Abstract
Background
The aim of this study was to investigate the protective effects of cucurbitacin B (CuB) on sepsis-induced acute lung injury (ALI) in rats.
Material/Methods
An ALI model was made by cecal ligation and puncture (CLP) in SD rats. Rats were randomly divided into 5 groups (n=15 per group): animals undergoing a sham CLP (sham group); animals undergoing CLP (CLP control group); animals undergoing CLP and treated with CuB at 1 mg/kg of body weight (bw) (low-dose CuB [L-CuB] group), animals undergoing CuB at 2 mg/kg of bw (mid-dose CuB [M-CuB] group); and animals undergoing CuB at 5 mg/kg of bw (high-dose CuB [H-CuB] group). Samples of blood and lung tissue were harvested at different time points (6, 12, and 24 hour post-CLP surgery) for the detection of indicators which represented ALI. Five rats were respectively sacrificed at each time point. Pathological changes of lung tissue were observed by H&E staining. Another 50 rats were distributed into the same five groups to record the 72 hour survival rates.
Results
Treatment with CuB significantly increased the blood gas PaO2 levels and decreased lung wet/dry (W/D) ratio (p<0.05). It significantly reduced protein concentration, accumulation of the inflammatory cells, and levels of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), (p<0.05), in the bronchoalveolar lavage fluid (BALF). Pulmonary pathological damage and survival rates at 72 hours were found to be effectively improved by CuB. In addition, CuB performed its pulmonary protection effects in a dose-depended manner.
Conclusions
CuB can effectively improve the pulmonary gas exchange function, reduce pulmonary edema, and inhibit the inflammatory response in the lung, revealing that CuB may serve as a potential therapeutic strategy for sepsis-induced ALI.
MeSH Keywords: Acute Lung Injury, Cucurbita, Inflammation Mediators, Pulmonary Edema, Sepsis
Background
Sepsis is a bacterial-infected systemic inflammatory response syndrome, and is one of the most frequent causes of mortality in intensive care units (ICUs), resulting in excessive tissue injury and death in approximately 30% to 50% of patients [1]. Because of pulmonary susceptibility, acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are frequent among the varieties of complications and multiple organ failures of sepsis [2], and are frequently responsible for death or disability of patients in ICUs [3]. However, the etiology and pathogenesis of ALI is complex, involving regulatory networks effected by different factors, including excessive and uncontrolled inflammatory response, oxidation, and anti-oxidation. Until recently, there has been no specific or effective preventive strategy for ALI, and no suitable therapeutic options existed, with treatment being largely supportive care. Currently, mechanical ventilation is still the main treatment for ARDS, but the mechanical stress produced by excessive mechanical ventilation is now recognized as an additional key contributor to lung inflammation and a vital determinant of lung injury [4,5]. Therefore, there is an urgent need for the development of new drugs to treat ALI. Drugs possessing anti-inflammatory and antioxidant properties have the potential to be effective against ALI.
Cucurbitacins are a group of diverse triterpenoid molecules originally extracted from the Cucurbitaceae plant family [6]. As one of the most potent and common members of cucurbitacins, cucurbitacin B (CuB) has been reported to have a variety of biological activities, including antitumor, hepatoprotective, anti-inflammatory, and antioxidant activities [7–9]. CuB is particularly well characterized in terms of its ability to inhibit tumor proliferation and migration. Zhang Meng et al. found that CuB inhibited proliferation in lung cancer cells and induced lung cancer cell apoptosis through cytochrome c release, Bcl-2 downregulation, and STAT3 pathway inhibition [10]. In accordance with Zhang Meng, other research has shown that CuB is a promising lead compound for the development of an anti-lung cancer drugs [11,12]. However, research about CuB for the treatment of ALI has not been reported. Considering the anti-inflammatory, antioxidant, and anti-lung cancer effects of CuB, we created a sepsis-induced ALI model using the method of cecal ligation and puncture (CLP) to investigate the potential treatment effects of CuB on an ALI model in rats.
Material and Methods
Animals and reagent
Male Sprague Dawley (SD) rats of 7–8 weeks of age, weighing between 200 and 220 g, were provided by the laboratory animal center of Jiangsu University. Animals were housed in the specific pathogen-free animal room with the temperature of 20°C to 26°C and relative humidity of 40% to 70%.
The animals were exposed to a 12 hour light/dark cycle and allowed free access to chow and water throughout the study. Cucurbitacin B (HPLC ≥98%) used in the study was purchased from Shanghai PureOne Biotechnology Co. Ltd. (Shanghai, China).
