Abstract
Background
Squamous cell carcinoma (SCC) is the second most common type of skin cancer, for which non- or mini-invasive treatment is of critical importance. 5-aminolevulinic acids based photodynamic therapy (ALA-PDT) is a mini-invasive approach that causes focal tumor cell injury, apoptosis, and necrosis through light sensitivity. The efficacy of combining ALA-PDT and surgery in treating SCC, however, has not been demonstrated.
Material/Methods
A total of 60 SCC patients were randomly assigned into attrition plus ALA-PDT group (experimental group) and single ALA-PDT treatment group (control group). Clinical efficacy, recurrence rate, and adverse effects were analyzed in conjunction with H&E staining and immunohistochemistry (IHC) staining for p53 expression.
Results
The overall effective rate of the experimental group was 73.3%, which was significantly higher than that of the control group (46.7%). The experimental group also had a lower recurrence rate (16.6% versus 30.0%, p<0.05). Similar rates of adverse effects existed between the two groups. After treatment, abnormal cells disappeared, while the p53 positive rate after treatment was elevated in the two groups (p<0.05 comparison of before and after treatment). The experimental group had a higher p53 positive rate compared to the control group (p<0.05).
Conclusions
Combined therapy of attrition with ALA-PDT significantly elevated the effective treatment rate and can decrease the recurrence rate with reliable safety in treating SCC, thus ALA-PDT can be used as an optimal plan for SCC treatment.
MeSH Keywords: Carcinoma, Squamous Cell; Recurrence; Treatment Outcome
Background
Squamous cell carcinoma (SCC), also called epidermal-like cancer or prickle cell carcinoma, is a type of malignant skin tumor derived from epidermal Malpighian cells [1,2]. SCC represents about 20% of non-melanoma skin tumors, second only in frequency of occurrence to basal cell carcinoma [3]. SCC frequently occurs on the skin of the head or face in elderly people. In recent years, its incidence in the younger population has increased [4,5]. Traditional treatments, including surgery and radio- chemo- and immune-therapy, may result in various injuries to the treated skin area (e.g., face) or to related organ functions [6,7]. Therefore, focal non-invasive or mini-invasive treatment has become a research priority for skin tumors.
Photodynamic therapy (PDT) is a novel technique using photodynamic effect to diagnose and treat diseases [8]. PDT is a light sensitization reaction involving oxygen molecules accompanied with biological effects, based on related photodynamic effects [9]. 5-aminolevulinic acid (ALA) is a common light sensitive reagent. PDT treatment process involves the excitation of the light sensitive reagent that has been absorbed into the tissue by applying a laser irradiating at specific wavelengths. The light sensitive reagent, when in excitation status, transduces the energy to peripheral oxygen molecules to generate reactive single oxygen molecules, which can then produce oxidative reactions. Moreover, adjacent biological macromolecules may produce cytotoxicity, further causing cell damage or apoptosis [10,11]. Molecular studies have found that photodynamic therapy can directly damage the DNA structure of tumor cells; chromosomes have been shown to be in a condensed status, with the inhibition of enzymes that interrupts the DNA template structure and functionality, thus inhibiting DNA synthesis [12,13]. Photodynamic effects also strongly inhibit transmembrane transport, directly causing tumor cell death [14]. Moreover, photodynamic effects can also cause destruction of endothelial cells of micro-vessels, accelerate the formation of platelet clots, and induce hypoxia and ischemia death of tumor cells. It can also destruct mesenchymal to prevent tumor recurrence and metastasis [15]. ALA-PDT is a combined mini-invasive therapy combining photosensitive reagents and respective light sources to generate free radicals and single oxygen molecules, both of which work as strong oxidants to damage focal tumor cells, and induce their apoptosis or necrosis [16]. The clinical effect of surgery combined with ALA-PDT in treating SCC, however, has not been well studied.
Material and Methods
Patient information
A total of 60 patients (32 males, 28 females, average age 56±7.2 years, range 43–78 years) who were diagnosed with SCC in Hangzhou Third People’s Hospital from June 2014 to January 2015 were recruited for this study. By pathological examination, there were 23, 17, and 20 cases of stage II, stage III, and stage IV SCC, respectively.
The study protocol was approved by the Research Ethics Committee of Hangzhou Third People’s Hospital, and all patients gave their informed consent before study the began.
Inclusive criteria
primary treatment patients without prior chemotherapy radiotherapy or biological therapy, or laser, frozen, or focal drug application. All primary skin lesions were derived from skin SCC; and those with higher difficulty or cosmetic requirement.
