Abstract
Layering a regenerative polymer scaffold on the surface of the heart, termed as a cardiac patch, has been proven to be effective in preserving cardiac function after myocardial infarction (MI). However, the placement of such a patch on the heart usually needs open-chest surgery, which is traumatic, therefore prevents the translation of this strategy into the clinic. We sought to device a way to apply a cardiac patch by spray painting in situ polymerizable biomaterials onto the heart with a minimally invasive procedure. To prove the concept, we used platelet fibrin gel as the “paint” material in a mouse model of MI. The use of the spraying system allowed for placement of a uniform cardiac patch on the heart in a mini-invasive manner without the need for sutures or glue. The spray treatment promoted cardiac repair and attenuated cardiac dysfunction after MI.
Keywords: : cardiac patch, platelet fibrin gel, myocardial infarction, regeneration
Introduction
Myocardial infarction (MI) is a major cause of death worldwide.1 It results in cardiomyocyte loss, myocardial remodeling, and the deterioration of cardiac function, which can eventually lead to heart failure.2 Current therapies, including conventional pharmacological agents and left ventricular (LV) assist devices can only delay the progression of the disease, and the shortage of heart donors is also a factor for innovating new therapies.3 Therefore, new therapies are needed to regenerate damaged hearts to overcome the poor prognosis of patients with heart failure.
The emergence of biomaterials and cardiac tissue engineering has started to provide promising alternatives. Over the last 5 years, the general biomaterial approaches to treatment of MI have remained the same: LV restraints, cardiac patches, or injectable hydrogel biomaterials.4 Layering a regenerative polymer scaffold on the surface of the heart, termed as a cardiac patch, has been proven to be effective in preserving cardiac function after MI.5 However, the placement of such a patch on the heart usually needs open-chest surgery, which is traumatic, therefore prevents the translation of this technology to the clinic. We sought to device a way to apply a cardiac patch onto the heart in a minimally invasive way. Inspired by the practice of spray painting used by construction industry, we hypothesize that in situ polymerizable biomaterials can be spray painted onto the surface of the heart to form a uniform layer of cardiac patch. To prove the concept, we used platelet fibrin gel as the “paint” material and used a biomaterials spray device in a mouse model of MI. Platelet fibrin gel is known for its ability to quickly aid in clot formation and has been used a biomaterial for cardiac repair in animal studies.3,4,6
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by Institutional Animal Care and Usage Committee at North Carolina State University. All surgeries were performed under isofluorane combined with oxygen, and all efforts were made to minimize suffering. Animals were euthanized through inhalation of CO2.
Preparation of platelet fibrin gel spray
A uniform platelet fibrin patch was formed by spraying platelet-rich plasma and calcium-containing media solution (through a double-lumen syringe, with the aid from compressed CO2. Platelet-rich plasma was derived from CD1 mice as previously described.7 The solutions were delivered through the home-designed spray set, which hold the two components in separate 1 mL syringes and provided simultaneous mixing and delivery. The ratio of platelet-rich plasma to calcium-containing media solution components was 1:1 as previously described.7 A tube carried compressed CO2 air of 10 PSI pressure to the syringe tip to create the spray of the two components onto the heart or onto in vitro cultured cells.
Characterization of the sprayed platelet fibrin patch
To determine the gelation time of platelet fibrin gel, we monitored gel formation over time by spraying platelet fibrin gel onto coverslips. A stress-controlled shear rheometer (AR-2000; TA Instruments) with 20 mm parallel disc geometry was used to evaluate the rheological properties of the gelation process. To visualize the morphological details of platelet fibrin gel, fresh platelet fibrin gel samples were prepared and fixed with 2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) for 1 h and then rinsed with cacodylate buffer three times (15 min each). The samples were then dehydrated in 35%, 50%, 70%, 80%, 95%, and 100% ethanol successively for 10 min each and dried in hexamethyldisilazane (Sigma Aldrich, MO). Scaffolds were sputter-coated with gold, and images were captured with a JEOL JSM-6380 LV (JEOL Ltd, Japan). To study whether this kind of platelet fibrin gel could release vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF)-1, hepatocyte growth factor (HGF), platelet-derived growth factor (PDGF), and transforming growth factor-beta (TGF-β), we collected conditioned media at day 2, 5, 9, and 14, and fresh media were added back into the culture plate well to be conditioned for next time points. Enzyme-linked immunosorbent assay kits were used to determine the expression of VEGF, IGF-1, HGF, PDGF, and TGF-β.
Cardiomyocyte biocompatibility with the sprayed platelet fibrin patch
To determine the biocompatibility of platelet fibrin patch to cardiomyocytes, we cultured fresh neonatal rat cardiomyocytes (NRCMs) from Sprague–Dawley (SD) rats. The NRCMs harvesting and culturing methods were entailed in previous studies.8,9 To distinguish if the mechanical force from spray operation has impact on cardiomyocytes, we cultured NRCMs on presprayed gel (NRCM on Spray) or sprayed gel directly onto preplated NRCMs (Spray on NRCM). We also cultured NRCMs on standard tissue culture plate (TCP) coated with fibronectin (BD Bioscience, CA) as the control. The percentage of viable NRCMs was determined by the LIVE/DEAD Viability/Cytotoxicity Kit (Invitrogen, CA), which quickly discriminates live from dead cells by simultaneously staining with green-fluorescent calcein-AM (live) and red-fluorescent ethidium homodimer-1 (EthD; dead). Cell morphology was characterized from the same images using the ImagePro software (Media Cybernetics, MD). Videos capturing cardiomyocyte contraction were taken at day 1 and 7.
Animal studies
The method to induce MI in mice was based on previous study.10 In brief, male CD1 mice were anesthetized with 3% isoflurane combined with oxygen inhalation. Under sterile conditions, the heart was exposed by a minimally invasive left thoracotomy, and acute MI was produced by permanent ligation of the left anterior descending coronary artery with metal clip dispensed by AutoSuture Surgiclip (Autosuture). Immediately after MI induction, the mice received one of the following four treatments: (1) MI + Platelet Fibrin Spray group: Spray of 200 μL platelet fibrin gel onto the heart immediately after MI; (2) MI + Fibrin Spray group: Spray of 200 μL fibrin gel onto the heart immediately after MI; (3) MI alone group: MI surgery without spray; and (4) SHAM group: thoracotomy only without AMI. After chest closure, animals were transferred to the care unit for recovery and received carprofen (5 mg/kg) once daily and buprenorphine (0.05 mg/kg) twice daily for up to 48 h. All animals underwent transthoracic echocardiography at the 4 h and 21 day, and the method is described in detail in the following part. A total of 30 animals were included into the study, and survival rate was recorded in treated group.
To identify the location and degradation of sprayed platelet fibrin patch in vivo, we prelabeled the platelet fibrinogen components by incubation with Texas Red-X succinimidyl ester (1 mg/mL [Invitrogen, Carlsbad, CA]) at 37°C for 0.5 h. Another cohorts of animals (n = 12) were induced MI and sprayed with labeled platelet fibrin gel on the MI surface. Following MI induction, one animal died due to bleeding, one animal died of sudden cardiac arrest, and one animal died of unknown reason. Thus, a total of nine animals were finally included in the analysis, and each three were sacrificed at day 0, 4, and 7 for fluorescent image and histology.
Cardiac function assessment by echocardiography
Animals underwent transthoracic echocardiography under 1.5% isofluorane–oxygen mixture anesthesia in supine position at the 4 h and 21 day. The procedure was performed by an animal cardiologist blind to the experimental design using a Philips CX30 ultrasound system couple with a L15 high-frequency probe. M-mode standard two-dimensional left parasternal long-axis echocardiographic examination was conducted. Ejection fraction (% EF) and fractional shortening (% FS) were used as cardiac function indicators. EF% was determined by left ventricular end-diastolic volume (LVEDV) and left ventricular end-systolic volume (LVESV), and the formula for calculation is (LVEDV − LVESV/LVEDV) × 100%. FS is determined by left ventricular end-diastolic diameter (LVEDD) and left ventricular end-systolic diameter (LVESD), and the formula for calculation is (LVEDD − LVESD/LVEDD) × 100%.
Heart morphometry and assessment of fibrosis
After the echocardiography study at day 21, animals were euthanized and hearts were harvested and frozen in OCT compound. Specimens were sectioned at 10 μm thickness from the apex to the ligation level with 100 μm intervals. Masson's trichrome staining was performed as described by the manufacturer's instructions (HT15 Trichrome Staining [Masson] Kit; Sigma-Aldrich). Images were acquired with a PathScan Enabler IV slide scanner (Advanced Imaging Concepts, Princeton, NJ). From the Masson's trichrome stained images, morphometric parameters, including viable myocardium and scar size were measured in each section with NIH ImageJ software. The percentage of viable myocardium as a fraction of the scar area (infarcted size) was quantified as described.11 Three selected sections were quantified for each animal.
Immunohistochemistry
For immunohistochemistry staining, heart cryosections were fixed with 4% paraformaldehyde, permeabilized, and blocked with Protein Block Solution (DAKO, Carpinteria, CA) containing 0.1% saponin (Sigma, St. Louis, MO), and then incubated with the following antibodies overnight at 4 °C: rabbit anti-von Willebrand factor (vWF, 1:200; Abcam), mouse anti-alpha-sarcomeric actin (1:200; Sigma). FITC- or Texas-Red secondary antibodies (1:100) were obtained from Abcam Company and used for the conjunction with these primary antibodies. For assessment of cell apoptosis, heart cryosections were incubated with TUNEL solution (Roche Diagnostics GmbH, Mannheim, Germany) and counterstained with DAPI (Life Technology, NY). Images were taken by an Olympus epifluorescence microscopy system. The images and analysis were obtained from the peri-infarct area.
Statistical analysis
All data are presented as mean ± standard deviation. Comparisons between any two groups were performed with two-tailed unpaired or paired Student's t-test. Comparisons among more than two groups were performed with one-way analysis of variance followed by post hoc Bonferroni correction. p < 0.05 was considered statistically significant.
Results
Characterization of the sprayed platelet fibrin patch in vitro
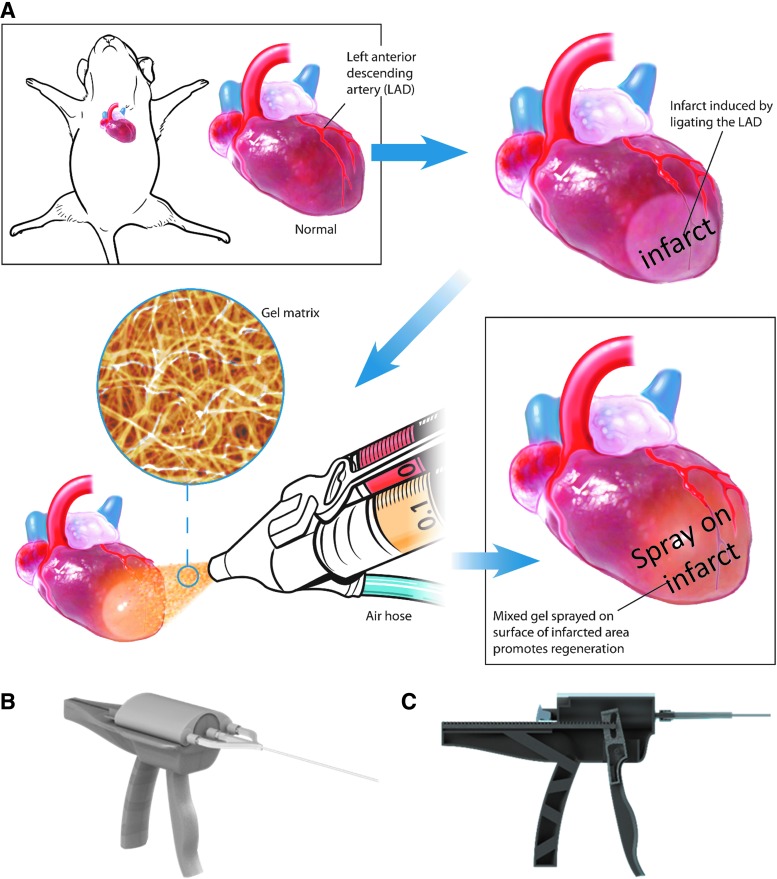
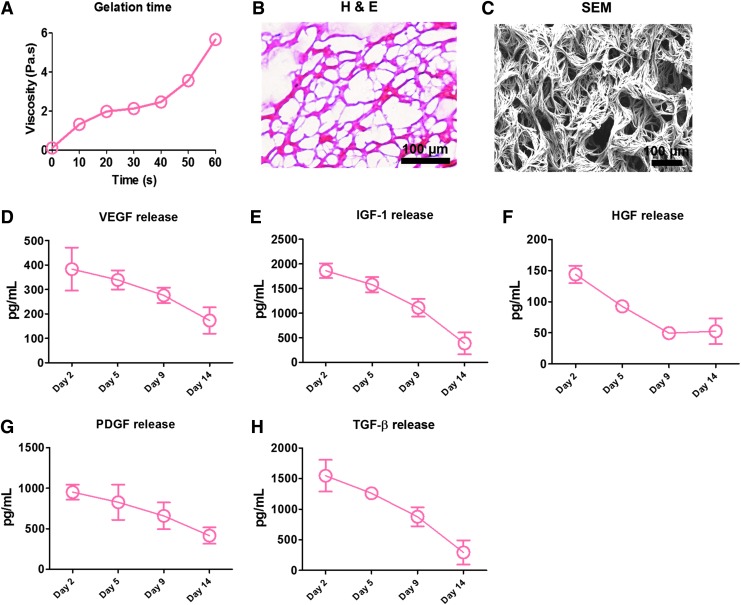
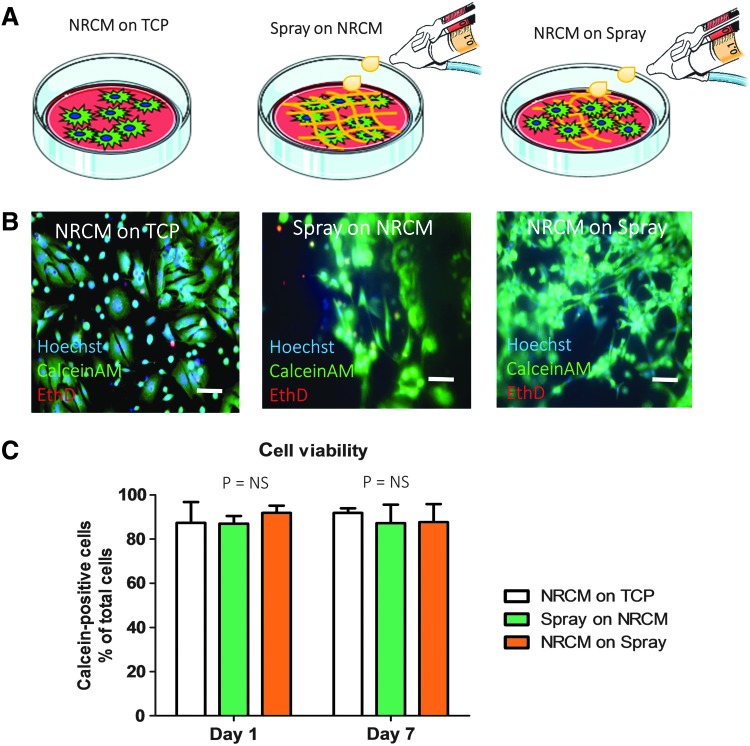
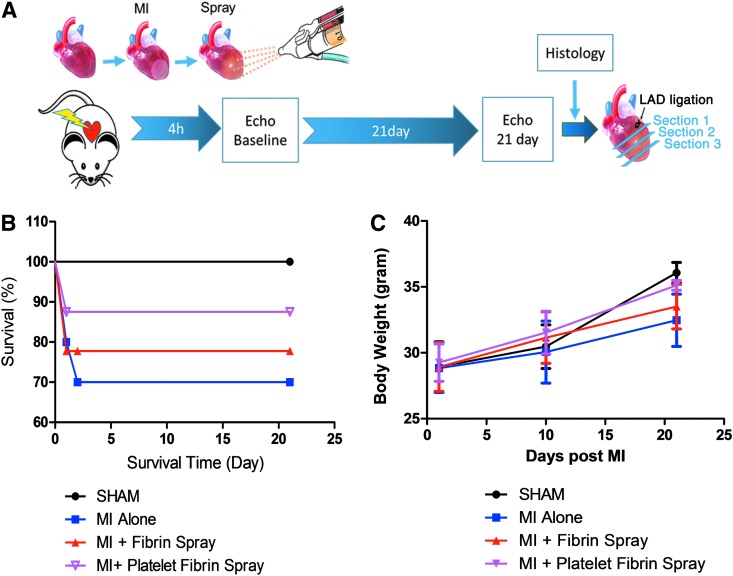
Figure 1 depicts a schematic of the spray device and the overall design of animal studies. In brief, platelet-rich plasma and calcium-containing media solution were packed in double-lumen syringe and sprayed out by compressed CO2. Immediately after the spray, in situ polymerization occurred to form a stable patch (Fig. 2A) on the glass slide. Hematoxylin–eosin staining (Fig. 2B) and scanning electron microscopy (Fig. 2C) revealed a fibrous structure of the sprayed platelet fibrin patch. Enzyme-linked immunosorbent assay revealed that the platelet fibrin gel released regenerative growth factors such as VEGF, IGF-1, HGF, PDGF, and TGF-β (Fig. 2D–H). To test whether the sprayed gel is compatible with cardiomyocytes, NRCMs were cultured on TCP or the sprayed platelet fibrin patch, and to evaluate if the pressure from the spray operation would affect the viability of NRCMs, we also sprayed platelet fibrin gel directly onto NRCMs (Fig. 3A). NRCMs exhibited elongated cell morphology in the sprayed platelet fibrin gel (Fig. 3B). Live/Dead assay revealed that NRCMs cultured with platelet fibrin gel exhibited a similar cell viability compared with those cultured on TCP at day 1 and 7 (Fig. 3C). In addition, NRCMs continued to beat spontaneously within the platelet fibrin gel (Supplementary Movies S1–S3; Supplementary Data are available online at www.liebertpub.com/tec).
FIG. 1.