Animal groups
Seventy-five rats were randomly divided into the following five groups (n=15 per group): a sham CLP group; a CLP control group; a low-dose CuB [L-CuB] group; a mid-dose CuB [M-CuB] group, and a high-dose CuB [H-CuB] group. Animals of the three CuB treatment groups received intraperitoneal injection with CuB at the dosages of 1, 2, and 5 mg/kg of bw, respectively. While the sham group and CLP control group were treated with 1% DMSO (Sigma) at the same volume. At 6, 12, and 24 hours after CLP surgery, six rats were respectively sacrificed to determine the study indicators and morphology of the lung tissue. Another 50 SD rats were distributed into the same five groups as above for the observation of survival rates.
ALI modeling
All animals with the exception of those in the sham group were treated to create a classic sepsis-induced ALI model by the method of cecal ligation and puncture (CLP). The rats underwent 12-hour deprivation of food but not water, and then were anesthetized with chloral hydrate anesthesia (0.3 mL/100 g of bw). After skin sterilization, a midline abdominal incision about 2–3 cm was made to expose the cecum, which was then ligated between the terminal and ileocecal valve. Then, an 18-gauge needle was used to puncture through the central segment of ligation, and a small amount of cecal contents was squeezed out through the puncture wound. Finally, the cecum was restored into the abdominal cavity and the surgical incision was sutured layer by layer. Comparatively, ceca of rats in the sham group were exposed and massaged as described earlier, but they were not ligated or punctured.
Measurement of PaO2 Levels and Lung W/D Ratio
Blood samples of 2 mL were obtained from rat aorta abdominals for arterial blood gas analyze by automatic blood gas analyzer (Compact3, ALV Company, Switzerland). Right upper pulmonary lobes were excised, blotted dry, and weighed (wet weight), and then placed in an oven at 70°C for 48 hours to obtain the dry weight. The ratio of wet lung weight to dry lung weight (lung W/D ratio) was calculated to assess tissue edema.
Histopathologic examination of lung
At 12-hours post-CLP, portions of the right lower pulmonary lobe were harvested and washed with saline solution. Then the lung tissue was immersed in 10% neutral-buffered formalin and processed routinely by embedding in paraffin. After being sliced into 4 um-thick sections, the paraffin-fixed tissue specimens were stained with hematoxylin and eosin (H&E staining) and then mounted on glass slides and viewed under a light microscopy (Olympus BX51, Japan).
Measurement of protein content, cytokine and cell counting in BALF
Rat left lungs were removed quickly from the thoracic cavity and lavaged using 0.5 mL saline through the bronchus alveolar, four times. Then the bronchoalveolar lavage fluid (BALF) was collected and centrifuged for 10 minutes at 2,500 rpm at room temperature. The supernatant of BALF was collected and temporarily stored at −80°C for the measurement of protein content, tumor necrosis factor-α (TNF-α),and interleukin-6 (IL-6) in BALF, while the sediment was used for leukocyte differential count. Protein contents in the supernatant of BALF were determined by automatic biochemical analyzer (TBA-40FR, Toshiba, Japan), and expressed in mg/mL. Levels of TNF-α and IL-6 were measured using enzyme-linked immunosorbent assay kits specific for rats (Wuhan Boster Biotechnology Co. Ltd., Wuhan, China). The BALF cell sediment were resuspended in 0.1 mL of saline, centrifuged onto slides and stained for eight minutes with Wright-Giemsa staining. Differential cell counts for neutrophils and lymphocyte were conducted through quantification of the slides by counting a total of 200 cells/slide at 40× magnification.
Survival rates examination
In a separate analysis, the survival rates of rats were recorded at 72 hours (n=10 per group), as described earlier, to observe whether CuB treatment would confer protection against sepsis-induced ALI. And pathologic autopsy was performed to confirm CuB’s definite effects on the ALI symptoms.
Statistical analysis
Statistical analysis of the data was performed by SPSS for Windows 17.0 and all data were expressed as means ±SD. Changes between samples were compared by Student’s t-test, and differences between groups were compared by one-way analysis of variance (ANOVA). The survival rate curve was analyzed by log-rank; p<0.05 was considered statistically significant.
Results
CuB improved pulmonary gas exchange by upregulating PaO2 levels
Arterial blood PaO2 level was evaluated in this study to represent the pulmonary gas exchange function. As showed in Figure 1A, the pulmonary gas exchange efficiency in the CLP control group and the three CuB treatment groups at all time points was significantly lower compared to the sham group, as determined by an obvious drop in blood gas PaO2 levels (all p<0.05). Compared to the CLP control group, the PaO2 levels of the CuB-treated groups were significantly increased (all p<0.05). These results demonstrated that CuB had an obvious improving effect on the gas exchange function of the lungs, and as the concentration of CuB was higher, the protection effect was stronger.
Figure 1.