Exclusive criteria
Those patients with severe light allergy, cardiac or cerebral vascular disease, severe liver or heart failure, other malignant tumor or hematologic disease, systemic immune disease, infectious disease, or inflammatory disease. Those patients who could not finish follow-up, died accidentally, or had incomplete clinical information.
Major equipment and reagent
Hydrochloric acid ammonium was purchased from Fudan Zhangjiang Pharmaceutical (China approval H20070027). Photodynamic therapy instrument was provided by Boji (China). LabSystem Version 1.3.1 microplate reader was purchased from Bio-Rad (USA). Rat anti-human p53 monoclonal antibody and anti-biotin goat anti-mouse IgG secondary antibody were purchased from Boster (China). IHC SP kit and H&E staining kit were purchased from Boster (China). ELISA kits for TNF-α and IL-6 were purchased from R&D (USA). Other common reagents were purchased from Sangon (China).
Patient grouping
Patients were randomly divided into two equal groups (n=30), including an experimental group which received combined treatment of surgery with ALA-PDT, and a control group which received ALA-PDT therapy. The control group included 17 males and 13 females (ages 44–76 years, average age 55±6.2 years). There were 11, 9, and 10 cases in stage II, stage III, and stage IV, respectively. In the experimental group, there were 15 males and 15 females (ages 43–78 years, average age 57±5.1 years). In this group there were 12, 8, and 10 cases in stage II, stage III, and stage IV, respectively. No significant difference of general clinical information existed between the two groups.
Treatment of patients
The control group received ALA-PDT treatment, including the application of 20% ALA on the skin lesion surface and surrounding 1 cm regions, followed by coverage of treated area with a plastic membrane. Patients were kept in the dark for 3–4 hours, followed by application of light treatment (635 nm wavelength) for 30 minutes at 100 J/cm2 using Ella photodynamic treatment instrument. The therapy consists of five treatments at 10–14 day intervals. In the experimental group, the tumor feature and site were determined by pathology examination. A skin incision was made at 0.5–1.0 cm around the tumor edge. Attrition was performed to remove tumors completely. ALA-PDT therapy was performed from 2 day after surgery using the same method used for the control group. Follow-ups were performed monthly after treatment via telephone or outpatient clinic visits, and follow-up lasted for 12–24 months (average follow-up 15±5 months).
H&E staining for tumor pathology
Tissue samples were fixed in 4% formalin and then dehydrated in gradient ethanol. Then the tissue was treated by xylene immersion, paraffin embedding, and sectioned into 5 μm thick slices. After drying, tissue sections were de-waxed, rehydrated in gradient ethanol, and stained by H&E method for observation under the microscope.
P53 expression of tumors by IHC staining
Tumor tissue sections were stained using SP kit for IHC staining: 10% normal goat serum was used to block endogenous peroxidase, followed by addition of p53 (1: 1,000) dilution. Tissue sections were incubated at 37°C for one hour, followed by PBS rinsing, three times. Biotin-labelled secondary goat anti-mouse IgG was applied and the tissue sections incubated for 20 minutes at room temperature, followed by three PBS rinses. Next, the tissue sections were incubated in SABC complex for 20 minutes at room temperature, followed by PBS rinsing, DAB-H2O2 development, and hematoxylin counter-staining. Tissue sections were then dehydrated in ethanol and immersed in xylene, followed by resin mounting of coverslips. Microscopic observation was performed on all prepared sections. Positive staining was identified by brown-yellow granular cell counting. Negative expression (−) was determined when no brown colored cells were observed. Weak positive expression (+) was identified as less than 25% of brown colored cells. Positive expression (++) was defined when there were more than 25–50% of brown colored cells. Strong positive expression (+++) was identified when there were more than 50% of brown colored cells.
Analysis of clinical treatment efficacy
Evaluation of clinical treatment efficacy included: complete remission (disappear of tumor, normal skin tissues by biopsy), partial remission (tumor shrinkage by more than 50%) and invalid (tumor shrinkage by less than 50%). Overall effective rate was calculated as complete remission case number + incomplete remission case number divided by the total number of cases. Recurrence was defined as newly generated lesion at the original treatment site, ulcer, or erosion. Adverse effects include focal redness, tissue edema, erosion, burning sensation, and pain.
Statistical analysis
SPSS19.0 software was used for statistical analysis. Measurement data are presented as mean ± standard deviation (SD). LSD test was used for between-group-comparison. Enumeration data is shown by percentage and was compared by chi-square test. A statistically significant difference was defined when p<0.05.
