Abstract
Background
To develop new strategies for identifying atopic dermatitis patients, a better understanding of the signs for chronic inflammatory status is needed. This study was designed to investigate whether neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are related to the severity of atopic dermatitis (AD) assessed by the Scoring Atopic Dermatitis (SCORAD) index.
Material/Methods
A retrospective study involving 80 AD patients and 45 healthy control subjects was performed. NLR, PLR, and the number of peripheral blood eosinophils were compared between AD patients and healthy controls, and correlations between these indexes and clinical characteristics were analyzed.
Results
NLR, PLR, and eosinophils in AD patients were all significantly higher than in healthy individuals. Among AD patients, NLR (p<0.001) and PLR (p<0.001), as contrasted with eosinophils (p=0.146), were correlated positively with SCORAD index. Additionally, an NLR level of 1.75 was determined as the predictive cut-off value of severe AD (SCORAD ≥51) (sensitivity 94.7%, specificity 58.6%, the area under the receiver-operating characteristic curve (AUROC) 0.778, p=0.001). For eosinophils, the sensitivity and specificity were 78.9% and 62.1%, respectively, and the AUROC was only 0.685 (p=0.032) in predicting high SCORAD.
Conclusions
NLR and PLR reflect inflammatory response and disease severity in AD patients.
MeSH Keywords: Dermatitis, Atopic; Eosinophils; Neutrophils
Background
Atopic dermatitis (AD) (also known as atopic eczema) is a chronic inflammatory skin disease with specific immune and inflammatory mechanisms. Although it most often starts in infancy and affects about 20% of children, AD is also highly prevalent in adults [1]. No specific laboratory or histological findings have been reported, so the diagnosis relies exclusively on sets of criteria [2] that require information obtained from patient history and physical examination. The relationship between AD and systemic inflammation has been reported in several studies [3–6]. Many different markers of inflammation have been used to assess inflammatory status in atopic dermatitis and to reflect disease activity, such as eosinophil counts or serum immunoglobulin E (IgE) levels [7–10].
The neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and similar parameters (e.g., red blood cell distribution width [RDW] and mean platelet volume [MPV]), which can be easily calculated using peripheral blood, have been examined as a new expression of the inflammatory biomarkers in many diseases [11–16]. Similarly, recent studies have shown that these elevated levels of systemic inflammatory markers (NLR, PLR, RDW, and MPV) are associated with severity of many diseases, such as cancer, psoriasis, systemic lupus erythematosus, and acne vulgaris [15,17–20]. Similar parameters, such as metastatic lymph node ratios, are new and emerging parameters for various cancers [21,22].
Nevertheless, to the best of our knowledge, only a limited number of studies have investigated NLR and PLR values in patients with atopic diseases (such as asthma and allergic rhinitis) [23,24]. The NLR and PLR have not yet been elucidated in patients with atopic dermatitis, not even the relationship between NLR, PLR levels, and severity in patients with AD. Therefore, we aimed to determine if NLR and PLR were related to the severity of AD.
Material and Methods
Study population
This retrospective study was performed at the First Hospital of Jilin University between January 2009 and April 2016. A total of 109 patients with atopic dermatitis were included. Patients with other chronic inflammatory diseases such as inflammatory bowel diseases, cardiovascular disorders, overt infections, hematological diseases, chronic liver or kidney diseases, and autoimmune disorders were excluded from the analysis. After exclusion, 80 patients were included in the final analysis. For comparison, 45 age- and sex-matched healthy controls were used. The study was conducted in accordance with the Declaration of Helsinki and was approved by the First Hospital of Jilin University Ethics Committee.
Clinical and laboratory assessments
Demographic information and laboratory data were collected and documented on a form by a clinician who was blinded to prevent bias.
Age, sex, white blood cell (WBC) count, neutrophils/lymphocytes ratio (NLR), neutrophil count, lymphocyte count, eosinophil count, platelet count (PLT), platelet-to-lymphocyte ratio (PLR), red blood cell distribution width (RDW), mean platelet volume (MPV), red blood cell distribution width-to-platelet ratio (RPR), high sensitive C reactive protein (hs-CRP), erythrocyte sedimentation rate (ESR), the total serum immunoglobulin E (IgE) levels, and Scoring Atopic Dermatitis Index (SCORAD) of the patients were recorded on admission. Disease severity was classified into 3 categories (below 24 as mild, between 25 and 50 as moderate, and above 51 as severe), according to the assessment at the patient’s last visit.
Statistical analysis
Continuous data are presented as mean ± standard deviation. Differences in the continuous variables between groups were determined by t test or the Mann-Whitney U test, as appropriate. Patient groups established according to SCORAD score were compared with one-way analysis of variance for multiple comparisons. When a significant difference between the 3 groups was observed, the Mann-Whitney U test was used for the determination of difference between pairs. The correlations between SCORAD and other parameters were evaluated with Pearson’s correlation test. Furthermore, the area under the receiver-operating characteristic curve (AUROC) was assessed to determine the sensitivity and specificity of NLR, eosinophils, and PLR in predicting a high SCORAD score. All statistical analyses were performed using SPSS 17.0 (SPSS Inc., Chicago, IL) and statistical significance was defined as a p value <0.05.
Results
Demographic, clinical characteristics and laboratory results of the study population are summarized in Table 1. Our study included 80 mild-to-severe atopic dermatitis patients (51 males, 29 females) and 45 healthy controls (28 males, 17 females). The mean ages of AD patients and controls were 7.3±3.5 and 6.8±2.7 years, respectively. There was no difference between the groups with regard to sex or age.
Table 1.
Clinical, demographic characteristics and laboratory results of the participants.
| Study group (n=80) | Control group (n=45) | p value | |
|---|---|---|---|
| Gender (Male/Female) | 51/29 | 28/17 | >0.05 |
| Age (years) | 7.3±3.5 | 6.8±2.7 | >0.05 |
| WBC count, 109/L | 8.49±2.56 | 6.06±1.78 | <0.001 |
| Neutrophils, 109/L | 4.67±1.90 | 3.48±1.27 | 0.0031 |
| Lymphocytes, 109/L | 2.71±1.54 | 1.99±0.77 | 0.0147 |
| NLR | 2.73±2.23 | 1.90±0.73 | 0.0086 |
| Eosinophils, 109/L | 0.33±0.38 | 0.15±0.12 | 0.0014 |
| PLT, 109/L | 290.00±97.60 | 232.83±65.07 | 0.0054 |
| PLR | 152.22±99.70 | 130.63±56.61 | 0.2654 |
| RDW, % | 13.52±1.20 | 13.00±1.01 | 0.0562 |
| MPV, fL | 10.20±1.04 | 10.61±0.86 | 0.1277 |
| RPR | 0.054±0.026 | 0.061±0.021 | 0.2204 |
| hs-CRP, mg/L | 5.56±5.03 | ||
| ESR, mm/1 h | 27±29 | ||
| Immunoglobulin E, IU/ml | 713±1240 | ||
| SCORAD | 59±34 |
WBC – white blood cell; NLR – neutrophils to lymphocytes ratio; PLT – platelet; PLR – platelet to lymphocyte ratio; RDW – red blood cell distribution width; MPV – mean platelet volume; RPR – red blood cell distribution width to platelet ratio; hs-CRP – high sensitive C reactive protein; ESR – erythrocyte sedimentation rate; SCORAD – Scoring Atopic Dermatitis Index.
We found that the patients with atopic dermatitis had significantly higher white blood cell counts, neutrophil counts, lymphocyte counts, NLR, eosinophil counts, and PLT than the healthy controls (Table 1), but there were no statistically significant differences between PLR, RDW, MPV, and RPR of patients and controls.
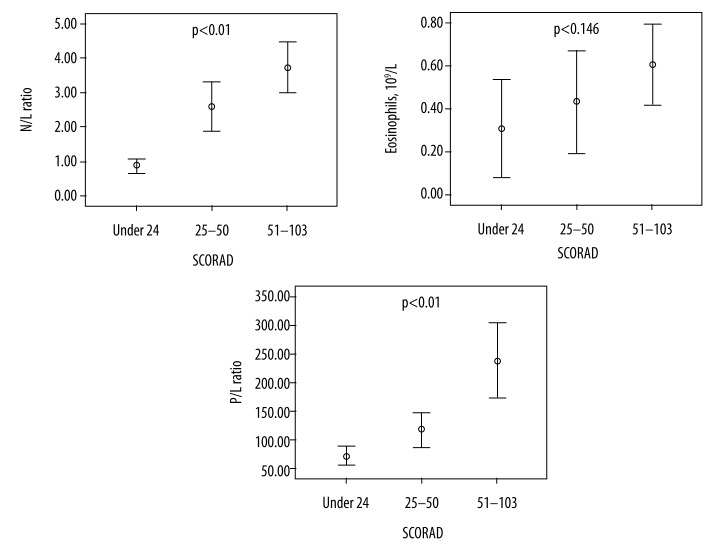
Table 2 provides the laboratory data and the differences among the 3 groups in each of the clinical measurements. In our study, 24 patients (30.0%) had mild atopic dermatitis, 30 (37.5%) had moderate atopic dermatitis, and 26 (32.5%) had severe AD. Neutrophil counts (p<0.01), lymphocyte counts (p<0.01), NLR (p<0.01), PLR (p<0.01), and ESR (p<0.05) according to SCORAD score subgroups were significantly different. Furthermore, NLR (p<0.01) and PLR (p<0.01) increased with SCORAD score as compared to eosinophils (Table 2, Figure 1).
Table 2.
Laboratory results of patients according to SCORAD.
| Group I (n=24) SCORAD (0–24) |
Group II (n=30) SCORAD (25–50) |
Group III (n=26) SCORAD (51–103) | p | G I–II p | G I–III p | G II–III p | |
|---|---|---|---|---|---|---|---|
| WBC count, 109/L | 8.14±1.75 | 9.56±3.22 | 8.68±3.55 | 0.248 | |||
| Neutrophils, 109/L | 2.88 ±1.05 | 6.25±2.81 | 5.71±2.27 | 0.000 | 0.001 | 0.007 | 0.826 |
| Lymphocytes, 109/L | 4.31±1.59 | 2.80±1.15 | 1.47±0.82 | 0.000 | 0.016 | 0.000 | 0.060 |
| NLR | 0.92±0.48 | 2.79±1.93 | 3.82±2.12 | 0.000 | 0.001 | 0.000 | 0.150 |
| Eosinophils, 109/L | 0.31±0.34 | 0.43±0.45 | 0.60±0.37 | 0.146 | |||
| PLT, 109/L | 288±107 | 296±102 | 278±70 | 0.910 | |||
| PLR | 75±31 | 118±47 | 238±97 | 0.000 | 0.203 | 0.000 | 0.000 |
| RDW, % | 13.75±1.15 | 13.13±0.96 | 13.61±1.18 | 0.351 | |||
| MPV, fL | 10.25±1.16 | 10.00±1.04 | 10.58±0.95 | 0.437 | |||
| RPR | 0.054 ± 0.019 | 0.051±0.022 | 0.056±0.028 | 0.881 | |||
| hs-CRP, mg/L | 2.52±2.01 | 7.38±6.90 | 6.21±4.67 | 0.080 | |||
| ESR, mm/1 h | 6±4.2 | 21±30 | 31±28 | 0.029 | 0.272 | 0.032 | 0.558 |
| Immunoglobulin E, IU/ml | 309±430 | 387±596 | 1032±1722 | 0.182 |
WBC – white blood cell; NLR – neutrophils to lymphocytes ratio; PLT – platelet; PLR – platelet to lymphocyte ratio; RDW – red blood cell distribution width; MPV – mean platelet volume; RPR – red blood cell distribution width to platelet ratio; hs-CRP – high sensitive C reactive protein; ESR – erythrocyte sedimentation rate; SCORAD – Scoring Atopic Dermatitis Index.
Figure 1.
N/L ratio, Eosinophils and P/L ratio of patients according to SCORAD.
Interestingly, for NLR, PLR, and eosinophils levels, each was found to be positively correlated with the other two (p<0.01) (Figure 2). Diagnostic accuracy of NLR, PLR, and eosinophils levels in relation to high SCORAD score (≥51) is depicted in Figure 3. The sensitivity and specificity of eosinophils were 78.9% and 62.1%, respectively, and the AUROC was 0.685 (p=0.032) (Table 3). The sensitivity and specificity of PLR were 63.2% and 75.9%, respectively, and the AUROC value was 0.679 (p=0.038). Nevertheless, an NLR level of 1.75 was determined as the predictive cut-off value of severe AD (sensitivity 94.7%, specificity 58.6%, AUROC 0.778) (p=0.001). Compared with preexisting indicators, NLR yielded a higher AUROC than eosinophil counts (0.685; P=0.032) in predicting high SCORAD.
Figure 2.
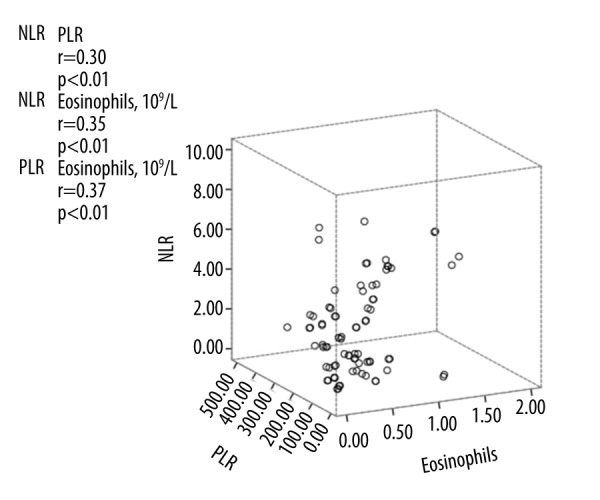
Correlation between N/L ratio, P/L ratio and Eosinophils.
Figure 3.
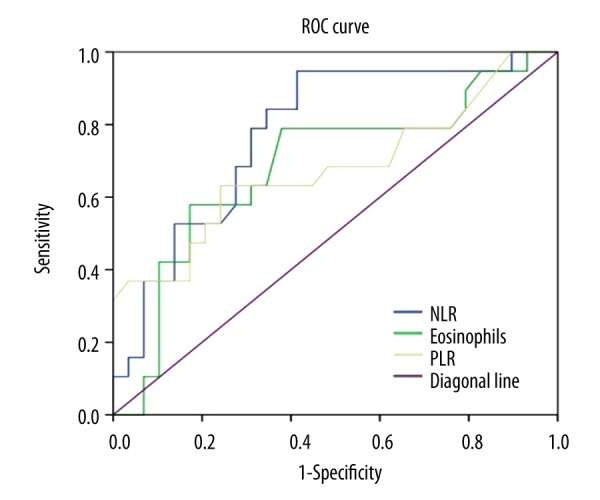
The receiver-operating characteristic curve showing the sensitivity and specificity of NLR, Eosinophils, and PLR with regard to high SCORAD.
Table 3.
Diagnostic accuracy of different formulate with regard to high SCORAD score.
| AUROC(95% CI) | p Value | Cut off | Sensitivity, % | Specificity, % | |
|---|---|---|---|---|---|
| NLR | 0.778 (0.643, 0.912) | 0.001 | 1.75 | 94.7 | 58.6 |
| Eosinophils, 109/L | 0.685 (0.524, 0.847) | 0.032 | 0.41 | 78.9 | 62.1 |
| PLR | 0.679 (0.513, 0.844) | 0.038 | 120.50 | 63.2 | 75.9 |
NLR – neutrophils to lymphocytes ratio; PLR – platelet to lymphocyte ratio; SCORAD – Scoring Atopic Dermatitis Index; AUROC – the area under the receiver-operating characteristic curve.
Discussion
Atopic dermatitis is a chronic and recurring allergic inflammatory skin disease frequently associated with peripheral eosinophilia [6, 8, 25, 26]. In this study, we investigated whether NLR and PLR were associated with the severity of childhood AD.
Uniformity and specificity are important when diagnosing AD, especially when doing clinical studies, otherwise risking misclassification. However, uniformity in the utilization of diagnostic criteria for AD, as well as validation, is lacking. To date, no study has determined whether quantification of eosinophils can truly serve as a criterion standard metric. Eosinophilia (i.e., more than 500 eosinophils per microliter of blood) has long been shown to be present in most patients with AD and is correlated with the disease activity [7,10,27,28], and has even served as a diagnostic parameter in differentiating allergic (extrinsic) AD from non-allergic (intrinsic) AD [10]. Our results correspond well with those of earlier studies noting that peripheral blood eosinophilia could be used as a diagnostic tool in recognizing atopic dermatitis [2,10,26,29]. However, we found that blood eosinophil count was not well correlated with disease activity, which differs from previous reports [9,28,30]. A possible explanation for this discrepancy is the that constituent ratio of the 3 subgroups of severity of AD has been changed through the exclusion criteria, and thus, as a retrospective study with a small number of patients, not all variable confounding factors could be included in statistical analysis.
Previous studies have revealed that AD is often associated with the later development of asthma, allergic sensitization, and allergic rhinitis, also known as “the atopic march” [31,32], but recent evidence has demonstrated that the development of a particular allergic disease does not necessarily follow the classical paradigm of the atopic march [33]. Moreover, the correlations seem to be much more complicated than that of one condition progressing into another [34–36]. Additionally, the severity of AD as evaluated with the SCORAD index is concomitant with the occurrence of asthma or allergic rhinitis [37]. In fact, an association between increased NLR, chronic systemic inflammation, and risk for asthma, allergic rhinitis, or even gastrointestinal diseases has been reported in the literature [21,23,24,38]. Furthermore, elevated NLR is associated with the severity of allergic rhinitis in children, which indicates that NLR can be used as an indicator of inflammation in allergic rhinitis [23]. Therefore, we speculate that uncontrolled inflammation, as is seen in AD, may cause an increase in NLR.
Although genetic factors play an important role in AD, the pathogenesis is attributable to specific immune and inflammatory mechanisms. Currently, NLR, as a widely available, inexpensive, easily derived, and reproducible laboratory marker, is used to quantify systemic inflammation. There are also reports of a positive correlation between the NLR and commonly used inflammatory markers [16,17]. We detected a positive correlation between NLR and absolute eosinophil counts, which are used as surrogate markers of disease activity, as in asthma, atopic dermatitis, allergic rhinitis, and conjunctivitis [39]. Unlike novel inflammatory biomarkers, the NLR is cost-effective and readily available, and it provides additional risk stratification beyond conventional risk scores. A better understanding of the role of NLR in AD may help to clarify the reasons for the development of different clinical subtypes (the extrinsic type and the intrinsic type), comorbidities, and why some patients have longer duration and insufficient responses to treatments, as inflammation is important not only in pathophysiology but also clinical outcomes of AD.
It is known that AD is generally more common in females [40]. However, recent studies have revealed a male predominance at younger ages [8,41]. In the present study, the sex ratio was 1.76: 1, with a male predominance, which was higher than in a previous report in which 68% of the study participants were male (sex ratio 1.5: 1) [8]. This is probably attributable to the high proportion of younger children.
The present study had several potential limitations. First, the sample size was relatively small and all the subjects were from the same center. Second, this study was designed as a retrospective study lacking longitudinal observation. Third, we did not collect enough results of skin prick tests or allergen-specific IgE, so we were unable to divide AD into extrinsic type and the intrinsic type according to the serum IgE levels and the presence or absence of allergen-specific IgE [42]. Finally, due to insufficient data, we did not explore the influence of treatment on NLR or PLR.