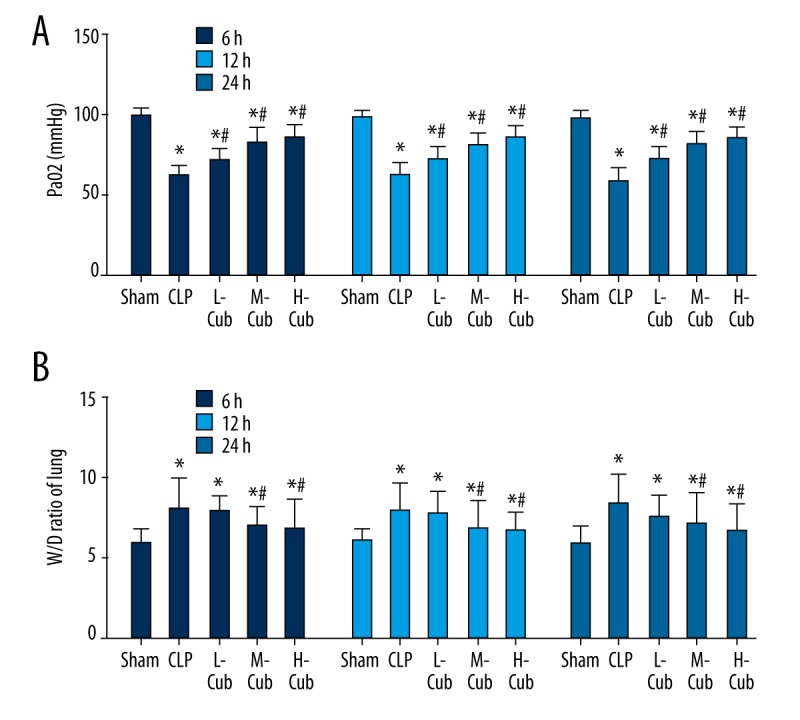
(A, B) CuB increased PaO2 levels and reduced the lung W/D ratio in CLP-induced ALI rats. Data are presented as mean ±SD, n=5. * P<0.05 compared to sham rats and # p<0.05, compared to CLP rats.
CuB reduced pulmonary edema by downregulating lung W/D ratio
The lung W/D ratio was evaluated in this study to indicate pulmonary edema. As shown in Figure 1B, the lung W/D ratios of the CLP control group and the three CuB treatment groups at all time points were significantly higher than the lung W/D ratios in the sham group (all p<0.05). When compared to the CLP control group, only the M-CuB and H-CuB groups had significantly reduced lung W/D ratios (all p<0.05), while the lung W/D ratios between the CLP group and the L-CuB group were not markedly different (all p>0.05). These results indicated that CuB can inhibit pulmonary edema by regulating the permeability of alveolar epithelial cells and capillaries.
CuB down-regulated protein content and cell counting in BALF
As demonstrated in Figure 2, the protein content and cell counts (total cells, neutrophils, and lymphocytes) of BALF in the CLP control group and the CuB-treated groups were always markedly higher than those in the sham group (all p<0.05), which indicated that, after lung injury, the obvious capillary permeability of the alveolar led to leakage of protein and infiltration of inflammatory cells. In regard to the effect of CuB, all the three CuB treatment groups showed varying degrees of decreasing effects on the protein content and cell counts of BALF (all p<0.05) compared to the CLP group.
Figure 2.