Results
Analysis of treatment efficacy using attrition combined ALA-PDT or single ALA-PDT for late stage SCC
We further analyzed the clinical effects of attrition combined with ALA-PDT or single ALA-PDT in treating late stage SCC. Results showed satisfactory effects of both methods. Combined therapy (experimental group) had significantly higher efficacy than the control group (p<0.05). Treatment of SCC at different stages exhibited various efficacies, which were decreased following disease progression (Table 1).
Table 1.
Analysis of clinical treatment efficacy using attrition combined with ALA-PDT vs. single ALA-PDT treatment.
| N | Complete remission | Partial remission | Ineffective | Overall effective rate (%) | |
|---|---|---|---|---|---|
| Control | 30 | 6 | 8 | 16 | 46.7 |
| Stage II | 11 | 3 | 4 | 4 | 63.6 |
| Stage III | 9 | 2 | 2 | 5 | 44.4 |
| Stage IV | 10 | 1 | 2 | 7 | 30.0 |
| Experimental | 30 | 17 | 5 | 8 | 73.3* |
| Stage II | 12 | 8 | 2 | 2 | 83.3* |
| Stage III | 8 | 5 | 1 | 2 | 75.0* |
| Stage IV | 10 | 4 | 2 | 4 | 60.0* |
p<0.05 compared to control group at the same stage.
Treatment of late stage SCC using attrition combining ALA-PDT or single ALA-PDT
We analyzed the recurrence of disease using attrition with ALA-PDT or single ALA-PDT in treating late stage SCC. Results showed that recurrence rates during post-operative follow-up periods were 16.6% and 30.0% for the experimental group and the control group, respectively (p<0.05). SCC at different disease stages had various recurrence potential, as higher recurrence rates occurred with more advanced SCC progression (Table 2).
Table 2.
Analysis of SCC recurrence using combined therapy or ALA-PDT.
| N | Recurrence (N) | Recurrent rate (%) | |
|---|---|---|---|
| Control | 30 | 9 | 30.0 |
| Stage II | 11 | 2 | 18.2 |
| Stage III | 9 | 3 | 33.3 |
| Stage IV | 10 | 4 | 40.0 |
| Experimental | 30 | 5 | 16.6* |
| Stage II | 12 | 1 | 8.33* |
| Stage III | 8 | 1 | 12.5* |
| Stage IV | 10 | 3 | 30.0* |
p<0.05 compared to control group at the same stage.
Adverse effects of combined therapy versus single ALA-PDT treatment
Follow-up analysis was used to observe the adverse effects of late stage SCC between the experimental group and the control group. Results showed certain adverse effects in both groups after treatment. For example, mild erosion was treated and healed, while other adverse effects were self-limiting. In the control group, there were three cases with focal redness, one case with edema, and two cases with burning sensation, resulting in the overall adverse effect rate at 20.0%. In the experimental group, there was one case with focal redness, one case with edema and one case with erosion, leading to the overall adverse effect rate at 16.6%. No infection was observed in either group. No significant differences existed between the two groups.
Pathological changes of combined therapy and single ALA-PDT in treating SCC
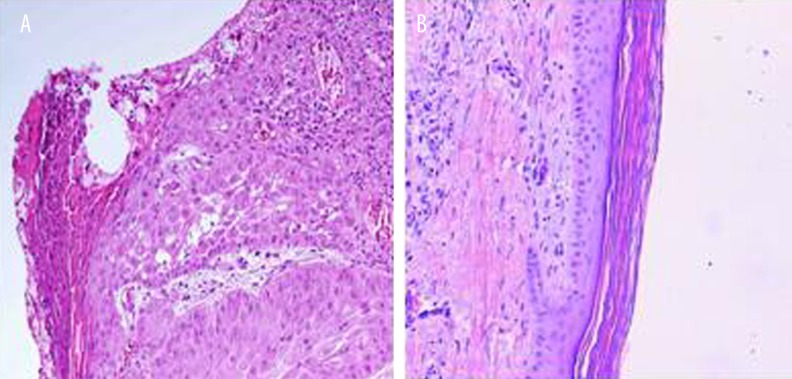
Further observations on post-operative treatment efficacy of both groups showed malignant ulcer and erosion in tumors before treatment. Epidermal and fiber-spindle like cells had infiltrative growth with grouped arrangement and formation of cell cones. Spindle cells showed staggered, bundled, or irregular arrangement, with diffused distribution. Higher heterogeneity existed in cells with multiple nuclear division and strong staining plus polymorphism. After treatment using either attrition plus ALA-PDT or single PDT, both ulcer and erosion of skin were significantly improved, with the disappearance of abnormal shaped cells in tumor tissues (Figures 1, 2).
