Abstract
The use of dual-task training paradigm to enhance postural stability in patients with balance impairments is an emerging area of interest. The differential effects of dual tasks and dual-task training on postural stability still remain unclear. A systematic review and meta-analysis were conducted to analyze the effects of dual task and training application on static and dynamic postural stability among various population groups. Systematic identification of published literature was performed adhering to Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines, from inception until June 2016, on the online databases Scopus, PEDro, MEDLINE, EMBASE, and SportDiscus. Experimental studies analyzing the effects of dual task and dual-task training on postural stability were extracted, critically appraised using PEDro scale, and then summarized according to modified PEDro level of evidence. Of 1,284 records, 42 studies involving 1,480 participants met the review’s inclusion criteria. Of the studies evaluating the effects of dual-task training on postural stability, 87.5% of the studies reported significant enhancements, whereas 30% of the studies evaluating acute effects of dual tasks on posture reported significant enhancements, 50% reported significant decrements, and 20% reported no effects. Meta-analysis of the pooled studies revealed moderate but significant enhancements of dual-task training in elderly participants (95% CI: 1.16–2.10) and in patients suffering from chronic stroke (−0.22 to 0.86). The adverse effects of complexity of dual tasks on postural stability were also revealed among patients with multiple sclerosis (−0.74 to 0.05). The review also discusses the significance of verbalization in a dual-task setting for increasing cognitive–motor interference. Clinical implications are discussed with respect to practical applications in rehabilitation settings.
Keywords: multitasking, fall, balance, cognition, rehabilitation, training, coordination
Introduction
Postural stability is an integral component of the motor control and coordination process of the body, which is required for preserving steadiness during static and dynamic activities.1 This component relies upon proprioceptive afferents and complex sensorimotor actions.2–4 Posture is mediated by both higher “controlled” and lower “automatic” levels of processing,5,6 implying the involvement of basal ganglia–cortical loop for higher level processing7 and brainstem synergies for lower level processing.8 Studies have suggested that any alleviation in conscious-controlled attention toward postural control increases the likelihood of disrupting coordination and stability,9,10 possibly, as a consequence of movement-specific reinvestment.9,11 The theory of reinvestment suggests that directing attention internally to control movement, which is usually automatic, can disrupt its performance.9,10 The theory also suggests that aging12 and neurological diseases9 are common conditions that increase reinvestment. Seidler et al13 reaffirmed these suggestions and associated physiological changes with aging and injury to loss in gray/white matter within the central nervous system, resulting in differential-reorganized cortical activation. Here, the authors suggested that differential cortical activation within the higher neural centers can affect task prioritization, further allowing increased conscious attention while carrying out cognitive or motor tasks.14
To resolve this issue, distracting dual tasks have been used in several studies.9,15–17 A dual task acutely directs the performer’s attention toward an external source of attention (eg, n-back, random letter generation tasks), while performing a primary task. According to the constrained action hypothesis, this attentional change might allow motor systems to function in an automatic manner, resulting in more effective performance.10 Practical applications for enhancing the automation of postural control have been demonstrated in studies evaluating complex motor skills,18,19 postural stability,17 and gait.15
However, with an increase in complexity, a subsequent increase in cognitive processing and eventually cognitive–motor interference has been reported.20–23 This increase in central interference adversely affects both cognitive and motor performance.6,23 Studies speculate that inhibition of cognitive and balance ability post dual-task inclusion can be because of the bottleneck and central sharing model theories.21,24 According to these theories, functioning of a neurological pathway mediating both cognitive and motor functions might be affected, when a continuous input as in a dual-task setting is directed with a primary task. This might adversely affect cognitive tasks or stability performance.
Similarly, a complexity-related decrease in cortical reciprocal inhibition in fall-prone population groups (elderly, patients with history of fall, with neurological diseases) has been identified as an important factor to promote postural instability.25,26 Studies suggest reduced gamma-aminobutyric acid B-mediated cortical inhibition27 and elevated muscular coactivation26,28 to be the primary reasons for this effect. Boisgontier et al,6 Ruffieux et al,26 and Smith et al29 in their review studies concluded that application of dual task on fall-prone population groups results in postural instability and poor cognitive performance. However, minimal effects of cognitive–motor interference have been reported in a few reviews for diseased fall-prone population groups, which theoretically should exhibit poorer cognitive resources as compared to their healthy older counterparts.30,31 Therefore, there is a need to determine specific factors that in terms of complexity for a cognitive or motor task might result in differential effects on stability.
Furthermore, studies have extensively mentioned the beneficial effects of motor,32,33 dual-task training,34–36 for enhancing cognitive and motor performance even in fall-prone population groups. Another important determinant that is commonly utilized to enhance stability and cognitive performance is physical exercise.32,33,37 The studies report these training maneuvers to be crucial for smoothening of various cognitive abilities and reducing cognitive–motor interference.38–40 Müller and Blischke41 suggested that the training allows modulation of consciousness-dependent motor activities to be more automatic, thereby reducing dual-task costs. Likewise, Bherer et al42 while reporting the beneficial effects in fall-prone population groups suggested freeing up of cognitive resources meant for monitoring performance to be the primary reason. The change in modulation of motor activity has been suggested to allow automatization by “structural displacement”,43,44 where a shift in the operation control of motor planning and executive control occurs from higher cognitive centers to basic noncognitive centers.45,46 This training maneuver has recently drawn a lot of interest as compared to its older counterpart and speculations persist as to which protocol overlays beneficial effects on postural stability among different population groups.47,48 Recent review studies evaluating the effects of dual-task training in elderly38,49 and population groups with neurological diseases50,51 conclusively report the beneficial effects of dual-task training for enhancing cognitive abilities and stability, whereas some review studies report no identifiable benefits.33,52 The studies also mentioned the increased heterogeneity of the training protocols within the studies to cause difficulties in identifying a specific method’s effectiveness. Wang et al,51 for instance, in their meta-analysis reported benefits of dual-task training on static stability, however, with considerable heterogeneity (I2: 88%). This review was an attempt to extend the efforts of the previous reviews and comparatively examine the effects of dual tasks, dual-task training methodologies on the postural stability of healthy and fall-prone population groups. The review also aimed to conduct meta-analysis across homogeneous groups for determining effective methodologies in terms of complexity and training methodologies for dual task and dual-task training scenarios.
Methods
This review was conducted according to the guidelines outlined in Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement.53
Data sources and search strategy
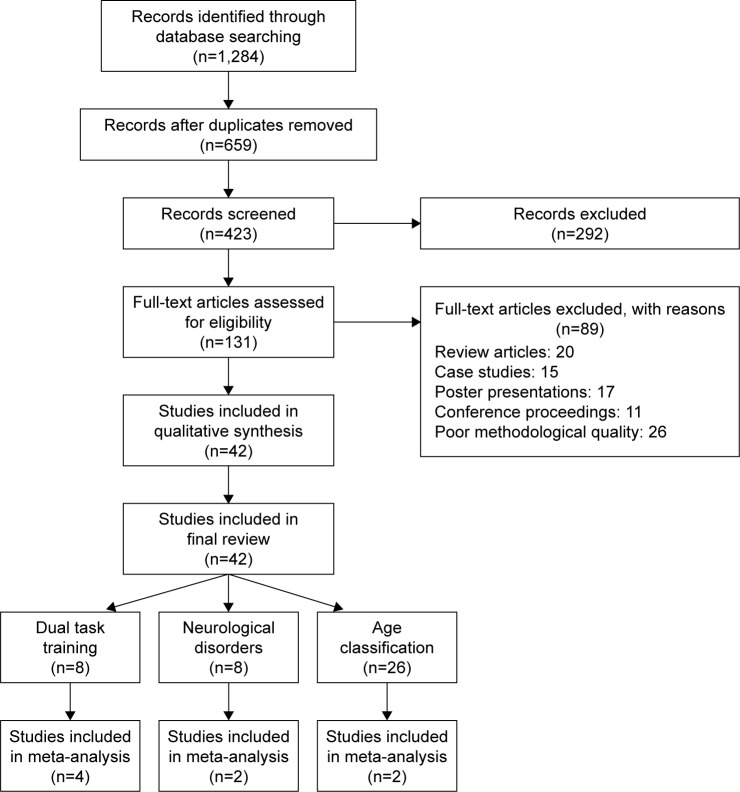
The databases Scopus, PEDro, SportDiscus, EMBASE, and MEDLINE were searched from inception until June 2016. The search was limited to the abovementioned databases due to access regulations of the university. Keywords for search strategy were included using medical subject headings (MeSH). An example of the search strategy for EMBASE database has been provided in Table S1. The inclusion criteria for the studies were as follows: 1) studies that were randomized controlled trials (RCTs), cluster RCTs, and controlled clinical trials (CCTs); 2) measurement of postural stability using highly valid and reliable methods (static and dynamic posturographic analyses, center of pressure, center of gravity analysis, sensory orientation test, Berg balance scale, time up and go test, star excursion balance test, modified star excursion balance test, and active movement extent discrimination apparatus); 3) dual tasks performed during the research were reliable and valid; 4) studies that scored ≥ 4 on the PEDro methodological quality scale; 5) experiments that were conducted on human participants; 6) published in a peer-reviewed academic journal; and 7) articles that were published in English language. Studies evaluating the abovementioned parameters in participants below the age of 18 years were not included, as development of postural control centers has been reported to take place during this developmental phase.54 Studies were excluded if they analyzed postural stability in a sitting position or while using a picture analysis software. All the studies identified during the search were independently screened (Figure 1) for eligibility by a primary researcher and every effort was undertaken to avoid subjective bias.55 Preliminary analysis for selection was performed by analyzing titles and abstracts, and, wherever necessary, the entire text of the article was studied. Where further clarification of the published data was required, the first researcher attempted to contact the respective authors. Bibliographic sections of all the articles were retrieved for evaluations. Citation search for all the included articles was performed using Web of Science. A classification of studies based on their experimental design56 and country of origin was also made (Supplementary material).
Figure 1.
Flow diagram illustrating studies for inclusion in the review study (PRISMA flow diagram).
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analysis.
Data extraction
Upon selection for review, the following data were extracted from each article: author, date of publication, selection criteria, sample size, sample description (gender, age, health status), intervention, dual-task, outcome measures, results, and conclusions. The data were then summarized and tabulated. Furthermore, classification of studies was made based on their experimental application,56 and the population groups were assessed.