Despite these limitations, we still found some meaningful correlations of NLR and PLR with inflammatory markers and severity in AD patients. In our study, we reached 3 main conclusions. First, NLR and PLR were increased in patients with atopic dermatitis compared with the control group. Second, elevated NLR and PLR levels were linked to increasing SCORAD, which proves they can reflect inflammatory response and disease severity in AD patients. Finally, NLR yielded a higher AUROC than peripheral blood eosinophil counts, while predicting high SCORAD. Even so, it should be kept in mind that NLR alone, without other variables, may not provide exact predictive information about disease severity in children with AD. We conclude that NLR should be evaluated with other conditions as mentioned above.
Conclusions
To the best of our knowledge, this is the first study to show positive correlations between NLR, PLR, and the severity of AD evaluated by using the SCORAD index. Our study shows that NLR, which is easily calculated using the differential WBC count, can be used as an indicator of the clinical severity of AD as recorded by the SCORAD index. Further prospective studies with larger cohorts are necessary to assess this issue.
Footnotes
Source of support: Departmental sources
Statement
This study received no funding.
Conflict of interest
There is no conflict of interest in this work.
References
- 1.Weidinger S, Novak N. Atopic dermatitis. Lancet. 2016;387:1109–22. doi: 10.1016/S0140-6736(15)00149-X. [DOI] [PubMed] [Google Scholar]
- 2.Brenninkmeijer EE, Schram ME, Leeflang MM, et al. Diagnostic criteria for atopic dermatitis: A systematic review. Br J Dermatol. 2008;158:754–65. doi: 10.1111/j.1365-2133.2007.08412.x. [DOI] [PubMed] [Google Scholar]
- 3.Bao L, Zhang H, Mohan GC, et al. Differential expression of inflammation-related genes in IL-4 transgenic mice before and after the onset of atopic dermatitis skin lesions. Mol Cell Probes. 2016;30:30–38. doi: 10.1016/j.mcp.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Werfel T, Biedermann T. Current novel approaches in systemic therapy of atopic dermatitis: Specific inhibition of cutaneous Th2 polarized inflammation and itch. Curr Opin Allergy Clin Immunol. 2015;15:446–52. doi: 10.1097/ACI.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 5.Tang TS, Bieber T, Williams HC. Are the concepts of induction of remission and treatment of subclinical inflammation in atopic dermatitis clinically useful? J Allergy Clin Immunol. 2014;133:1615–25.e1. doi: 10.1016/j.jaci.2013.12.1079. [DOI] [PubMed] [Google Scholar]
- 6.Mu Z, Zhao Y, Liu X, et al. Molecular biology of atopic dermatitis. Clin Rev Allergy Immunol. 2014;47:193–218. doi: 10.1007/s12016-014-8415-1. [DOI] [PubMed] [Google Scholar]
- 7.Dhar S, Malakar R, Chattopadhyay S, et al. Correlation of the severity of atopic dermatitis with absolute eosinophil counts in peripheral blood and serum IgE levels. Indian J Dermatol Venereol Leprol. 2005;71:246–49. doi: 10.4103/0378-6323.16615. [DOI] [PubMed] [Google Scholar]
- 8.Park SY, Oh S, Kim EJ, et al. Utility of eosinophil cationic protein levels in the diagnosis of intrinsic atopic dermatitis. Acta Derm Venereol. 2014;94:333–34. doi: 10.2340/00015555-1715. [DOI] [PubMed] [Google Scholar]
- 9.Lee KY, Cho KJ, Kim YT, Kim JT. Serum eosinophil-derived neurotoxin in childhood atopic dermatitis: A useful marker of disease activity? Ann Allergy Asthma Immunol. 2009;102:532–34. doi: 10.1016/S1081-1206(10)60131-7. [DOI] [PubMed] [Google Scholar]
