancho JM, Sánchez L, Astudillo A. Epigenetic repression of ROR2 has a Wnt-mediated, pro-tumourigenic role in colon cancer. Mol Cancer. 2010;9(1):170. doi: 10.1186/1476-4598-9-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma SSQ, Srivastava S, Llamosas E, Hawkins NJ, Hesson LB, Ward RL, Ford CE. ROR2 is epigenetically inactivated in the early stages of colorectal neoplasia and is associated with proliferation and migration. BMC Cancer. 2016;16(1):508. doi: 10.1186/s12885-016-2576-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma SSQ, Henry CE, Llamosas E, Higgins R, Daniels B, Hesson LB, Hawkins NJ, Ward RL, Ford CE. Validation of specificity of antibodies for immunohistochemistry: the case of ROR2. Virchows Arch. 2016:1–10. doi: 10.1007/s00428-016-2019-5. [DOI] [PubMed] [Google Scholar]
- 53.Balakrishnan A, Goodpaster T, Randolph-Habecker J, Hoffstrom BG, Jalikis FG, Koch LK, Berger C, Kosasih PL, Rajan A, Sommermeyer D. Analysis of ROR1 protein expression in human cancer and normal tissues. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-16-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables