Abstract
Objective: To examine the effectiveness of intradermal botulinum toxin type A injection in improving skin texture and midface lift while reducing pore size and sebum production, as well as investigate the differences in effectiveness between onabotulinumtoxinA and abobotulinumtoxinA using intradermal and intramuscular injection methods. Design: A 16-week, single-blind, split-face, randomized study. Each patient served as their own control, receiving onabotulinumtoxinA and abobotulinumtoxinA randomized to either the left or right side of the face. Patients received intradermal botulinum toxin type A injections at Week 0 and intramuscular botulinum toxin type A injections at Week 2. Participants: Ten women aged 35 to 65 years who exhibited static rhytids in the glabellar and periorbital area. Measurements: The primary endpoint was efficacy of split-face treatment of intradermal and intramuscular onabotulinumtoxinA and abobotulinumtoxinA as assessed by a blinded evaluator using baseline and post-treatment photographs. The secondary endpoints included safety as assessed by adverse events and patient satisfaction measured by questionnaires completed at baseline and post-treatment. Results: Intradermal injection of botulinum toxin type A led to a statistically significant improvement in skin texture (p=0.004) while also resulting in mild midface lift (p=0.024), but did not provide a significant reduction of pore size and sebum production. There was no statistically significant difference between onabotulinumtoxinA and abobotulinumtoxinA when injected intradermally or intramuscularly. Conclusion: Intradermal injection of botulinum toxin type A appears to be a safe and effective therapy that provides an improvement in facial skin texture and midface lift. Registry: clinicaltrials.gov (ID#: NCT02907268).
BOTULINUM TOXIN TYPE A (BTXA) injections have been the leading nonsurgical cosmetic procedure since the year 2000.1 The American Society for Aesthetic Plastic Surgery reported over 4.2 million BTXA procedures performed in 2015, with an 18.9-percent increase from 2014.1 BTXA is noted for its ability to target the physical effects of aging. With age and the associated decrease in skin elasticity, repeated muscle contractions cause hyperfuctional facial lines, particularly in the glabellar and periorbital regions.2–3 In the midface region, biological changes of facial muscles and gravity cause tear trough formation, a deepening of the nasolabial fold, and the depressor muscles to become more pronounced than the levators, resulting in drooping in the midcheek groove.4 Facial lines have an impact on an individual’s self-esteem, perception of attractiveness, social interactions, and body image.5 Dissatisfaction with the external effects of aging can be treated by BTXA injections to relax facial muscles and inhibit hyperfunctional facial lines. This procedure results in a younger looking face, thus decreasing the physical, psychological, and social effects of facial aging.5–9
The label-indicated method of administration of BTXA involves intramuscular injection. However, medical professionals have begun investigating the off-label intradermal administration. Intradermal injection of BTXA is becoming an increasingly popular technique used for facial rejuvenation, focused on correcting the imbalanced downward pull of the depressors in the midface.4 Intradermal injections allow for a relaxation of the depressors, the platysma, and lateral fibers of the orbicularis oculi.4 Injections specifically to the platysma and lateral fibers of the orbicularis oculi also increase the lift provided by the levators.4 Together, these effects of intradermal injections have shown to result in midface lift.4 Intradermal BTXA injections have also shown to result in a statistically significant improvement of wrinkles, significantly lower sebum production, a good level of patient satisfaction, and patient-reported improvement of skin oiliness and facial pores.10–13 Increased collagen production has been noted, which may contribute to anti-aging effects seen and serve as a possible advantage of BTXA intradermal injection.12 Given the novelty of the off-label intradermal injection of BTXA, research on the effectiveness of this treatment is limited. Although studies have noted the possible effects and advantages of this administration, the results have been inconclusive to date.
There are currently three United States Food and Drug Administration (FDA)-approved BTXA products: onabotulinumtoxinA (Botox®, Allergan, Inc, Irvine, California), abobotulinumtoxinA (Dysport®, Ipsen Biopharm Ltd., Wrexham, United Kingdom), and incobotulinumtoxinA (Xeomin®, Merz Pharmaceuticals, Frankfurt, Germany). OnabotulinumtoxinA was approved by the FDA in 2002, abobotulinumtoxinA was approved by the FDA in 2009, and incobotulinumtoxinA was approved by the FDA in 2010. This study will examine onabotulinumtoxinA and abobotulinumtoxinA. Studies of onabotulinumtoxinA and abobotulinumtoxinA revealed a statistically significant improvement in glabellar, crow’s feet, and forehead lines in the treatment group compared to the placebo group based on both investigator and patient assessments.14–24 OnabotulinumtoxinA and abobotulinumtoxinA are well-tolerated with low rates of adverse events.14,15,17–20,22–23,25–26 There have been no reported long-term adverse events or safety issues of either product.14,22–23,26 The median duration of effect of both treatments has been shown to be 3 to 4 months as evaluated by investigators, patients, and blinded assessors.22,23,27–29 Results comparing the effects of onabotulinumtoxinA and abobotulinumtoxinA administered intramuscularly are inconclusive and there is no research comparing the toxins administered intradermally. Studies have found that there is not a statistically significant difference between abobotulinumtoxinA and onabotulinumtoxinA in the same patients acting as their own control in terms of duration of action, efficacy, and safety.30 In contrast, other studies have shown that abobotulinumtoxinA displayed a greater improvement, earlier onset, and longer duration of improvement in a higher percentage of individuals when compared with onabotulinumtoxinA.31–35
Our study aims to examine the effectiveness of intradermal injections of onabotulinumtoxinA and abobotulinumtoxinA in improving midface lift, reducing pore size, decreasing sebum production, and improving skin texture. The secondary objective is to determine if there is a detectable difference between onabotulinumtoxinA and abobotulinumtoxinA when injected either intradermally or intramuscularly, as well as examine the safety and treatment-related patient satisfaction of the injections.
MATERIALS AND METHODS
Ten female participants aged 35 to 65 years who exhibited static rhytids in the glabellar and periorbital areas were enrolled in this study. Participants were asked to maintain the same skin care regimen throughout the study and four weeks prior to baseline, as well as adhere to study procedures while attending all sessions within the timeline of the study. All participants were asked to sign the informed consent form prior to performing any study procedures. Participants of childbearing potential were asked to use an effective form of contraception, and those who were currently pregnant or breastfeeding were excluded from the study. Additional exclusion criteria included sensitivity to BTXA, a known allergy to cow’s milk protein, active use of anti-aging products containing retinol, and any treatment in one year prior to baseline, including BTXA injections in the face or neck, facial soft tissue filler, laser, ultrasound technology and/or radio frequency on the face or neck, and treatment with isotretinoin or oral acne medications. The study protocol followed the ethical guidelines of the Declaration of Helsinki and was approved by an independent human research review committee.
The 10 enrolled participants were randomized into two possible treatment groups. The first treatment group received onabotulinumtoxinA on the right side of their face and abobotulinumtoxinA on the left side of their face. The second treatment group received abobotulinumtoxinA on the right side of their face and onabotulinumtoxinA on the left side of their face. The patient was blinded as to which side received what injection. The schedule of assessments and procedures for the study can be found in Table 1.
Table 1.
Schedule of assessment and procedures for the duration of the study
| ASSESSMENT/PROCEDURE | SCREENING | WEEK 0 | WEEK 2 | WEEK 4 | WEEK 16 |
|---|---|---|---|---|---|
| Informed consent | X | ||||
| Eligibility | X | X | |||
| Medical history | X | ||||
| Patient questionnaire | X | X | X | X | |
| Adverse events | X | X | X | X | |
| Digital camera photos (3 angles) | X | X | X | X | |
| Visia photos | X | X | X | X | |
| Vectra 3D photos | X | X | X | X | |
| Sebutape patches | X | X | |||
| Randomization | X | ||||
| Botox/Dysport ID | X | ||||
| Botox/Dysport IM | X | ||||
| Blinded Evaluator Assessments | X | X | X |
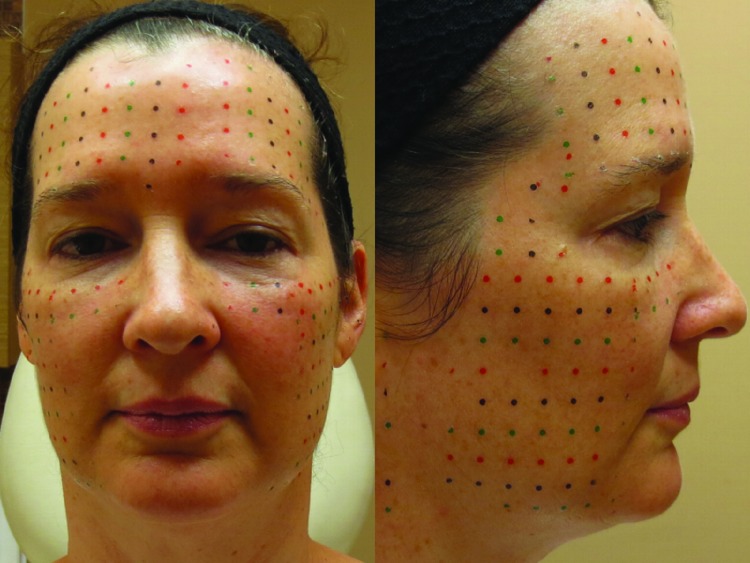
Baseline treatment at Week 0 included the intradermal injection of onabotulinumtoxinA on one side of the face and abobotulinumtoxinA on the other, determined by a premade randomization scheme, in a regular 1 cm2 grid across the cheeks and forehead (Figure 1). Patients were treated at Week 2 with traditional intramuscular injections consisting of onabotulinumtoxinA on the same side of their face as at Week 0 and abobotulinumtoxinA on the other side. Each patient was treated in the glabellar region with the same product used on the right side of their face for consistency, as separating the small procerus muscular area into left and right could not be assessed effectively.
Figure 1.
Typical markings at 1cm2 for intradermal administration
For intradermal injection, 100 units of onabotulinumtoxinA was reconstituted with 5cc of saline and 300 units of abobotulinumtoxinA was reconstituted with 6cc of saline. For intramuscular injection, 100 units of onabotulinumtoxinA was reconstituted with 1cc of saline and 300 units of abobotulinumtoxinA was reconstituted with 1.2cc of saline. The average overall ratio of onabotulinumtoxinA to abobotulinumtoxinA injected was 1:2.5. Patients were treated based on individual need, and thus the treatment volumes were not controlled or standardized. The average number of units of onabotulinumtoxinA and abobotulinumtoxinA administered intradermally and intramuscularly is shown in Table 2.