Overall study design. (A) Schematic showing the spray painting of biomaterials on the heart after myocardial infarction. (B, C) Design of a spray painting device for regenerative biomaterials.
FIG. 2.
Characterization of the sprayed platelet fibrin patch in vitro. (A) Mixing platelet-rich plasma with calcium-containing media solution in compressed air by the spray set resulted in a stable gel formation in less than 1 min; (B) Hematoxylin–eosin staining revealed a fibrous structure of the sprayed platelet fibrin gel; (C) Representative scanning electron microscopy images of the sprayed platelet fibrin gel; (D–H) Enzyme-linked immunosorbent assay (ELISA) of the concentrations of vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF)-1, hepatocyte growth factor (HGF), platelet-derived growth factor (PDGF), and transforming growth factor-beta (TGF-β) from sprayed platelet fibrin gel conditioned media at different time points (n = 3 per time points). Scale bar = 100 μm. H & E, hematoxylin and eosin.
FIG. 3.
Cardiomyocyte biocompatibility with the sprayed platelet fibrin patch. (A) Schematic graphs showing neonatal rat cardiomyocytes (NRCMs) were cultured on presprayed gel (NRCMs on Spray) or sprayed gel directly onto preplated NRCM (Spray on NRCMs) or cultured NRCMs on standard tissue culture plate (NRCMs on TCP) as control; (B, C) Calcein (live)/EthD (dead) staining reveals a distinct morphology and no significant difference for viability of NRCMs grown with platelet fibrin gel compared with those grown on TCP at day 1 and 7 in culture (n = 3). Scale bar = 10 μm. “NS” indicates p > 0.05 when compared to each other by one-way analysis of variance followed by post hoc Bonferroni correction.
