Abstract
Intentional facial expression of emotion is critical to healthy social interactions. Patients with neurodegenerative disease, particularly those with right temporal or prefrontal atrophy, show dramatic socioemotional impairment. This was an exploratory study examining the neural and behavioral correlates of intentional facial expression of emotion in neurodegenerative disease patients and healthy controls. One hundred and thirty three participants (45 Alzheimer's disease, 16 behavioral variant frontotemporal dementia, 8 non-fluent primary progressive aphasia, 10 progressive supranuclear palsy, 11 right-temporal frontotemporal dementia, 9 semantic variant primary progressive aphasia patients and 34 healthy controls) were video recorded while imitating static images of emotional faces and producing emotional expressions based on verbal command; the accuracy of their expression was rated by blinded raters. Participants also underwent face-to-face socioemotional testing and informants described participants' typical socioemotional behavior. Patients' performance on emotion expression tasks was correlated with gray matter volume using voxel-based morphometry (VBM) across the entire sample. We found that intentional emotional imitation scores were related to fundamental socioemotional deficits; patients with known socioemotional deficits performed worse than controls on intentional emotion imitation; and intentional emotional expression predicted caregiver ratings of empathy and interpersonal warmth. Whole brain VBMs revealed a rightward cortical atrophy pattern homologous to the left lateralized speech production network was associated with intentional emotional imitation deficits. Results point to a possible neural mechanisms underlying complex socioemotional communication deficits in neurodegenerative disease patients.
Keywords: Frontotemporal dementia, Emotion, Social functioning, Empathy, Facial expression
Highlights
-
•
A rightward atrophy pattern homologous to the leftward "speech production network" predicted patients' ability to intentionally imitate emotional facial expressions.
-
•
Neurodegenerative disease patients with core deficits in social functioning show deficits in the ability to intentionally imitate facial expressions of emotion.
-
•
Intentional emotion imitation deficits were related to more fundamental deficits in emotion reading and general emotional expressiveness.
-
•
Emotion imitation deficits predicted caregivers’ ratings of interpersonal warmth and empathy.
Generating intentional emotional facial expressions is a highly complex, socially learned form of human communication (Bavelas and Chovil, 1997, Niedenthal and Brauer, 2012), and is distinct from spontaneous expression of emotion. Intentional expressions of emotion convey emotional meaning and can also mask underlying intentions and feelings during a social interaction (e.g., deception, manipulation, displays of social decorum, deliberate expressions of empathy). They may be modulated to influence the behavior of others or signal one's level of desire for social contact.
Intentional emotional expression is neurologically distinct from spontaneous emotional expression and intentional non-emotional facial expressions (e.g., blowing out a match), and may involve predominantly cortical and particularly right lateralized structures. The few resting state studies that have explored the neural substrates of intentional emotional expression in healthy adults have identified an assembly of cortical regions that are involved in higher order emotional and cognitive processes such as self-conscious emotion (pregenual anterior cingulate) (Sturm et al., 2013), hedonic value representation (ventral medial prefrontal cortex) (O'Doherty, 2004), sensorimotor processing and stable attentional control (frontal operculum/frontoinsula) (Dosenbach et al., 2007), deliberate motor sequencing (presupplementary motor area; SMA), and affective empathy (the superior temporal sulcus) (Nair et al., 2003, Rankin et al., 2006, Hennenlotter et al., 2005, Hopf et al., 1992, Lee et al., 2006, Rinn, 1984, van der Gaag et al., 2007). Whereas spontaneous facial expression of emotion is predominantly associated with subcortical regions (Matsumoto and Lee, 1993), the association of cortical areas with intentional emotional expression in healthy adults suggest that this behavior involves higher order regulatory functions. Yet compared to emotion comprehension and spontaneous emotional expression abilities, very little is known about the neural substrates and behavioral correlates of intentional facial expression of emotion.
An inability to modulate emotional facial expressions can have negative ramifications for an individual's socioemotional environment, and has been linked to depression, schizophrenia (Berenbaum and Rotter, 1992), flattened affect (Trémeau et al., 2014), and lower levels of trait empathy in healthy adults (Williams et al., 2013). Changes in socioemotional comportment, such as deficits in spontaneous emotional expression, empathy, emotion reading, and blunted affect are also seen in patients with behavioral variant frontotemporal dementia (bvFTD), right temporal frontotemporal dementia (rtvFTD), semantic variant primary progressive aphasia (svPPA), as well as Alzheimer's disease (AD) (Fernandez-Duque et al., 2010, Kumfor and Piguet, 2012, Rosen et al., 2002, Irish et al., 2014, Kamminga et al., 2015, Seeley et al., 2005), though the ability to intentionally express emotions has not been systematically studied in these populations. While primary deficits in spontaneous emotional expression and emotion reading may affect intentional emotional expression abilities in the patient groups described above, they are distinct from deficits in intentional emotional expression. For example, not all patients with spontaneous expression deficits lack the ability to generate emotional expression on cue (e.g., Parkinson's patients) and not all patients who lack deliberate facial expression abilities show reduced capacity for spontaneous emotional expression (e.g., “opercular syndrome”) (Borod, 1992, Rinn, 1984, Rinn, 2007). Thus, whether patient groups show true deficits in intentional emotional expression must be studied alongside careful examination of other socioemotional abilities. Neurodegenerative patients with socioemotional deficits provide an invaluable, naturally occurring human lesion model for understanding complex and mechanistic relationships among socioemotional human behaviors and the neural substrates that underpin them.
This was an exploratory study using a lesion model with the primary aim to determine whether deficits in intentional facial expression of emotion corresponded to damage to neural structures shown to underpin intentional facial expression of emotion in healthy populations. As a secondary aim, we investigated the degree to which neurodegenerative disease patients display specific deficits in intentional facial expression of emotion and examined the role of other concurrent socioemotional deficits in patients' ability to intentionally express facial emotion. We measured participants' ability to intentionally generate emotional facial expressions under two conditions: a verbal command condition and a picture imitation condition. To identify the structural correlates of the ability to intentionally simulate emotion, we performed a brain-behavior analysis across the whole sample by correlating performance on these tasks with gray matter volume using voxel-based morphometry (VBM), without regard to diagnostic group membership. Then, we examined emotion simulation in the distinct neurodegenerative syndromes, including how performance in these tasks related to other socioemotional abilities.