Table 2.
Average number of units administered to each patient
| onabotulinumtoxinA units(u) in one half of face | abobotulinumtoxinA units (u) in other half of face | |
|---|---|---|
| Average per patient at Week 0 (intradermal) | 50 | 125 |
| Average per patient at Week 2 | 26.5 | 64.5 |
| Total Average per patient | 76.5 | 189.5 |
Photographs were taken using Visia Complexion Analysis System (Canfield Scientific, Inc, Parsippany, New Jersey), Vectra® 3D (Canfield Scientific, Inc, Parsippany, New Jersey), and a handheld digital camera. Photos were taken at Weeks 0, 2, 4, and 16. All patients consented to the reproduction of recognizable photographs. A blinded evaluator assessed the right and left side of the participants’ faces using baseline and post-treatment photographs to evaluate changes in the following categories: severity of wrinkles, pore size, skin texture, skin tightness, degree of lift or droop, and sebum production. Changes in sebum levels were measured by image analysis of Sebutape (CuDerm, Dallas, Texas) patches on the right and left forehead prior to treatment at the baseline visit and at Week 2.
Patient satisfaction was measured using a self-reported satisfaction questionnaire completed at baseline and compared to patient questionnaire results post-treatment at Weeks 2, 4, and 16. The questionnaire was used to assess overall wrinkle severity and facial lift. Patients also self-assessed the right and left side of their face separately for wrinkle satisfaction, oil production on the forehead and cheek, pore size satisfaction, texture satisfaction, and skin tightness satisfaction.
Statistical Package for the Social Science (SPSS) was used for the statistical analysis of blinded evaluator analyses, self-reported patient assessments, and Sebutape patch assessments. The significance level was set at p<0.05. The Wilcoxon signed rank test was used for all analyses except for those in the glabellar region, in which case the Mann-Whitney U test was used to account for that area being treated with only one of the products.
RESULTS
Ten women participated in the study, with ages ranging from 44 to 60 years (M=51.1 years). All adverse events reported for both products and injection techniques were mild, and those related to study treatment included injection site reactions or drooping, which resolved over time. Participants were assessed based on the following criteria outlined in Figure 2: wrinkles (A), wrinkles (C), wrinkles (D), wrinkles (E), wrinkles (Glabella), skin texture, skin tightness, lift, sebum production, and pore size in both the forehead and cheek area.
Figure 2.
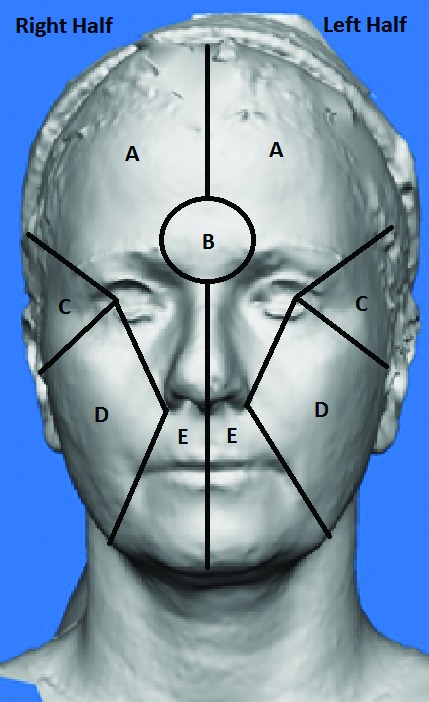
Facial regions for the assessment of wrinkles
The overall results of the blinded assessments are presented in Table 3. The overall effect of treatment was assessed by averaging the scores of both sides of the patients’ faces. Assessments completed at Weeks 2, 4, and 16 were compared to baseline assessments at Week 0. Week 2 presents the assessment results two weeks after intradermal injection, Week 4 presents the assessment results two week after intramuscular injection, and Week 16 presents the assessment 14 weeks after the last injection.
Table 3.