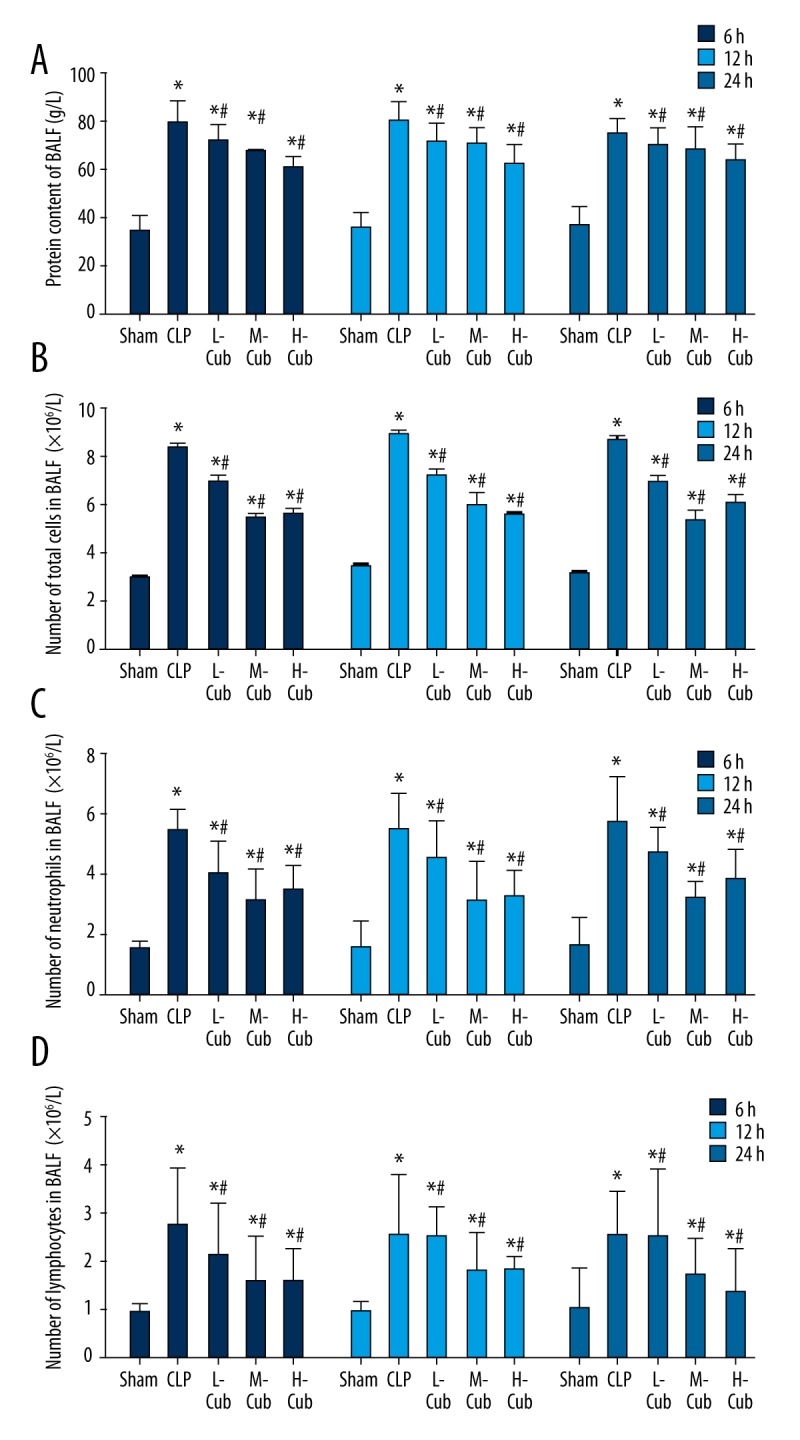
(A–D) CuB decreased the protein content and cell (total cells, neutrophils and lymphocytes) counting in the BALF of CLP-induced ALI rats. Data are presented as mean ±SD, n=5; * p<0.05 compared to sham rats and # p<0.05 compared to CLP rats.
CuB inhibited pulmonary inflammation by downregulating cytokines in BALF
Levels of TNF-α and IL-6 were determined by ELISA assay to investigate the effects of CuB on BALF cytokine secretion. Figure 3 shows that CLP caused a significant increase in the expression of both TNF-α and IL-6 compared to the sham group (all p<0.05). All of the three CuB treatment groups had significantly reduced levels of TNF-α and IL-6 (all p<0.05) in a dose-dependent manner, showing that, by reducing BALF cytokine secretion, CuB could suppress the inflammation response of lung.
Figure 3.
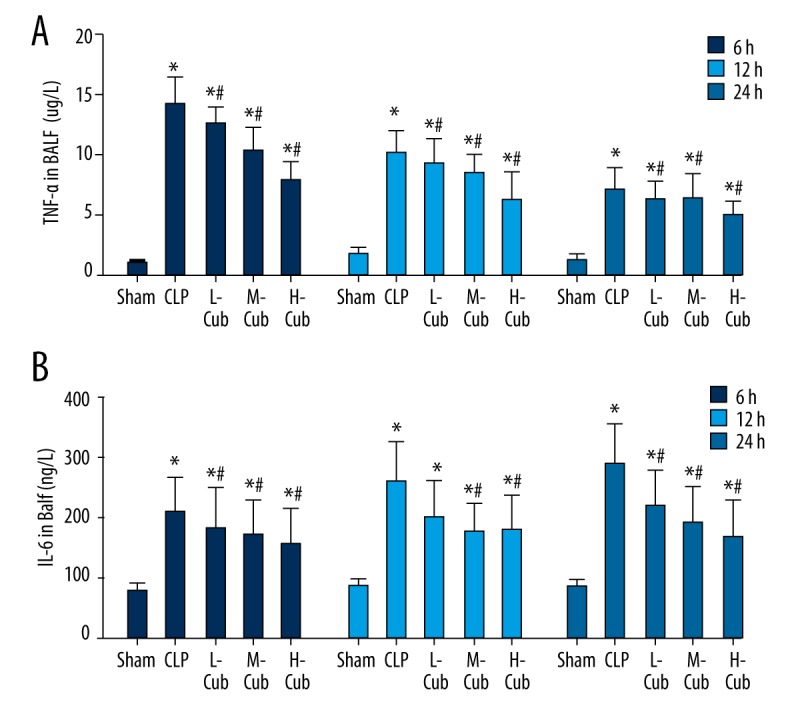
(A, B) CuB inhibited TNF-α and IL-6 expression in the BALF of CLP-induced ALI rats. Data are presented as mean ±SD, n=5; * p<0.05 compared to sham rats and # p<0.05 compared to CLP rats.