1. Methods
1.1. Intentional emotional expression task
133 participants (45 with possible or probable Alzheimer's disease (AD), 16 patients diagnosed with behavioral-variant frontotemporal dementia (bvFTD), 11 with right temporal frontotemporal dementia (rtvFTD), 9 with semantic variant primary progressive aphasia (svPPA), 8 with non-fluent primary progressive aphasia (nfvPPA), 10 with progressive supranuclear palsy (PSP), and 19 healthy older adults (NC) participated in this study. bvFTD patients were diagnosed according to FTDC criteria (Rascovsky et al., 2011), non-fluent and semantic variant met new International PPA criteria (Gorno-Tempini et al., 2011), right temporal frontotemporal patients (rtvFTD) were classified according to Josephs et al. (Josephs et al., 2009) (i.e., patients were considered rtvFTD if they met bvFTD or semantic variant PPA criteria, but had predominant right temporal atrophy, which was confirmed through visual inspection of structural MRI) and the AD patients met the National Institute on Ageing-Alzheimer's Association criteria (McKhann et al., 2011). Patients' diagnoses were determined by a multidisciplinary team of neurologists, neuropsychologists and nurses, following thorough neurological, neuropsychological and neuroimaging assessments. Please see Table 1 for demographic characteristics of the sample.
Table 1.
Demographic group characteristics and behavioral scores.
| Mean (SD) | AD n = 44 | bvFTD n = 14 | nfPPA n = 8 | PSP n = 10 | rtvFTD n = 11 | svPPA n = 9 | NC n = 34 | F = | P-value | η2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 65.5(8.6) | 59.7 (6.7)⁎⁎ | 68.4(9.8)⁎ | 66.2 (5.0) | 59.7 (9.9)⁎⁎ | 63.9 (5.7) | 68.6 (6.4) | 3.56 | < 0.01 | 0.15 |
| Sex (M/F) | 23/21 | 6/8 | 5/3 | 3/7 | 6/5 | 3/6 | 11/23 | χ2 = 5.99 | 0.43 | 0.05 |
| Education | 15.9 (2.4)⁎⁎ | 16.5 (3.1) | 18.1(2.0) | 16.2 (2.9) | 16.1 (2.7) | 15.7 (2.2) | 17.8 (0.4) | 2.8 | < 0.05 | 0.12 |
| CDR | 0.8(0.4)⁎⁎⁎ | 1.2 (0.6)⁎⁎⁎ | 0.5 (0.4)⁎ | 0.7 (0.3)⁎⁎ | 1.2(0.8)⁎⁎⁎ | 0.9 (0.7)⁎⁎ | 0 (0.0) | 19.7 | < 0.001 | 0.51 |
| MMSE | 23.0 (5.4)⁎⁎⁎ | 25.1(3.9)⁎ | 26.4 (3.9) | 27.5 (1.6) | 25.2(3.7)⁎ | 22.2(1.0)⁎⁎⁎ | 29.2(0.7) | 8.13 | < 0.001 | 0.28 |
| GDS† | 6.3(4.9) | 5.6 (7.8) | 5.3(5.6) | 8.5 (5.4) | 7.9(8.9) | 7.7(6.1) | 5 (2.4) | 1.48 | 0.21 | 0.05 |
| Emotion Imitation† | 4(0.2) | 3.1(0.4)⁎ | 3.8(0.5) | 4.3(0.6) | 2.3(0.5)⁎⁎ | 4.4 (0.5) | 4.8 (0.3) | 3.53 | < 0.01 | 0.16 |
| Verbal Command† | 4.5(0.2) | 3.9(0.4) | 3.9(0.5) | 5(0.5) | 3.2(0.4) | 3.9(0.5) | 4.8 (0.3) | 2.66 | 0.05 | 0.14 |
| Total IEET† | 4.6(1.2) | 3.5(1.5) | 4.3(1) | 4.4(1.1) | 3(1.9)⁎⁎ | 4.3(1.1) | 4.5 (0.7) | 3.63 | < 0.01 | 0.17 |
| Spontaneous (NI/I/SI)† | 34/2/8 | 8/0/4 | 6/2/0 | 0/1/8⁎⁎ | 5/0/2 | 6/0/1 | 34/3/0 | χ2 = 58.01 | < 0.001 | 0.40 |
| TASIT-EET† | 9.4 (2.3)⁎⁎ | 7.1 (3.3)⁎⁎⁎ | 10.1 (2.6) | 9.9 (2.6) | 6.4 (2.5)⁎⁎⁎ | 7.8 (1.5)⁎⁎ | 11.4 (1.5) | 10.2 | < 0.001 | 0.33 |
| NEO Warmth† | 30.5 (5.6) | 31.8 (6.7) | 22.0 (8.2) | 32.2 (3.6) | 30.6 (6.9) | 29.5 (6.3) | 31.2 (6.8) | 1.84 | 0.10 | 0.10 |
| IRI - EC† | 27.9 (6.5) | 16.3 (7.1)⁎⁎⁎⁎ | 26.8 (5.0) | 22.0 (6.7)⁎ | 17.1 (4.4)⁎⁎⁎⁎ | 27.1 (6.3) | 28.3 (4.3) | 10.7 | < 0.0001 | 0.38 |
AD = Alzheimer's disease; bvFTD = behavioral variant frontotemporal dementia; nfvPPA = non-fluent variant primary progressive aphasia (PPA); PSP = progressive supranuclear Palsy; rtvFTD = right temporal FTD; svPPA = semantic variant PPA; NC = healthy older controls; CDR = Clinical Dementia Rating; MMSE = Mini-Mental State Examination; GDS = Geriatric Depression Scale; IEET = Intentional Emotional Expression Task; Spontaneous = spontaneous expression; NI = not impaired, I = impaired, SI = severely impaired; TASIT-EET = The Awareness of Social Inference Test – Emotion Evaluation Subtest; NEO = Neuroticism-Extraversion-Openness Inventory: IRI-EC = Interpersonal Reactivity Index – Empathetic Concern subscale; † = Controlled for age, sex and MMSE. Group mean is significantly different from control subjects mean (⁎P < 0.05; ⁎⁎P < 0.01; ⁎⁎⁎P < 0.001; ⁎⁎⁎⁎P < 0.0001) using Dunnett's post hoc t-tests.
Patients were recruited from a dementia specialty clinic and were excluded if they had a Clinical Dementia Rating (CDR) score > 2 or were not fluent in English. Normal controls (NCs) were recruited though newspaper advertisements and from a local senior community center. Inclusion criteria for normal controls included a normal neurological exam, CDR score = 0, Mini-mental state exam score ≥ 28/30, and delayed memory performance ≥ 25th percentile in both verbal and visual-spatial domains.
All participants were required to have an informant who could answer questions about their behavior on their behalf. Informants were typically a relative who lived with the participant, and were required to have known the participant for > 5 years. The participants and their informants signed an institutional review-board-approved research consent form to participate in the study. The research was approved by the University of California, San Francisco Committee for Human Resource Independent Review Board.
