Abstract
Objective: Professionals have periodically expressed concern that atypical antipsychotics may cause cognitive blunting in treated patients. In this study, we report data from a double-blind, randomized, controlled study of stimulant plus placebo versus combined stimulant and risperidone to evaluate the effects of the atypical antipsychotic on attention and short-term memory.
Methods: A total of 165 (n = 83 combined treatment; n = 82 stimulant plus placebo) children with attention-deficit/hyperactivity disorder and severe physical aggression, aged 6–12 years, were evaluated with Conners' Continuous Performance Test (CPT-II) and the Wechsler Intelligence Scale for Children-III (WISC) Digit Span subscale at baseline, after 3 weeks of stimulant-only treatment, and after six additional weeks of randomized treatment (stimulant+placebo vs. stimulant+risperidone).
Results: At 3 weeks, improvement on CPT-II performance (Commissions and Reaction Time Standard Error; p < 0.001) and on Digit Span memory performance (p < 0.006) was noted for the full sample. At study week 9, no difference in CPT-II or Digit Span performance was observed between the randomized groups (ps = 0.41 to 0.83).
Conclusions: Similar to other studies, we found no deleterious effects on attention and short-term memory associated with short-term use of risperidone. NCT00796302.
Keywords: : Risperidone, CNS stimulants, attention-deficit/hyperactivity disorder, conduct disorder, oppositional defiant disorder
Introduction
Psychostimulant treatment (e.g., methylphenidate, amphetamine, dextroamphetamine) has been a mainstay of attention-deficit/hyperactivity disorder (ADHD) treatment in youth for the last five decades; clinically, stimulants appear to enhance sustained attention and effort on assigned tasks, to reduce task-irrelevant restlessness, and to improve behavioral noncompliance and oppositional behavior (Barkley et al. 1999). One set of robust findings is that (1) children with ADHD perform poorly on vigilance tasks that measure sustained attention and distractibility and (2) stimulant medication offsets many of these apparent deficits (Douglas 1972; Douglas et al. 1988; Jacobvitz et al. 1990; Losier et al. 1996; Rubia et al. 2007; Riccio et al. 2001).
Indeed, this line of investigation into deficits of attention and impulse control eventually led to a reconceptualization of the condition from one that emphasized hyperactivity (e.g., Hyperkinetic Reaction of Childhood; Diagnostic and Statistical Manual of Mental Disorders [DSM-II]; American Psychiatric Association 1968) to one that emphasizes attention deficits and distractibility (e.g., disorder name changed to Attention Deficit Disorder; Diagnostic and Statistical Manual of Mental Disorders, 3rd ed. [DSM-III]; American Psychiatric Association 1980; Lange et al. 2010). Stimulant medicines have also been shown to have positive, but more modest effects on short-term memory (including digit memorization) and sundry other cognitive effects (Barkley 1977; Aman 1980; Barkley et al. 1999; Epstein et al. 2006; Epstein et al. 2011).
Still, stimulants alone may not address the problem behaviors often comorbid with ADHD; thus, stimulant medications are increasingly being prescribed concomitantly with other psychotropic medications in the Western society. Duffy et al. (2005) found that 52% of 392 2- to 17-year-olds in a network of American psychiatric practices were receiving concomitant psychotropic drugs. Comer et al. (2010) monitored the multiclass drug prescription among 3466 youth presenting for outpatient visits and involving use of psychotropic medications. For youth with a diagnosed mental disorder, the rate of multiclass prescription rose from 22% (1996 to 1999) to 32% (2004 to 2007). In particular, the coprescription of ADHD medication and antipsychotic was prominent (adjusted odds ratio = 6.2).
Although behavioral improvement is typically the reason that stimulant medications are augmented with antipsychotic medications, it is important that such behavioral gains not come at the expense of physical or cognitive well-being. There is ongoing concern about weight gain and secondary metabolic syndrome with atypical antipsychotics, including risperidone (Newcomer 2005; Correll et al. 2009). There are some references in the literature to “cognitive blunting” with antipsychotic treatment (Voruganti et al. 2007; Howard et al. 2011). Although the term cognitive blunting is rarely defined concretely, it is often understood to be the result of sedation, which is a prominent side effect of antipsychotics.
However, the literature base on the cognitive effects of antipsychotics on cognitive performance is quite limited. We are aware of only four studies that examined the effects of risperidone on cognition. Troost et al. (2006) assessed 14 children with pervasive developmental disorders and irritable behavior. When the children were switched from risperidone to placebo (n = 7), divided attention was significantly regressed, whereas performance improved further for children maintained on risperidone (n = 7); measures of focused attention showed no change. Pandina et al. (2007) summarized attention and memory outcomes from two randomized 6-week trials of risperidone for children with disruptive behavior disorders, finding no meaningful differences in short-term memory or continuous performance task scores. Interestingly, long-term risperidone-only treatment was associated with significant improvement over time (consistent with development and maturation) (Pandina et al. 2007).
Aman et al. (2008) assessed 38 children with autism and severe behavioral disturbance before and during 8 weeks of treatment with risperidone on tests of sustained attention, verbal learning, hand–eye coordination, and spatial memory. Most measures showed no effect of risperidone, whereas the participants performed better with risperidone on a cancellation task (more correct detections) and on recognition of previously presented words. Aman et al. (2009) compared risperidone and placebo in a crossover study that involved 16 children with IQ <84 (the majority with borderline IQ) who had been maintained on risperidone for disruptive behavior disorders. Accuracy was unaffected on two measures of short-term memory, and omission and commission errors were unchanged on a continuous performance task (CPT); however, hand steadiness was improved with risperidone on a measure of static tremor. Importantly, the participants in two of these studies had previously shown behavior changes in response to medication (Troost et al. 2006; Aman et al. 2009), which raises the question of whether performance was secondarily improved solely because of better behavioral compliance rather than any direct effect on cognition.
Finally, the Treatment of Early-Onset Schizophrenia Spectrum disorders study demonstrated positive but modest improvements in neurocognitive domains such as fine motor speed and inhibitory control following antipsychotic intervention (77 children receiving olanzapine, risperidone, and molindone were combined for the analysis) (Frazier et al. 2012).
Only one study has compared directly the effects of stimulants and antipsychotic medications on cognitive performance in children with ADHD. Werry and Aman (1975) compared placebo, methylphenidate, low-dose haloperidol (0.025 mg/kg·d), and high-dose haloperidol (0.05 mg/kg·d) in children with an average IQ and hyperkinetic reaction and/or unsocialized aggressive reaction (per DSM-III criteria). There was some suggestion of impaired short-term memory with the high dose of haloperidol, but impulsive responding on a vigilance task was actually improved with low-dose haloperidol. Aman et al. (1991) compared placebo, methylphenidate, and thioridazine (1.75 mg/d) in 27 children with low IQs (mean = 54) and disruptive behavior (mostly ADHD; n = 26). No effects, beneficial or adverse, were observed with thioridazine on measures of IQ, visual perception, short-term memory, attention span, or static tremor; methylphenidate caused improvements on several tests.
We recently completed a clinical trial (Treatment of Severe Childhood Aggression [TOSCA]) examining the effects of augmenting stimulant treatment plus parent training (STIM+PT) with risperidone in 168 children with ADHD, severe physical aggression, and a Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (DSM-IV-TR) (American Psychiatric Association 2000) disruptive behavior disorder. Augmenting STIM+PT with risperidone resulted in lower scores on parent ratings of total disruptive behavior, reduced reactive aggression, and increased social competence as rated by their parents (Aman et al. 2014). We also assessed attention and short-term memory while treated with STIM+PT and after random assignment to augmentation of treatment with risperidone.
The present report (1) examined the effects of STIM+PT on attention and short-term memory and (2) compared the effect of Basic treatment (stimulant+PT+placebo) versus Augmented treatment (stimulant+PT+risperidone) on attention and short-term memory. We hypothesized that performance would improve when the subjects received open-label STIM+PT and that blinded augmentation of STIM+PT treatment with risperidone would not have significant detrimental effects on performance.
Materials and Methods
Participants
The study design and methods are described in detail elsewhere (Farmer et al. 2011; Aman et al. 2014). Briefly, inclusion criteria were as follows: age 6–12 years, inclusive; DSM-IV-TR diagnosis of ADHD (any subtype); evidence of severe physical aggression; DSM-IV-TR diagnosis of conduct disorder (CD) or oppositional defiant disorder (ODD); and a Clinical Global Impressions Severity score of four or greater for aggression. Exclusion criteria were full-scale IQ below 71; neurologic or medical disorder that contraindicated medication; DSM-IV-TR autism spectrum disorder, psychotic disorder, eating disorder, or mood disorder; current use of psychotropic medication from which discontinuation presented significant risk; suicidal ideation; and family history of type 2 diabetes in at least two first-degree relatives. Other concomitant psychotropic treatments were not allowed during the study.
One hundred sixty-eight children were randomized in a 1:1 ratio to Basic (placebo added) or Augmented (risperidone added) [see Aman et al. (2014) for CONSORT diagram]. Three subjects (n = 1 Basic, n = 2 Augmented) had no data on the cognitive measures at any time point and were excluded from all analyses. Basic demographics of the final sample (n = 165) appear in Table 1.
Table 1.
Demographic Characteristics
| Total (n = 165) | Basic (n = 83) | Augmented (n = 82) | |
|---|---|---|---|
| Age | 8.94 ± 2.01 | 8.78 ± 1.97 | 9.10 ± 2.05 |
| IQ | 97.11 ± 13.88 | 97.10 ± 13.92 | 97.12 ± 13.91 |
| Male, n (%) | 128 (78) | 64 (77) | 64 (78) |
| Diagnosis, n (%) | |||
| ODD | 121 (73) | 61 (73) | 60 (73) |
| CD | 44 (27) | 22 (27) | 22 (27) |
| Race, n (%) | |||
| White | 98 (61) | 47 (57) | 51 (62) |
| Black | 67 (41) | 36 (44) | 31 (38) |
| Non-Hispanic | 154 (93) | 77 (93) | 77 (94) |
| Mother's employment, n (%) | |||
| Full or part-time | 85 (52) | 45 (54) | 40 (49) |
| Homemaker | 21 (13) | 10 (12) | 11 (13) |
| Othera | 59 (36) | 28 (34) | 31 (38) |
| Mother's education, n (%) | |||
| Bachelor's/advanced degree | 38 (23) | 24 (30) | 14 (17) |
| Some college/associate's degree | 70 (42) | 38 (46) | 32 (39) |
| High school or less | 56 (34) | 20 (24) | 36 (44) |
| Household income, n (%) | |||
| <20,000 | 61 (37) | 33 (40) | 28 (34) |
| 20,001–40,000 | 35 (21) | 16 (19) | 19 (23) |
| >40,000 | 69 (42) | 34 (41) | 35 (43) |
| Received randomized medication?b, n (%) | |||
| Yes | 145 (88) | 77 (93) | 68 (83) |
| No (stimulant responder) | 7 (4) | 3 (4) | 4 (5) |
| No (dropped out) | 13 (8) | 3 (4) | 10 (12) |
All participants received stimulant and parent training; beginning at week 3, participants randomized to Augmented received additional blinded risperidone and those randomized to Basic received blinded placebo.
Other employment included unemployment, disabled, retired, student, or other (open-ended).
Randomization occurred at baseline, before 3 weeks of open-label stimulant treatment. At the end of week 3 through week 7, subjects who were not excellent responders to stimulant (“stimulant responder”) were administered the second drug to which they had been randomized (risperidone or placebo).
ODD, oppositional-defiant disorder; CD, conduct disorder.
This study was approved by the Institutional Review Boards at the four participating sites (Ohio State University, Case Western Reserve University, University of Pittsburgh, and Stony Brook University). Parents or guardians signed consent forms, and all participants gave assent before enrollment.
Design
This was a 9-week clinical trial comprising 3 weeks of open-label treatment followed by 6 weeks of randomized, double-blind placebo-controlled treatment. All participants, irrespective of randomization, received ongoing PT, and open-label psychostimulant (usually osmotic release oral system [OROS] methylphenidate; STIM) was titrated to an optimal dose during the first 3 weeks of the study.
After 3 weeks, subjects who were judged not to be excellent clinical responders to methylphenidate (normalization on the primary outcome measure of disruptive behavior and CGI-Improvement rating of 1) began treatment with the second medication to which they had been randomized at baseline (risperidone or placebo). PT+STIM responders were evaluated clinically at each subsequent visit, and if they no longer met the stringent response criteria, the randomized medication (i.e., risperidone or placebo) was added. Within the Augmented group, five subjects did not receive the second medication until week 4, one at week 5, and two at week 7. Eight subjects (n = 3 in the Basic group and n = 5 in the Augmented group) were PT+STIM responders and did not receive the second medication during the trial, but are included in analyses based on the intent-to-treat principle. The mean dose of risperidone in the Augmented group at week 9 was 1.7 ± 0.75 mg/day. Mean doses of stimulant were 44.8 ± 14.6 mg/day for Basic compared with 46.1 ± 16.8 mg/day for Augmented (p = 0.88).
Measures
In addition to the parent report outcome measures described elsewhere (Aman et al. 2014; Gadow et al. 2014), we administered task-oriented measures of cognitive performance at Baseline, week 3, and week 9 (endpoint).
Conners' Continuous Performance Test-II (CPT-II)
The CPT-II (Conners and Staff 2000) was completed with a desktop computer in a quiet room at each site. The CPT-II is a computerized measure of inattentiveness, impulsivity, sustained attention, and vigilance; it consists of 360 letters that appear on a computer screen, one at a time, for ∼250 milliseconds followed by an interstimulus interval (ISI) of 1, 2, or 4 seconds. Each ISI condition occurred for 20 consecutive trials. The three ISI conditions were block randomized so that all three ISI conditions would occur every three blocks, but in a random order. Subjects were required to press the spacebar when any letter except the letter X appeared on the screen. The X appeared on 10% of trials. The total CPT-II takes ∼14 minutes to complete.
CPT-II outcome variables included reaction times (RTs) to correct responses (Hit RT), standard error of HRT (RTSE), commission errors, and omission errors. Errors of omission occurred when participants failed to respond on trials containing target letters (all non-X letters). Errors of commission occurred when subjects pressed the spacebar in the presence of the X.
Although termed a continuous performance task, Conners' CPT-II uses a go/no-go paradigm with frequently appearing go-signals (i.e., non-X letters) and infrequently appearing no-go-signals (i.e., Xs). The go/no-go paradigm develops a prepotent response and tests the participant's ability to inhibit this prepotent response during no-go trials (i.e., refrain from responding to the X).
Digit span
The Digit Span subscale of the Wechsler Intelligence Scales for Children, Third Edition (Wechsler 1991), is a direct assessment of the child's short-term recall and ability to manipulate verbal information in working memory. Digit Span Forward required subjects to repeat numbers in the same sequence as read by the examiner. Digit Span Backwards required the subjects to state the numbers in the reverse order of that given by the examiner. Numbers were presented at a rate of one per second, with subjects required to respond with the numeral string immediately afterward. Each test session lasted ∼5–7 minutes. Each number series consisted of two trials, which were each scored 0 or 1. Age-based scaled scores (M = 10, SD = 3) for the sum total of digits forward and digits backward, as well as the raw digits forward and digits backwards scores (for which there are no standardized scores), were used as outcome measures.
Statistical analyses
CPT-II administrations with rates of combined omission/commission error above 50% were excluded from the analyses as invalid data. This was to eliminate outliers; as such, participants were unlikely to have understood and/or complied with the task.
A constrained longitudinal (cLDA) mixed model with repeated measures was used, wherein baseline and postbaseline data were modeled as dependent variables. An unstructured variance covariance matrix was assumed for the correlated measures within each subject.
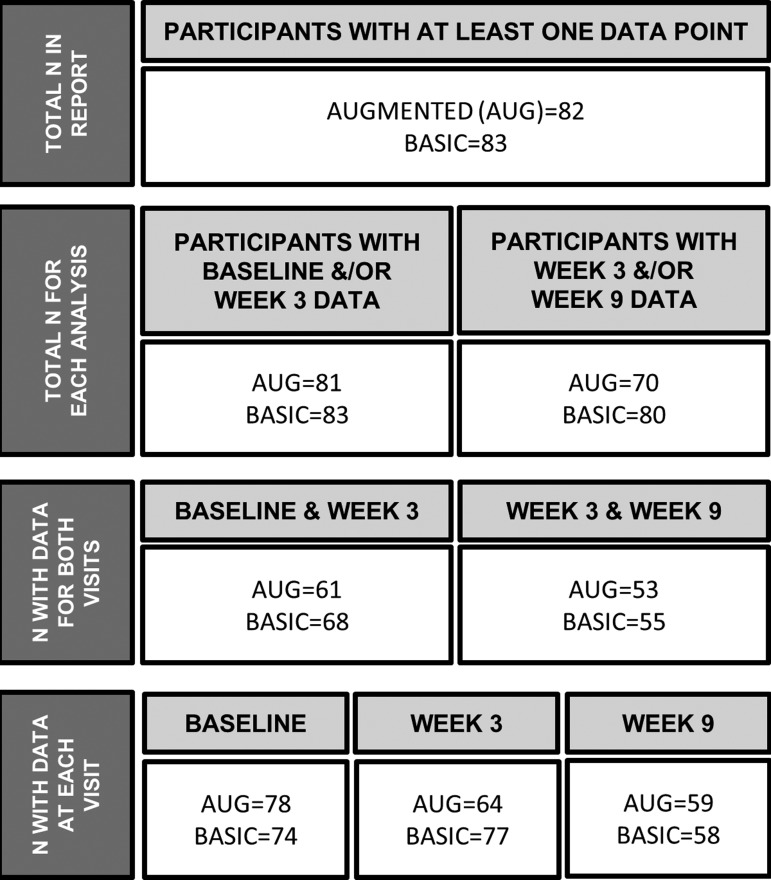
The mixed model used maximum likelihood estimation, which accommodates missing data on the outcome variable. This means that any participant with either data point was included in the analysis (a full accounting of sample size is found in Fig. 1). However, the missing at random assumption is not directly testable, so sensitivity analyses were also conducted wherein subjects with missing data were excluded. Most missing data (see Results section) were due to failure to administer the test or because of subject refusal. Importantly, demographic differences between subjects with the full complement of data (n = 100) and those missing at least one data point (n = 65) were not clinically meaningful. Members of the Augmented and Basic groups were equally likely to have the full complement of data (n = 50 in each group). Participants with all data were significantly older than those with some missing data [M ± standard deviation (SD) = 9.21 ± 1.88 years vs. 8.53 ± 2.15 years; F (1,163) = 4.55, p = 0.03; Cohen's d = 0.34 (0.03–0.65)] and had significantly higher IQ [M ± SD = 99.31 ± 14.53 vs. 93.72 ± 12.15; F (1,163) = 6.60, p = 0.01; Cohen's d = 0.41 (0.09–0.73)]. Participants with no missing data were also more likely to be white (68% vs. 49%, χ2 = 5.81, p = 0.02), but did not differ on other demographic variables or indicators of socioeconomic status.
FIG. 1.
Numbers of participants and valid CPT-II administrations used in each analysis. BASIC = stimulant+parent training+placebo. AUGMENTED (AUG) = stimulant+parent training+risperidone. The total number of participants included in this report, with at one CPT-II administration for at least one analysis (Baseline to week 3 and week 3 to 9), appears on the top row. For each set of analyses, participants with at least one data point were included (second row). Participants without data at either visit for each analysis were excluded. The third row reports the number of participants with data from both visits in each analysis, and the fourth row shows the simple counts of participants with valid CPT-II administrations at each visit. CPT-II, Conners' Continuous Performance Test, Second Edition.
Data were analyzed in two parts. First, the full sample was assessed for the change in the cognitive measures during the STIM+PT phase of the trial (Baseline to week 3). Thus, the mixed model contained fixed effects for time and site only. We hypothesized a significant effect of time (i.e., Baseline to week 3) for all neuropsychological outcomes, which would be consistent with a stimulant treatment effect, and an abundance of studies showing positive stimulant effects on cognitive performance in children with ADHD.
Second, the effect of randomized group (Augmented vs. Basic) was assessed for the period of double-blind treatment (week 3 to 9). We controlled for differences in risperidone dosage by including a fixed effect for weekly mg/kg dose, centered at the mean. The mixed model also contained fixed effects for time, group, and site. Because this was a cLDA model, wherein both week 3 and 9 values were modeled as dependent variables, the randomized groups were constrained to equality at week 3. Therefore, in this pre- and postmodel, the main effect of group is mathematically equivalent to the difference between groups at week 9, controlling for week 3 scores.
Third, we conducted sensitivity analyses excluding individuals with missing data. Fourth, we ran a second set of sensitivity analyses for the double-blind phase. These comparisons were run without the five children who were excellent stimulant responders and therefore never received the randomized risperidone. In other words, although the primary analysis model accounted for the zero dose of risperidone, we performed these additional tests to ensure that any blunting of performance by risperidone would not be masked by children randomized to risperidone who did not actually receive it.
For some variables, the residuals did not appear to be normally distributed, and the variable was subsequently square-root transformed (CPT-II Omissions and Reaction Time SE). Effect sizes were calculated as Cohen's d (with pooled standard deviation for between-group differences). Alpha was set to 0.05 for all comparisons. All data analyses were conducted in SAS-STAT Version 9.3 (SAS Institute 2012).
Results
The total number of scheduled CPT administrations for the 165 subjects across three visits was 504; 410 (81%) of these were successful. The following issues resulted in data loss: sessions where the number of correct responses across all trials was below 50% (n = 4); subject refusal (n = 23; one session each); equipment failure (n = 14); visit cut short/parent refusal (n = 4); early termination from the study (n = 44); and other (n = 5). The majority of subjects had a valid CPT at all three visits (n = 100, 61%) or two visits (n = 45, 27%). The numbers of valid CPT-II administrations are shown in Figure 1. Given our sample size, we determined that we were capable of detecting moderate between-group effect size (Cohen's d ∼ 0.5) at week 9 with 80% power and alpha of 0.05.