Figure 1.
General change of SCC after treated using attrition plus ALA-PDT or single ALA-PDT. (A) One case of stage IV SCC; (B) After attrition plus ALA-PDT combine treatment; (C) One case of stage IV SCC; (D) After single ALA-PDT treatment.
Figure 2.
Pathological change of SCC after treated using attrition plus ALA-PDT or single ALA-PDT. (A) Pathology of SCC before treatment; (B) Pathology of SCC after attrition plus ALA-PDT combine treatment or single ALA-PDT treatment.
p53 expression after combined or single treatment of SCC
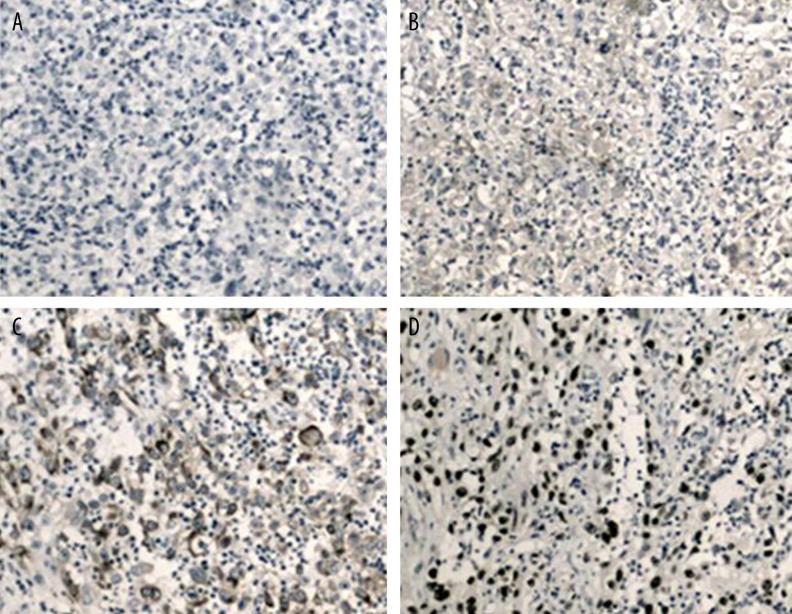
IHC staining was used to test for p53 expression after combined treatment of attrition plus ALA-PDT or single ALA-PDT. Results showed decreased p53 expression in SCC tissues before treatment. Both combined and single treatment significantly elevated p53 positive expression rate (p<0.05 compared to before treatment). In the experimental group, however, there was a more potent elevation of p53 positive rate compared to the control group (p<0.05, Figure 3, Table 3).
Figure 3.
P53 expression in SCC by IHC staining (×200). (A) Negative expression of p53; (B) Weak positive expression; (C) Positive expression; (D) Strong positive expression.
Table 3.
Analysis of p53 expressoin after combined treatment or single ALA-PDT treatment.
| Before treatment | After treatment | |||||
|---|---|---|---|---|---|---|
| (−) | (+ – +++) | % | (−) | (+ – +++) | % | |
| Control | 22 | 8 | 26.7 | 15 | 15 | 50.0# |
| Experiment | 23 | 7 | 23.3 | 8 | 22 | 73.3*# |
p<0.05 compared to control group;
p<0.05 compared to those before treatment.
Discussion
For larger area or higher cosmetic requirements of the SCC treatment due to potential post-operative lesions, inconsistent skin color after graft, organ translocation or deformation, or procedural effects on facial surface, mini- or invasive surgery has become the new perspective in treating skin malignant tumors. PDT can destruct the genetic structure of tumor cells, and cause clotting or necrosis of chromosomes, thus inhibiting DNA synthesis, decreasing the bioactivity of DNA synthase, inhibiting membrane transportation, causing vascular endothelial cell injury, inducing tumor cell apoptosis, facilitating ischemia necrosis, and suppressing tumor metastasis or recurrence. PDT has high specificity in SCC treatment, as it can focally kill tumor cells without damaging normal tissue or cells [17–19].