2. Methods
2.1. Intentional emotional expression task
Our intentional emotional expression task consisted of two conditions: a verbal command condition followed by a picture imitation condition. In the verbal command condition, participants were verbally prompted to produce six distinct emotional facial expressions (happiness, disgust, fear, anger, sadness, and surprise (e.g., “Now, show me on your face what it looks like to be happy”)). In the picture imitation condition, participants were asked to imitate pictures of six different emotional faces (e.g., “Now make the face/facial expression shown in this picture”). The gender of the model depicted in the pictures was random and the emotion depicted was randomly ordered. The performance was videotaped and later viewed by two independent judges who were blind to patients' diagnoses. Raters categorically coded the accuracy of each emotional facial expression based on an abbreviated version of the Emotion Facial Action Coding System (FACS) (Ekman and Friesen, 1978). Categorical coders rated expression as inaccurate = 0, not sure = 1, or accurate = 2. ‘Not sure’ and inaccurate scores were collapsed to one scores (inaccurate = 0), and accurate was recoded 1. A certified FACS coder also rated videos according to FACS rating system for reliability purposes. Once reliability was achieved between categorical coders and the FACS coder, accuracy scores were summed across trials (range 0–6). Interrater reliability, determined by intraclass Shrout-Fleiss correlation coefficients, was excellent (> 0.75 for all variables). An overall emotion expression score was also derived by averaging the verbal command and imitation sum totals.
2.2. Neuroexam and neuropsychology assessment
To controls for more fundamental deficits in facial motor function, neurologist ratings of orobuccal apraxia and spontaneous facial expressiveness were considered. To assess orobuccal apraxia, patients were asked to imitate 1–2 orobuccal gestures (e.g., “show me how you brush your teeth”). Orobuccal apraxia was scored as either present (1) or absent (0). General spontaneous facial expressiveness was rated on a 4-point scale by the examiner (0 = normal, 4 = severe loss of spontaneous expression).
2.3. Additional socioemotional testing
2.3.1. Face-to-face
Emotion reading was tested using The Awareness of Social Inference Test-Emotion Evaluation Test (TASIT-EET), which consists of 14 brief (~ 20s) video clips of actors depicting dynamic, multimodal expressions of emotion (happiness surprise, anger, sadness, fear, disgust, and neutral) using semantically neutral scripts (McDonald et al., 2003). Once the video ended, the participant selected the perceived emotion from a list of responses displayed on the video screen (range = 0–14).
2.3.2. Caregiver reports:
Interpersonal warmth was assessed using the warmth facet of the NEO-Five Factor Inventory – Extraversion subscale (NEO-PI) (Costa and McCrae, 1992). The warmth facet is an 8-item subscale under the domain of extraversion which captures aspects of friendliness, spontaneous expression, and warmth (e.g., “S/he is known as a warm and friendly person”), and is answered on a 5-point (1 = low; 5 = high) Likert scale (8–40 range).
Empathy was measured using the Interpersonal Reactivity Index (IRI) - empathic concern (EC) subscale (Davis, 1983). The IRI-EC is a 7-item subscale of the IRI that measures the tendency to be emotionally affected and concerned about others in distress (e.g., “seeing someone being take advantage of makes the patient feel protective towards them”). Informants rated how well each statement reflected the current behavior of the study participant on a 5-point scale of (1 = does not describe at all; 5 = describes very well; 7–35 range).
2.4. Voxel-based morphometry
All patients had a structural MRI scan on a 3 T, Magnetom VISION system (Siemens Inc., Iselin, N.J.) equipped with a standard quadrature head coil. A volumetric magnetization prepared rapid gradient echo MRI (MPRAGE, TR/TE/TI = 10/4/300 milliseconds) was used to obtain T1-weighted images of the entire brain, with 15-degree flip angle, coronal orientation perpendicular to the double spin echo sequence, 1.0 × 1.0 mm2 in-plane resolution and 1.5 mm slab. All imaging was done within 3 months of the experimental session at UCSF. Scans were checked for motion artifact and those with excessive movement were not preprocessed for structural VBM analysis.
VBM preprocessing and analysis were performed using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/) and SPM8 software package (www.fil.ion.uc.ac.uk/spm/software/spm8). The images were visually inspected for artifacts, bias-corrected, and tissue classified (gray matter, white matter, cerebrospinal fluid segments). This was followed by spatial normalization of the segmented images to MNI space with a 1.0 mm cubic resolution using affine and nonlinear transformations with the diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL) method, as implemented in the toolbox (Ashburner and Friston, 2005, Ashburner, 2007). The DARTEL method was also used to create a customized template from older healthy controls (n = 300). In all preprocessing steps, default parameters of the VBM8 toolbox were used, with the exception of using the light clean-up procedure in the morphological filtering step. The spatially normalized, segmented, and modulated gray matter images were smoothed with an 8-mm FWHM isotropic Gaussian kernel.
2.5. Statistical analyses
Because extreme general facial expressiveness deficits were rare in our sample (n = 5), clinical observer rating of general facial expressiveness was parameterized from a 4-pt. to a 3-pt. ordinal variable in order to normalize the frequency distribution of scores (scores of 3 and 4 were collapsed). General linear models (GLMs) were carried out in R (3.1.3). Age, gender, education, and disease severity were included as standard confounds in each of the analyses described below. To determine whether intentional emotion expression performance differed by group, GLMs were conducted, with standard Dunnett-Hsu post hoc tests comparing each diagnostic group to the NC group (Table 1).
To determine whether group performance on the intentional emotional expression tasks was predicted by more fundamental motor and socioemotional deficits, GLMs were conducted using motor (i.e., orobuccal apraxia and clinical ratings of general expressiveness) and socioemotional (i.e., depression, emotion reading) scores as predictors, controlling for age and disease severity. Finally, to determine if failure of the intentional emotional tasks predicted empathic concern and warmth ratings by caregivers, expression scores were converted into z-scores using healthy controls as the standardization sample and a cutoff for failure was placed at z = − 1.35 (which corresponds to the threshold for clinical neuropsychological impairment), and t-tests were conducted comparing the pass/fail groups.
2.6. Voxel Based Morphometry (VBM)
To identify structural anatomic correlates of intentional expression scores, covariates-only (multiple regression design) analyses were used correlating intentional emotional expression scores to gray matter atrophy across the entire sample. Age, gender, MMSE, and total intracranial volume (TIV) were entered as covariates into all designs. The resulting statistical parametric (SPM) map was thresholded at voxel-wise P < 0.001, and then corrected for multiple comparisons at P < 0.05 based on cluster extent and a custom-fit error distribution determined by 1000 permutations of the data (Wilson et al., 2010). Resulting SPM T-maps were superimposed on the Montreal Neurological Institute (MNI) single subject brain using automated anatomical labeling (AAL) included in the MRIcron software package (http://www.sph.sc.edu/comd/rorden/mricro.html).