- 10.Jenerowicz D, Czarnecka-Operacz M, Silny W. Peripheral blood eosinophilia in atopic dermatitis. Acta Dermatovenerol Alp Pannonica Adriat. 2007;16:47–52. [PubMed] [Google Scholar]
- 11.Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther. 2016;14:573–77. doi: 10.1586/14779072.2016.1154788. [DOI] [PubMed] [Google Scholar]
- 12.Balta S, Celik T, Mikhailidis DP, et al. The relation between atherosclerosis and the neutrophil-lymphocyte ratio. Clin Appl Thromb Hemost. 2016;22(5):405–11. doi: 10.1177/1076029615569568. [DOI] [PubMed] [Google Scholar]
- 13.Ayca B, Akin F, Celik O, et al. Platelet to lymphocyte ratio as a prognostic marker in primary percutaneous coronary intervention. Platelets. 2015;26:638–44. doi: 10.3109/09537104.2014.968117. [DOI] [PubMed] [Google Scholar]
- 14.Peng W, Li C, Zhu WJ, et al. Prognostic value of the platelet to lymphocyte ratio change in liver cancer. J Surg Res. 2015;194:464–70. doi: 10.1016/j.jss.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Kim DS, Shin D, Lee MS, et al. Assessments of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in Korean patients with psoriasis vulgaris and psoriatic arthritis. J Dermatol. 2016;43:305–10. doi: 10.1111/1346-8138.13061. [DOI] [PubMed] [Google Scholar]
- 16.Sen BB, Rifaioglu EN, Ekiz O, et al. Neutrophil to lymphocyte ratio as a measure of systemic inflammation in psoriasis. Cutan Ocul Toxicol. 2013;33:223–27. doi: 10.3109/15569527.2013.834498. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Chen Y, Yang X, et al. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were associated with disease activity in patients with systemic lupus erythematosus. Int Immunopharmacol. 2016;36:94–99. doi: 10.1016/j.intimp.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Qin B, Ma N, Tang Q, et al. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod Rheumatol. 2016;26:372–76. doi: 10.3109/14397595.2015.1091136. [DOI] [PubMed] [Google Scholar]
- 19.Rifaioglu EN, Bülbül Şen B, Ekiz Ö, Cigdem Dogramaci A. Neutrophil to lymphocyte ratio in Behçet’s disease as a marker of disease activity. Acta Dermatovenerol Alp Pannonica Adriat. 2014;23:65–67. [PubMed] [Google Scholar]
- 20.Seckin HY, Bas Y, Takci Z, Kalkan G. Effects of isotretinoin on the inflammatory markers and the platelet counts in patients with acne vulgaris. Cutan Ocul Toxicol. 2016;35:89–91. doi: 10.3109/15569527.2015.1021927. [DOI] [PubMed] [Google Scholar]
- 21.Isik A, Peker K, Firat D, et al. Importance of metastatic lymph node ratio in non-metastatic, lymph node-invaded colon cancer: A clinical trial. Med Sci Monit. 2014;20:1369–75. doi: 10.12659/MSM.890804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isik A, Okan I, Firat D, et al. A new prognostic strategy for gastric carcinoma: albumin level and metastatic lymph node ratio. Minerva Chir. 2014;69:147–53. [PubMed] [Google Scholar]
- 23.Dogru M, Evcimik MF, Cirik AA. Is neutrophil-lymphocyte ratio associated with the severity of allergic rhinitis in children? Eur Arch Otorhinolaryngol. 2016;273(10):3175–78. doi: 10.1007/s00405-015-3819-y. [DOI] [PubMed] [Google Scholar]
- 24.Dogru M, Yesiltepe Mutlu RG. The evaluation of neutrophil-lymphocyte ratio in children with asthma. Allergol Immunopathol (Madr) 2016;44(4):292–96. doi: 10.1016/j.aller.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Leiferman KM. Eosinophils in atopic dermatitis. Allergy. 1989;44(Suppl 9):20–26. [PubMed] [Google Scholar]