Blinded assessment results overall
| Week | Variable | M | SD | p |
|---|---|---|---|---|
| 2 | Wrinkles (A) | -0.45 | 0.49721 | .034* |
| Wrinkles (C) | -0.3 | 0.63246 | 0.102 | |
| Wrinkles (D) | 0.317 | 0.21082 | 0.157 | |
| Wrinkles (E) | -0.1 | 0.31623 | 0.317 | |
| Wrinkles (Glabella) | -0.1 | 0.31623 | 0.317 | |
| Skin Texture | 3.95 | 0.36893 | 0.004* | |
| Skin Tightness | 3.1 | 0.31623 | 0.317 | |
| Lift | 3.35 | 0.66875 | 0.024* | |
| Sebum Production | 1 | 0 | 1 | |
| Pore Size: Forehead | 1.1 | 0.31623 | 0.317 | |
| Pore Size: Cheek | 1.1 | 0.31623 | 0.317 | |
| 4 | Wrinkles (A) | -0.55 | 0.4378 | 0.015* |
| Wrinkles (C) | -0.5 | 0.66667 | 0.041* | |
| Wrinkles (D) | -0.05 | 0.15811 | 0.317 | |
| Wrinkles (E) | -0.1 | 0.31623 | 0.317 | |
| Wrinkles (Glabella) | -0.6 | 0.5164 | 0.014* | |
| Skin Texture | 4.05 | 0.15811 | 0.002* | |
| Skin Tightness | 3.1 | 0.21082 | 0.157 | |
| Lift | 4.05 | 0.79757 | 1 | |
| Sebum Production | 1 | 0 | 1 | |
| Pore Size: Forehead | 0.9 | 0.31623 | 0.317 | |
| Pore Size: Cheek | 0.9 | 0.31623 | 0.317 | |
| 16 | Wrinkles (A) | -0.6667 | 0.43301 | 0.014* |
| Wrinkles (C) | -0.4444 | 0.76830 | 0.102 | |
| Wrinkles (D) | -0.0556 | 0.16667 | 0.317 | |
| Wrinkles (E) | -0.1111 | 0.33333 | 0.317 | |
| Wrinkles (Glabella) | -.6667 | .50000 | 0.014* | |
| Skin Texture | 3.7778 | .71200 | 0.026* | |
| Skin Tightness | 3.1667 | 0.35355 | 0.180 | |
| Lift | 4.1667 | 1.06066 | 0.726 | |
| Sebum Production | 1.0000 | 0.00000 | 1.000 | |
| Pore Size: Forehead | 0.8889 | 0.33333 | .317 | |
| Pore Size: Cheek | 0.8889 | 0.33333 | 0.317 |
Notes: (*) significant at the 0.05 level. Data from one of the 10 patients was removed at Week 16. All variables are measured in terms of change from Week 0. All scores were computed by averaging across right and left side of patients’ faces. For all variables, p corresponds to the p value of the Wilcoxon signed rank test comparing the median of each variable with the “no change” score. The “no change” scores are: 0 for wrinkles-related variables, 3 for Texture and Tightness, 4 for Lift, and 1 for Sebum Production and Pore Size. M: median; SD: standard deviation
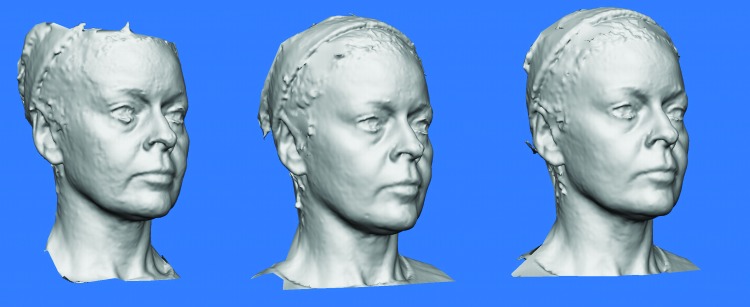
As seen in Table 3, Week-2 assessment of the intradermal injection of BTXA showed a statistically significant improvement from baseline in overall skin texture (p=0.004), mild lift (p=0.024), and improvement in wrinkles of the forehead (Wrinkles A) (p=0.034). At Week 4, the intramuscular injection of BTXA led to a statistically significant improvement from baseline of wrinkles of the forehead (Wrinkles A) (p=0.015), around the eyes (Wrinkles C) (p=0.041), and the glabella (Wrinkles Glabella) (p=0.014), as well as skin texture (p=0.002). Week 16 analysis displayed similar results with statistically significant improvements from baseline in wrinkles on the forehead (Winkles A) (p=0.014), in the glabellar region (Wrinkles Glabella) (p=0.014), and skin texture (p=0.026). No significant changes were observed for sebum production or pore size (either on forehead or on cheek) at Weeks 2, 4, or 16 compared to Week 0. The significant improvement in skin texture can be seen from the grey-scale 3D Vectra photography, as shown in Figures 3, 4, 5, and 6. The texture improvement was seen in 100 percent of patients at two weeks post-intradermal injection.
Figure 3.
Grey-scale 3D Vectra Photographic Evidence from Patient 04. A) Vectra 3D photo of patient 04 at baseline; B) Vectra 3D Photos of patient 04 at Week 2 (post-intradermal). Improved skin texture. Slight decrease in nasolabial folds, marionette lines, and jowls. Increased distance between lateral canthus and inferior border of lateral brow; C) Vectra 3D Photos of patient 04 at Week 4 (post-intramuscular). Added improvement in glabellar and periorbital lines.
Figure 4.
Grey-scale 3D Vectra Photographic Evidence from Patient 05. A) Vectra 3D photo of patient 05 at baseline; Vectra 3D Photos of patient 05 at Week 2 (post-intradermal). Improved skin texture. Slight decrease in nasolabial folds, marionette lines. Increased distance between lateral canthus and inferior border of lateral brow; C) Vectra 3D Photos of patient 05 at Week 4 (post-intramuscular). Added improvement of glabellar and periorbital lines.
Figure 5.
Grey-scale 3D Vectra Photographic Evidence from Patient 06. A) Vectra 3D photo of patient 06 at baseline; B) Vectra 3D Photos of patient 06 at Week 2 (post-intradermal). Improved skin texture. Slight decrease in nasolabial folds, marionette lines. C) Vectra 3D Photos of patient 06 at Week 4 (post-intramuscular). Added improvement in glabellar and periorbital lines.
Figure 6.
Grey-scale 3D Vectra Photographic Evidence from Patient 08. A) Vectra 3D photo of patient 08 at baseline; B) Vectra 3D Photos of patient 08 at Week 2 (post-intradermal). Improved skin texture. Slight decrease in nasolabial folds, marionette lines, and jowls. Increased distance between lateral canthus and inferior border of lateral brow; C) Vectra 3D Photos of patient 08 at Week 4 (post-intramuscular). Added improvement in glabellar and periorbital lines.