Quality and risk of bias assessment
The quality of the studies was assessed using the PEDro methodological quality scale. The scale consists of eleven items addressing external validity, internal validity, and interpretability. The PEDro scale can detect potential bias with fair to good reliability57 and is a valid measure of the methodological quality of trials. A blinded rating of the methodological quality of the studies was carried out by the primary reviewer. Ambiguous issues were discussed between reviewers, and consensus was reached. For the included CCTs, a scoring of 9–10, 6–8, and 4–5 was considered to be of “excellent”, “good”, and “fair” quality,58 respectively. Likewise, the level of evidence was suggested to be of level 1a (strong) if more than one RCT (≥ 6), 1b if one RCT (≥ 6), and 2 if one RCT (<6), or CCTs with similar methodological approaches were consistent with the results.58 With differential results among paired groups of studies, the result of the study(s) with higher PEDro score was given more consideration. Inadequate randomization, nonblinding of assessors, no intention to treat analysis, and no measurement of compliance were major threats to biasing.2
Data analysis
This systematic review also included a random-effect meta-analysis approach to develop a better understanding of the incorporated interventions. A narrative synthesis of the findings structured around the intervention, population characteristics, methodological quality (Table 1), and the type of outcome is provided. Likewise, summaries of intervention effects for each study were provided in a tabular form (Table S1). A meta-analysis was conducted between pooled studies using comprehensive meta-analysis (CMA V 3.0; Englewood, NJ, USA). Heterogeneity between the studies was assessed using I2 statistics. The data in this review were systematically distributed and for each available variable pooled, dichotomous data were analyzed and forest plots with 95% confidence intervals (CIs) are reported. The effect sizes were adjusted and reported as Hedge’s g. Thresholds for interpretation of effect sizes were as follows: a standard mean effect size of 0 means no change, negative effect size means a negative change, mean effect size of <0.1 is considered a small effect, 0.1–0.3 a medium effect and >0.30 a large effect.59,60 Interpretation of heterogeneity via I2 statistics was as follows: 0–40% might not be significant, 30%–60% represents moderate heterogeneity, 50%–90% represents substantial heterogeneity, and 75%–100% represents considerable heterogeneity. Meta-analysis reports including heterogeneity among studies were evaluated to determine the reason of heterogeneity, and the included studies were then pooled separately and analyzed again. The alpha level was set at 95%.
Table 1.
Studies analyzing the effects of dual-task training and dual tasks on posture
| Study | Research aim | Sample description | PEDro score and (level of evidence) | Research design | Postural analysis | Dual-task (C: cognitive, Cm: cognitive–motor and M: motor components) | Conclusion | |
|---|---|---|---|---|---|---|---|---|
| Dual-task training | ||||||||
| Choi et al (2015)34 | Assess the effects of DT training on postural stability among participants suffering from subacute stroke | 8 F, 12 M (59±12) DT training (10), Ct (10) | 5 (1b) | Postural stability assessed post DT training for 30 min, five times a week for 4 weeks | Sway analysis and Berg balance scale | Cm: computer-based cognitive memory test | Significantly enhanced postural stability post DT training DT performance significantly enhanced post DT training | |
| An et al (2014)48 | Assess the effects of DT training on postural stability among participants suffering from chronic stroke | 33 chronic stroke patients Motor (11), cognitive (11), motor–cognitive (12) training group | 4 (1b) | Postural stability assessed post DT training for 30 min, three times a week for 8 weeks | Sway analysis, time up and go test and functional reach test | C: mental arithmetic task Cm: Stroop, verbal analogical and counting backward task | Significantly enhanced postural stability in motor–cognitive training group as compared to motor or cognitive alone training group | |
| Kim et al (2013)62 | Assess the effects of DT training on postural stability among participants suffering from chronic stroke | VUDT: 5 F, 7 M (52±2) VDT: 8 F, 5 M (59±3) UDT: 9 F, 4 M (57±3) |
4 (1b) | Postural stability assessed post DT training for 30 min, three times a week for 8 weeks with/without unstable base, visual restriction, dual task | Sway analysis, functional reach test and Berg balance scale | Cm: trial making and Stroop task | Significantly enhanced postural stability post DT training especially in VUDT group DT performance significantly enhanced post DT training in VUDT group | |
| Hiyamizu et al (2012)36 | Assess the effects of DT training on postural stability among healthy elderly participants | Elderly: DT training – 10 F, 7 M (72±5) DT: 16 F, 3 M (71±4) |
7 (1b) | Postural stability assessed post DT training twice a week for 3 months (ST) with/without ST, EO/EC | Berg balance scale, activity-based confidence scale | Cm: visual search, verbal fluency, calculation task and Stroop task M: strength and balance training task | No significant difference in postural sway after DT training DT performance significantly enhanced post DT training | |
| Buragadda et al (2012)61 | Assess the effects of DT training on postural stability among elderly participants with balance impairments | 30 participants | 4 (1b) | Postural stability assessed post DT training (variable, fixed priority), 45 min session, three times a week for 4 weeks | Chair stand test, functional reach test, time up and go test | Cm: word spelling task and memory task | Significantly enhanced postural stability post variable priority DT training as compared to fixed priority | |
| Li et al (2010)63 | Assess the effects of DT training on postural stability among healthy elderly participants | DT training: 7 F, 3 M (74±5) Ct: 6 F, 4 M (77±7) |
4 (1b) | Static, dynamic posture, and mobility stability assessed post DT training during five sessions separated by 2 days | Single support balance, sway analysis, and sit to stand test | Cm: n-back task | Significantly enhanced postural stability during single and double support dynamic balance DT performance significantly enhanced post DT training | |
| Silsupadol et al (2009)35 | Assess the effects of DT training on postural stability among elderly participants | ST training: 7 F (74±7) DT training (fixed): 6 F (74±6) DT training (variable): 4 F (76±4) |
6 (1b) | Postural stability assessed post DT training (variable, fixed priority), 45 min session, three times a week for 4 weeks | Berg balance scale, activity-based confidence scale | Cm: random letter generation task | Significantly enhanced postural stability post variable priority DT training as compared to fixed priority DT training | |
| Pellecchia (2005)81 | Assess the effects of DT training on postural stability | 9 F, 9 M (18–46) | 5 (1b) | Postural stability assessed during single and DT training conditions, within three sessions | Sway analysis | Cm: counting backward in three tasks | Significantly enhanced postural stability post DT training as compared to single task training condition, DT performance significantly enhanced post DT training No difference in DT performance post DT training |
|
| Neurological disorder | ||||||||
| Jacobi et al (2015)91 | Assess the effects of DT on postural stability among healthy and participants suffering from DCD | Healthy: 10 F, 10 M (58±11) DCD: 10 F, 10 M (58±11) |
4 (2) | Static and dynamic postural stability assessed while EO/EC, with platform stable/unstable, with/without DT | Sensory organization test | Cm: verbal working memory task | Significantly reduced postural stability in participants with DCD as compared to healthy participants DT performance significantly reduced | |
| Prosperini et al (2015)66 | Assess the effects of DT on postural stability among healthy and participants suffering from MS | Healthy: 30 F, 16 M (39±9) MS: 60 F, 32 M (39±10) |
4 (2) | Postural stability assessed with/without EO/EC, DT | Sway analysis | Cm: Stroop word color task | Significantly reduced postural stability in participants with MS as compared to healthy participants DT performance significantly reduced in MS patients | |
| Andrade et al (2014)22 | Assess the effects of DT on postural stability among participants suffering from AD, PD, and healthy participants | AD: 9 F, 3 M (72±5) PD: 7 F, 6 M (71±6) Healthy: 7 F, 6 M (66±4) |
4 (2) | Postural stability assessed with/without DT | Sway analysis | Cm: counting backward task | Significantly reduced postural stability with ST performance in AD, PD participants as compared to their healthy counterparts DT performance significantly reduced in patients with AD and PD | |
| Boes et al (2012)21 | Assess the effects of DT on postural stability among participants suffering from MS | MS: mild – 17 F, 2 M (46±13) Moderate: 24 F, 3 M (58±7) |
6 (2) | Postural stability assessed with/without DT | Sway analysis | Cm: word list generation task | Significantly reduced postural stability in participants classified in moderate MS as compared to mild MS group | |
| Negahban et al (2011)65 | Assess the effects of DT on postural stability among healthy and participants suffering from MS | Healthy: 15 F, 8 M (31±7) MS: 15 F, 8 M (32±7) |
6 (2) | Postural stability assessed on rigid/foam surface, while EO/EC, with/without DT | Sway analysis | C: silent backward counting task | Significantly enhanced postural stability in MS and healthy participants DT performance not affected in patients with MS as compared to healthy participants | |
| Holmes et al (2010)94 | Assess the effects of DT on postural stability among healthy elderly and participants suffering from PD | Healthy: 4 F, 8 M (62±8) PD: 4 F, 8 M (64±9) |
5 (2) | Postural stability assessed with/without DT | Sway analysis | Cm: numeral recitation and monologue generation task | Significantly enhanced postural stability observed in participants affected by PD as compared to healthy controls with/without DT, especially in monologue generation task | |
| Marchese et al (2003)92 | Assess the effects of DT on postural stability among healthy participants and participants suffering from PD | Healthy: 7 F, 13 M (60±7) PD: 8 F, 16 M (66±7) |
5 (2) | Postural stability assessed with/without EO/EC, DT | Sway analysis | Cm: calculation task M: thumb opposition task | Reduced postural stability observed in participants affected by PD as compared to healthy controls with/without DT and motor tasks during eyes closed/open. With PD Fa performing significantly poorer | |
| Morris et al (2000)93 | Assess the effects of DT on postural stability among healthy and participants suffering from idiopathic PD (with/without history of fall) | PD fall: 8 F, 7 M (67±8) PD no fall: 8 F, 7 M (68±7) Healthy: 8 F, 7 M (68±7) |
5 (2) | Postural stability assessed with/without internal/external perturbation, with/without DT | Internal and external perturbations to center of mass | Cm: verbal recital task | Significantly reduced postural stability with DT in PD fall-prone > nonfall-prone > healthy participants | |
| Age classification | ||||||||
| Lanzarin et al (2015)20 | Assess the effects of DT on postural stability among healthy young participants | Young: 10 F, 10 M (25±4) | 5 (2) | Postural stability assessed with/without EO/EC, with/without DT | Sensory organization test | C: mental arithmetic task | Significantly reduced postural stability during ST, while EO and EO with oscillating platform | |
| Bergamin et al (2014)68 | Assess the effects of DT on postural stability among healthy young and elderly participants | Young: 15 F, 15 M (23±1) Elderly: 15 F, 15 M (72±5) |
4 (2) | Postural sway assessed with/without DT | Sway analysis | C: mental arithmetic task Cm: spatial memory and counting backward aloud task | Significantly enhanced postural stability during mental arithmetic, spatial memory task Significantly poor stability during counting backward aloud task | |
| Hwang et al (2013)77 | Assess the effects of DT on postural stability among healthy young participants | 9 F, 11 M (28±4) | 5 (2) | Postural stability assessed with/without vibration, with/without DT | Sway analysis | Cm: verbal and nonverbal task with hand-held button press | Significantly enhanced postural stability during nonverbal tasks, but less during verbal tasks | |
| Haggerty et al (2012)74 | Assess the effects of DT on postural stability among healthy elderly participants | Elderly: 4 F, 6 M (74±4) | 5 (2) | Postural stability assessed with/without VTf, DT | Sway analysis and biofeedback | Cm: verbal auditory and push button dual task | Significant enhancement of postural stability when DT performed with VTf as compared to DT alone DT performance significantly reduced | |
| Mak et al (2011)78 | Assess the effect of delayed visual feedback and DT on posture among healthy young and elderly participants | Young: 10 F, 5 M (24±3) Elderly: 5 F, 10 M (age not specified) |
4 (2) | Posture stability assessed with EO, delayed visual feedback, with/without DT | Sway analysis | C: mental arithmetic task | Significantly enhanced postural stability in young participants. Significantly reduced postural stability in elderly participants | |
| Resch et al (2011)17 | Assess the effects of ST on postural stability among healthy young participants | Young: 10 F, 10 M (20±1) | 5 (2) | Postural stability assessed by using SOT with/without DT | Sensory organization test | Cm: auditory switch task | Significantly enhanced postural control DT performance significantly reduced | |
| Doumas et al (2008)73 | Assess the effects of DT on postural stability and vice versa among healthy young and elderly participants | Young: 10 F, 8 M (21±2) Elderly: 10 F, 8 M (71±3) |
4 (2) | Postural stability assessed with/without DT | Sway analysis | Cm: n-back task | Significantly reduced postural stability for elderly participants in sway reference somatosensory condition. No difference in postural stability of young participants DT performance significantly reduced in elderly participants during sway reference somatosensory condition | |
| Ramenzoni et al (2007)82 | Assess the effects of DT on postural stability among healthy young participants | Young: 10 F, 13 M (28–25 y) | 5 (2) | Postural stability assessed with/without DT, during encoding and rehearsal with combination of verbal and visual interference | Sway analysis | Cm: verbal and visual cognitive task | Significantly reduced postural stability during encoding of verbal and visual task as compared to rehearsal period DT performance significantly reduced during verbal and visual tasks | |
| Donker et al (2007)16 | Assess the effects of DT on postural stability among healthy young participants | Young: 20 F, 10 M (19–30 y) | 5 (2) | Postural stability assessed while EO/EC and with/without DT | Sway analysis | Cm: uttering name backwards task | Significant enhancement of postural stability when DT performed with eyes closed | |
| Swan et al (2007)85 | Assess the effects of DT on postural stability among healthy female young participants | Young: 98 F (18–27 y) | 4 (2) | Postural stability assessed with/without DT | Sway analysis | Cm: Brook spatial and nonsense memory task | Significant enhancement in postural stability with enhanced DT difficulty. No effect of difficulty enhancement in balance task DT performance significantly reduced with increased complexity of DT | |
| Vuillerme et al (2006)89 | Assess the effects of DT on postural stability among healthy young participants | Young: 9 M (23±1) | 4 (2) | Postural stability assessed with/without EO/EC, ST | Sway analysis | Cm: probe reaction time task | Significantly enhanced postural stability during EO, closed DT as compared to EC | |
| Huxhold et al (2006)79 | Assess the effects of ST, DT on postural stability among healthy young and elderly participants | Young: 10 F, 10 M (24±2) Elderly: 9 F,10 M (69±3) |
4 (2) | Postural stability assessed with/without ST and DT | Sway analysis | Cm: digit choice reaction time, two back digit working memory, two back spatial working memory and watching digit conditions task | Significantly enhanced postural stability in both age groups with simple DT DT performance significantly reduced in elderly participants during digit and spatial two back tasks |
|
| Swan et al (2004)86 | Assess the effects of DT on postural stability among healthy young and elderly participants | Young: 18 F (19–25 y) Elderly: 15 F (60–74 y) |
5 (2) | Postural stability assessed under, with/without, ST and EO/EC | Sway analysis | Cm: Brooks spatial and nonspatial task | Significantly enhanced postural stability for both age groups DT performance significantly reduced in elderly as compared to young participants | |
| Pellecchia (2003)76 | Assess the effects of DT on postural stability among healthy young participants | Young: 10 F, 10 M (18–30 y) | 4 (2) | Postural stability assessed with/without DT | Sway analysis | Cm: digit reversal task, counting backward in twos and counting backward task | Significantly reduced postural stability with DT DT performance significantly reduced as DT complexity increased |
|
| Brauer et al (2002)69 | Assess the effects of ST on postural stability among healthy young and elderly participants with and without history of fall | Young: 5 F, 10 M (22±5) Elderly: Nfa – 4 F, 11 M (72±6) Fa – 6 F, 7 M (79±6) |
5 (2) | Postural stability assessed with sudden movement at the balance platform, with/without DT | Postural recovery via sway analysis | Cm: vocal reaction time task | Reduced postural stability in elderly participants (Fa) and young participants during DT as compared to elderly (Nfa). Also poor recovery by Fa with DT and limited effect of DT on Nfa and young participants DT performance significantly reduced among elderly Fa as compared to elderly Nfa and young participants |
|
| Andersson et al (2002)67 | Assess the effects of DT, calf stimulation, and self-balance focus on postural stability among healthy participants | Exp 1: 17 F, 13 M (27±8) Exp 2: 10 F, 10 M (30±8) |
4 (2) | Postural stability assessed with (Exp 1)/without (Exp 2) mental task, ie, focus on balance, with/without DT | Sway analysis | C: silent backward counting task | Significantly reduced postural stability during DT DT performance significantly reduced with balance pertubations |
|
| Dault et al (2001)72 | Assess the effects of DT on postural stability among healthy young participants | Young: 12 F, 12 M (20–40 y) | 5 (2) | Static and dynamic postural stability assessed with/without DT | Sway analysis | Cm: Stroop word color task | Significantly reduced postural stability when dynamic stability assessed with ST performance. No significant difference in postural sway during static ST performance DT performance significantly reduced with increased complexity of DT task | |
| Hunter and Hoffman (2001)75 | Assess the effects of DT on postural stability among healthy young participants | Young: 15 F, 15 M (24 y) | 4 (2) | Postural stability assessed with modulation of eye movement and modality of presentation of DT | Sway analysis and video- motion analysis | Cm: visual and auditory cognitive task | Significantly reduced postural stability within visual condition DT performance unaffected | |
| Melzer et al (2001)80 | Assess the effects of DT on postural stability among healthy young and elderly participants | Young: 20 (26±3) Elderly: 20 (77±2) |
5 (2) | Postural stability assessed with/without narrow/wide BoS DT and EMG | Sway analysis | Cm: modified stroop test | Significantly reduced postural stability among elderly participants during DT performance and narrow BoS. Enhancement in stability during DT performance in young participants | |
| Teasdale and Simoneau (2001)88 | Assess the effects of DT on postural stability among healthy young and elderly participants | Young: 5 F, 3 M (24±0) Elderly: 2 F, 6 M (68±0) |
4 (2) | Postural stability assessed with/without DT | Sway analysis | Cm: probe reaction time task | Significantly reduced postural stability as compared to young participants DT performance significantly reduced in elderly as compared to younger participants | |
| Brauer et al (2001)70 | Assess the effects of DT on postural stability among healthy young and elderly participants with and without history of fall | Elderly: Nfa – 5 F, 9 M (72±6) Fa – 6 F, 7 M (79±6) |
5 (2) | Postural stability assessed with sudden movement at the balance platform, with/without DT | Sway analysis | Cm: vocal reaction time task | Significantly reduced postural stability in elderly participants (Fa) during DT as compared to elderly (Nfa). Also poor recovery by Fa with DT and no effect of DT on Nfa DT performance significantly reduced in elderly Fa participants as compared to Nfa elderly participants | |
| Shumway-Cook and Woollacott (2000)83 | Assess the effects of DT on postural stability among healthy young and elderly participants with and without history of fall | Young: 3 F, 15 M (34±8) Elderly: Nfa – 4 F, 14 M (74±6) Fa – 3 F, 15 M (85±6) |
5 (2) | Postural stability assessed with balance disturbances, with/without EO/EC, somatosensory input, DT | Sway analysis | Cm: choice reaction time auditory task | Significantly reduced postural stability among elderly participants Fa as compared to young and elderly Nfa participants during DT DT performance unaffected, similar in younger and elderly participants | |
| Marsh and Geel (2000)79 | Assess the effects of DT on postural stability among healthy young and elderly participants | Young: 14 F (25±2) Elderly: 16 F (71±3) |
5 (2) | Postural stability assessed, with EO/EC, with/without DT | Sway analysis | Cm: vocal reaction time task | Significantly reduced postural stability among elderly participants as compared to young participants DT performance significantly reduced in elderly as compared to younger participants | |
| Brown et al (1999)71 | Assess the effects of DT on postural stability among healthy young and elderly participants | Young: 5 F, 10 M (25±5) Elderly: 3 F, 7 M (78±4) |
5 (2) | Postural stability assessed with balance disturbances, with/without DT | Postural recovery via sway analysis and video-motion capturing | Cm: backward digit recall task | Reduced postural stability among elderly as compared to young participants during balance disturbances | |
| Shumway-Cook et al (1997)84 | Assess the effects of DT on postural stability among healthy young and elderly participants with and without history of fall | Young: 10 F, 10 M (31±6) Elderly: Nfa – 11 F, 9 M (74±6) Fa – 13 F, 7 M (78±8) |
5 (2) | Postural stability assessed on flat and compliant surfaces, with and without DT | Sway analysis | Cm: sentence completion, language processing, visual perception, and judgment of line orientation task | Significantly reduced postural stability in elderly participants (Fa) during dual tasks on both surfaces as compared to young participants No significant effect on young and elderly (Nfa) on flat surface under simple DT DT performance significantly reduced in Fa as compared to young participants |
|
| Teasdale et al (1993)87 | Assess the effects of DT among healthy young and elderly participants | Young: 8 M (24±0) Elderly: 3 F, 6 M (71±0) |
4 (2) | Postural stability assessed while making postural adjustments, with/without DT | Sway analysis | Cm: auditory reaction time task | Significantly reduced postural stability among elderly participants as compared to young participants during DT DT performance significantly decreased in elderly as compared to younger participants | |
Notes: Significant: P<0.05; nonsignificant: P>0.05. Data presented as mean ± standard deviation.
Abbreviations: AD, Alzheimer’s disease; BoS, base of support; Ct, control group; DCD, degenerative cerebellar disorder; DT, acute dual-task application; Exp, experimental group; EMG, electromyography; EO, eyes open; EC, eyes closed; F, female; Fa, history of fall; M, male; MS, multiple sclerosis; Nfa, no history of falls; PD, Parkinson’s disease; SOT, sensory orientation test; ST, single task; UDT, unstable base with dual-task; VUDT, visual restriction and unstable base with dual task; VDT, visual restriction with dual task; VTf, vibro-tactile feedback.
Results
Characteristics of included studies
The initial search yielded 1,284 studies, which on implementing the inclusion/exclusion criteria were reduced to 42 (Figure 1). Data from the included studies are summarized in Table 1. Of the 42 studies, three were RCTs,34–36 and 39 were CCTs. Eight studies evaluated the effects of dual-task training on postural stability.34–36,48,61–64 Eight studies evaluated the effects of dual tasks on participants suffering from neurological diseases, such as degenerative cerebellar disorder, Parkinson’s disease, and multiple sclerosis.21,65,66 Twenty-six studies evaluated the effects of dual tasks on postural stability among healthy young and/or elderly participants.16,17,20,67–89 Within these 26 studies, 14 studies compared the effects between young and elderly participants, eleven studies evaluated only young and one study evaluated only elderly participants.
Participants
Of the included studies, 33 studies incorporated mixed-gender participant groups.16–18,20–22,36,61–67,69–78,81–84,87,88,90–94 Four studies incorporated only female participants,35,79,85,86 and two studies incorporated only male participants.34,89 Three studies did not specify the gender of the included participants.48,61,80 The included studies provided data on 1,480 participants (n=796 females/581 males). Descriptive statistics related to the age (mean ± standard deviation) of the participants were tabulated across the studies. Three studies provided the median age of participants,75,87,88 and five studies mentioned the age of participants in range.16,81,82,85,86
Risk of bias within studies
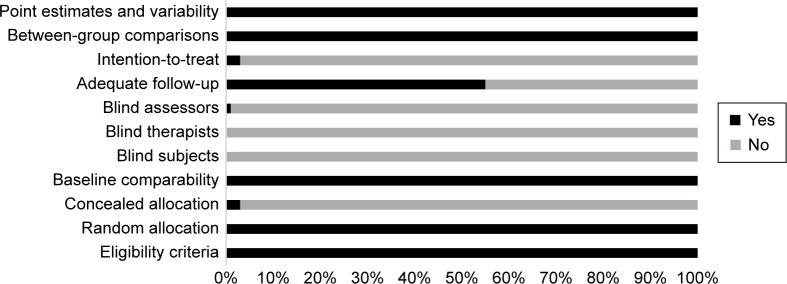
In order to efficiently reduce the risks of bias, the studies had to score ≥ 4 on PEDro scale to be included in the review. The criteria for research studies to be included in the review were limited to gold standard RCTs, cluster RCTs, and CCTs. The individual scores attained by the studies using the PEDro scale are reported in Tables 1 and S2. The average PEDro score for the 42 included studies was computed to be 4.7 out of 10, indicating fair quality of the overall studies. One study scored 7,36 three studies scored 6,21,35,65 20 studies scored 5,16,17,20,34,69–72,74,77,79–84,86,92–95 and 18 studies scored 4.22,48,61–63,66–68,73,75,76,78,81,85,87–89,91
Risk of bias across studies
Common methodological shortfalls observed in this review were inadequate concealment, intention-to-treat, nonblinding of participants, therapists, and assessors. One study reported blinding of assessors and confirmed intention-to-treat the included participants. Furthermore, only two studies confirmed concealed allocation of subjects.35,36 The authors could not interpret concealed allocation of participants in three studies,65,82,85 and, therefore, no points were awarded to the studies. The overall risk of bias for quality assessment within studies is illustrated in Figure 2.
Figure 2.
Risk of bias across studies.
Meta-analysis
The evaluation of research studies via meta-analysis requires strict inclusion criteria to efficiently limit the heterogeneity.96 However, among the pooled group of studies the authors observed unexplained heterogeneity, suggesting incorporation of a random-effect meta-analysis under such conditions. The researchers added that a random-effect meta-analysis involves an assumption that the estimated effects in various studies are unidentical but follow some distribution. Therefore, studies analyzing similar variables were pooled, and a random-effect meta-analysis was conducted across four categories (dual-task training: elderly participants, dual task: multiple sclerosis, young, old). The main reason for not including the statistical approach within the studies was major differences in training duration, assessment methods, age/gender, complexity of dual tasks, and lack of descriptive statistics within the manuscript. The descriptive statistics mentioned within illustrative figures were not included in the study. The authors included ten studies in the meta-analysis which incorporated evaluation of postural stability in participants similar at baseline and were evaluated with similar methodological approaches, but with different dual tasks. The aim of such analysis was to demonstrate the differential effects of complexity of dual tasks on postural stability. Additionally, the reasons for specific studies are mentioned subsequently.
Dual-task training
Eight studies under this category analyzed the effects of dual-task training on postural stability, whereas four studies, in two different categories, were included in the meta-analysis.35,48,61,62 From the nonincluded studies, one study analyzed the effects of dual-task training on subacute stroke patients,34 two studies analyzed the effects on elderly participants,36,63 and one study on younger participants.64 The two studies analyzing the effects of dual-task training on elderly participants considerably differed based on training duration and incorporated dual tasks. Hiyamizu et al36 incorporated Stroop task with a dual-task training duration of two sessions per week for 3 months; however, Li et al63 used an n-back task with a training duration spread over five sessions with a 2-day gap within each session.
Neurological impairment
Eight studies under this category analyzed the effects of dual task on postural stability of participants affected by neurological disorders. Two studies analyzing the effects of dual task on multiple sclerosis were included in the meta-analysis. However, the third study despite having similar variables could not be included as the descriptive statistics were not available in the text and were not obtained even after contacting the corresponding author. Similarly, three other studies analyzing patients affected by Parkinson’s disease could not be included due to lack of descriptive statistics.22,92,93 Only one study analyzed the effects of dual tasks on postural stability of participants affected from degenerative cerebellar disorder.91
Young and elderly participants
Twenty-six studies under this category analyzed the effects of dual task on young and/or elderly participants. Four studies analyzing the effects of dual task on young and elderly participants were included in the meta-analysis.17,20,80,94 Thirteen studies analyzing similar variables in terms of age and dual tasks were not included in the meta-analysis as they did not include descriptive statistics explicitly, but in figures, ie, bar diagrams.16,67,68,72,75,76,79,82,85–89 Shumway-Cook and Woollacott83 and Shumway-Cook et al84 evaluated the effects of dual task on postural stability during balance perturbations in participants predisposed to falls, their healthy counterparts, and young participants, while the studies differed in terms of utilized dual tasks. Mak et al,78 on the contrary, included a rather novel aspect of visual feedback during standing and utilized dual tasks in conjugation with this feedback approach. Hwang et al77 also utilized one leg standing as compared to the counterpart studies, which utilized a basic two-legged standing under different conditions. Brauer et al69,70 analyzed postural recovery post balance perturbation with dual tasks among participants predisposed to falls, their healthy counterparts, and young participants with similar dual tasks. Likewise, Brown et al71 also utilized a similar approach and effective comparisons could have been drawn between studies to evaluate the effects of dual task on postural stability. Due to lack of descriptive statistics, and not heterogeneity, the studies could not be included in the analysis.
Outcomes
The results suggest clear evidence for a positive impact of dual-task training for enhancing postural stability among fall-prone elderly population groups and participants affected from stroke. A negative impact of dual tasks was observed in studies evaluating the effects of dual tasks on postural stability among fall-prone population groups affected by neurological disorders and/or with prior history of fall, as compared to their younger healthier counterparts.
Meta-analysis report
Dual-task training
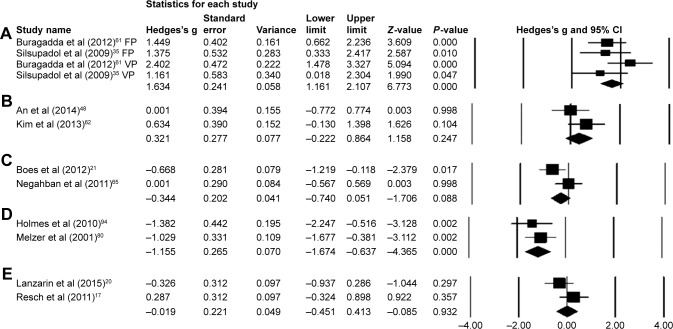
Eight studies evaluated the effects of dual-task training on postural stability.34–36,48,61–64 One RCT34 and two CCTs evaluated the effects of dual-task training on postural stability in subacute and chronic stroke patients, respectively. Two RCTs35,36 and three CCTs evaluated the effects of dual-task training on elderly and young participants. Significant enhancements in postural stability were reported in one good35 and six fair-quality studies.34,48,61–64 However, one good-quality study reported no significant enhancements in postural stability. A random-effect meta-analysis was conducted across two categories. First, two studies evaluated the effects of fixed and variable priority dual-task training on postural stability among elderly population groups.35,61 A random letter generation task was utilized during the training phase which lasted for a 45-min session, three times a week for 4 weeks. Scores from Berg balance scale were utilized to assess the postural stability. Upon analysis, a large effect size was observed (Hedge’s g: 1.63), and 95% CI (1.16–2.10) was reported in the positive domain, demonstrating a beneficial effect of variable task priority within dual-task training to enhance postural stability (Figure 3A). Heterogeneity tests reported negligible heterogeneity (I2: 20.26%, P<0.01). Moreover, the studies were then reevaluated on the basis of fixed and variable priority dual-task training. In the condition of fixed priority dual-task training, upon analysis, a large effect size was observed (Hedge’s g: 1.42) and 95% CI (0.79–2.05) in the positive domain. Similarly, in the condition of variable priority dual-task training, a large effect size was observed (Hedge’s g: 1.91) and 95% CI (1.19–2.63) in the positive domain. Thereby, demonstrating a beneficial effect of variable priority over fixed priority dual-task training method.
Figure 3.
Forest plot illustrating individual studies evaluating the effects of (A) dual-task training with fixed (FP) and variable (VP) priority in elderly participants, (B) dual-task training in elderly participants affected from stroke, (C) dual-task in postural stability of participants affected from multiple sclerosis, (D) dual-task in postural stability of elderly participants, (E) dual-task in postural stability of young participants.
Notes: Adjusted effect sizes; Hedge’s g (boxes), and 95% CI (whiskers) are presented, demonstrating repositioning errors for individual studies. Diamond represents pooled effect sizes and 95% CI. A negative mean difference indicates a favorable outcome for control groups; a positive mean difference indicates a favorable outcome for experimental groups.
Abbreviation: CI, confidence interval.
Second, two studies analyzing the effects of dual-task training on postural stability among patients affected from chronic stroke were included in the meta-analysis.48,62 The studies utilized a similar dual-task training duration phase of a 30-min session, three times a week for 8 weeks. Postural stability in the studies was assessed using functional reach test. Upon analysis, a large effect size was observed (Hedge’s g: 0.32), and 95% CI (−0.22 to 0.86) cm was reported in the positive domain, demonstrating a beneficial effect of within dual-task training to enhance postural stability (Figure 3B). Heterogeneity tests reported negligible heterogeneity (I2: 23.2%, P=0.24). The studies according to the PEDro methodological scale computed an average score of 4.8, indicating the average quality of the studies to be fair.
Neurological impairments
Eight studies evaluating the effects of dual-task performance on postural stability among participants affected by neurological disorders, such as cerebellar disorder, Parkinson’s disease,22,92–94 and multiple sclerosis,21,65,66,91 were included in the review. Significant enhancements in postural stability were reported in one good65 and one fair-quality study.94 Additionally, five fair-quality studies reported a significant reduction in postural stability among individuals affected by Parkinson’s disease,22,93,94 multiple sclerosis,66 and degenerative cerebellar disorder.91 One good-quality study reported a reduction in postural stability (not significant) among participants affected by Parkinson’s disease.92 Five studies evaluated the comparative effects between healthy participants and participants affected by neurological disorders,66,91,92,94 but one study evaluated the comparison between participants affected by mild and moderate multiple sclerosis.21 Also, two studies evaluated the inclusion of stable and unstable surfaces for maintaining postural stability while performing a dual task.65,91
A random-effect meta-analysis was conducted across one category, for evaluation of the effects of dual task on multiple sclerosis.21,65 Even though the two included studies conducted the tests using different dual tasks, the methodology and included participants were similar at baseline. The meta-analysis comprehensively demonstrated the differential effects of complexity of dual tasks on postural stability, ie, where on the one hand silent backward counting task improved the postural stability of the participants with multiple sclerosis, on the other hand incorporating word list generation task, incorporated by Boes et al,21 adversely impacted postural stability. Upon analysis, a large effect size was observed (Hedge’s g: −0.34) and 95% CI (−0.74 to 0.05) cm was reported marginally in the negative domain, demonstrating a differential effect of dual-task complexity on the postural stability of participants with multiple sclerosis (Figure 3C). Heterogeneity tests reported considerable heterogeneity (I2: 63.6%, P=0.08). The increased heterogeneity could be attributed to the differential complexity of dual tasks within the studies, which according to Vuillerme and Vincent97 might affect the outcome of the primary task. According to PEDro methodological scale, the studies overall scored an average of 4.8, indicating the quality of the studies to be fair.