Voxel-based morphometry studies in neurodegenerative disease patients are susceptible to co-atrophy artifacts, wherein regions that are not directly related to a task or behavior are appear as significant in VBM due to disease specific patterns of co-atrophy. For example, a study examining memory task performance in AD patients may correctly identify medial temporal regions but erroneously identify posterior cingulate regions because medial temporal and posterior cingulate regions have similar onset and slope of atrophy in AD. In order to perform an error-check to control for potential co-atrophy effects, we parameterized each level of diagnosis and entered these additional diagnostic group variables into the design matrix as confounding covariates. The results of this analysis identify regions of atrophy as significantly related to intentional emotion scores only if they appear in more than one diagnostic group. However, these results must be considered in light of the main effects results as it will fail to identify any region legitimately related to intentional expression scores but is atrophied only in a single diagnostic group. (please see (Sollberger et al., 2009) and (Rankin et al., 2009) for rationale and additional methodological details). To examine the degree to which the two intentional expression conditions rely on overlapping regions, the two T maps were overlaid using MRIcron (http://www.mccauslandcenter.sc.edu/CRNL/) on an average brain.
3. Results
3.1. Group demographics and behavioral results
Omnibus analyses of covariance using GLMs with an alpha level of < 0.05 revealed significant differences in age, education and disease severity scores across diagnostic groups (see Table 1). Age was not correlated with performance on our tasks (imitation r2 = 0.01, P = 0.15; verbal command total r2 = 0.007, P = 0.17; total r2 = 0.01, P = 0.11), nor was education (r2 = 0.008, P = 0.17; verbal command r2 = 0.04, P = 0.07; total r2 = 0.02, P = 0.09); however, each of these variables was included as a potential confound in later analyses. Orobuccal apraxia was present in three participants in our sample (1 AD, 2 bvFTD), therefore these participants were excluded from further behavioral and imaging analyses (n = 130 final sample). After controlling for age, education, and disease severity, we found that diagnostic groups significantly differed on intentional emotion imitation scores, as well as the overall expression score, though not on the emotional expression from verbal command scores. Post hoc pairwise comparisons using Dunnett-Hsu showed that bvFTDs (P = 0.040) and rtvFTD (P = 0.002) patients were significantly impaired on the imitation task compared to controls, and that rtvFTD (P = 0.001) patients were significantly impaired on the overall emotion expression task compared to controls whereas bvFTDs showed a non-significant trend (P = 0.058; see Table 1).
GLMs revealed that neurologist's rating of patients' general facial expressiveness (imitation – F = 8.24, P < 0.0001, η2 = 0.16; verbal command – F = 31.2, P < 0.0001, η2 = 0.54; total – F = 22.26, P < 0.0001, η2 = 0.42), and emotion reading (imitation – r2 = 0.13, P = 0.003, η2 = 0.11; verbal command – r2 = 0.12, P = 0.005, η2 = 0.08; total – r2 = 0.17, P = 0.0003, η2 = 0.14) abilities predicted emotion expression scores on both tasks.
Comparing participants who failed the imitation task to those who did not, we found that those who failed the task were rated significantly lower in empathic concern (P = 0.049) and warmth (P = 0.02) by caregivers than those who did not; however, we found no difference on ratings of empathic concern or warmth between participants who failed the verbal command condition and those who did not.
3.2. VBM results
Overall deficits in intentional emotional facial expressions (verbal + imitation) corresponded with volume loss in the bilateral central (CO) and frontal opercula (FO), anterior insula (AI), medial orbitofrontal cortex (mOFC), ventral medial prefrontal (vmPFC), and the R > L inferior frontal gyrus (IFG), dorsal anterior cingulate (dACC), precentral gyrus, putamen thalamus, medial temporal lobe, and medial temporal gyrus (all results pFWE < 0.05). Basic volumetric summaries of each diagnostic group are presented in Supplementary Materials.
Inability to accurately generate emotional facial expression elicited from a verbal cue corresponded with volume in the L > R operculum, AI, and IFG (Table 2). Inability to intentionally imitate emotional expression from a photo was associated with predominant right-lateralized volume loss in the CO, FO, AI, IFG, and the vmPFC/mOFC. It was also associated with the right supramarginal gyrus (SMG), putamen, thalamus, precentral gyrus, superior temporal pole, nucleus accumbens, and caudate (all results pFWE < 0.05; Table 3, Fig. 1).
Table 2.
Voxel-based morphometry analyses of deliberate expression of emotion from verbal cue in the whole sample.
| Brain region | Peak MNI Coordinates |
T-value (PFWE < 0.05) | ||
|---|---|---|---|---|
| x | y | z | ||
| L frontal operculum | − 40 | 27 | 1 | 5.38 |
| L anterior insula | − 39 | 4 | 5 | 5.20 |
| L mOFC | − 25 | 22 | − 21 | 5.18 |
| R frontal operculum | 42 | 9 | 5 | 5.12 |
R = right; L = left; mOFC = medial orbitofrontal cortex.
Table 3.
Voxel-based morphometry analyses of deliberate imitation of emotion in the whole sample.
| Brain region | Peak MNI coordinates |
T-value (PFWE < 0.05) | ||
|---|---|---|---|---|
| x | y | z | ||
| R central operculum⁎ | 42 | 4 | 4 | 7.46 |
| R anterior insula⁎ | 42 | 6 | 1 | 7.36 |
| R frontal operculum⁎ | 42 | 9 | 4 | 7.24 |
| R mOFC⁎ | 18 | 27 | − 16 | 6.76 |
| R inferior frontal gyrus⁎ | 33 | 25 | − 6 | 6.60 |
| R supramarginal gyrus⁎ | 61 | − 25 | 25 | 6.26 |
| R parietal operculum | 61 | − 25 | 23 | 6.20 |
| R vmPFC | 9 | 34 | − 22 | 6.01 |
| R putamen | 31 | 4 | 2 | 5.88 |
| R thalamus | 10 | − 11 | 2 | 5.46 |
| R precentral gyrus | 55 | 6 | 16 | 5.43 |
| R superior temporal pole | 55 | 9 | − 3 | 5.40 |
| R nucleus accumbens | 63 | − 7 | 5 | 5.38 |
| R postcentral gyrus | 63 | − 4 | 11 | 5.23 |
| R caudate | 15 | 13 | 4 | 5.17 |
| R supplementary motor area | 9 | 24 | 32 | 5.06 |
| L anterior insula⁎ | − 37 | 9 | 1 | 5.87 |
| L frontal operculum⁎ | − 39 | 9 | 2 | 5.74 |
| L anterior cingulate | − 1 | 42 | − 4 | 5.44 |
| L mOFC | − 24 | 39 | − 18 | 5.30 |
| L mPFC | 58 | 28 | 74 | 5.07 |
MNI = Montreal Neurological Institute.
⁎ / bold text = regions to survive coatrophy error check; R = right; L = left; OFC = orbitofrontal cortex; rmPFC = rostromedial prefrontal cortex; dlPFC = dorsolateral prefrontal cortex; dmPFC = dorsomedial prefrontal cortex.