Additionally, a comparison was conducted to assess differences in effectiveness between onabotulinumtoxinA and abobotulinumtoxinA based on blinded assessments (Table 4). Results showed that there were no significant differences in any of the measurements when comparing onabotulinumtoxinA with abobotulinumtoxinA with p values ranging from 0.083 through 1.000. Based on blinded assessment results, 80 percent of the patients displayed equal improvement overall at Week 2 from baseline and 90 percent improved equally at Week 4 from baseline.
Table 4:
Blinded assessment results by treatment
| Week | Variable | Treatment | ||||
|---|---|---|---|---|---|---|
| Botox (n=5) | Dysport (n=5) | |||||
| M | SD | M | SD | p | ||
| 2 | Wrinkles (A) | -0.50 | 0.53 | -0.40 | 0.52 | 0.317 |
| Wrinkles (C) | -1.00 | 0.45 | 0.00 | 0.00 | 0.317 | |
| Wrinkles (D) | -0.30 | 0.67 | -0.30 | 0.67 | 1.000 | |
| Wrinkles (E) | -0.10 | 0.32 | -0.10 | 0.32 | 1.000 | |
| Wrinkles (Glabella) | -0.10 | 0.32 | -0.10 | 0.32 | 1.000 | |
| Skin Texture | 4.00 | 0.47 | 3.90 | 0.32 | 0.317 | |
| Skin Tightness | 3.20 | 0.63 | 3.00 | 0.00 | 0.317 | |
| Lift | 3.35 | 0.67 | 3.40 | 0.70 | 0.317 | |
| Sebum Production | 1.00 | 0.00 | 1.00 | 0.00 | 1.000 | |
| Pore Size: Forehead | 1.10 | 0.32 | 1.10 | 0.32 | 1.000 | |
| Pore Size: Cheek | 1.10 | 0.32 | 1.10 | 0.32 | 1.000 | |
| 4 | Wrinkles (A) | -0.60 | 0.52 | -0.50 | 0.53 | 0.564 |
| Wrinkles (C) | -1.00 | 0.55 | -1.00 | 0.55 | 1.000 | |
| Wrinkles (D) | -0.50 | 0.71 | -0.50 | 0.71 | 1.000 | |
| Wrinkles (E) | 0.00 | 0.00 | -0.10 | 0.32 | 0.317 | |
| Wrinkles (Glabella) | -0.10 | 0.32 | -0.10 | 0.32 | 1.000 | |
| Skin Texture | 4.10 | 0.32 | 3.10 | .00 | 0.317 | |
| Skin Tightness | 3.10 | 0.32 | 0.21082 | 0.32 | 1.000 | |
| Lift | 4.20 | 0.92 | 3.90 | 0.74 | .083 | |
| Sebum Production | 1 | 0.00 | 0 | 0.00 | 1.000 | |
| Pore Size: Forehead | 0.90 | 0.32 | 0.90 | 0.32 | 1.000 | |
| Pore Size: Cheek | 0.90 | 0.32 | 0.90 | 0.32 | 1.000 | |
| 16 | Wrinkles (A) | -0.67 | 0.50 | -0.67 | 0.50 | 1.000 |
| Wrinkles (C) | -1.00 | 0.45 | -1.00 | 0.58 | 1.000 | |
| Wrinkles (D) | -0.44 | 0.88 | -0.44 | 0.73 | 1.000 | |
| Wrinkles (E) | 0.00 | 0.00 | -0.11 | 0.33 | 0.317 | |
| Wrinkles (Glabella) | -0.11 | 0.33 | -0.11 | 0.33 | 1.000 | |
| Skin Texture | 3.89 | 0.78 | 3.67 | 0.71 | 0.157 | |
| Skin Tightness | 3.22 | 0.44 | 3.11 | 0.33 | 0.317 | |
| Lift | 4.22 | 1.09 | 4.11 | 1.05 | 0.317 | |
| Sebum Production | 1.00 | 0.00 | 1.00 | 0.00 | 1.000 | |
| Pore Size: Forehead | 0.89 | 0.33 | 0.89 | 0.33 | 1.000 | |
| Pore Size: Cheek | 0.89 | 0.33 | 0.89 | 0.33 | 1.000 | |
Notes: Data from one of the ten patients was removed at Week 16. All variables are measured in terms of change from Week 0. For all variables except Wrinkles (Glabella), p corresponds to the p value of the Wilcoxon signed rank test comparing Botox- and Dysport-treated sides. For Wrinkles (Glabella), p corresponds to the p value of Mann-Whitney U test, because each patient’s glabella was treated either with Botox or Dysport (not both). M: median; SD: standard deviation
Finally, effectiveness of onabotulinumtoxinA and abobotulinumtoxinA was compared using patient questionnaires (Table 5). Consistent with blinded assessment results, no significant differences were found between onabotulinumtoxinA- and abobotulinumtoxinA-treated sides for any of the variables at Weeks 2, 4, or 16, with p values ranging from 0.83 to 1.000. Additionally, 50 percent of patients reported that, overall, both sides had improved equally at Week 2 from baseline and 70 percent of patients reported equal improvement at Week 4 from baseline. Patients reported a good level of satisfaction after both intradermal and intramuscular injections of BTXA in terms of wrinkles, pore size, skin tightness, and skin texture. Overall, 75 percent of patients reported an increase in satisfaction at Week 2 compared to baseline and 85 percent of patients reported an increase in satisfaction at Week 4 compared to baseline.