Young and elderly
Twenty-six studies evaluated the effects of dual-task performance on postural stability among young, elderly, young/elderly, and participants with/without history of falls.16,17,20,67–80,82–89 Eleven fair-quality studies evaluated the effects of dual tasks on young participants.16,17,20,67,72,75,77,81,82,85,89 Four fair-quality studies reported significant enhancements in postural stability,16,17,77,85 whereas seven fair-quality studies reported significant reduction in postural stability.20,67,72,75,81,82,89
Two fair-quality studies evaluated the effects of dual tasks on elderly participants.70,74 Both the studies reported a significant reduction in postural stability post dual-task intervention.
Thirteen fair-quality studies compared the effects of dual tasks between young and elderly participants.68,69,71,73,76,78–80,83,84,86–88 Four studies included a comparison between elderly participants with/without history of falls.69,70,83,84 Three studies reported significant enhancements in postural stability among both young and elderly participants.68,76,86 Eight studies reported significant reductions in postural stability of elderly participants as compared to younger participants where enhancements in postural stability were observed.21,73,79,80,83,84,87,88 Two studies reported reduced postural stability (nonsignificant) among elderly participants; however, enhancements were observed in younger counterparts. Similarly, significantly reduced posturasl stability was reported for participants with prior history of fall as compared to their healthy counterparts.69,70,83,84 A random-effect meta-analysis was conducted across two categories for evaluation of the effects of dual task on healthy young participants. The two studies analyzed the postural stability using sensory orientation test; however, differential dual tasks were incorporated in the review.17,20 The methodology and included participants were similar at baseline. The meta-analysis comprehensively demonstrated the differential effects of complexity of dual tasks on postural stability, ie, where on the one hand auditory switch task improved the postural stability of the participants,17 on the other hand, incorporating a complex mental arithmetic task adversely impacted postural stability among young participants. Upon analysis, a trivial effect size was observed (Hedge’s g: −0.02) and 95% CI (−0.45 to 0.41)% was reported marginally in the negative domain, demonstrating a differential effect of dual-task complexity on the postural stability of young participants (Figure 3E). Heterogeneity tests reported considerable heterogeneity (I2: 48.2%, P=0.93), which could possibly be related to the differential complexity of the dual tasks incorporated within the studies. A second random-effect meta-analysis was conducted to evaluate the effects of dual task on elderly participants. The two studies analyzed the postural stability using length of center of pressure path; however, different dual tasks were included in the studies. Despite the complexity, these cognitive tasks demonstrated detrimental effects of dual tasks on postural stability of elderly participants. The methodology and included participants were similar at baseline. Upon analysis, a large effect size was observed (Hedge’s g: −1.15) and 95% CI (−1.67 to −0.63) cm was reported considerably in the negative domain, demonstrating a negative effect of dual-task complexity on the postural stability of elderly participants (Figure 3). Heterogeneity tests reported negligible heterogeneity (I2: 0%, P>0.01). The studies according to the PEDro methodological scale computed an average score of 4.2, indicating the average quality of the studies to be fair.
Discussion
This systematic review aimed to extend our understanding of the effects of dual tasks and dual-task training on static and dynamic postural stability among healthy and fall-prone population groups. Beneficial effects of dual-task training on postural stability of participants especially with poor balance capabilities were observed in this review. A PEDro 1b level of evidence and random-effect meta-analysis demonstrated the beneficial effects of dual-task training for enhancing postural stability among fall-prone population groups.
The review observed beneficial effects of dual-task training in studies analyzing patients affected from sub-acute34 and chronic stroke.48,62 The studies reported patients affected from stroke to possess considerable impairments in their cognitive–motor domain. Because of this, altered weight distribution has been reported in stroke patients while maintaining static and dynamic postures.98 However, An et al48 and Kim et al62 performed a dual-task training regime (30-min session, three times a week for 8 weeks) and reported beneficial effects on postural stability even for conditions with visual restriction and/or unstable base when presented with dual tasks. These enhancements were also evident in the meta-analysis where enhancements in functional reach test (Hedge’s g: 0.32) and 95% CI (−0.22 to 0.86) cm were observed. The authors justified the beneficial effects by suggesting prevention of tipping effect.48 This review, however, believes training could have possibly allowed skill acquisition for the cognitive and motor task while making the use of reactive forces, which in turn has been shown to reduce active muscular contraction.99 This can possibly aid in reduction of muscular coactivation and muscle guarding-related decrements in postural stability.6 A meta-analysis conducted by Wang et al51 also reported similar beneficial effects among stroke patients; 95% CI (0.54–5.21).
Furthermore, Silsupadol et al35 and Buragadda et al61 in their respective studies demonstrated a differential aspect of dual-task training with variable task prioritization. Meta-analysis revealed a beneficial effect of 95% CI (1.19–2.63) in variable priority as compared to 95% CI (0.79–2.05) in the fixed priority condition. The authors in their respective studies also reported enhancements in cognitive task performance, rate of learning, and ability to maintain skill level during follow-up period. Silsupadol et al35 interestingly affirmed the enhancements obtained because of dual-task training toward the task integration hypothesis, which states better development of task coordination skills following practicing with two tasks together. Likewise, Kramer et al100 in their study reported similar benefits during variable priority training and suggested that participants under variable priority conditions can learn to coordinate between two tasks during training. The authors speculated that the processing demand needed to perform a task was less when the attention was divided between two tasks. Moreover, the authors also reported a training effect during a 3-month follow-up within the variable priority condition as compared to fixed priority condition.35 According to Shigematsu et al,101 the training phase with a motor component enhances neural functioning and reduces response latency by effectively recruiting postural muscles resulting in improved sensory information processing. The review also identified radiological evidence by Erickson et al,102 which suggested enhanced cerebral hemodynamics in dorsolateral prefrontal cortex within the dual-task training group, and associated this effect with improved performance. In addition, certain centers of the brain associated with dual-task processing showed less activation posttraining, implying reduced processing demands posttraining.102 Some studies have also implied this training maneuver to act as a cognitive therapy for patients with attentional deficits and cognitive impairments.34,52 Furthermore, this review identified dual-task training regimes to also allow benefits in cognitive performance.38,52 According to Hiyamizu et al36 and Wollesen and Voelcker-Rehage,38 enhancements in cognitive performance might lead toward smoothening of cognitive activities while maintaining static and dynamic postures, resulting in preventing falls. The authors of the present review also believe that the enhancements in stability and dual-task performance are highly associated with the findings of Wolpert et al103 and Masters and Maxwell.9 In the present study, the initial phase of learning is suggested to be more cognitively driven as compared to the later stages of learning, which in a dual-task training setting might get more fluent and independent. Our results are in line with previously conducted systematic reviews, where dual-task training has been reported to enhance postural stability and cognitive performance.38,49,50,52 However, this review is the first to reveal beneficial effects of dual-task training in a meta-analysis and a level of evidence analysis.
This review observed detrimental effects of dual tasks on postural stability for the participants with higher predisposition to fall. For instance, complexity-associated reduction in postural stability was reported for patients affected with multiple sclerosis21 and Parkinson’s disease.93 Researchers suggest incorporation of two underlying theories for this detrimental effect, ie, bottleneck and capacity model theories.21,104 Boes et al21 suggested that since the patients with neurological impairments such as multiple sclerosis, stroke, Parkinson’s disease, and elderly participants have cognitive deficits, it is possible that the neurological capacity for these patients would be even less in terms of the aforementioned models. However, the findings of systematic reviews conducted by Learmonth et al31 and Wajda and Sosnoff30 concluded minimal effects of cognitive–motor interferences on postural stability for patients with multiple sclerosis and their healthy counterparts. The meta-analysis conducted by Learmonth et al31 revealed a small effect size of −0.11.
Furthermore, explaining the factors causing additional balance discrepancies in patients with parkinsonism, Bohnen et al105 and Andrade et al22 discussed that the dopaminergic and cholinergic pathways play a significant role in stabilizing the control of posture. These pathways play an important role in affecting the prioritization of posture and dual tasks within the central sharing model. The review conducted by Dirnberger and Jahanshahi106 supported these results and pointed out the considerable reduction in dopaminergic neuron in posterior putamen, anterior striatum, limbic nuclei, and neocortical extensions.107,108 As mentioned earlier, the basal ganglia–cortical network is involved in managing the “conscious” aspects of postural stability.6 Therefore, it might play an extensive role in causing considerable cognitive–motor interferences to reduce dual-task performance and postural stability and even promote posture “second” strategy.109 Marchese et al92 added that the dual task, ie, calculation, motor sequence of thumb opposition task, might have caused the Parkinson’s patients to shift their attention, further leading to disturbed conscious control and reduced stability. Interestingly, one study analyzing patients with parkinsonism revealed beneficial effects of dual-task application. The authors from the study suggested that the patients constrained their posture for directing attention toward the dual task, which ironically also enhanced their posture. However, the authors of the review argue that factors of complexity within a dual task have played a role for enhancing stability, ie, reduced anterior posterior sway during nonspeech conditions.
Brauer et al69,70 and Shumway-Cook et al84 reported postural stability and its recovery to be poorer among participants with prior history of fall as compared to their healthy counterparts, while performing a dual task (verbal reaction to auditory tone task and sentence completion with visual perception tasks). Radiological evidence by Herath et al110 and Szameitat et al111 reported the involvement of cortical areas along inferior frontal sulcus, middle frontal gyrus, and the intraparietal sulcus while performing auditory and visual reaction dual tasks. Therefore, suggesting that superimposing a dual task over already weak reorganized cortical structures may impart more stress and adversely impact postural stability.14 The findings of the present review are in line with recent review studies,6,26 where poor postural stability was also observed in fall-prone population groups as compared to their healthy younger counterparts.
Interestingly, the review found differential effects of dual tasks in studies evaluating healthy young participants and participants with balance deficits. For instance, researchers such as Vuillerme et al,89 Ramenzoni et al,82 Pellecchia,64 and Lanzarin et al20 reported detrimental effects of dual tasks on young participants; on the other hand Donker et al,16 Bergamin et al,68 Huxhold et al,76 Mak et al,78 Resch et al,17 and Hwang et al77 reported beneficial effects even among fall-prone elderly participants. In addition, beneficial effects of the dual-task application were also observed in participants with multiple sclerosis65 and Parkinson’s disease.94 Conventionally, according to published reports fall-prone population groups experience poor postural stability under the influence of higher information processing constraints. However, this review observed these differential results and suggests an inverse correlation between the complexity of the dual tasks and the postural stability. Researchers suggest that according to the Yerkes–Dodson law a U-shaped relation between cognitive demand and postural sway might reflect the level of arousal associated with dual cognitive task demand,76 thereby suggesting an increase in postural sway with added complexity in a cognitive task.
Jacobi et al91 analyzed the postural stability of ataxic and healthy controls using a verbal working memory task. The authors reported less center of pressure sway with reduced dual-task complexity for the ataxic group during a sensory orientation test. According to the authors, the involvement of cerebellum in both cognitive and motor tasks can result in increased interference,112 thereby affecting dual-task and postural performance. Also, the role of cerebellum has been reported especially during the performance of dual tasks while maintaining executive control including working memory, language, and visuospatial information.113 Radiological evidence also demonstrates increased BOLD (blood oxygen level dependent) response in the cerebellar vermis and anterior lobe while simultaneous performance of cognitive–motor tasks.114 This review also observed articulation as a major factor for complexity in terms of a dual task, yielding differential effects upon postural stability. Bensoussan et al,115 Marchese et al,92 and Yardley et al,116 for instance, reported detrimental effects of aloud verbal, arithmetic tasks on postural stability. On the contrary, Negahban et al65 and Lanzarin et al20 reported beneficial effects of nonverbal tasks on postural stability of fall-prone participants. Literature analysis revealed research studies identifying commonly used dual tasks such as verbal recital, n-back, and counting backward to be considered as more cognitively driven.117 This review also observed the studies to ignore the verbal and hearing component incorporated in a dual-task paradigm. A functional magnetic resonance imaging analysis by Behroozmand et al118 revealed the involvement of bilateral superior temporal gyrus, Heschl’s gyrus, precentral gyrus, supplementary motor area, Rolandic operculum, postcentral gyrus, putamen insula, and right inferior frontal gyrus during speech production.119 Moreover, the authors mentioned that speech production is also followed by a feedback error detection system in the sensory cortex that again activates the motor areas for speech adjustments, therefore suggesting the auditory feedback as an additional factor for increasing complexity in a dual-task setting.