Results of the first set of analyses examining change during the STIM+PT are shown in Table 2. Statistically significant improvement of modest size was observed between baseline and week 3 on CPT commission errors and RTSE. A small but statistically significant improvement was found after 3 weeks of STIM+PT treatment on the Digit Span standard score, apparently driven by improvement in performance on digits backwards. Sensitivity analyses to evaluate the influence of missing data, wherein subjects with missing data (n = 65) were excluded, showed no measurable differences from the planned analyses.
Table 2.
Improvement on Most Cognitive Measures During STIM+PT, N = 164
| Baseline M (SE) | Week 3 M (SE) | F (1,160) | p | Cohen's d (95% CI) | |
|---|---|---|---|---|---|
| Omissionsa | 4.87 (0.16) | 4.70 (0.17) | 1.04 | 0.31 | 0.08 (−0.09, 0.25) |
| Comissions | 27.37 (0.51) | 24.56 (0.68) | 28.15 | <0.001 | 0.35 (0.23, 0.49) |
| Reaction time (ms) | 466.13 (7.51) | 464.01 (9.45) | 0.08 | 0.78 | 0.02 (−0.11, 0.15) |
| Reaction time SEa (ms) | 4.34 (0.08) | 4.03 (0.10) | 13.17 | <0.001 | 0.28 (0.13, 0.42) |
| Digit span (standard score) | 9.54 (0.23) | 10.12 (0.25) | 7.81 | 0.006 | 0.18 (0.07, 0.30) |
| Digits forward (raw score) | 7.91 (0.18) | 8.14 (0.19) | 2.86 | 0.09 | 0.10 (−0.02, 0.22) |
| Digits backward (raw score) | 3.51 (0.13) | 3.89 (0.14) | 11.14 | 0.001 | 0.33 (0.20, 0.47) |
Sample size reflects subjects with at least one data point in the analysis. Estimated marginal means with standard error were calculated from mixed model, controlling for site.
Square-root transformed.
CI, confidence interval.
Results of analyses examining treatment effects from week 3 to 9 indicated no positive or negative effect of added risperidone on any cognitive measure (Table 3). All effect sizes were near-to-zero. Two sets of sensitivity analyses were performed. First, subjects with missing data (n = 65) were excluded; the pattern of results did not differ from the planned analyses. Second, subjects who were randomized to, but did not receive, risperidone (n = 5) were excluded. Again, these results did not differ from the planned analyses (full results of sensitivity analyses available on request from CAF).
Table 3.
No Differences at Endpoint in Attention and Memory Outcomes Between Randomized Groups, N = 150
| Basic M (SE) | Augmented M (SE) | F (1,146) | p | Cohen's d (95% CI) | |
|---|---|---|---|---|---|
| Omissionsa | 4.75 (0.19) | 4.57 (0.40) | 0.14 | 0.71 | 0.07 (−0.25, 0.39) |
| Comissions | 24.09 (0.83) | 25.34 (1.21) | 0.69 | 0.41 | −0.14 (−0.46, 0.18) |
| Reaction time (ms) | 464.57 (10.67) | 455.52 (14.35) | 0.52 | 0.47 | 0.08 (−0.24, 0.40) |
| Reaction time SEa (ms) | 3.98 (0.10) | 3.90 (0.17) | 0.14 | 0.71 | 0.07 (−0.25, 0.39) |
| Digit span (standard score) | 10.37 (0.28) | 10.03 (0.60) | 0.24 | 0.62 | 0.09 (−0.23, 0.41) |
| Digits forward (raw score) | 8.21 (0.20) | 8.30 (0.35) | 0.05 | 0.83 | −0.04 (−0.36, 0.28) |
| Digits backward (raw score) | 3.96 (0.16) | 4.05 (0.31) | 0.06 | 0.80 | −0.04 (−0.36, 0.28) |
Basic = stimulant+parent training+placebo. Augmented = stimulant+parent training+risperidone. Sample size reflects subjects with at least one data point in the analysis. Estimated marginal means with standard error were calculated from the mixed model, controlling for site and mean-centered mg/kg dose of risperidone. In the double-blind analyses, the groups (Augmented and Basic) were constrained to equality at week 3. The effect of time in the week 3 to 9 analyses was nonsignificant for all outcomes.
Square-root transformed.
Discussion
When children diagnosed with disruptive behavior disorder (i.e., ODD or CD), comorbid ADHD, and severe aggression are treated with a combined treatment strategy of stimulant medication and parent training for 3 weeks, they showed improved performance on several neuropsychological indices, including go/no-go task commission errors, RT, and RT variability, as well as Digits Backward on the Wechsler Intelligence Scale for Children (WISC) Digit Span task. These neuropsychological outcomes are indicators of various attentional abilities, including response inhibition, working memory, processing speed, and sustained attention, and have been shown to be sensitive to stimulant treatment in previous studies (Epstein et al. 2006). Augmentation of this treatment strategy with 6 weeks of risperidone did not appreciably improve or worsen cognitive performance on any neuropsychological task performance measure.
As noted earlier, the findings for the Baseline to week 3 comparison are confounded in that all subjects initiated the STIM+PT treatment during that time. Thus, it is unknown whether the passage of time alone (i.e., practice and maturation), PT, STIM, test variability, or some combination of all of these effects was responsible for the changes. In some ways, the source of the change is not as important as the fact that changes did occur. This is a demonstration that the cognitive measures were indeed capable of detecting change.