Table 5:
Patient satisfaction results by treatment
| Week | Variable | Treatment | ||||
|---|---|---|---|---|---|---|
| Botox | Dysport | |||||
| M | SD | M | SD | p | ||
| 2 | Wrinkles | 1.90 | 1.52 | 1.70 | 1.49 | 0.705 |
| Forehead: Oily | -0.30 | 1.57 | -0.70 | 1.70 | 0.157 | |
| Cheek: Oily | 0.40 | 1.51 | 0.30 | 1.34 | 0.317 | |
| Pore Size | 2.00 | 2.00 | 1.80 | 1.81 | 0.458 | |
| Texture | 1.40 | 0.97 | 1.70 | 1.25 | 0.083 | |
| Tightness | 1.30 | 0.95 | 1.60 | 1.26 | 0.180 | |
| 4 | Wrinkles | 2.00 | 1.83 | 2.20 | 1.32 | 0.480 |
| Forehead: Oily | -0.40 | 1.26 | -0.60 | 1.51 | 0.157 | |
| Cheek: Oily | 0.40 | 1.71 | 0.20 | 1.62 | 0.157 | |
| Pore Size | 2.60 | 1.78 | 2.50 | 1.78 | 0.655 | |
| Texture | 2.20 | 1.55 | 2.20 | 1.48 | 1.000 | |
| Tightness | 2.00 | 1.15 | 1.80 | 1.55 | 0.414 | |
| 16 | Wrinkles | 1.44 | 1.42 | 1.33 | 1.73 | 0.564 |
| Forehead: Oily | 0.00 | 1.00 | -0.11 | 1.05 | 0.317 | |
| Cheek: Oily | 0.33 | 1.41 | 0.22 | 1.39 | 0.317 | |
| Pore Size | 1.67 | 1.87 | 1.56 | 2.13 | 0.564 | |
| Texture | 0.89 | 1.96 | 1.00 | 2.00 | 0.317 | |
| Tightness | 0.78 | 1.20 | 1.22 | 1.56 | 0.102 | |
Notes: Data from one of the 10 patients was removed at Week 16. All variables are measured in terms of change from Week 0. For all variables, p corresponds to the p value of the Wilcoxon signed rank test comparing Botox- and Dysport-treated sides. M: median; SD: standard deviation
DISCUSSION
The main objective of this study was to determine the effectiveness of intradermal BTXA injection in improving skin texture and lift while also reducing sebum production and pore size. This study found intradermal BTXA injection resulted in significant improvement in skin texture while also providing a mild lifting effect. However, in contrast to previous results, the results of our study found that there was no significant effect on sebum production or pore size when BTXA was injected intradermally.
The results obtained after intramuscular injections were similar to results after intradermal injections alone; a significant improvement of wrinkles of the forehead (Wrinkles A) and skin texture was seen at all time points. However, an additional improvement of wrinkles around the eyes (Wrinkles C) and wrinkles of the glabella was seen post-intramuscular injection, which was not seen post-intradermal injection. The significant midface lift seen post-intradermal injection at Week 2 is no longer seen post-intramuscular injections, suggesting this is a possible advantage of the intradermal technique alone.
This study also examined the difference of onabotulinumtoxinA and abobotulinumtoxinA when injected either intradermally or intramuscularly. This study found no significant difference between onabotulinumtoxinA and abobotulinumtoxinA when using the same injection approach. Moreover, the same patterns were observed in the improvement of wrinkles for both onabotulinumtoxinA and abobotulinumtoxinA from Week 0 until Week 16. Therefore, the effect of onabotulinumtoxinA and abobotulinumtoxinA did not differ when injected either intradermally or intramuscularly. The lack of significant difference between the effectiveness of onabotulinumtoxinA and abobotulinumtoxinA was validated by the patients’ responses on the questionnaire, which indicated no significant difference in satisfaction with a particular side at any time point.
Limitations. The results of the study are limited by the use of a small sample size. Thus, an insignificant p value could either mean that there was in fact no difference noted, or the sample size was too small in order to detect a difference. In order to have a more comprehensive understanding of intradermal BTXA injections as well as the differences between onabotulinumtoxinA and abobotulinumtoxinA, future studies should consider expanding the sample size and assemble a more representative sample. Lastly, the blinded evaluator made subjective assessments based on the appearance of the photos provided, thus not having an objective scale of measurement is another limitation of this study.
CONCLUSION
In conclusion, there was no significant difference in effectiveness assessed by both patient and blinded investigator between onabotulinumtoxinA and abobotulinumtoxinA when administered intradermally and intramuscularly. The results also show that the intradermal injection of BTXA did not reduce pore size and did not reduce sebum production. However, the results of intradermal injections did show a significant improvement in skin texture, while also providing a mild lift. The clinical significance of this textural change is not clear, and further studies could be conducted to explore the ways in which this finding can be applied in clinical practice. Overall, this study adds value to the much needed research base of the intradermal and intramuscular injection of two botulinum toxin type A products.