Fig. 1.
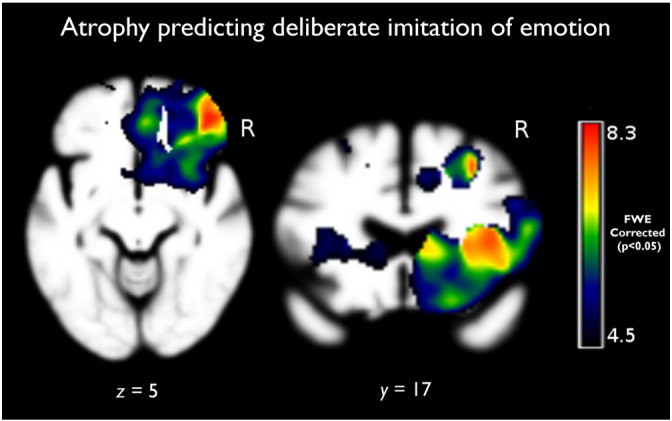
Results of the main effects analysis of the deliberate imitation where atrophy negatively correlated with the ability to deliberately imitate emotion depicted in a photograph, adjusting for age, gender, Mini-Mental State Examination (MMSE), and total intracranial volume (TIV; PFWE < 0.05).
3.2.1. Error check
In a shared effects analysis we controlled for group membership to identify brain-behavior relationships that appear in more than one diagnostic group, providing supportive evidence that they reflect generalizable brain-behavior relationships and ruling out the alternative that they an artifact resulting from correlated atrophy patterns within a single group. We found that the bilateral insula and the R operculum, mOFC/vmPFC, IFG, SMG and FO remained significant after the co-atrophy adjusted analysis of intentional emotion imitation (i.e., photo imitation); no regions survived this correction for the verbal command condition.
4. Discussion
The primary aim of this study was to determine whether a neural network mediating intentional expression of emotion in healthy adults was also reflected in a lesion model with neurodegenerative disease patients. We also investigated whether neurodegenerative diagnostic groups differed according to their ability to intentionally express facial emotion; and whether intentional expression was related to other socioemotional functions. The inability to intentionally imitate emotion was predicted by atrophy in a predominantly right hemisphere fronto-opercular network homologous to the left hemisphere “speech production network” found compromised in patients with apraxic motor speech disorders (Mandelli et al., 2014). The ability to intentionally express emotions from a verbal cue, conversely, was predicted by a set of predominantly left-sided structures that partially overlaps with this speech production network. Together, these results suggest a specific neural basis for clinical deficits in intentional motor facial expression of emotion, suggesting an “emotional apraxia” network neurologically homologous to the speech production network.
Consistent with prior resting state studies of an intentional expression network that has been identified in healthy adults (Braadbaart et al., 2014), which we refer to as the emotional apraxia network, we found that deficits in intentional imitation of emotional expression were predicted by volume loss in cortical regions including the R > L operculum (CO/FO), insula, mOFC, IFG and SMG. This network mirrors the “speech production network” that has been identified as pathogenic in patients with apraxic motor speech disorders (Mandelli et al., 2014), and suggests that intentional motor facial expression of emotion is a very similar process. Broadly, the regions we observed in our patients – the bilateral operculum, insula and the right SMG, right IFG, and mOFC– play important roles in interoception, stimulus-driven attention, facial movements, and cortical modulation during successful task control (Wild et al., 2006, Dosenbach et al., 2007, Craig, 2009, García-Cordero et al., 2016). These are also key regions that comprise the purported “mirror neuron system” (MNS), responsible for the embodied expression of others' goal-directed actions (Iacoboni et al., 2005). However, intentional emotional expression arguably involves more complex, higher-order processes beyond the kind of action-perception mapping that is often cited as an essential emergent property of the MNS.
4.1. Neural representations of intentional emotion imitation
We found that the right SMG played a key role in intentional emotion imitation, which is consistent with findings that show that this region plays a complex role in action-perception mapping, including generating internal models of one's own outward facial expressions. The SMG is a multimodal association area which integrates audio, visual, and somatosensory information, and has been associated with “generating, testing, and correcting internal predictions about external sensory events” (Decety and Lamm, 2007). The left SMG is linked to somatosensory feedback and error correction during speech production, and the right SMG likely plays a similar role in modeling visuospatial-based motor actions. The right SMG, along with the right IFG (also implicated in our task), has been associated with the ability to discriminate one's own face from others’ (Uddin et al., 2005) as well as the ability to maintain a “poker face” (Vanderhasselt et al., 2013). This suggests that a key function of this region may be the online encoding and updating of visuospatial representations, including the internal representation of one's own outward expressive appearance.
Other frontal structures that played an important role in the intentional emotional expression circuit included the right IFG, which mediates inhibitory responding and attentional control (Hampshire et al., 2010); and the mOFC which is involved in encoding and updating predicted value representations of goals (Hare et al., 2010). Also, the AI and FO, which are tightly coactivating regions involved respectively in integrating diverse interoceptive and emotional information (Craig, 2009, García-Cordero et al., 2016), and modulating cortical activity that results in controlled and successful task performance (Dosenbach et al., 2007, Higo et al., 2011). A recent study by García-Cordero et al. (García-Cordero et al., 2016) found that the insula, operculum, and inferior frontal gyrus mediate the ability to accurately track interoceptive information across neurodegenerative patient groups, and in bvFTD specifically, interoceptive accuracy is particularly related to insula damage. In addition to the insula, the FO also has strong connections to the SMG (Higo et al., 2011). When they compared the neural circuitry mediating observation versus imitation of emotional faces, van der Gaag and colleagues (van der Gaag et al., 2007) found that the IFG and SMG (as well as the SMA) were proportionally more active during observation than imitation of emotional faces, whereas the operculum was more strongly activated during imitation. Arguably, the IFG, the vmPFC/mOFC, operculum, and the SMG mediate feed-forward functions in which internal representations guide the ability to accurately attend to, model and control expressions, based on multimodal perceptual feedback mediated by the insula.
Additional regions, including the cerebellum and fusiform face area (FFA), are also activated in healthy adults during intentional emotion imitation (Carr et al., 2003, Hennenlotter et al., 2005, van der Gaag et al., 2007), but were not found in analyses of our patients. This may be because the role these structures play in intentional emotion imitation is more secondary and non-causal. The FFA mediates emotion reading abilities (Sabatinelli et al., 2011), and the same is true of the cerebellum (Herbert et al., 2009, Ferrucci et al., 2012), thus these regions are likely to show strong activations in healthy controls in a task-based functional imaging paradigms involving emotion. However, in studies of autism spectrum disorder as well as in typically developing children, emotion recognition is not a condition of successful emotion imitation (Ham et al., 2011). The ability to accurately identify emotions explained only small proportion of the variance in our participants' imitation scores, while other abilities such as motor control appeared to be more essential to intentional emotion imitation in patients.