Yardley et al116 speculated the interaction between muscular control of speech-associated respiration and posture to cause perturbation in posture. The authors compared complex articulated, mental tasks while analyzing postural stability and reported beneficial effects on stability in the absence of articulation. This present review also suggests that the reinvolvement of higher motor centers during speech production in a dual task might possibly result in central interference, which might impact the person’s dual task and stability performance. This review also adds to the existing knowledge that dual-task paradigms involving only a mental component, such as mental arithmetic task, might also include a motor component. As mentioned earlier, hearing also incorporates activation of cortical structures, precisely bilateral superior temporal gyrus, and Heschl’s gyrus.118 The phase of instructions might activate this cortical pathway and can add to the certain amount of complexity in the dual-task scenario, which although trivial might result in considerable adverse effects in fall-prone population groups. This review did not find any study that analyzed the effects of dual-task posture in the absence of auditory information, ie, via noise canceling headphones, white noise; therefore possibly explaining the reduction in stability for studies employing nonverbal dual tasks.20,67
In summary, a systematic review was conducted across five online academic search databases: Scopus, PEDro, MEDLINE, EMBASE, and SportDiscus. A total of 1,284 articles were incorporated in our initial search, which later on implementing our inclusion criteria were reduced to 42 (Figure 1). The meta-analysis conducted on studies suggested beneficial effects of dual-task training with variable priority for enhancing postural stability, especially among elderly participants. Moreover, an inverse relation was observed between the complexity of dual task and postural stability. This review also observed an articulation component within a dual task to be a component of added complexity, which further might enhance cognitive–motor interference in fall-prone population groups. This study also reveals detrimental effects of complex dual tasks among population groups with a higher predisposition to fall, as compared to their healthy counterparts.
Strengths
This present review is the first to analyze and compare the effects of dual-task training and dual task on postural stability. Respective authors of the included papers were contacted for additional descriptive data or information. The review conformed to PRISMA guidelines in all applicable areas. A meta-analysis and a PEDro level of evidence were included for the studies included in this present review. The data used to compute the meta-analysis were used from the descriptive statistics and not identified from figures to reduce the incidence of bias. This present review was also an effort to address the limitations pertained by previously conducted reviews. For instance, a few of the previous systematic reviews carried out the search across few academic databases. For instance, Ruffieux et al26 conducted the search across two academic databases, Boisgontier et al6 across three databases, and Agmon et al49 across four academic databases. This present review identified five widely utilized and reputed academic databases and continuously updated the data over a duration of 9 months. Additionally, few keyword search terms were identified as a possible limitation factor in the previous systematic reviews. However, during our literature search the authors utilized a broad variety of MeSH keyword search terms (Supplementary material), which might have increased the possibilities of including a wide array of studies. The meta-analysis carried out in this present review is the first to evaluate the effects of dual-task training on elderly participants. However, it also aimed to replicate previous findings, while addressing the increased heterogeneity.
Limitations
Several limitations persisted in the systematic review, which are to be considered while interpreting the results. The average quality of the included studies according to PEDro methodological quality scale was found to be 4.7, indicating a fair quality of the studies. A high risk of bias prevailed because of the limited number of RCTs. The restriction of search strategy limited to English language, exclusion of conference proceedings and observational studies might have resulted in omission of relevant research. Inability to retrieve descriptive statistics from the respective studies and including fewer studies in the meta-analysis was also a major limitation of this study.
This present study did not impose restrictions on the type of included dual task, in order to analyze the differential effects of complexity of dual task. Therefore, a higher chance of biasing and differential outcomes can be expected. Likewise, the systematic difference between the population group base statistics related to age, weight, gender, and disease severity led to difficulty in comparing studies. A majority of the incorporated studies had a small sample size, which generates a high possibility of a type II error.120 The conclusions derived in the review based on incorporation of dual-task training in rehabilitation protocol are based on limited research.
Future directions
Future studies should focus on combining easier, nonverbal dual tasks in training during rehabilitation. Neuroimaging studies can provide additional insights for mechanisms involved during execution of nonverbal dual tasks. The review also suggests training fall-prone population groups to prioritize balance, ie, posture “first” in complex fall-prone environments; for instance escalators, narrow alleyways.121 Likewise, nonverbal tasks utilized during activity of daily living can be analyzed in dual-task training regimes. Together, real-life implications can be drawn from these studies.
Acknowledgments
The publication of this article was funded by the Open Access fund of Leibniz Universität Hannover. The authors would like to thank Dr Matthew W Driller, Prof Dr Rich SW Masters for their constructive comments. The coauthor expresses sincere gratitude toward Education New Zealand and the University of Waikato for awarding research scholarships.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wikstrom EA, Tillman MD, Smith AN, Borsa PA. A new force-plate technology measure of dynamic postural stability: the dynamic postural stability index. J Athl Train. 2005;40(4):305–309. [PMC free article] [PubMed] [Google Scholar]
- 2.Ghai S, Driller M, Ghai I. Effects of joint stabilizers on proprioception and stability: a systematic review and meta-analysis. Phys Ther Sport. 2016 doi: 10.1016/j.ptsp.2016.05.006. In press. [DOI] [PubMed] [Google Scholar]
- 3.Goble DJ, Coxon JP, Wenderoth N, Van Impe A, Swinnen SP. Proprioceptive sensibility in the elderly: degeneration, functional consequences and plastic-adaptive processes. Neurosci Biobehav Rev. 2009;33(3):271–278. doi: 10.1016/j.neubiorev.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Vaugoyeau M, Viel S, Amblard B, Azulay J, Assaiante C. Proprioceptive contribution of postural control as assessed from very slow oscillations of the support in healthy humans. Gait Posture. 2008;27(2):294–302. doi: 10.1016/j.gaitpost.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Raftopoulos A. Cognitive Penetrability of Perception: Attention, Action, Strategies, and Bottom-Up Constraints. New York, NY: Nova Publishers; 2005. [Google Scholar]
- 6.Boisgontier MP, Beets IA, Duysens J, Nieuwboer A, Krampe RT, Swinnen SP. Age-related differences in attentional cost associated with postural dual tasks: increased recruitment of generic cognitive resources in older adults. Neurosci Biobehav Rev. 2013;37(8):1824–1837. doi: 10.1016/j.neubiorev.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs J, Horak F. Cortical control of postural responses. J Neural Transm. 2007;114(10):1339–1348. doi: 10.1007/s00702-007-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honeycutt CF, Gottschall JS, Nichols TR. Electromyographic responses from the hindlimb muscles of the decerebrate cat to horizontal support surface perturbations. J Neurophysiol. 2009;101(6):2751–2761. doi: 10.1152/jn.91040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masters RSW, Maxwell J. The theory of reinvestment. Int Rev Sport Exer Psychol. 2008;1(2):160–183. [Google Scholar]
- 10.Wulf G, McNevin N, Shea CH. The automaticity of complex motor skill learning as a function of attentional focus. Q J Exp Psychol A. 2001;54(4):1143–1154. doi: 10.1080/713756012. [DOI] [PubMed] [Google Scholar]
- 11.Masters RSW. Knowledge, knerves and know-how: the role of explicit versus implicit knowledge in the breakdown of a complex motor skill under pressure. Br J Psychol. 1992;83(3):343–358. [Google Scholar]
- 12.Schaefer S, Schellenbach M, Lindenberger U, Woollacott M. Walking in high-risk settings: do older adults still prioritize gait when distracted by a cognitive task? Exp Brain Res. 2015;233(1):79–88. doi: 10.1007/s00221-014-4093-8. [DOI] [PubMed] [Google Scholar]
- 13.Seidler RD, Bernard JA, Burutolu TB, et al. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev. 2010;34(5):721–733. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talelli P, Ewas A, Waddingham W, Rothwell J, Ward N. Neural correlates of age-related changes in cortical neurophysiology. Neuroimage. 2008;40(4):1772–1781. doi: 10.1016/j.neuroimage.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaefer S, Jagenow D, Verrel J, Lindenberger U. The influence of cognitive load and walking speed on gait regularity in children and young adults. Gait Posture. 2015;41(1):258–262. doi: 10.1016/j.gaitpost.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Donker SF, Roerdink M, Greven AJ, Beek PJ. Regularity of center-of-pressure trajectories depends on the amount of attention invested in postural control. Exp Brain Res. 2007;181(1):1–11. doi: 10.1007/s00221-007-0905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Resch JE, May B, Tomporowski PD, Ferrara MS. Balance performance with a cognitive task: a continuation of the dual-task testing paradigm. J Athl Train. 2011;46(2):170. doi: 10.4085/1062-6050-46.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beilock SL, Carr TH. On the fragility of skilled performance: what governs choking under pressure? J Exp Psychol Gen. 2001;130(4):701–725. [PubMed] [Google Scholar]
- 19.Ghai S, Driller MW, Masters RS. The influence of below-knee compression garments on knee-joint proprioception. Gait Posture. 2016 Aug 9; doi: 10.1016/j.gaitpost.2016.08.008. Epub. [DOI] [PubMed] [Google Scholar]
- 20.Lanzarin M, Parizzoto P, Libardoni TDC, Sinhorim L, Tavares GMS, Santos GM. The influence of dual-tasking on postural control in young adults. Fisioter Pesquisa. 2015;22(1):61–68. [Google Scholar]
- 21.Boes MK, Sosnoff JJ, Socie MJ, Sandroff BM, Pula JH, Motl RW. Postural control in multiple sclerosis: effects of disability status and dual task. J Neurol Sci. 2012;315(1):44–48. doi: 10.1016/j.jns.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Andrade LPD, Rinaldi NM, Coelho FGDM, Tanaka K, Stella F, Gobbi LTB. Dual task and postural control in Alzheimer’s and Parkinson’s disease. Motriz Rev Ed Fís. 2014;20(1):78–84. [Google Scholar]