The changes from end of week 3 to 9 reflect a true experimental comparison (Basic vs. Augmented). In these comparisons, none of the variables showed treatment effects, leading to the conclusion that measurable changes in cognition were not occurring in the context of this portion of the clinical trial. Our present findings regarding the combination of risperidone and stimulant are consistent with the majority of reports on the cognitive effects of antipsychotic monotherapy in children with various psychiatric disorders, which found no meaningful effects (Aman et al. 1991; Aman et al. 2009) or improvements (Werry and Aman 1975; Troost et al. 2006; Aman et al. 2008).
Clearly, the most common result in these studies of antipsychotic monotherapy and psychomotor performance has been one of no change relative to placebo control. One may wonder why there is such a common belief that antipsychotics will produce “dulling” in patients so treated. Antipsychotics are commonly used to manage highly disruptive behavior in children with autism spectrum disorders, disruptive disorders (oppositional defiant disorder, conduct disorder), bipolar disorder, and in youth with positive symptoms of schizophrenia. It is perhaps tempting to speculate that a behaviorally typical child would have an adverse cognitive response if given clinical doses of an antipsychotic drug and then, by extension, to expect the same in patients with psychiatric disorders. However, this would be a mistake, as atypical antipsychotic drugs can have a variety of poorly specified effects, such as the reduction of emotional lability, hyperactivity, and agitation sometimes associated with these conditions. Thus, it should not be surprising that the net cognitive effect of antipsychotic monotherapy in children with severe disruptive behavior differs from what one would expect in a typical child.
This study had a number of limitations. First, the PT+STIM period from Baseline through week 3 was uncontrolled, and we are therefore limited in our ability to attribute improvements to intervention. Second, we experienced some loss of data because of attrition, equipment failure, refusal, and so forth. As a result, the sample containing all cognitive data had slightly older participants (by about 8 months), with slightly higher IQs (by about six points), who were more likely to be white (68% vs. 49%). This may limit the generalizability of our findings, although we do not find these differences to be very compelling. Although we have no data to support this, it is possible that attrition was differentially related to side effects or diminished cognitive performance. Third, because of the way that our data were captured and saved in our database, we did not have the raw, trial-by-trial CPT data. This limited our ability to compute alternative indicators of task performance (e.g., ex-Gaussian estimators), and we analyzed only summary measures.
Fourth, while the comparison of Basic and Augmented addressed important clinical questions regarding combined pharmacotherapy, it was not identical to a comparison of placebo versus risperidone, as unknown drug interactions may have been at work. Fifth, not all children randomized to Augmented treatment actually received risperidone. This is because several participants had an excellent response to PT+STIM alone and did not require additional treatment. In keeping with the intent-to-treat principle, our initial analysis included all participants in their originally assigned treatment groups, but controlled for the actual dose of risperidone received (the effect was nonsignificant in all cases). Furthermore, we also performed a sensitivity analysis without the five participants who did not receive risperidone, to ensure that we were not diluting the effect of risperidone. Sensitivity analyses, with these STIM-monotherapy children excluded, had no appreciable effect on outcomes.
Finally, although inattention, impulsiveness, and short-term memory were assessed in this study, many other cognitive functions of high interest (e.g., long-term memory, problem solving, set-shifting, executive function) were not. Furthermore, these laboratory-based measures may only partially reflect impairment in a real-world setting.
The study also has several strengths. The sample size (N = 165 with at least some data) was relatively large for this type of clinical sample and is substantially larger than most other studies of antipsychotic effects on cognition. Given this sample size, we would have been able to detect a clinically significant effect (d = 0.5) with 80% power. However, the between-group effect sizes were all close to zero (range, d = −0.14 to 0.09).
The use of a randomized controlled trial in this difficult-to-ascertain clinical population is novel, as this population is frequently encountered in the clinical setting, but is rarely involved in research. Finally, the import of these data is bolstered by the reports of dramatic increases in combined pharmacotherapy in child and adolescent psychiatry.
In summary, concomitant treatment with psychostimulant and antipsychotic is on the rise, especially for children with severe behavior profiles. The results of this multisite, randomized, controlled clinical trial demonstrated that the combination of parent training, stimulant, and risperidone was moderately more effective than parent training and stimulant alone in improving disruptive behaviors (Aman et al. 2014). In the current study, we found no evidence of deleterious attention or memory effects after the first 6 weeks of treatment.
Conclusion
Consistent with substantial other literature in childhood psychopharmacology, we found no evidence of antipsychotic interference with sustained attention, impulsive responding, or short term memory in children with disruptive behavior disorders. Our findings extend previous reports in that the antipsychotic therapy was adjunctive to stimulant treatment.
Clinical Significance
Our data failed to indicate any cognitive effects with combined stimulant and risperidone therapy, after 6 weeks of treatment, in children identified and treated for severe physical aggression. We cannot discount the possibility of any cognitive effects whatsoever or for indefinite periods of exposure, but clinicians may find the null effects of combined treatment on cognition to be reassuring. Likewise, the lack of effects observed here, together with generally benign findings in other studies of antipsychotic monotherapy, may be reassuring to parents, guardians, and practitioners alike.