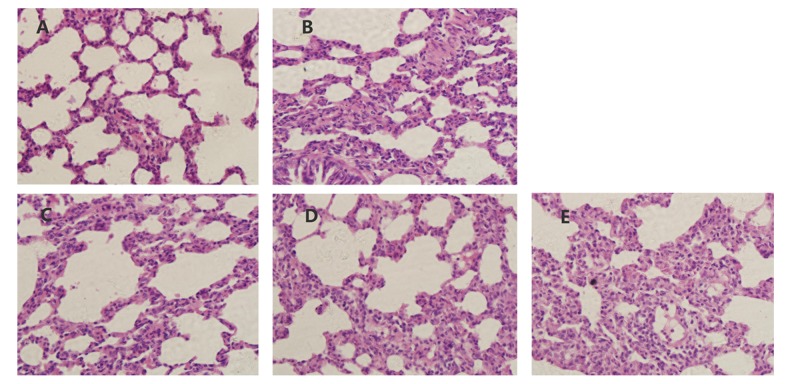
Effects of CuB on lung histopathologic changes
Histopathological examination showed that the lung tissue of rats in the sham group was morphologically normal, while in the CLP group severe damage of the pulmonary alveoli structure was observed with the appearance of histopathological changes, such as pulmonary edema, destruction of pulmonary alveoli structure, and inflammation cell infiltration (Figure 4A, 4B). Varying degrees of pathologic improvement were observed in the CuB-treated groups suggesting that CuB can decrease many of the symptoms of ALI (Figure 4C–4E).
Figure 4.
CuB ameliorated histopathological changes in lung tissues of CLP-induced ALI rats (12 hours after CLP).
Effects of CuB on 72 hour survival rate in rats
As shown in Table 1, only 10% of rats in the CLP control group survived within 72 hours compared to 100% of in the sham group (p<0.05). Conversely, the 72 hour survival rates of the L-CuB (40%), M-CuB (60%), and H-CuB (60%) were all significantly higher compared to the CLP group (p<0.05). These results suggest that CuB could effect the mortality rate of rats with ALI.
Table 1.
CuB increased 72 h survival rates of CLP-induced ALI rats (n=10 for each group).
| Group | Sham | CLP | L-CuB | M-CuB | H-CuB |
|---|---|---|---|---|---|
| 72 h survival rates | 100%a | 10%d | 40%c | 60%b | 60%b |
Different superscripts denote significantly different values (p<0.05).
In addition, the autopsy results showed obvious symptoms existed, such as hemorrhagic odor exudation in the abdominal cavity, intestinal flatulence, swelling, darkening, necrosis, adhesion at cecal of ligation end, and marked pulmonary congestion, and that compared to the CLP group, the CuB treatment groups had obviously attenuated these symptoms, which suggested CuB had a protective effect on sepsis ALI rats.
Discussion
Acute lung injury (ALI), clinically characterized by respiratory distress and intractable hypoxemia, is a common clinical syndrome secondary to severe trauma, infection, shock, and major surgery [13]. ALI/ARDS associated with sepsis is a major cause of high morbidity and mortality in critical ill patients, and is the first step to developing multiple organ dysfunction syndrome (MODS) [14]. Currently, natural products have been reported to have positive effects and evident advantages in preventing and treating ALI [14–16]. Accumulated evidence shows that CuB may have a potential protective effect on lung tissue [8–10]. Hence, the present study aimed to investigate whether CuB can effectively attenuate and treat sepsis-induced ALI. Animals models used in our study were established by the method of cecal ligation and puncture (CLP), which is recognized as the standard clinical sepsis model. According to our results, we founded that CuB improved the pulmonary gas exchange function (upregulating PaO2) and reduced pulmonary edema (downregulating lung W/D ratio and BALF’s protein content). In addition, CuB also effectively inhibited inflammatory reactions in the lung by decreasing the inflammatory cells of BALF and reducing the cytokine levels. Here we discuss CuB’s ALI protection effects related to these three aspects.