Footnotes
Disclosure:The authors report no relevant conflicts of interest.
REFERENCES
- 1.The American Society for Aesthetic Plastic Surgery. Cosmetic Surgery National Data Bank Statistics. 2015. [Accessed June 23, 2016]. http://www.surgery.org/sites/default/files/ASAPS-Stats2015.pdf [DOI] [PubMed]
- 2.Friedman O. Changes associated with the aging face. Facial Plast Surg Clin North Am. 2005;13:371–380. doi: 10.1016/j.fsc.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Beer K, Beer J. Overview of facial aging. Facial Plast Surg. 2009;25(5):281–284. doi: 10.1055/s-0029-1243075. [DOI] [PubMed] [Google Scholar]
- 4.Petchngaovilai C. Midface lifting with botulinum toxin: intradermal technique. J Cosmet Dermatol. 2009;8(4):312–316. doi: 10.1111/j.1473-2165.2009.00467.x. [DOI] [PubMed] [Google Scholar]
- 5.Koblenzer CS. Psychologic aspects of aging and the skin. Clin Dermatol. 1996;14(2):171–177. doi: 10.1016/0738-081x(95)00152-6. [DOI] [PubMed] [Google Scholar]
- 6.Charles Finn J, Cox SE, Earl ML. Social implications of hyperfunctional facial lines. Dermatol Surg. 2003;29(5):450–455. doi: 10.1046/j.1524-4725.2003.29112.x. [DOI] [PubMed] [Google Scholar]
- 7.Sommer B, Zschocke I, Bergfeld D, et al. Satisfaction of patients after treatment with botulinum toxin for dynamic facial lines. Dermatol Surg. 2003;29(5):456–460. doi: 10.1046/j.1524-4725.2003.29113.x. [DOI] [PubMed] [Google Scholar]
- 8.Dayan SH, Arkins JP, Patel AB, Gal TJ. A double-blind, randomized, placebo-controlled health-outcomes survey of the effect of botulinum toxin type A injections on quality of life and self-esteem. Dermatol Surg. 2010;36(Suppl 4):2088–2097. doi: 10.1111/j.1524-4725.2010.01795.x. [DOI] [PubMed] [Google Scholar]
- 9.Trindade de Almeida A, Carruthers J, Cox SE, et al. Patient satisfaction and safety with aesthetic onabotulinumtoxinA after at least 5 years: a retrospective cross-sectional analysis of 4,402 glabellar treatments. Dermatol Surg. 2015;41(Suppl 1):S19–S28. doi: 10.1097/DSS.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 10.Chang SP, Tsai HH, Chen WY, et al. The wrinkles soothing effect on the middle and lower face by intradermal injection of botulinum toxin type A. Int J Dermatol. 2008;47(12):1287–1294. doi: 10.1111/j.1365-4632.2008.03895.x. [DOI] [PubMed] [Google Scholar]
- 11.Rose AE, Goldberg DJ. Safety and efficacy of intradermal injection of botulinum toxin for the treatment of oily skin. Dermatol Surg. 39(3):443–448. doi: 10.1111/dsu.12097. [DOI] [PubMed] [Google Scholar]
- 12.Jeon IK, Chang SE, Park GH, Roh MR. Comparison of microneedle fractional radiofrequency therapy with intradermal botulinum toxin a injection for periorbital rejuvenation. Dermatology. 2013;227(4):367–372. doi: 10.1159/000356162. [DOI] [PubMed] [Google Scholar]
- 13.Shah AR. Use of intradermal botulinum toxin to reduce sebum production and facial pore size. J Drugs Dermatol. 2008;7(9):847–850. [PubMed] [Google Scholar]
- 14.Nahai F, Lorenc P, Kenkel JM, et al. A review of onabotulinumtoxinA (Botox) Aesthet Surg J. 2013;33(1 Suppl):9S–12S. doi: 10.1177/1090820X12474629. [DOI] [PubMed] [Google Scholar]
- 15.Nahai F, Lorenc P, Kenkel JM, et al. A review of abobotulinumtoxinA (Dysport) Aesthet Surg J. 2013;33(1 Supplement):13S–17S. doi: 10.1177/1090820X12474632. [DOI] [PubMed] [Google Scholar]
- 16.Carruthers A, Bruce S, Cox SE, et al. OnabotulinumtoxinA for treatment of moderate to severe crow’s feet lines: a review. Aesthet Surg J. 2016;36(5):591–597. doi: 10.1093/asj/sjw025. [DOI] [PubMed] [Google Scholar]
- 17.Moers-Carpi M, Carruthers J, Fagien S, et al. Efficacy and safety of onabotulinumtoxinA for treating crow’s feet lines alone or in combination with glabellar lines: a multicenter, randomized, controlled trial. Dermatol Surg. 2015;41(1):102–112. doi: 10.1097/DSS.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 18.Carruthers A, Bruce S, de Coninck A, et al. Efficacy and safety of onabotulinumtoxinA for the treatment of crows feet lines: a multicenter, randomized, controlled trial. Dermatol Surg. 2014;40(11):1181–1190. doi: 10.1097/DSS.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 19.Solish N, Rivers JK, Humphrey S, et al. Efficacy and safety of onabotulinumtoxinA treatment of forehead lines: a multicenter, randomized, dose-ranging controlled trial. Dermatol Surg. 2016;42(3):410–419. doi: 10.1097/DSS.0000000000000626. [DOI] [PubMed] [Google Scholar]