In addition to PDT targeted tumor cell killing effect, the selection of an effective light sensitive reagent is of critical importance, as the reagent can specifically targeted tumor cells via absorbing and emitting photons at specific wavelengths to accelerate killing targeted cells by photo polymerization effects. ALA is a second generation photosensitive reagent, and is the precursor in hemoglobin synthesis. Besides strong photosensitive activity, it also overcomes some of the weakness of first generation reagent, including photo-allergy and insufficient function on target cells. By using the endogenous light sensitive activity of ALA absorbed by cells with higher proliferative activity such as tumor cells, ALA can aggregate selectively to induce tumor cell apoptosis [20,21]. For those SCC patients who are unwilling to accept surgery, ALA-PDT has satisfactory efficacy on tissue pathology, with advantages including ease, safety, effectiveness, small adverse effects, and better retention of cosmetic effects. The curative rate of primary and original SCC cases is high, including certain cases of complete cure. For late stage SCC, however, ALA-PDT had unfavorable efficacy, mainly due to limited penetrative depth of PDT (around 0.6 cm) [22]. Moreover, a study has shown the correlation between PDT efficacy and tumor size, infiltrative depth, and malignancy [23]. Therefore, in our study, attrition was first performed on SCC at stage II–IV, to reduce tumor size and infiltrative depth, followed by ALA-PDT under the full effective range as a focal anesthesia only. Results showed the satisfactory efficacy using attrition combined with ALA-PDT or single ALA-PDT in treating late stage SCC. The combined therapy, however, had improved overall effective rates compared to the single treatment, and decreased recurrence rate. Comparison among different stages of SCC showed decreased effective rates with SCC progression and higher recurrence rates. Minor adverse effects were found in both methods but without the requirement of adverse effect treatment and no difference between groups. Pathology examination showed significant improvement in tumor pathology after either treatment, without scars or facial injury. Further analysis of apoptotic protein p53 also showed elevated positive rates of p53 expression in both groups after treatment, with a more potent increase in the experimental group.
Conclusions
Attrition combined with ALA-PDT in treating SCC effectively improved overall effectiveness rates, and reduced recurrence rates with reliability and safety. Due to this confirmed treatment effect, ALA-PDT can be considered as one of the selective plans for SCC treatment, with promising application in clinics.
Footnotes
Source of support: Research supported by Hangzhou Health Department of Science and Technology Plan Item (NO. 2016A28)
Disclosure of conflict of interest
None.
References
- 1.Gnanajothy R, Warren GW, Okun S, Peterson LL. A combined modality therapeutic approach to metastatic anal squamous cell carcinoma with systemic chemotherapy and local therapy to sites of disease: Case report and review of literature. J Gastrointest Oncol. 2016;7(3):E58–63. doi: 10.21037/jgo.2015.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barlow BR, Marshall RV, Wofford JD. Giant cutaneous squamous cell carcinoma requiring emergent embolization. JAAD Case Rep. 2016;2(3):216–18. doi: 10.1016/j.jdcr.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertino G, Sersa G, De Terlizzi F, et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: Results of the treatment of skin cancer. Eur J Cancer. 2016;63:41–52. doi: 10.1016/j.ejca.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Fu T, Aasi SZ, Hollmig ST. Management of high-risk squamous cell carcinoma of the skin. Curr Treat Options Oncol. 2016;17(7):34. doi: 10.1007/s11864-016-0408-2. [DOI] [PubMed] [Google Scholar]
- 5.Koneru B, Shi Y, Munaweera I, et al. Radiotherapeutic bandage for the treatment of squamous cell carcinoma of the skin. Nucl Med Biol. 2016;43(6):333–38. doi: 10.1016/j.nucmedbio.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Burillo-Martinez S, Maroñas-Jimenez L, Palencia-Pérez SI, et al. Failure of photodynamic therapy (PDT) in 3 patients with folliculitis decalvans. J Am Acad Dermatol. 2016;74(4):e69–70. doi: 10.1016/j.jaad.2015.09.075. [DOI] [PubMed] [Google Scholar]