Air exchange is the most important function of the lung, which is extremely sensitive to ischemic hypoxia. In the lung injury model, the release of cytokines always leads to micro-circulation damage and oxygen carrying capacity decrease of pulmonary vascular tissue, which results in local hypoxia. Oxidative stress indicators, such as reactive oxygen species (ROS) and hypoxia inducible factor-1α (HIF-1α), will be excessively produce and overexpressed under hypobaric hypoxia environments. Excess ROS production would directly damage lung tissue by lipid peroxidation, DNA oxidative damage, and protein denaturation. Meanwhile, ROS can also launch the systemic inflammatory response and induce serious ALI by activating several signaling pathways and inflammation mediators [17,18]. HIF-1α is the oxysensitive subunit of hypoxia inducible factor (HIF)-1, which is a transcription factor induced by hypoxia. Under ROS or hypoxic conditions, HIF-1α is stabilized, and translocates into the nucleus, and upregulates the target gene expression for regulation of oxygen homeostasis [19]. HIF-1α is believed to be crucial in the pathogenesis of pulmonary edema and lung injury, and many drugs have been proven to alleviate ALI of different types by inhibiting the HIF-1α signaling pathway [20–22]. CuB has been shown to have great potential to reduce the oxidative stress indicators as mentioned earlier. It has been reported that CuB can scavenge several free radicals, as well as increase the level of N-acetylcysteine, a well-known antioxidant [9,23]. Moreover, research has also found that CuB markedly decreased the accumulation of HIF-1α protein dose-dependently, and prevented the expression of HIF-1 target genes [24,25]. In this study, PaO2 levels were evaluated to determine the pulmonary oxygenation and gas exchange function. Our results showed that CuB dose-dependently increased PaO2 levels, suggesting that CuB may improve pulmonary local hypoxia by inhibiting oxidative stress indicators such as ROS and HIF-1α, and increasing oxygen carrying capacity.
As the inevitable pathological symptoms of ALI/ARDS, pulmonary edema is often caused by integrity violation of the alveolar capillary barrier, which is considered the key link in the pathogenesis of sepsis. The destruction of vascular endothelial and alveolar epithelium can enhance permeability and further lead to excessive accumulation of protein-rich fluid in the pulmonary alveoli. There are a lot of factors which can increase the permeability of the alveolar capillary barrier. First, vascular endothelial growth factor (VEGF), which promotes the proliferation of endothelial cells and angiogenesis, can increase the capillary permeability of the body as a whole [26]. It has been reported that the effect of VEGF on increasing vascular permeability was 50,000 times stronger than that of histamine [27]. Second, the body in a pathological condition will release a large number of inflammatory cytokines, such as TNF-α, which will induce damage and apoptosis of endothelial cells, and further increase the permeability of the alveolar capillary barrier [28,29]. Fortunately, CuB seems to have the ability to decrease the permeability of the alveolar capillary barrier. RT-PCR analysis has shown that CuB inhibited the mRNA expression level of VEGF in a dose-dependent manner [30]. Xu Biao et al. founded that CuB inhibited the liver fibrosis by reducing the expression of VEGF [31]. CuB has also been reported to have anti-inflammatory activity. Our present study results showed that CuB significantly decreased W/D ratio and the protein content in BALF, and our pulmonary histopathological examination showed that CuB could obviously alleviate alveolar epithelial cell edema, decrease the percolate in alveolar cavity, as well as narrow the alveolar wall. These results suggest that, by reducing the cytokines like VEGF and TNF-α, CuB may decrease the permeability of the alveolar capillary barrier and thereby have a pulmonary protective effect.
Recently, systemic inflammatory response caused by a disequilibrium between the pro-inflammatory and anti-inflammatory reactions has been confirmed as the key of ALI pathogenesis. In sepsis, inflammatory cell activation and neutrophil infiltration, which is induced by diffuse damage of the alveolar capillary barrier, is the pathological basis of severe inflammation and visceral injury. Then activated inflammatory cells can overexpress a variety of inflammatory mediators and cytokines, such as TNF-α and IL-6, which play an important role in the maintenance and enhancement of the inflammatory response. As a pro-inflammatory factor produced from monocytes and macrophages, the leading role of TNF-α is neutrophil recruitment and inflammatory mediator release, which will further produce cascade amplification of the inflammatory process and finally cause ALI and MODS. IL-6 is a chemokine which also contributes much to the development of ALI [32]. Additionally, NF-κB has been proven to play a central role in the inflammatory response, participating in the transcription of many cytokines and inflammatory mediators (TNF-α, IL-6, MCP1, etc.). Blocking the NF-κB signaling pathway can alleviate lung injury and the inflammatory response in LPS-induced ALI rats. Coincidentally, accumulated evidence demonstrates that CuB has significant anti-inflammatory effects. Kim et al. founded that CuB blocked the LPS-activated release of pro-inflammatory mediators, such as TNF-α and IL-6, without any cytotoxicity [8]. JinHR et al. demonstrated that CuB inhibited the expression of NF-κB reporter gene and NF-κB target genes in a dose-dependent manner and suppressed the transactivation activity of RelA/p65 subunit of NF-κB [33]. Our study results showed that CuB significantly decreased cell counts (total cells, neutrophils, and lymphocytes) of BALF, and dramatically lowered the cytokine levels of TNF-α and IL-6 in BALF. In addition, pulmonary morphology results showed that CuB could dose-dependently reduce inflammatory cell infiltration. From these results, we can see that CuB may perform ALI treatment effects by blocking the release of inflammatory factors and inhibiting the activation of NF-κB pathway.