Characterization of sprayed platelet fibrin patch on post-MI hearts
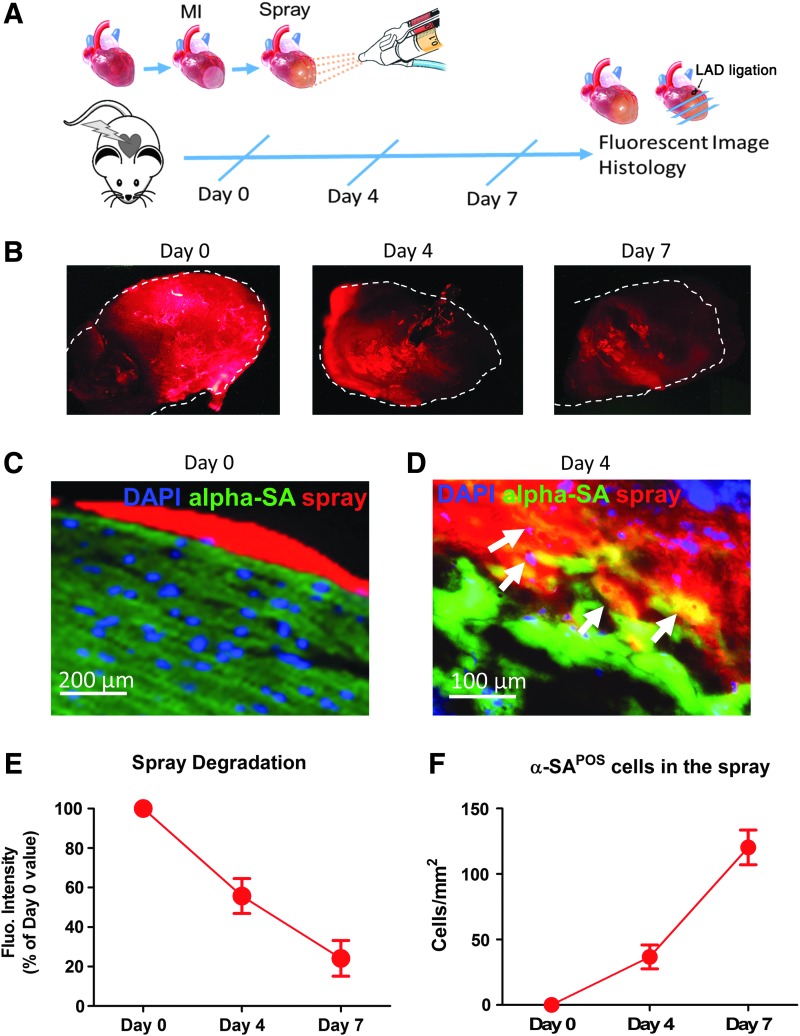
To identify the location and degradation of sprayed platelet fibrin patch in vivo, we prelabeled platelet fibrin gel with Texas Red-X succinimidyl ester (Invitrogen) to enable histological detection.12,13 We then evaluated the sprayed platelet fibrin patch in a mouse model of MI (Fig. 4A). The spray resulted in a uniform platelet fibrin patch on the surface of the heart (Fig. 4B, C), and degradation of the patch was observed over the time (Fig. 4E). Cryosections of the hearts enabled ready identification of the platelet fibrin gel by Texas Red-X epifluorescence (Fig. 4C, D). Sprayed gel was infiltrating into the myocardium (Fig. 4D, F).
FIG. 4.
Characterization of sprayed platelet fibrin patch on post-MI hearts. (A) Schematic design of sprayed labeled platelet fibrin patch in a mouse model of MI. Animals were euthanized at subsequent time point and hearts were harvested for fluorescent image and sliced for histology; (B) Animals were sacrificed at day 0, day 4, and 7, gross pictures showed a uniform platelet fibrin patch labeled with Texas Red-X epifluorescence ester on the surface of the MI heart at subsequent time points; (C) cryosection of the heart enabled ready identification of the platelet fibrin patch by Texas Red-X epifluorescence at day 0. Scale bar =200 μm; (D) Heart cryosections were stained with 4′, 6-diamidino-2-phenylindole (DAPI) for nuclei, and alpha-sarcomeric actin (alpha-SA) for cardiomyocytes, (arrows) indicating sprayed gel was infiltrating into the myocardium since day 4. Scale bar = 100 μm; (E) To calculate the percentage of degradation over time, the sprayed gel area at days 4 and 7 were measured and then normalized to the day 0 sprayed gel area; (F) Quantitation of cardiomyocytes integrated by the sprayed patch at different time points. MI, myocardial infarction.
Sprayed platelet fibrin patch attenuates LV remodeling and preserves cardiac function
A total of 30 animals were enrolled into the study for exploring the beneficial effects of sprayed cardiac patch (Fig. 5A). The survival rate was calculated by the record of survival animals, and 21-day post-MI was set as humane endpoints (Fig. 5B). In the MI alone group, two animals died of bleeding at day 1 post-MI, and one animal died of sudden cardiac arrest at day 2 post-MI. In animals treated with sprayed fibrin gel group, two mice died of bleeding at day 1 post-MI. Also, in animals treated with sprayed platelet fibrin gel group, only one mouse died of bleeding at day 1 post-MI. All other animals survived. Therefore, a total of 24 animals were finally included in the analysis (cardiac function and histopathology), distributed as follows: 6 in the SHAM group, 6 in the MI alone group, 6 in the MI + Fibrin Spray group, and 6 in the MI + Platelet Fibrin Spray group. Body weights of these survived animals were also recorded at subsequent time points to evaluate the life quality of mice after different treatments (Fig. 5C).
FIG. 5.
Overall study design of functional study for platelet fibrin patch on post-MI heart. (A) Schematic design of sprayed platelet fibrin patch on mice heart with MI, animals were sacrificed after 4 h and 21day echocardiography detection, following harvested hearts and sliced into three sections as illustrated. (B) Survival rate was calculated in mice post-MI with different treatment. (C) Body weight changes of CD1 mice were recorded at various time points in groups with different treatment.
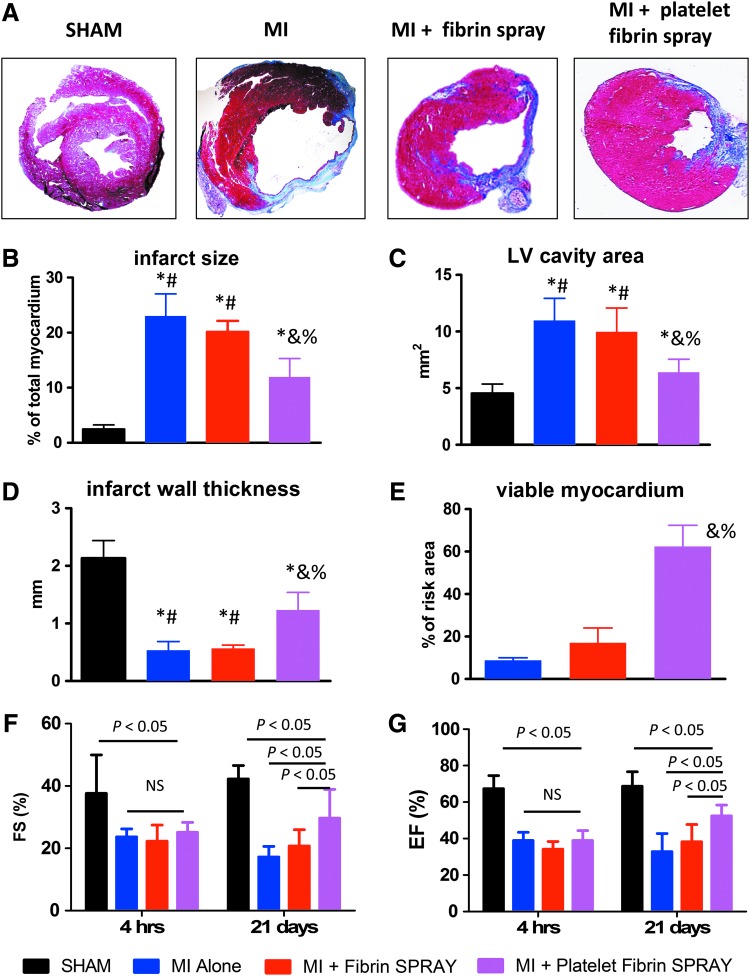
Masson's trichrome staining showed simultaneous detection of scar (blue) and healthy myocardial (red) tissues, and platelet fibrin patch-treated hearts exhibited attenuated LV remodeling and less abnormal heart morphology (Fig. 6A). Concomitantly, quantitative morphometry reflected that platelet fibrin patch-treated hearts exhibited smaller infarct size (Fig. 6B), smaller LV cavities (Fig. 6C), thicker infarcted walls (Fig. 6D), and more viable myocardium (Fig. 6E), indicating the ability of platelet fibrin patch to attenuate adverse LV remodeling.
FIG. 6.
Sprayed platelet fibrin patch attenuates LV remodeling and preserves cardiac function. (A) Representative Masson's trichrome-stained heart sections obtained from hearts 21 days after surgery. Scar tissue and viable myocardium are identified by blue and red stains respectively; (B–E) Quantitative analysis of infarct size, LV cavity area, infarct wall thickness, and viable tissue percentages from the Masson's trichrome images (n = 3 animals per group); (F, G) Left ventricular fraction shortening (LVFS) and left ventricular ejection fraction (LVEF) were measured by echocardiography at baseline (4 h post-MI) and 21 days afterward in SHAM, MI alone, MI + Fibrin Spray, and MI + Platelet Fibrin Spray groups (n = 6 animals per group); *p < 0.05 when compared with the SHAM group; #p < 0.05 when compared with MI + Platelet Fibrin Spray group; &p < 0.05 when compared with MI Alone group; %p < 0.05 when compared with MI + Fibrin Spray group. LV, left ventricular.