Acknowledgments
This study was supported by grants from National Institute of Mental Health (NIMH) to The Ohio State University (R01 MH077907), Case Western Reserve University (R01 MH077750), the University of Pittsburgh (R01 MH077676), and Stony Brook University (R01MH 077997). The project was supported by a National Institutes of Health (NIH) General Clinical Research Center grant M01RR10710 (State University of New York-Stony Brook) and Clinical and Translational Science Awards from the National Center for Advancing Translational Sciences: grants UL1TR000090 (The Ohio State University) and UL1 RR024153 and UL1TR000005 (University of Pittsburgh). The content is solely the responsibility of the authors and does not necessarily represent the official views of the respective National Centers for Advancing Translational Sciences or the NIH. The authors thank Ms. Taylor Wong (Ohio State University) for technical support in researching and locating relevant published research for this article.
Disclosures
Dr. M.G.A. has received research contracts, consulted with, or served on advisory boards of Biomarin Pharmaceuticals, Bristol-Myers Squibb, Cog State, Confluence Pharmaceutica, Coronado Biosciences, Forest Research, Hoffmann-La Roche, Johnson and Johnson, MedAvante Inc., Novartis, Pfizer, ProPhase LLC, and Supernus Pharmaceuticals. Dr. K.D.G. is a shareholder in Checkmate Plus, publisher of the Child and Adolescent Symptom Inventory-4R. Dr. O.G.B. has received royalties from Routledge Press and acted as a consultant for Ezra Innovations and PRIME CME. Dr. L.E.A. has received research funding from Curemark, Forest, Lilly, Neuropharm, Novartis, Noven, and Shire (as well as NIH and Autism Speaks) and has consulted or been on advisory boards for Neuropharm, Novartis, Noven, Organon, Roche, Seaside Therapeutics, Shire, Tris Pharma, Pfizer, and Gowlings. Dr. N.K.M. has received research support from Forest Research, GlaxoSmithKline, Eli Lilly and Co., Lundbeck, Merck, the NIH, Novartis, Otsuka, Pfizer, Rhodes Pharmaceuticals, Roche, Shire, Stanley Medical Research Institute, Sunovion, and Supernus Pharmaceuticals. Dr. V.R.R. has received research support from GlaxoSmithKline, Merck/Schering Plough, the NIMH, Covance/Otsuka, and Pfizer. Dr. S.B. has received research support from Supernus Pharmaceuticals. Dr. R.L.F. receives or has received research support, acted as a consultant, and/or served on a speaker's bureau for Alcobra, American Academy of Child & Adolescent Psychiatry, American Physician Institute, American Psychiatric Press, AstraZeneca, Bracket, Bristol-Myers Squibb, CogCubed, Cognition Group, Coronado Biosciences, Dana Foundation, Elsevier, Forest, GlaxoSmithKline, Guilford Press, Johns Hopkins University Press, Johnson and Johnson, Jubilant Clinsys, KemPharm, Lilly, Lundbeck, Merck, NIH, Neurim, Novartis, Noven, Otsuka, Oxford University Press, Pfizer, Physicians Postgraduate Press, Purdue, Rhodes Pharmaceuticals, Roche, Sage, Shire, Sunovion, Supernus Pharmaceuticals, Transcept Pharmaceuticals, Validus, and WebMD. Drs. J.N.E., C.A.F., D.J.K., B.S.G.M., and J.S., and Ms. H.K. report no conflicts of interest.
References
- Aman MG: Psychotropic drugs and learning problems—A selective review. J Learn Disabil 13:87–97, 1980 [DOI] [PubMed] [Google Scholar]
- Aman MG, Bukstein OG, Gadow KD, Arnold LE, Molina BS, McNamara NK, Rundberg-Rivera EV, Li X, Kipp H, Schneider J, Butter EM, Baker J, Sprafkin J, Rice RR, Jr., Bangalore SS, Farmer CA, Austin AB, Buchan-Page KA, Brown NV, Hurt EA, Grondhuis SN Findling RL: What does risperidone add to parent training and stimulant for severe aggression in child attention-deficit/hyperactivity disorder? J Am Acad Child Adolesc Psychiatry 53:47–60, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman MG, Hollway JA, Leone S, Masty J, Lindsay R, Nash P, Arnold LE: Effects of risperidone on cognitive-motor performance and motor movements in chronically medicated children. Res Dev Disabil 30:386–396, 2009 [DOI] [PubMed] [Google Scholar]
- Aman MG, Hollway JA, McDougle CJ, Scahill L, Tierney E, McCracken JT, Arnold LE, Vitiello B, Ritz L, Gavaletz A, Cronin P, Swiezy N, Wheeler C, Koenig K, Ghuman JK, Posey DJ: Cognitive effects of risperidone in children with autism and irritable behavior. J Child Adolesc Psychopharmacol 18:227–236, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aman MG, Marks RE, Turbott SH, Wilsher CP, Merry SN: Methylphenidate and thioridazine in the treatment of intellectually subaverage children: Effects on cognitive-motor performance. J Am Acad Child Adolesc Psychiatry 30:816–824, 1991 [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 2nd ed. (DSM-II). Washington, DC: American Psychiatric Association, 1968 [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 3rd ed. (DSM-III). Washington, DC: American Psychiatric Association, 1980 [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association, 2000 [Google Scholar]
- Barkley R, DuPaul G, Connor D: Stimulants. In: Practitioner's Guide to Psychoactive Drugs for Children and Adolescents. Edited by Werry J, Aman M. New York: Plenum Press, 1999, pp. 213–247 [Google Scholar]
- Barkley RA. A review of stimulant drug research with hyperactive children. J Child Psychol Psychiatry 18:137–165, 1977 [DOI] [PubMed] [Google Scholar]