4.2. Clinical associations in neurodegenerative disease
We found that the ability to intentionally imitate emotional expressions was most impaired in the neurodegenerative disease patient groups whose cardinal symptoms involve socioemotional deficits, i.e., bvFTD and rtvFTD patients, though no patient group showed abnormally reduced ability to intentionally express emotion on the basis of a verbal command. Patient groups did not differ on the accuracy of intentional emotional expressions elicited from verbal command even though patient groups with known language deficits were included in the analysis. This may be because the stimulus prompts consisted of high frequency words (e.g., “happy”), therefore language deficits did not significantly impact patients' overall performance on this task. However, intentional imitation of emotion may be more challenging for patients due to the diversity of socioemotional abilities involved in the task. Across all patient groups, participants' ability to intentionally imitate emotions was predicted by general facial expressiveness and emotion reading; and patient groups with cardinal deficits in warmth and emotional empathy – rtvFTD and bvFTD patients (Rankin et al., 2006, Rascovsky et al., 2011; Sollberger et al., 2009) – were significantly impaired on the intentional imitation task compared to controls. Consistent with prior literature, PSP patients' spontaneous expressiveness was severely impaired in our sample (Behrman et al., 1969, Romano and Colosimo, 2001, Ahmed et al., 2008), however PSP patients' intentional facial expression was comparable to controls. This finding further provides further support that intentional and spontaneous facial expressions of emotion are distinct social behaviors likely mediated by distinct neural substrates.
We also found that patients who failed the intentional emotional imitation task were rated less empathically concerned and warm by caregivers. Ours is among few studies to examine the relationship between intentional emotional imitation and trait empathy. Williams and colleagues (Williams et al., 2013) found similar results testing healthy adults' ability to accurately imitate complex emotional blends; they found that greater ability to intentionally imitate emotions was related to trait empathy scores. The authors attributed this relationship to variability in mapping action-simulations to motor plans mediated by the mirror neuron system. While this is common view, emotional empathy is a complex, multifactorial construct that involves emotion regulation abilities in addition to the capacity for action-perception mapping (Decety and Michalska, 2010). The fact that we found regions implicated in cognitive control and attention suggests that emotion regulation, not simply action-perception mapping, is involved in intentional emotional imitation. Thus, to better understand the mechanistic relationship between intentional emotional imitation and empathy, careful delineation of empathy-related processes, such as self-other distinction, emotional regulation, and attention should be carefully considered.
5. Limitations
Our small sample sizes among select patient groups with rare neurodegenerative disease were a clear limitation to the study. While larger sample sizes are ideal, our study can at least provide qualitative but valuable clinical information on patient groups with known socioemotional and expressive deficits. Further, diagnostic groups in our study differed somewhat according to age and education. We included these variables as standard confounds in our analyses, and the educational differences across our groups were clinically negligible and were unlikely to impact our results. However, differences in age across the patient groups is a results of divergent age of onset in these syndromes, and future studies may want to control for this factor by limiting their studies to only one or two patient groups. Another limitation is that spontaneous forms of expression were measured indirectly via the neurologists' observation of impairment. Though our findings are consistent with previous studies that found reduced facial expressivity in rtvFTD (Edwards-Lee et al., 1997), future studies may wish to directly compare spontaneous expression evoked under experimental conditions to intentional emotional expression. Additionally, intentional expression was coded categorically to heighten rater reliability, though this approach may have reduced sensitivity to variability among patients, which could be an informative target for future studies.
6. Conclusions
We found that the ability to imitate emotional facial expressions was linked to a right lateralized cortical network homologous to the left-sided speech production network compromised in aphasic patients. This right-sided network may perform an analogous role to the speech network in paralinguistic socioemotional communication, as its regions have also been implicated in attention, error monitoring, and motor control. Intentional emotional expression impairments were predicted by deficits in general expressiveness and emotion reading across neurodegenerative patients, and bvFTD and rtvFTD patients' imitation abilities were significantly impaired compared to controls. Finally, neurodegenerative patients who failed the imitation task were rated less empathic and warm by caregivers. Intentional emotional expression is an important feature of social communication, and these findings may establish mechanistic model for emotional apraxia in neurodegenerative patients.
The following is the supplementary data related to this article.
Volumetric summary of diagnostic groups as a proportion of total intracranial volume
Acknowledgements
This research was supported by grants from NIH (5K23 AG021606, 1R01AG029577, P01AG019724, P50AG023501) and The Larry L. Hillblom Foundation (#2002/2J, 2007/2I) in affiliation with UCSF.
References
- Ahmed Z., Josephs K.A., Gonzalez J., DelleDonne A., Dickson D.W. Clinical and neuropathologic features of progressive supranuclear palsy with severe pallido-nigro-luysial degeneration and axonal dystrophy. Brain J. Neurol. 2008;131(Pt 2):460–472. doi: 10.1093/brain/awm301. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. NeuroImage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Bavelas J.B., Chovil N. The Psychology of Facial Expression. 1997. Faces in dialogue; pp. 334–346. [Google Scholar]
- Behrman S., Carroll J.D., Janota I., Matthews W.B. Progressive supranuclear palsy. Clinico-pathological study of four cases. Brain J. Neurol. 1969;92(3):663–678. doi: 10.1093/brain/92.3.663. [DOI] [PubMed] [Google Scholar]
- Berenbaum H., Rotter A. The relationship between spontaneous facial expressions of emotion and voluntary control of facial muscles. J. Nonverbal Behav. 1992;16(3):179–190. [Google Scholar]