To evaluate the therapeutic benefits of platelet fibrin gel spray treatment, we also explored its ability to preserve or improve cardiac function in post-MI hearts. Both LVEF and LVFS at baseline (4 h post-MI) showed a similar decrease in MI alone group or MI + Fibrin/Platelet Fibrin SPRAY group when compared with SHAM group, indicating a comparable degree of initial injury. Over the next 3 weeks, both LVEF and LVFS declined in the MI alone group or preserved in the MI + Fibrin Spray group, but were improved in the MI + Platelet Fibrin SPRAY group (Fig. 6F, G). These results suggested that the sprayed platelet fibrin patch is sufficient to attenuate the loss of cardiac function after experimental MI.
Sprayed fibrin patch promotes endogenous repair and attenuates myocardial apoptosis
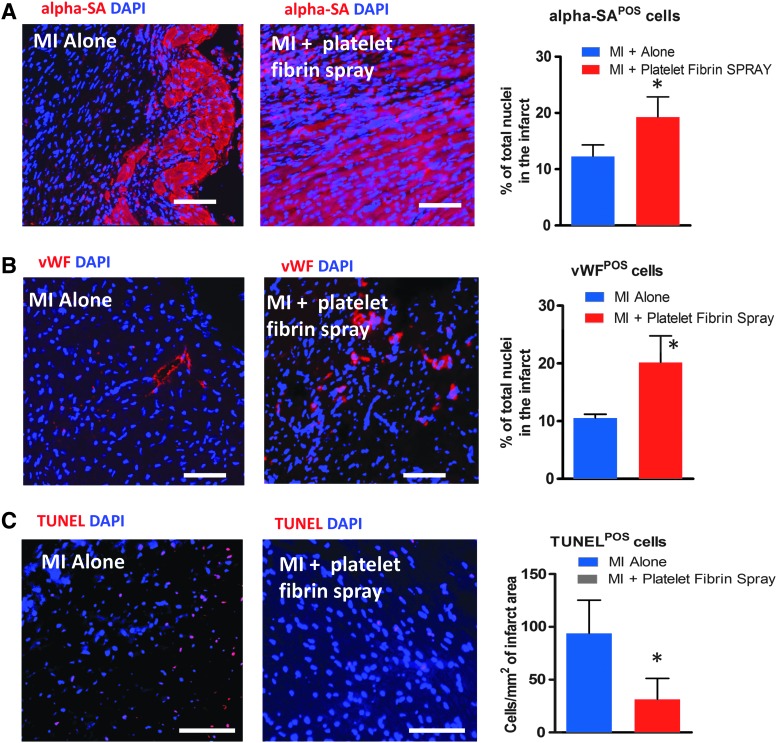
To investigate whether the sprayed platelet fibrin patch could promote endogenous repair, we stained heart section for alpha-sarcomeric actin (cardiomyocytes) or vWF (endothelial cells) 3 weeks post-MI. More cardiomyocytes (Fig. 7A; red) and endothelial cells (Fig. 7B; red) were detected residing in the infarcted area in MI treated with sprayed platelet fibrin patch compared with the MI alone group. Quantitative analysis also confirmed those findings (Fig. 7A, B bar graphs). TUNEL staining and analysis confirmed fewer apoptotic nuclei in the spray-treated hearts than in nontreated hearts (Fig. 7C; red nuclei), suggesting that the spray treatment could inhibit cell apoptosis after MI.
FIG. 7.
Sprayed platelet fibrin patch promotes endogenous repair and attenuates tissue apoptosis. (A) Cardiomyocytes stained with alpha sarcomeric actin (α-SA) (red) in the hearts 21 days after MI surgery. The numbers of α-SA positive cardiomyocytes were quantified (n = 3 animals per group). (B) Endothelial cells stained with von Willebrand factor (vWF) (red) in the hearts 21 days after MI surgery. The numbers of vWF-positive endothelial cells were quantified (n = 3 animals per group). (C) Representative fluorescent micrographs showing the presence of TUNEL+ apoptotic cells (red) in the hearts of MI alone and MI + Platelet Fibrin Spray groups. The numbers of apoptotic-positive cells were quantified (n = 3 animals per group). Scale bars = 100 μm. *p < 0.05 when compared with the treated with “MI Alone.”
Discussion
MI represents a major cause of death and generates significant socioeconomic costs.14 After MI, adult mammalian hearts only retain negligible potency for repair and regeneration. In contrast, sustained cardiomyocyte death and scaring following injury can eventually lead to heart failure.15
To date, approaches to cardiac tissue engineering range from injectable hydrogel to construction of complex cardiac constructs.16 Intramuscular injections have variable outcomes due to low biomaterials retention at the site of injection.17 Implanting engineered cardiac patch represents a promising strategy for cardiac tissue engineering.5,18,19 However, the placement of such a patch on the heart usually requires open-chest surgery and suturing in the heart. Such procedures are traumatic and time consuming.