- Comer JS, Olfson M, Mojtabai R: National trends in child and adolescent psychotropic polypharmacy in office-based practice, 1996–2007. J Am Acad Child Adolesc Psychiatry 49:1001–1010, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK. MHS Staff: Conners' Continuous Performance Test II (CPT II V. 5). North Tonawanda, NY: Multi-Health Systems, Inc., 2000, pp. 1–16 [Google Scholar]
- Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK: Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 302:1765–1773, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas V, Barr R, Amin K, O'Neill M, Britton B: Dosage effects and individual responsivity to methylphenidate in attention deficit disorder. J Child Psychol Psychiatry 29:453–475, 1988 [DOI] [PubMed] [Google Scholar]
- Douglas VI. Stop, look and listen: The problem of sustained attention and impulse control in hyperactive and normal children. Can J Behav Sci 4:259–282, 1972 [Google Scholar]
- Duffy FF, Narrow WE, Rae DS, West JC, Zarin DA, Rubio-Stipec M, Pincus HA, Regier DA: Concomitant pharmacotherapy among youths treated in routine psychiatric practice. J Child Adolesc Psychopharmacol 15:12–25, 2005 [DOI] [PubMed] [Google Scholar]
- Epstein JN, Brinkman WB, Froehlich T, Langberg JM, Narad ME, Antonini TN, Shiels K, Simon JO, Altaye M: Effects of stimulant medication, incentives, and event rate on reaction time variability in children with ADHD. Neuropsychopharmacology 36:1060–1072, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JN, Keith Conners C, Hervey AS, Tonev ST, Eugene Arnold L, Abikoff HB, Elliott G, Greenhill LL, Hechtman L, Hoagwood K: Assessing medication effects in the MTA study using neuropsychological outcomes. J. Child Psychol Psychiatry 47:446–456, 2006 [DOI] [PubMed] [Google Scholar]
- Farmer CA, Arnold LE, Bukstein OG, Findling RL, Gadow KD, Li X, Butter EM, Aman MG: The treatment of severe child aggression (TOSCA) study: Design challenges. Child Adolesc Psychiatry Ment Health 5:36, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier JA, Giuliano AJ, Johnson JL, Yakutis L, Youngstrom EA, Breiger D, Sikich L, Findling RL, McClellan J, Hamer RM: Neurocognitive outcomes in the treatment of early-onset schizophrenia spectrum disorders study. J Am Acad Child Adolesc Psychiatry 51:496–505, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, Arnold LE, Molina BSG, Findling RL, Bukstein OG, Brown NV, McNamara NK, Rundberg-Rivera EV, Li X, Kipp HL, Schneider J, Farmer CA, Baker JL, Sprafkin J, Rice RR, Bangalore SS, Butter EM, Buchan-Page KA, Hurt EA, Austin AB, Grondhuis SN, Aman MG: Risperidone added to parent training and stimulant medication: Effects on attention-deficit/hyperactivity disorder, oppositional defiant disorder, conduct disorder, and peer aggression. J Am Acad Child Adolesc Psychiatry 53:948–959, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard P, Twycross R, Shuster J, Mihalyo M, Wilcock A: Antipsychotics. J Pain Symptom Manage 41:956–965, 2011 [DOI] [PubMed] [Google Scholar]
- Jacobvitz D, Sroufe LA, Stewart M, Leffert N: Treatment of attentional and hyperactivity problems in children with sympathomimetic drugs: A comprehensive review. J Am Acad Child Adolesc Psychiatry 29:677–688, 1990 [DOI] [PubMed] [Google Scholar]
- Lange KW, Reichl S, Lange KM, Tucha L, Tucha O: The history of attention deficit hyperactivity disorder. Atten Defic Hyperact Disord 2:241–255, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losier BJ, McGrath PJ, Klein RM: Error patterns on the continuous performance test in non‐medicated and medicated samples of children with and without ADHD: A meta‐analytic review. J. Child Psychol Psychiatry 37:971–987, 1996 [DOI] [PubMed] [Google Scholar]
- Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects. CNS drugs 19:1–93, 2005 [DOI] [PubMed] [Google Scholar]
- Pandina GJ, Bilder R, Harvey PD, Keefe RS, Aman MG, Gharabawi G: Risperidone and cognitive function in children with disruptive behavior disorders. Biol Psychiatry 62:226–234, 2007 [DOI] [PubMed] [Google Scholar]
- Riccio CA, Waldrop JJ, Reynolds CR, Lowe P: Effects of stimulants on the continuous performance test (CPT). J Neuropsychiatry Clin Neurosci 13:326–335, 2001 [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith A, Taylor E: Performance of children with attention deficit hyperactivity disorder (ADHD) on a test battery of impulsiveness. Child Neuropsychol 13:276–304, 2007 [DOI] [PubMed] [Google Scholar]
- SAS Institute: SAS Version 9.3. Cary, NC: SAS Institute, Inc, 2012 [Google Scholar]
- Troost PW, Althaus M, Lahuis BE, Buitelaar JK, Minderaa RB, Hoekstra PJ: Neuropsychological effects of risperidone in children with pervasive developmental disorders: a blinded discontinuation study. J Child Adolesc Psychopharmacol 16:561–573, 2006 [DOI] [PubMed] [Google Scholar]
- Voruganti L, Awad A, Parker G, Forrest C, Usmani Y, Fernando M, Senthilal S: Cognition, functioning and quality of life in schizophrenia treatment: Results of a one-year randomized controlled trial of olanzapine and quetiapine. Schizophr Res 96:146–155, 2007 [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children—Third edition (WISC-III). San Antonio, TX: Psychological Corporation, 1991 [Google Scholar]
- Werry JS, Aman MG: Methylphenidate and haloperidol in children: Effects on attention, memory, and activity. Arch Gen Psychiatry 32:790–795, 1975 [DOI] [PubMed] [Google Scholar]