- Borod J.C. Interhemispheric and intrahemispheric control of emotion: a focus on unilateral brain damage. J. Consult. Clin. Psychol. 1992;60(392317414):339–348. doi: 10.1037//0022-006x.60.3.339. [DOI] [PubMed] [Google Scholar]
- Braadbaart L., De Grauw H., Perrett D., Waiter G.D., Williams J. The shared neural basis of empathy and facial imitation accuracy. NeuroImage. 2014;84:367–375. doi: 10.1016/j.neuroimage.2013.08.061. [DOI] [PubMed] [Google Scholar]
- Carr L., Iacoboni M., Dubeau M., Mazziotta J.C., Lenzi G.L. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc. Natl. Acad. Sci. U. S. A. 2003;100(9):5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa P.T.J., McCrae R.R. 1992. The NEO-PI-R Professional Manual. [Google Scholar]
- Craig A.D. How do you feel – now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Davis M.H. Measuring individual differences in empathy: evidence for a multidimensional approach. J. Pers. Soc. Psychol. 1983;44:113–126. [Google Scholar]
- Decety J., Michalska K.J. Neurodevelopmental changes in the circuits underlying empathy and sympathy from childhood to adulthood. Dev. Sci. 2010;13(6):886–899. doi: 10.1111/j.1467-7687.2009.00940.x. [DOI] [PubMed] [Google Scholar]
- Decety J., Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. The neuroscientist: a review journal bringing neurobiology. Neurol. Psychiatr. 2007;13(6):580–593. doi: 10.1177/1073858407304654. [DOI] [PubMed] [Google Scholar]
- Dosenbach N.U., Fair D.A., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.A., Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E., Schlaggar B.L., Petersen S.E. Distinct brain networks for adaptive and stable task control in humans. Proc. Natl. Acad. Sci. U. S. A. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P., Friesen W.V. 1978. The Facial Affect Coding System. [Google Scholar]
- Edwards-Lee T., Miller B.L., Benson D.F., Cummings J.L., Russell G.L., Boone K., Mena I. The temporal variant of frontotemporal dementia. Brain. 1997;120:1027–1140. doi: 10.1093/brain/120.6.1027. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque D., Hodges S.D., Baird J.A., Black S.E. Empathy in frontotemporal dementia and Alzheimer's disease. J. Clin. Exp. Neuropsychol. 2010;32(3):289–298. doi: 10.1080/13803390903002191. [DOI] [PubMed] [Google Scholar]
- Ferrucci R., Giannicola G., Rosa M., Fumagalli M., Boggio P.S., Hallett M., Zago S., Priori A. Cerebellum and processing of negative facial emotions: cerebellar transcranial DC stimulation specifically enhances the emotional recognition of facial anger and sadness. Cognit. Emot. 2012;26(5):786–799. doi: 10.1080/02699931.2011.619520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Cordero I., Sedeño L., de la Fuente L., Slachevsky A., Forno G., Klein F., Lillo P., Ferrari J., Rodriguez C., Bustin J., Torralva T., Baez S., Yoris A., Esteves S., Melloni M., Salamone P., Huepe D., Manes F., García A.M., Ibañez A. Feeling, learning from and being aware of inner states: interoceptive dimensions in neurodegeneration and stroke. Philos. Trans. R. Soc. B. 2016;371(1708) doi: 10.1098/rstb.2016.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Hillis A.E., Weintraub S., Kertesz A., Mendez M., Cappa S.F., Ogar J.M., Rohrer J.D., Black S., Boeve B.F., Manes F., Dronkers N.F., Vandenberghe R., Rascovsky K., Patterson K., Miller B.L., Knopman D.S., Hodges J.R., Mesulam M.M., Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham H.S., Bartolo A., Corley M., Rajendran G., Szabo A., Swanson S. Exploring the relationship between gestural recognition and imitation: evidence of dyspraxia in autism spectrum disorders. J. Autism Dev. Disord. 2011;41(1):1–12. doi: 10.1007/s10803-010-1011-1. [DOI] [PubMed] [Google Scholar]
- Hampshire A., Chamberlain S.R., Monti M.M., Duncan J., Owen A.M. The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage. 2010;50(3):1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Camerer C.F., Knoepfle D.T., Rangel A. Value computations in ventral medial prefrontal cortex during charitable decision making incorporate input from regions involved in social cognition. J. Neurosci. 2010;30(2):583–590. doi: 10.1523/JNEUROSCI.4089-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennenlotter A., Schroeder U., Erhard P., Castrop F., Haslinger B., Stoecker D., Lange K.W., Ceballos-Baumann A.O. A common neural basis for receptive and expressive communication of pleasant facial affect. NeuroImage. 2005;26(2):581–591. doi: 10.1016/j.neuroimage.2005.01.057. [DOI] [PubMed] [Google Scholar]
- Herbert C., Ethofer T., Anders S., Junghofer M., Wildgruber D., Grodd W., Kissler J. Amygdala activation during reading of emotional adjectives–an advantage for pleasant content. Soc. Cogn. Affect. Neurosci. 2009;4(1):35–49. doi: 10.1093/scan/nsn027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo T., Mars R.B., Boorman E.D., Buch E.R., Rushworth M.F. Distributed and causal influence of frontal operculum in task control. Proc. Natl. Acad. Sci. U. S. A. 2011;108(10):4230–4235. doi: 10.1073/pnas.1013361108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf H.C., WM M.D., Hopf N.J. Localization of emotional and volitional facial paresis. Neurology. 1992;42(10):1918. doi: 10.1212/wnl.42.10.1918. [DOI] [PubMed] [Google Scholar]
- Iacoboni M., Molnar-Szakacs I., Gallese V., Buccino G., Mazziotta J.C., Rizzolatti G. Grasping the intentions of others with one's own mirror neuron system. PLoS Biol. 2005;3(3) doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish M., Hodges J.R., Piguet O. Right anterior temporal lobe dysfunction underlies theory of mind impairments in semantic dementia. Brain J. Neurol. 2014;137(Pt 4):1241–1253. doi: 10.1093/brain/awu003. [DOI] [PubMed] [Google Scholar]
- Josephs K.A., Whitwell J.L., Knopman D.S., Boeve B.F., Vemuri P., Senjem M.L., Parisi J.E., Ivnik R.J., Dickson D.W., Petersen R.C., Jack C.R., Jr. Two distinct subtypes of right temporal variant frontotemporal dementia. Neurology. 2009;73(18):1443–1450. doi: 10.1212/WNL.0b013e3181bf9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamminga J., Kumfor F., Burrell J.R., Piguet O., Hodges J.R., Irish M. Differentiating between right-lateralised semantic dementia and behavioural-variant frontotemporal dementia: an examination of clinical characteristics and emotion processing. J. Neurol. Neurosurg. Psychiatry. 2015;86(10):1082–1088. doi: 10.1136/jnnp-2014-309120. [DOI] [PubMed] [Google Scholar]
- Kumfor F., Piguet O. Disturbance of emotion processing in frontotemporal dementia: a synthesis of cognitive and neuroimaging findings. Neuropsychol. Rev. 2012;22(3):280–297. doi: 10.1007/s11065-012-9201-6. [DOI] [PubMed] [Google Scholar]