- 20.Dayan S, Coleman WP, Dover JS, et al. Effects of onabotulinumtoxinA treatment for crow’s feet lines on patient-reported outcomes. Dermatol Surg. 2015;41(Suppl 1):S67–S74. doi: 10.1097/DSS.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 21.Kane MA, Brandt F, Rohrich RJ, et al. Evaluation of variable-dose treatment with a new U.S. botulinum toxin type A (Dysport) for correction of moderate to severe glabellar lines: results from a Phase 3, randomized, double-blind, placebo-controlled study. Plast Reconstr Surg. 2009;124(5):1619–1629. doi: 10.1097/PRS.0b013e3181b5641b. [DOI] [PubMed] [Google Scholar]
- 22.Moy R, Maas C, Monheit G, et al. Long-term safety and efficacy of a new botulinum toxin type A in treating glabellar lines. Arch Facial Plast Surg. 2009;11(2):77–83. doi: 10.1001/archfacial.2009.5. [DOI] [PubMed] [Google Scholar]
- 23.Brandt F, Swanson N, Baumann L, Huber B. Randomized, placebo-controlled study of a new botulinum toxin type A for treatment of glabellar Lines: Efficacy and Safety. Dermatol Surg. 2009;35(12):1893–1901. doi: 10.1111/j.1524-4725.2009.01235.x. [DOI] [PubMed] [Google Scholar]
- 24.Hexsel D, Brum C, Porto MD, et al. Full-face injections of variable total doses of abobotulinum toxin type A: a randomized, phase IV clinical trial of safety and efficacy. J Drugs Dermatol. 2013;12(12):1356–1362. [PubMed] [Google Scholar]
- 25.Carruthers A, Sadick N, Brandt F, et al. Evolution of facial aesthetic treatment over five or more years: a retrospective cross-sectional analysis of continuous onabotulinumtoxinA treatment. Dermatol Surg. 2015;41(6):693–701. doi: 10.1097/DSS.0000000000000340. [DOI] [PubMed] [Google Scholar]
- 26.Cohen JL, Schlessinger J, Cox S, Lin X the Reloxin Investigational Group. An analysis of the long-term safety data of repeat administrations of botulinum neurotoxin type A-ABO for the treatment of glabellar lines. Aesthetic Surg J. 2009;29(6 Suppl):S43–49. doi: 10.1016/j.asj.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Baumann L, Dayan S, Connolly S, et al. Duration of clinical efficacy of onabotulinumtoxinA in crow’s feet lines: results from two multicenter, randomized, controlled trials. Dermatol Surg. 2016;42(5):598–607. doi: 10.1097/DSS.0000000000000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glogau R, Kane M, Beddingfield F, et al. OnabotulinumtoxinA: a meta-analysis of duration of effect in the treatment of glabellar lines. Dermatol Surg. 2012;38(11):1794–1803. doi: 10.1111/j.1524-4725.2012.02582.x. [DOI] [PubMed] [Google Scholar]
- 29.Bonaparte JP, Ellis D. Alterations in the elasticity, pliability, and viscoelastic properties of facial skin after injection of onabotulinum toxin A. JAMA Facial Plast Surg. 2015;17(4):256–263. doi: 10.1001/jamafacial.2015.0376. [DOI] [PubMed] [Google Scholar]
- 30.Michaels BM, Csank GA, Ryb GE, et al. Prospective randomized comparison of onabotulinumtoxinA (Botox) and abobotulinumtoxinA (Dysport) in the treatment of forehead, glabellar, and periorbital wrinkles. Aesthet Surg J. 2012;32(1):96–102. doi: 10.1177/1090820X11430685. [DOI] [PubMed] [Google Scholar]
- 31.Kassir R, Kolluru A, Kassir M. Triple-blind, prospective, internally controlled comparative study between abobotulinumtoxinA and onabotulinumtoxinA for the treatment of facial rhytids. Dermatol Ther. 2013;3(2):179–189. doi: 10.1007/s13555-013-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu KC, Nettar KD, Bapna S, et al. Split-face double-blind study comparing the onset of action of onabotulinumtoxinA and abobotulinumtoxinA. Arch Facial Plast Surg. 2012;14(3):198–204. doi: 10.1001/archfacial.2011.1142. [DOI] [PubMed] [Google Scholar]
- 33.Nestor MS, Ablon GR. Comparing the clinical attributes of abobotulinumtoxinA and onabotulinumtoxinA utilizing a novel contralateral frontalis model and the frontalis activity measurement standard. J Drugs Dermatol. 2011;10(10):1148–1157. [PubMed] [Google Scholar]
- 34.Nestor MS, Ablon GR. Duration of action of abobotulinumtoxinA and onabotulinumtoxinA: a randomized, double-blind study using a contralateral frontalis model. J Clin Aesthet Dermatol. 2011;4(9):43–49. [PMC free article] [PubMed] [Google Scholar]
- 35.Nettar KD, Yu KC, Bapna S, et al. An internally controlled, double-blind comparison of the efficacy of onabotulinumtoxinA and abobotulinumtoxinA. Arch Facial Plast Surg. 2011;13(6):380–386. doi: 10.1001/archfacial.2011.37. [DOI] [PubMed] [Google Scholar]