- 7.Qidwai A, Khan S, Md S, et al. Nanostructured lipid carrier in photodynamic therapy for the treatment of basal-cell carcinoma. Drug Deliv. 2016;23(4):1476–85. doi: 10.3109/10717544.2016.1165310. [DOI] [PubMed] [Google Scholar]
- 8.Sotiriou E, Apalla Z, Ioannides D. Complete resolution of a squamous cell carcinoma of the skin using intralesional 5-aminolevulinic acid photodynamic therapy intralesional PDT for SCC. Photodermatol Photoimmunol Photomed. 2010;26(5):269–71. doi: 10.1111/j.1600-0781.2010.00531.x. [DOI] [PubMed] [Google Scholar]
- 9.Jerjes W, Upile T, Betz CS, et al. The application of photodynamic therapy in the head and neck. Dent Update. 2007;34(8):478–80. 483–84, 486. doi: 10.12968/denu.2007.34.8.478. [DOI] [PubMed] [Google Scholar]
- 10.Zhu TC, Parsai EU, Orton CG. Point/counterpoint. PDT is better than alternative therapies such as brachytherapy, electron beams, or low-energy x rays for the treatment of skin cancers. Med Phys. 2011;38(3):1133–35. doi: 10.1118/1.3512802. [DOI] [PubMed] [Google Scholar]
- 11.Ibbotson SH. An overview of topical photodynamic therapy in dermatology. Photodiagnosis Photodyn Ther. 2010;7(1):16–23. doi: 10.1016/j.pdpdt.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Li Q. One squamous cell carcinoma with a tumor thickness of 5.5 mm was successfully treated with photodynamic therapy. Int J Dermatol. 2011;50(4):492–94. doi: 10.1111/j.1365-4632.2009.04388.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee TY, Cheon YK, Shim CS. Photodynamic therapy in patients with advanced hilar cholangiocarcinoma: Percutaneous cholangioscopic versus peroral transpapillary approach. Photomed Laser Surg. 2016;34(4):150–56. doi: 10.1089/pho.2015.3989. [DOI] [PubMed] [Google Scholar]
- 14.Gao W, Wang Z, Lv L, et al. Photodynamic therapy induced enhancement of tumor vasculature permeability using an upconversion nanoconstruct for improved intratumoral nanoparticle delivery in deep tissues. Theranostics. 2016;6(8):1131–44. doi: 10.7150/thno.15262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gozali MV, Yi F, Zhang JA, et al. Photodynamic therapy inhibit Fibroblast Growth Factor-10 induced keratinocyte differentiation and proliferation through ROS in Fibroblast Growth Factor Receptor-2b pathway. Sci Rep. 2016;6:27402. doi: 10.1038/srep27402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge X, Liu J, Shi Z, et al. Inhibition of MAPK signaling pathways enhances cell death induced by 5-Aminolevulinic acid-photodynamic therapy in skin squamous carcinoma cells. Eur J Dermatol. 2016;26(2):164–72. doi: 10.1684/ejd.2015.2725. [DOI] [PubMed] [Google Scholar]
- 17.Akram Z, Al-Shareef SA, Daood U, et al. Bactericidal efficacy of photodynamic therapy against periodontal pathogens in periodontal disease: A systematic review. Photomed Laser Surg. 2016;34(4):137–49. doi: 10.1089/pho.2015.4076. [DOI] [PubMed] [Google Scholar]
- 18.von Felbert V, Bauerschlag D, Maass N, et al. A specific photoimmunotheranostics agent to detect and eliminate skin cancer cells expressing EGFR. J Cancer Res Clin Oncol. 2016;142(5):1003–11. doi: 10.1007/s00432-016-2122-7. [DOI] [PubMed] [Google Scholar]
- 19.Maur M, Toss A, Dominici M, et al. Impressive response to dose-dense chemotherapy in a patient with NUT midline carcinoma. Am J Case Rep. 2015;16:424–29. doi: 10.12659/AJCR.893879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cabete J, Rafael M, Cravo M, et al. Long-term recurrence of nonmelanoma skin cancer after topical methylaminolevulinate photodynamic therapy in a dermato-oncology department. An Bras Dermatol. 2015;90(6):846–50. doi: 10.1590/abd1806-4841.20154080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jambusaria-Pahlajani A, Ortman S, Schmults CD, Liang C. Sequential curettage, 5-fluorouracil, and photodynamic therapy for field cancerization of the scalp and face in solid organ transplant recipients. Dermatol Surg. 2016;42(Suppl 1):S66–72. doi: 10.1097/DSS.0000000000000589. [DOI] [PubMed] [Google Scholar]
- 22.Gandhi SA, Kampp J. Skin cancer epidemiology, detection, and management. Med Clin North Am. 2015;99(6):1323–35. doi: 10.1016/j.mcna.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Yang Y, Yang Y, Lu Y. Surgery combined with topical photodynamic therapy for the treatment of squamous cell carcinoma of the lip. Photodiagnosis Photodyn Ther. 2016;14:170–72. doi: 10.1016/j.pdpdt.2016.04.008. [DOI] [PubMed] [Google Scholar]