- 23.Montero-Odasso M, Bergman H, Phillips NA, Wong CH, Sourial N, Chertkow H. Dual-tasking and gait in people with mild cognitive impairment. The effect of working memory. BMC Geriatr. 2009;9(1):41. doi: 10.1186/1471-2318-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16(1):1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- 25.Hortobágyi T, del Olmo MF, Rothwell JC. Age reduces cortical reciprocal inhibition in humans. Exp Brain Res. 2006;171(3):322–329. doi: 10.1007/s00221-005-0274-9. [DOI] [PubMed] [Google Scholar]
- 26.Ruffieux J, Keller M, Lauber B, Taube W. Changes in standing and walking performance under dual-task conditions across the lifespan. Sports Med. 2015;45(12):1739–1758. doi: 10.1007/s40279-015-0369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujiyama H, Hinder MR, Schmidt MW, Garry MI, Summers JJ. Age-related differences in corticospinal excitability and inhibition during coordination of upper and lower limbs. Neurobiol Aging. 2012;33(7):e1481–e1484. doi: 10.1016/j.neurobiolaging.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 28.Fujita H, Kasubuchi K, Wakata S, Hiyamizu M, Morioka S. Role of the frontal cortex in standing postural sway tasks while dual-tasking: a functional near-infrared spectroscopy study examining working memory capacity. Biomed Res Int. 2016;2016:7053867. doi: 10.1155/2016/7053867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith E, Cusack T, Blake C. The effect of a dual task on gait speed in community dwelling older adults: a systematic review and meta-analysis. Gait Posture. 2016;44:250–258. doi: 10.1016/j.gaitpost.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Wajda DA, Sosnoff JJ. Cognitive-motor interference in multiple sclerosis: a systematic review of evidence, correlates, and consequences. Biomed Res Int. 2015;2015:720856. doi: 10.1155/2015/720856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Learmonth YC, Ensari I, Motl RW. Cognitive motor interference in multiple sclerosis: insights from a systematic quantitative review. Arch Phys Med Rehabil. 2016 Aug 16; doi: 10.1016/j.apmr.2016.07.018. Epub. [DOI] [PubMed] [Google Scholar]
- 32.Zanotto T, Bergamin M, Roman F, et al. Effect of exercise on dual-task and balance on elderly in multiple disease conditions. Curr Aging Sci. 2014;7(2):115–136. doi: 10.2174/1874609807666140328095544. [DOI] [PubMed] [Google Scholar]
- 33.Gobbo S, Bergamin M, Sieverdes JC, Ermolao A, Zaccaria M. Effects of exercise on dual-task ability and balance in older adults: a systematic review. Arch Gerontol Geriatr. 2014;58(2):177–187. doi: 10.1016/j.archger.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Choi JH, Kim BR, Han EY, Kim SM. The effect of dual-task training on balance and cognition in patients with subacute post-stroke. Ann Rehabil Med. 2015;39(1):81–90. doi: 10.5535/arm.2015.39.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silsupadol P, Shumway-Cook A, Lugade V, et al. Effects of single-task versus dual-task training on balance performance in older adults: a double-blind, randomized controlled trial. Arch Phys Med Rehabil. 2009;90(3):381–387. doi: 10.1016/j.apmr.2008.09.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hiyamizu M, Morioka S, Shomoto K, Shimada T. Effects of dual task balance training on dual task performance in elderly people: a randomized controlled trial. Clin Rehabil. 2012;26(1):58–67. doi: 10.1177/0269215510394222. [DOI] [PubMed] [Google Scholar]
- 37.Gomez-Pinilla F, Hillman C. The influence of exercise on cognitive abilities. Compr Physiol. 2013;3(1):403–428. doi: 10.1002/cphy.c110063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wollesen B, Voelcker-Rehage C. Training effects on motor–cognitive dual-task performance in older adults. Eur Rev Aging Phys Act. 2013;11(1):5. [Google Scholar]
- 39.Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown S, Bennett E. The role of practice and automaticity in temporal and nontemporal dual-task performance. Psychol Res. 2002;66(1):80–89. doi: 10.1007/s004260100076. [DOI] [PubMed] [Google Scholar]
- 41.Müller H, Blischke K. Grundlagen der Sportpsychologie. [Basics of sports psychology] Limpert Verlag; Wiesbaden: 2009. German. [Google Scholar]
- 42.Bherer L, Kramer AF, Peterson MS, Colcombe S, Erickson K, Becic E. Training effects on dual-task performance: are there age-related differences in plasticity of attentional control? Psychol Aging. 2005;20(4):695. doi: 10.1037/0882-7974.20.4.695. [DOI] [PubMed] [Google Scholar]
- 43.Heuer H. Motor Learning as a Process of Structural Constriction and Displacement. Berlin: Springer; 1984. pp. 295–305. [Google Scholar]
- 44.Blischke K, Reiter C. Bewegungsautomatisierung durch Doppeltätigkeits-Üben. [Movement automation by double action practicing.] Spectrum der Sportwissenschaften. 2002;14:8–29. [Google Scholar]
- 45.Blischke K. Automatisierung einer großmotorischen Kalibrierungsaufgabe durch Prozeduralisierung. [Automation of a large-scale calibration task by proceduralization] Psychol Sport. 2001;8:19–38. [Google Scholar]
- 46.Blischke K, Wagner F, Zehren B, Brueckner S. Dual-task practice of temporally structured movement sequences augments integrated task processing, but not automatization. J Human Kinet. 2010;25:5–15. [Google Scholar]
- 47.Bherer L, Kramer AF, Peterson MS, Colcombe S, Erickson K, Becic E. Transfer effects in task-set cost and dual-task cost after dual-task training in older and younger adults: further evidence for cognitive plasticity in attentional control in late adulthood. Exp Aging Res. 2008;34(3):188–219. doi: 10.1080/03610730802070068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.An H-J, Kim J-I, Kim Y-R, et al. The effect of various dual task training methods with gait on the balance and gait of patients with chronic stroke. J Phys Ther Sci. 2014;26(8):1287–1291. doi: 10.1589/jpts.26.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agmon M, Belza B, Nguyen HQ, Logsdon RG, Kelly VE. A systematic review of interventions conducted in clinical or community settings to improve dual-task postural control in older adults. Clin Interv Aging. 2014;9:477–492. doi: 10.2147/CIA.S54978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fritz NE, Cheek FM, Nichols-Larsen DS. Motor-cognitive dual-task training in persons with neurologic disorders: a systematic review. J Neurol Phys Ther. 2015;39(3):142–153. doi: 10.1097/NPT.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang XQ, Pi YL, Chen BL, et al. Cognitive motor interference for gait and balance in stroke: a systematic review and meta-analysis. Eur J Neurol. 2015;22(3):555–e37. doi: 10.1111/ene.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pichierri G, Wolf P, Murer K, de Bruin ED. Cognitive and cognitive-motor interventions affecting physical functioning: a systematic review. BMC Geriatr. 2011;11(1):1. doi: 10.1186/1471-2318-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 54.Steindl R, Ulmer H. Standstabilität im kindes-und jugendalter. [Stability in childhood and adolescence] HNO. 2004;52(5):423–430. doi: 10.1007/s00106-003-0928-5. [DOI] [PubMed] [Google Scholar]
- 55.Centre for Reviews and Dissemination . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. Centre for Reviews and Dissemination. York: York Publishing Services Ltd; 2009. [Google Scholar]
- 56.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5. Wiley Online Library; 2008. [Accessed August 3, 2016]. Available from: http://handbook.cochrane.org. [Google Scholar]
- 57.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–721. [PubMed] [Google Scholar]
- 58.Teasell RW, Foley NC, Bhogal SK, Speechley MR. Evidence-Based Review of Stroke Rehabilitation. Topics in Stroke Rehabilitation. 2003;10(1):29–58. doi: 10.1310/8YNA-1YHK-YMHB-XTE1. [DOI] [PubMed] [Google Scholar]
- 59.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 60.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009] The Cochrane Collaboration; 2008. [Google Scholar]
- 61.Buragadda S, Alyaemni A, Melam GR, Alghamdi MA. Effect of dual-task training (fixed priority-versus-variable priority) for improving balance in older adults. World Appl Sci J. 2012;20(6):884–888. [Google Scholar]
- 62.Kim D, Ko J, Woo Y. Effects of dual task training with visual restriction and an unstable base on the balance and attention of stroke patients. J Phys Ther Sci. 2013;25(12):1579–1582. doi: 10.1589/jpts.25.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li KZ, Roudaia E, Lussier M, Bherer L, Leroux A, McKinley P. Benefits of cognitive dual-task training on balance performance in healthy older adults. J Gerontol A Biol Sci Med Sci. 2010;65(12):1344–1352. doi: 10.1093/gerona/glq151. [DOI] [PubMed] [Google Scholar]
- 64.Pellecchia GL. Dual-task training reduces impact of cognitive task on postural sway. J Mot Behav. 2005;37(3):239–246. doi: 10.3200/JMBR.37.3.239-246. [DOI] [PubMed] [Google Scholar]
- 65.Negahban H, Mofateh R, Arastoo AA, et al. The effects of cognitive loading on balance control in patients with multiple sclerosis. Gait Posture. 2011;34(4):479–484. doi: 10.1016/j.gaitpost.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 66.Prosperini L, Castelli L, Sellitto G, et al. Investigating the phenomenon of “cognitive-motor interference” in multiple sclerosis by means of dual-task posturography. Gait Posture. 2015;41(3):780–785. doi: 10.1016/j.gaitpost.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 67.Andersson G, Hagman J, Talianzadeh R, Svedberg A, Larsen HC. Effect of cognitive load on postural control. Brain Res Bull. 2002;58(1):135–139. doi: 10.1016/s0361-9230(02)00770-0. [DOI] [PubMed] [Google Scholar]
- 68.Bergamin M, Gobbo S, Zanotto T, et al. Influence of age on postural sway during different dual-task conditions. Front Aging Neurosci. 2014;6:271–277. doi: 10.3389/fnagi.2014.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brauer S, Woollacott M, Shumway-Cook A. The influence of a concurrent cognitive task on the compensatory stepping response to a perturbation in balance-impaired and healthy elders. Gait Posture. 2002;15(1):83–93. doi: 10.1016/s0966-6362(01)00163-1. [DOI] [PubMed] [Google Scholar]
- 70.Brauer SG, Woollacott M, Shumway-Cook A. The interacting effects of cognitive demand and recovery of postural stability in balance-impaired elderly persons. J Gerontol A Biol Sci Med Sci. 2001;56(8):M489–M496. doi: 10.1093/gerona/56.8.m489. [DOI] [PubMed] [Google Scholar]
- 71.Brown LA, Shumway-Cook A, Woollacott MH. Attentional demands and postural recovery: the effects of aging. J Gerontol A Biol Sci Med Sci. 1999;54(4):M165–M171. doi: 10.1093/gerona/54.4.m165. [DOI] [PubMed] [Google Scholar]
- 72.Dault MC, Geurts AC, Mulder TW, Duysens J. Postural control and cognitive task performance in healthy participants while balancing on different support-surface configurations. Gait Posture. 2001;14(3):248–255. doi: 10.1016/s0966-6362(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 73.Doumas M, Smolders C, Krampe RT. Task prioritization in aging: effects of sensory information on concurrent posture and memory performance. Exp Brain Res. 2008;187(2):275–281. doi: 10.1007/s00221-008-1302-3. [DOI] [PubMed] [Google Scholar]