Conclusions
In summary, this study demonstrated that, relying on the strong anti-inflammatory and antioxidant abilities, CuB supplementation can effectively improve the pulmonary gas exchange function, reduce pulmonary edema, and inhibit the inflammatory response in the lung. This research revealed that CuB may serve as a potentially useful therapeutic strategy for sepsis-induced ALI.
Footnotes
Source of support: Departmental sources
Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Schlichting D, McCollam JS. Recognizing and managing severe sepsis: A common and deadly threat. South Med J. 2007;100:594–600. doi: 10.1097/SMJ.0b013e31804aa29f. [DOI] [PubMed] [Google Scholar]
- 2.Matuschak GM, Lechner AJ. Acute lung injury and the acute respiratory distress syndrome: pathophysiology and treatment. Mo Med. 2010;107:252–58. [PMC free article] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 4.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–49. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 5.Slutsky AS, Tremblay LN. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med. 1998;157:1721–25. doi: 10.1164/ajrccm.157.6.9709092. [DOI] [PubMed] [Google Scholar]
- 6.Liu T, Zhang M, Zhang H, et al. Combined antitumor activity of cucurbitacin B and docetaxel in laryngeal cancer. Eur J Pharmacol. 2008;587:78–84. doi: 10.1016/j.ejphar.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 7.El Naggar el MB, Chalupová M, Pražanová G, et al. Hepatoprotective and proapoptotic effect of Ecballium elaterium on CCl4-induced hepatotoxicity in rats. Asian Pac J Trop Med. 2015;8:526–31. doi: 10.1016/j.apjtm.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Kim M, Park SY, Jin ML, et al. Cucurbitacin B inhibits immunomodulatory function and the inflammatory response in macrophages. Immunopharmacol Immunotoxicol. 2015;37:473–80. doi: 10.3109/08923973.2015.1085065. [DOI] [PubMed] [Google Scholar]
- 9.Tannin-Spitz T, Bergman M, Grossman S. Cucurbitacin glucosides: Antioxidant and free-radical scavenging activities. Biochem Biophys Res Commun. 2007;364:181–86. doi: 10.1016/j.bbrc.2007.09.075. [DOI] [PubMed] [Google Scholar]
- 10.Zhang M, Bian ZG, Zhang Y, et al. Cucurbitacin B inhibits proliferation and induces apoptosis via STAT3 pathway inhibition in A549 lung cancer cells. Mol Med Rep. 2014;10:2905–11. doi: 10.3892/mmr.2014.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva IT, Carvalho A, Lang KL, et al. In vitro and in vivo antitumor activity of a novel semisynthetic derivative of cucurbitacin B. PLoS One. 2015;10:e0117794. doi: 10.1371/journal.pone.0117794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shukla S, Khan S, Kumar S, et al. Cucurbitacin B alters the expression of tumor-related genes by epigenetic modifications in NSCLC and inhibits NNK-induced lung tumorigenesis. Cancer Prev Res (Phila) 2015;8:552–62. doi: 10.1158/1940-6207.CAPR-14-0286. [DOI] [PubMed] [Google Scholar]
- 13.Wang SY, Li ZJ, Wang X, et al. Effect of ulinastatin on HMGB1 expression in rats with acute lung injury induced by sepsis. Genet Mol Res. 2015;14:4344–53. doi: 10.4238/2015.April.30.7. [DOI] [PubMed] [Google Scholar]
- 14.Xiao X, Yang M, Sun D, Sun S. Curcumin protects against sepsis-induced acute lung injury in rats. J Surg Res. 2012;176:31–39. doi: 10.1016/j.jss.2011.11.1032. [DOI] [PubMed] [Google Scholar]
- 15.Yan X, Wu L, Li B, et al. Cyanidin-3-O-glucoside attenuates acute lung injury in sepsis rats. J Surg Res. 2015;199:592–600. doi: 10.1016/j.jss.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Sun L, Liu S, et al. Effect of emodin on Aquaporin 5 expression in rats with sepsis-induced acute lung injury. J Tradit Chin Med. 2015;35:679–84. doi: 10.1016/s0254-6272(15)30159-x. [DOI] [PubMed] [Google Scholar]