- 26.Simon D, Braathen LR, Simon HU. Eosinophils and atopic dermatitis. Allergy. 2004;59:561–70. doi: 10.1111/j.1398-9995.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 27.Kagi MK, Joller-Jemelka H, Wuthrich B. Correlation of eosinophils, eosinophil cationic protein and soluble interleukin-2 receptor with the clinical activity of atopic dermatitis. Dermatology. 1992;185:88–92. doi: 10.1159/000247419. [DOI] [PubMed] [Google Scholar]
- 28.Uehara M, Izukura R, Sawai T. Blood eosinophilia in atopic dermatitis. Clin Exp Dermatol. 1990;15:264–66. doi: 10.1111/j.1365-2230.1990.tb02086.x. [DOI] [PubMed] [Google Scholar]
- 29.Jenerowicz D, Czarnecka-Operacz M, Silny W. Selected eosinophil proteins as markers of inflammation in atopic dermatitis patients. Acta Dermatovenerol Croat. 2006;14:73–80. [PubMed] [Google Scholar]
- 30.Coclici SE, Bozomitu LI, Mindru DE, et al. Atopic dermatitis – clinical epidemiology and immunological correlations. Rev Med Chir Soc Med Nat Iasi. 2016;120:40–47. [PubMed] [Google Scholar]
- 31.Spergel JM. From atopic dermatitis to asthma: the atopic march. Ann Allergy Asthma Immunol. 2010;105:99–106. doi: 10.1016/j.anai.2009.10.002. quiz 7–9, 17. [DOI] [PubMed] [Google Scholar]
- 32.Zheng T, Yu J, Oh MH, Zhu Z. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res. 2011;3:67–73. doi: 10.4168/aair.2011.3.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barberio G, Pajno GB, Vita D, et al. Does a ‘reverse’ atopic march exist? Allergy. 2008;63:1630–32. doi: 10.1111/j.1398-9995.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- 34.Scadding GK. Further marches: allergic and non-allergic. Clin Exp Allergy. 2007;37:485–87. doi: 10.1111/j.1365-2222.2007.02675.x. [DOI] [PubMed] [Google Scholar]
- 35.Spergel JM. Epidemiology of atopic dermatitis and atopic march in children. Immunol Allergy Clin North Am. 2010;30:269–80. doi: 10.1016/j.iac.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Kazama I, Miura C, Nakajima T. Nonsteroidal anti-inflammatory drugs quickly resolve symptoms associated with EBV-induced infectious mononucleosis in patients with atopic predispositions. Am J Case Rep. 2016;17:84–88. doi: 10.12659/AJCR.895399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Celakovska J, Bukac J. The severity of atopic dermatitis evaluated with the SCORAD index and the occurrence of bronchial asthma and rhinitis, and the duration of atopic dermatitis. Allergy Rhinol (Providence) 2016;7:8–13. doi: 10.2500/ar.2016.7.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isik A, Deniz Firat Y, Peker K, et al. How could such a wide piece of tree root pass through the narrow pyloric orifice? An extremely rare case. Am J Case Rep. 2014;15:284–87. doi: 10.12659/AJCR.890713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furuta GT, Atkins FD, Lee NA, Lee JJ. Changing roles of eosinophils in health and disease. Ann Allergy Asthma Immunol. 2014;113:3–8. doi: 10.1016/j.anai.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tokura Y. Extrinsic and intrinsic types of atopic dermatitis. J Dermatol Sci. 2010;58:1–7. doi: 10.1016/j.jdermsci.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Kim DS, Lee JH, Lee KH, Lee MG. Prevalence and severity of atopic dermatitis in Jeju Island: a cross-sectional study of 4,028 Korean elementary schoolchildren by physical examination utilizing the three-item severity score. Acta Derm Venereol. 2012;92:472–74. doi: 10.2340/00015555-1410. [DOI] [PubMed] [Google Scholar]
- 42.Park JH, Choi YL, Namkung JH, et al. Characteristics of extrinsic vs. intrinsic atopic dermatitis in infancy: correlations with laboratory variables. Br J Dermatol. 2006;155:778–83. doi: 10.1111/j.1365-2133.2006.07394.x. [DOI] [PubMed] [Google Scholar]