- 74.Haggerty S, Jiang L-T, Galecki A, Sienko KH. Effects of biofeedback on secondary-task response time and postural stability in older adults. Gait Posture. 2012;35(4):523–528. doi: 10.1016/j.gaitpost.2011.10.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hunter MC, Hoffman MA. Postural control: visual and cognitive manipulations. Gait Posture. 2001;13(1):41–48. doi: 10.1016/s0966-6362(00)00089-8. [DOI] [PubMed] [Google Scholar]
- 76.Huxhold O, Li S-C, Schmiedek F, Lindenberger U. Dual-tasking postural control: aging and the effects of cognitive demand in conjunction with focus of attention. Brain Res Bull. 2006;69(3):294–305. doi: 10.1016/j.brainresbull.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 77.Hwang JH, Lee C-H, Chang HJ, Park D-S. Sequential analysis of postural control resource allocation during a dual task test. Ann Rehabil Med. 2013;37(3):347–354. doi: 10.5535/arm.2013.37.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mak L, Yeh TT, Boulet J, Cluff T, Balasubramaniam R. Interaction between delayed visual feedback and secondary cognitive tasks on postural control in older adults. Science & Motricité. 2011;74:81–88. [Google Scholar]
- 79.Marsh AP, Geel SE. The effect of age on the attentional demands of postural control. Gait Posture. 2000;12(2):105–113. doi: 10.1016/s0966-6362(00)00074-6. [DOI] [PubMed] [Google Scholar]
- 80.Melzer I, Benjuya N, Kaplanski J. Age-related changes of postural control: effect of cognitive tasks. Gerontology. 2001;47(4):189–194. doi: 10.1159/000052797. [DOI] [PubMed] [Google Scholar]
- 81.Pellecchia GL. Postural sway increases with attentional demands of concurrent cognitive task. Gait Posture. 2003;18(1):29–34. doi: 10.1016/s0966-6362(02)00138-8. [DOI] [PubMed] [Google Scholar]
- 82.Ramenzoni VC, Riley MA, Shockley K, Chiu C-YP. Postural responses to specific types of working memory tasks. Gait Posture. 2007;25(3):368–373. doi: 10.1016/j.gaitpost.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 83.Shumway-Cook A, Woollacott M. Attentional demands and postural control: the effect of sensory context. J Gerontol A Biol Sci Med Sci. 2000;55(1):M10. doi: 10.1093/gerona/55.1.m10. [DOI] [PubMed] [Google Scholar]
- 84.Shumway-Cook A, Woollacott M, Kerns KA, Baldwin M. The effects of two types of cognitive tasks on postural stability in older adults with and without a history of falls. J Gerontol A Biol Sci Med Sci. 1997;52(4):M232–M240. doi: 10.1093/gerona/52a.4.m232. [DOI] [PubMed] [Google Scholar]
- 85.Swan L, Otani H, Loubert PV. Reducing postural sway by manipulating the difficulty levels of a cognitive task and a balance task. Gait Posture. 2007;26(3):470–474. doi: 10.1016/j.gaitpost.2006.11.201. [DOI] [PubMed] [Google Scholar]
- 86.Swan L, Otani H, Loubert PV, Sheffert SM, Dunbar GL. Improving balance by performing a secondary cognitive task. Br J Psychol. 2004;95(1):31–40. doi: 10.1348/000712604322779442. [DOI] [PubMed] [Google Scholar]
- 87.Teasdale N, Bard C, LaRue J, Fleury M. On the cognitive penetrability of posture control. Exp Aging Res. 1993;19(1):1–13. doi: 10.1080/03610739308253919. [DOI] [PubMed] [Google Scholar]
- 88.Teasdale N, Simoneau M. Attentional demands for postural control: the effects of aging and sensory reintegration. Gait Posture. 2001;14(3):203–210. doi: 10.1016/s0966-6362(01)00134-5. [DOI] [PubMed] [Google Scholar]
- 89.Vuillerme N, Isableu B, Nougier V. Attentional demands associated with the use of a light fingertip touch for postural control during quiet standing. Exp Brain Res. 2006;169(2):232–236. doi: 10.1007/s00221-005-0142-7. [DOI] [PubMed] [Google Scholar]
- 90.de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55(2):129–133. doi: 10.1016/s0004-9514(09)70043-1. [DOI] [PubMed] [Google Scholar]
- 91.Jacobi H, Alfes J, Minnerop M, Konczak J, Klockgether T, Timmann D. Dual task effect on postural control in patients with degenerative cerebellar disorders. Cerebellum Ataxias. 2015;2(1):6. doi: 10.1186/s40673-015-0025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marchese R, Bove M, Abbruzzese G. Effect of cognitive and motor tasks on postural stability in Parkinson’s disease: a posturographic study. Mov Disord. 2003;18(6):652–658. doi: 10.1002/mds.10418. [DOI] [PubMed] [Google Scholar]
- 93.Morris M, Iansek R, Smithson F, Huxham F. Postural instability in Parkinson’s disease: a comparison with and without a concurrent task. Gait Posture. 2000;12(3):205–216. doi: 10.1016/s0966-6362(00)00076-x. [DOI] [PubMed] [Google Scholar]
- 94.Holmes J, Jenkins M, Johnson AM, Adams S, Spaulding S. Dual-task interference: the effects of verbal cognitive tasks on upright postural stability in Parkinson’s disease. Parkinsons Dis. 2010;2010:696492. doi: 10.4061/2010/696492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Teasell R, Marshall S, Cullen N, et al. Evidence Based Review of Moderate to Severe Acquired Brain Injury. Toronto, ON: Ontario Neurotrauma Foundation; 2005. [Google Scholar]
- 96.Bolier L, Haverman M, Westerhof GJ, Riper H, Smit F, Bohlmeijer E. Positive psychology interventions: a meta-analysis of randomized controlled studies. BMC Public Health. 2013;13(1):119. doi: 10.1186/1471-2458-13-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vuillerme N, Vincent H. How performing a mental arithmetic task modify the regulation of centre of foot pressure displacements during bipedal quiet standing. Exp Brain Res. 2006;169(1):130–134. doi: 10.1007/s00221-005-0124-9. [DOI] [PubMed] [Google Scholar]
- 98.Tyson SF, Hanley M, Chillala J, Selley A, Tallis RC. Balance disability after stroke. Phys Ther. 2006;86(1):30–38. doi: 10.1093/ptj/86.1.30. [DOI] [PubMed] [Google Scholar]
- 99.Vereijken B, Whiting H, Beek W. A dynamical systems approach to skill acquisition. Q J Exp Psychol. 1992;45(2):323–344. doi: 10.1080/14640749208401329. [DOI] [PubMed] [Google Scholar]
- 100.Kramer AF, Larish JF, Strayer DL. Training for attentional control in dual task settings: a comparison of young and old adults. J Exp Psychol Appl. 1995;1(1):50. [Google Scholar]
- 101.Shigematsu R, Okura T, Nakagaichi M, et al. Square-stepping exercise and fall risk factors in older adults: a single-blind, randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2008;63(1):76–82. doi: 10.1093/gerona/63.1.76. [DOI] [PubMed] [Google Scholar]
- 102.Erickson KI, Colcombe SJ, Wadhwa R, et al. Training-induced functional activation changes in dual-task processing: an FMRI study. Cereb Cortex. 2007;17(1):192–204. doi: 10.1093/cercor/bhj137. [DOI] [PubMed] [Google Scholar]
- 103.Wolpert DM, Diedrichsen J, Flanagan JR. Principles of sensorimotor learning. Nat Rev Neurosci. 2011;12(12):739–751. doi: 10.1038/nrn3112. [DOI] [PubMed] [Google Scholar]
- 104.Tombu M, Jolicoeur P. All-or-none bottleneck versus capacity sharing accounts of the psychological refractory period phenomenon. Psychol Res. 2002;66(4):274–286. doi: 10.1007/s00426-002-0101-x. [DOI] [PubMed] [Google Scholar]
- 105.Bohnen N, Müller M, Koeppe R, et al. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009;73(20):1670–1676. doi: 10.1212/WNL.0b013e3181c1ded6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dirnberger G, Jahanshahi M. Executive dysfunction in Parkinson’s disease: a review. J Neuropsychol. 2013;7(2):193–224. doi: 10.1111/jnp.12028. [DOI] [PubMed] [Google Scholar]
- 107.Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. N Engl J Med. 1988;318(14):876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- 108.Kalaitzakis ME, Pearce RK. The morbid anatomy of dementia in Parkinson’s disease. Acta Neuropathol. 2009;118(5):587–598. doi: 10.1007/s00401-009-0597-x. [DOI] [PubMed] [Google Scholar]
- 109.Yogev-Seligmann G, Hausdorff JM, Giladi N. Do we always prioritize balance when walking? Towards an integrated model of task prioritization. Mov Disord. 2012;27(6):765–770. doi: 10.1002/mds.24963. [DOI] [PubMed] [Google Scholar]
- 110.Herath P, Klingberg T, Young J, Amunts K, Roland P. Neural correlates of dual task interference can be dissociated from those of divided attention: an fMRI study. Cereb Cortex. 2001;11(9):796–805. doi: 10.1093/cercor/11.9.796. [DOI] [PubMed] [Google Scholar]
- 111.Szameitat AJ, Schubert T, Müller K, Von Cramon DY. Localization of executive functions in dual-task performance with fMRI. J Cogn Neurosci. 2002;14(8):1184–1199. doi: 10.1162/089892902760807195. [DOI] [PubMed] [Google Scholar]
- 112.Schmahmann JD, Pandyat DN. The cerebrocerebellar system. Int Rev Neurobiol. 1997;41:31–60. doi: 10.1016/s0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- 113.Timmann D, Daum I. Cerebellar contributions to cognitive functions: a progress report after two decades of research. Cerebellum. 2007;6(3):159–162. doi: 10.1080/14734220701496448. [DOI] [PubMed] [Google Scholar]
- 114.Wu T, Liu J, Hallett M, Zheng Z, Chan P. Cerebellum and integration of neural networks in dual-task processing. Neuroimage. 2013;65:466–475. doi: 10.1016/j.neuroimage.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bensoussan L, Viton J-M, Schieppati M, et al. Changes in postural control in hemiplegic patients after stroke performing a dual task. Arch Phys Med Rehabil. 2007;88(8):1009–1015. doi: 10.1016/j.apmr.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 116.Yardley L, Gardner M, Leadbetter A, Lavie N. Effect of articulatory and mental tasks on postural control. Neuroreport. 1999;10(2):215–219. doi: 10.1097/00001756-199902050-00003. [DOI] [PubMed] [Google Scholar]
- 117.Kane MJ, Conway AR, Miura TK, Colflesh GJ. Working memory, attention control, and the N-back task: a question of construct validity. J Exp Psychol Learn Mem Cogn. 2007;33(3):615. doi: 10.1037/0278-7393.33.3.615. [DOI] [PubMed] [Google Scholar]
- 118.Behroozmand R, Shebek R, Hansen DR, et al. Sensory–motor networks involved in speech production and motor control: an fMRI study. Neuroimage. 2015;109:418–428. doi: 10.1016/j.neuroimage.2015.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Parkinson AL, Flagmeier SG, Manes JL, Larson CR, Rogers B, Robin DA. Understanding the neural mechanisms involved in sensory control of voice production. Neuroimage. 2012;61(1):314–322. doi: 10.1016/j.neuroimage.2012.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Freiman JA, Chalmers TC, Smith H, Jr, Kuebler RR. The importance of beta, the type II error and sample size in the design and interpretation of the randomized control trial: survey of 71 negative trials. N Engl J Med. 1978;299(13):690–694. doi: 10.1056/NEJM197809282991304. [DOI] [PubMed] [Google Scholar]
- 121.Bloem BR, Grimbergen YA, van Dijk JG, Munneke M. The “posture second” strategy: a review of wrong priorities in Parkinson’s disease. J Neurol Sci. 2006;248(1):196–204. doi: 10.1016/j.jns.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 122.Ramsey L, Winder RJ, McVeigh JG. The effectiveness of working wrist splints in adults with rheumatoid arthritis: a mixed methods systematic review. J Rehabil Med. 2014;46(6):481–492. doi: 10.2340/16501977-1804. [DOI] [PubMed] [Google Scholar]