- 17.Pi J, Zhang Q, Fu J, et al. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl Pharmacol. 2010;244:77–83. doi: 10.1016/j.taap.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 19.Kenneth NS, Rocha S. Regulation of gene expression by hypoxia. Biochem J. 2008;414:19–29. doi: 10.1042/BJ20081055. [DOI] [PubMed] [Google Scholar]
- 20.Jiang H, Huang Y, Xu H, et al. Inhibition of hypoxia inducible factor-1α ameliorates lung injury induced by trauma and hemorrhagic shock in rats. Acta Pharmacol Sin. 2012;33:635–43. doi: 10.1038/aps.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kallet RH, Matthay MA. Hyperoxia acute lung injury. Respir Care. 2013;58:123–41. doi: 10.4187/respcare.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SY, Li MH, Shi LS, et al. Rhodiola crenulata extract alleviates hypoxia pulmonary edema in rats. Evid Based Complement Alternat Med. 2013;2013:718–39. doi: 10.1155/2013/718739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yasuda S, Yogosawa S, Izutani Y, et al. Cucurbitacin B induces G2 arrest and apoptosis via a reactive oxygen species-dependent mechanism in human colon adenocarcinoma SW480 cells. Mol Nutr Food Res. 2010;54:559–65. doi: 10.1002/mnfr.200900165. [DOI] [PubMed] [Google Scholar]
- 24.Ma J, Zi Jiang Y, Shi H, et al. Cucurbitacin B inhibits the translational expression of hypoxia-inducible factor-1α. Eur J Pharmacol. 2014;723:46–54. doi: 10.1016/j.ejphar.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Dat NT, Jin X, Hong YS, Lee JJ. An isoaurone and other constituents from Trichosanthes kirilowii seeds inhibit hypoxia-inducible factor-1 and nuclear factor-kappaB. J Nat Prod. 2010;73:1167–69. doi: 10.1021/np900820p. [DOI] [PubMed] [Google Scholar]
- 26.Abrahamov D, Erez E, Tamariz M, et al. Plasma vascular endothelial growth factor level is a predictor of the severity of postoperative capillary leaksyndrome in neonates undergoing cardiopulmonary bypass. Pediatr Surg Int. 2002;18:54–59. doi: 10.1007/s003830200012. [DOI] [PubMed] [Google Scholar]
- 27.Samoto K, Pemg GC, Ehtesham M, Liu Y, et al. A herpes simplex virus type1 mutant deleted for gamma34.5 and LAT kills glioma cells in vitro and is inhibited for in vivo reactivation. Cancer Gene Ther. 2001;8:269–77. doi: 10.1038/sj.cgt.7700306. [DOI] [PubMed] [Google Scholar]
- 28.Yang D, Xie P, Guo S, Li H. Induction of MAPK phosphatase-1 by hypothermia inhibits TNF-alpha-induced endothelial barrier dysfunction and apoptosis. Cardiovasc Res. 2010;85:520–29. doi: 10.1093/cvr/cvp323. [DOI] [PubMed] [Google Scholar]
- 29.Medvedev AE, Kopydlowski KM, Vogel SN. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: Dysregulation of cytokine, chemokine, and toll-like receptor 2 and 4 gene expression. J Immunol. 2000;164:5564–74. doi: 10.4049/jimmunol.164.11.5564. [DOI] [PubMed] [Google Scholar]
- 30.Ma J, Zi Jiang Y, Shi H, et al. Cucurbitacin B inhibits the translational expression of hypoxia-inducible factor-1α. Eur J Pharmacol. 2014;723:46–54. doi: 10.1016/j.ejphar.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Biao X, Qiao Xia T. [Effect of cucurbitacin B on the expression of VEGF and oxidative stress in the liver fibrosis tissue due to schistosom a japonicum infection]. Pharmacology and Clinics of Chinese Materia Medica. 2009;25:33–36. [in Chinese] [Google Scholar]
- 32.Mizuno N, Naruse S, Kitagawa M, et al. Effects of phospholipase A2 inhibitors on Ca2+ oscillations in pancreatic acinar cells. Pancreas. 2000;20:77–83. doi: 10.1097/00006676-200001000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Jin HR, Jin X, Dat NT, Lee JJ. Cucurbitacin B suppresses the transactivation activity of RelA/p65. J Cell Biochem. 2011;112:1643–50. doi: 10.1002/jcb.23078. [DOI] [PubMed] [Google Scholar]