- Lee T.W., Josephs O., Dolan R.J., Critchley H.D. Imitating expressions: emotion-specific neural substrates in facial mimicry. Soc. Cogn. Affect. Neurosci. 2006;1(2):122–135. doi: 10.1093/scan/nsl012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelli M.L., Caverzasi E., Binney R.J., Henry M.L., Lobach I., Block N., Amirbekian B., Dronkers N., Miller B.L., Henry R.G., Gorno-Tempini M.L. Frontal white matter tracts sustaining speech production in primary progressive aphasia. J. Neurosci. 2014;34(29):9754–9767. doi: 10.1523/JNEUROSCI.3464-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto D., Lee M. Consciousness, volition, and the neuropsychology of facial expressions of emotion. Conscious. Cogn. 1993;2(3):237–254. [Google Scholar]
- McDonald S., Flanagan S., Rollins J., Kinch J. TASIT: a new clinical tool for assessing social perception after traumatic brain injury. J. Head Trauma Rehabil. 2003;18(3):219–238. doi: 10.1097/00001199-200305000-00001. [DOI] [PubMed] [Google Scholar]
- McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Kawas C.H., Klunk W.E., Koroshetz W.J., Manly J.J., Mayeux R. The diagnosis of dementia due to Alzheimer's disease: recommendations from the national institute on aging-Alzheimer's association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedenthal P.M., Brauer M. Social functionality of human emotion. Annu. Rev. Psychol. 2012;63(1):259–285. doi: 10.1146/annurev.psych.121208.131605. [DOI] [PubMed] [Google Scholar]
- Nair D.G., Purcott K.L., Fuchs A., Steinberg F., Kelso J.A.S. Cortical and cerebellar activity of the human brain during imagined and executed unimanual and bimanual action sequences: a functional MRI study. Cogn. Brain Res. 2003;15(3):250–260. doi: 10.1016/s0926-6410(02)00197-0. [DOI] [PubMed] [Google Scholar]
- O'Doherty J.P. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr. Opin. Neurobiol. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Rankin K.P., Salazar A.M., Gorno-Tempini M., Pavlic D., Stanley C.M., Glenn S., Weiner M., Miller B.L. Detecting sarcasm from paralinguistic cues: anatomic and cognitive correlates in neurdegenerative disease. NeuroImage. 2009;47(4):2005–2015. doi: 10.1016/j.neuroimage.2009.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin K.P., Gorno-Tempini M., Allison S.C., Stanley C.M., Glenn S., Weiner M.W., Miller B.L. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129:2945–2956. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J., van Swieten J.C., Seelaar H., Dopper E.G., Onyike C.U., Hillis A.E., Josephs K.A., Boeve B.F., Kertesz A., Seeley W.W., Rankin K.P., Johnson J.K., Gorno-Tempini M.L., Rosen H., Prioleau-Latham C.E., Lee A., Kipps C.M., Lillo P., Piguet O., Rohrer J.D., Rossor M.N., Warren J.D., Fox N.C., Galasko D., Salmon D.P., Black S.E., Mesulam M., Weintraub S., Dickerson B.C., Diehl-Schmid J., Pasquier F., Deramecourt V., Lebert F., Pijnenburg Y., Chow T.W., Manes F., Grafman J., Cappa S.F., Freedman M., Grossman M., Miller B.L. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain J. Neurol. 2011;134(Pt 9):2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn W.E. The neuropsychology of facial expression: a review of the neurological and psychological mechanisms for producing facial expressions. Psychol. Bull. 1984;95(1):52. [PubMed] [Google Scholar]
- Rinn W.E. Emotional facial expression in parkinson's disease: a response to bowers (2006) J. Int. Neuropsychol. Soc. 2007;13(04):721–722. doi: 10.1017/S1355617707070944. [DOI] [PubMed] [Google Scholar]
- Rosen H.J., Perry R.J., Murphy J., Kramer J.H., Mychack P., Schuff N., Weiner M., Levenson R.L., Miller B.L. Emotion comprehension in the temporal variant of frontotemporal dementia. Brain. 2002;125:2286–2295. doi: 10.1093/brain/awf225. [DOI] [PubMed] [Google Scholar]
- Romano S., Colosimo C. Procerus sign in progressive supranuclear palsy. Neurology. 2001;57(10):1928. doi: 10.1212/wnl.57.10.1928. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D., Fortune E.E., Li Q., Siddiqui A., Krafft C., Oliver W.T., Beck S., Jeffries J. Emotional perception: meta-analyses of face and natural scene processing. NeuroImage. 2011;54(3):2524–2533. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Bauer A.M., Miller B.L., Gorno-Tempini M., Kramer J.H., Weiner M., Rosen H.J. The natural history of temporal variant frontotemporal dementia. Neurology. 2005;64(8):1384–1390. doi: 10.1212/01.WNL.0000158425.46019.5C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollberger M., Stanley C.M., Wilson S.M., Gyurak A., Beckman V., Growdon M., Jang J., Weiner M.W., Miller B.L., Rankin K.P. Neural basis of interpersonal traits in neurodegenerative diseases. Neuropsychologia. 2009;47(13):2812–2827. doi: 10.1016/j.neuropsychologia.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm V.E., Sollberger M., Seeley W.W., Rankin K.P., Ascher E.A., Rosen H.J., Miller B.L., Levenson R.W. Role of right pregenual anterior cingulate cortex in self-conscious emotional reactivity. Soc. Cogn. Affect. Neurosci. 2013;8(4):468–474. doi: 10.1093/scan/nss023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trémeau F., Malaspina D., Duval F., Corrêa H., Hager-Budny M., Coin-Bariou L., Macher J., Gorman J.M. Facial expressiveness in patients with schizophrenia compared to depressed patients and nonpatient comparison subjects. Am. J. Psychiatr. 2014 doi: 10.1176/appi.ajp.162.1.92. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q., Kaplan J.T., Molnar-Szakacs I., Zaidel E., Iacoboni M. Self-face recognition activates a frontoparietal “mirror” network in the right hemisphere: an event-related fMRI study. NeuroImage. 2005;25(3):926–935. doi: 10.1016/j.neuroimage.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Vanderhasselt M.A., Kuhn S., De Raedt R. 'Put on your poker face': neural systems supporting the anticipation for expressive suppression and cognitive reappraisal. Soc. Cogn. Affect. Neurosci. 2013;8(8):903–910. doi: 10.1093/scan/nss090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Gaag C., Minderaa R.B., Keysers C. Facial expressions: what the mirror neuron system can and cannot tell us. Soc. Neurosci. 2007;2(3–4):179–222. doi: 10.1080/17470910701376878. [DOI] [PubMed] [Google Scholar]
- Williams J.H., Nicolson A.T., Clephan K.J., de Grauw H., Perrett D.I. 2013. A Novel Method Testing the Ability to Imitate Composite Emotional Expressions Reveals an Association with Empathy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S.M., Henry M.L., Besbris M., Ogar J.M., Dronkers N.F., Jarrold W., Miller B.L., Gorno-Tempini M.L. Connected speech production in three variants of primary progressive aphasia. Brain J. Neurol. 2010;133(Pt 7):2069–2088. doi: 10.1093/brain/awq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild B., Rodden F.A., Rapp A., Erb M., Grodd W., Ruch W. Humor and smiling: cortical regions selective for cognitive, affective, and volitional components. Neurology. 2006;66(6):887–893. doi: 10.1212/01.wnl.0000203123.68747.02. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Volumetric summary of diagnostic groups as a proportion of total intracranial volume