The present study reported a novel way to deliver a cardiac patch onto the heart with a minimally invasive way. Platelet-rich plasma and calcium-containing media solution were packed separately and passed through a double-lumen syringe until the two met at the nozzle tip where compressed air was used to spray the mixture onto the heart. The mixture then formed a stable platelet fibrin gel by in situ polymerization. This spray method can be applied to other types of biomaterials. We used platelet fibrin gel as the model biomaterial in this study because it is well known for its biocompatibility and has been widely applied in regenerative medicine.17,20 Previous studies have also demonstrated that fibrin glue could improve the survival of injectable skeletal myoblasts, bone marrow cells, marrow-derived cardiac stem cells, adipose-derived stem cells, and human induced pluripotent stem cell-derived cardiomyocytesin MI hearts.21–26 Furthermore, injection of fibrin alone (without cells) could preserve cardiac function after MI and prevent negative LV remodeling.27 Also, our previous report has demonstrated the positive role of platelet fibrin gel in cardiac repair after MI.13
In the present study, we first demonstrated that a stable platelet fibrin gel patch can be formed by this spray method (Fig. 2A). To better understand the beneficial effects exerted by sprayed platelet fibrin gel, we demonstrated that sprayed platelet fibrin gel is nontoxic to cardiomyocytes (Fig. 3) and could stay on the heart but degrade over the time (Fig. 4). The sprayed cardiac patch contained interconnected pores (Fig. 2B, C) and could release paracrine factors like VEGF, IGF-1, HGF, PDGF, and TGF-β (Fig. 2D–H), which have been shown to promote cardiac repair.11,28 It has been well known that VEGF is proangiogenic and IGF-1 stimulates mitogenesis, promotes differentiation, and inhibits apoptosis in the heart.29,30 HGF promotes cell proliferation, motility, morphogenesis, and angiogenesis and also provides tissue protection after injury.31 Also, previous studies have demonstrated that factors such as VEGF, IGF-1, and HGF could contribute to tissue repair through their proangiogenic and antiapoptotic roles.32–34 More particularly, VEGF, IGF-1, and HGF could regulate the therapeutic effects of stem cells on injured heart by the aforementioned mechanisms.35,36 On the contrary, the contribution of sprayed platelet fibrin patch to infarct repair is likely even more important. In terms of endogenous cell regeneration, neovascularization and antiapoptosis, such actions are consistent with the reduced scar fibrosis, attenuated LV remodeling and improved cardiac function post-MI (Fig. 6). Most likely, paracrine effects have been the most plausible mechanism, by which platelet fibrin gel exerted its beneficial effects, which has been broadly investigated.7,13 Once sprayed on top of the infarcted heart, the cardiac patch secretes regenerative factors to promote angiomyocardiogenesis and reduce apoptosis (Fig. 7).
Conclusion
In summary, the use of this spraying system allowed for placement of cardiac patch without the need for surgeries and sutures. Spray painting of biomaterials on the surface of the heart provides an effective strategy for cardiac repair after MI.
Supplementary Material
Acknowledgment
This work was supported by funding from National Institute of Health HL123920, NC State University Chancellor's Faculty Excellence Program, NC State Chancellor's Innovation Fund, University of North Carolina General Assembly Research Opportunities Initiative grant, and National Natural Science Foundation of China 81370216 and 81570274, Science and Technology Innovation Team Support Project of Henan Province 14IRTSTHN018, and Innovation Team of Science and Technology Project of Henan Province. Junnan Tang is supported by China Scholarship Council.
Disclosure Statement
No competing financial interests exist.
References
- 1.Murray C.J., and Lopez A.D. Mortality by cause for eight regions of the World: Global Burden of Disease Study. Lancet 349, 1269, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Awada H.K., Hwang M.P., and Wang Y. Towards comprehensive cardiac repair and regeneration after myocardial infarction: aspects to consider and proteins to deliver. Biomaterials 82, 94, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christman K.L., and Lee R.J. Biomaterials for the treatment of myocardial infarction. J Am Coll Cardiol 48, 907, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Rane A.A., and Christman K.L. Biomaterials for the treatment of myocardial infarction: a 5-year update. J Am Coll Cardiol 58, 2615, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann W.H., Melnychenko I., Wasmeier G., Didie M., Naito H., et al. . Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med 12, 452, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Shen D., Tang J., Hensley M.T., Li T., Caranasos T.G., Zhang T., et al. . Effects of matrix metalloproteinases on the performance of platelet fibrin gel spiked with cardiac stem cells in heart repair. Stem Cells Transl Med 5, 793, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng K., Shen D., Smith J., Galang G., Sun B., et al. . Transplantation of platelet gel spiked with cardiosphere-derived cells boosts structural and functional benefits relative to gel transplantation alone in rats with myocardial infarction. Biomaterials 33, 2872, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandergriff A.C., Hensley M.T., Cheng K., et al. . Isolation and cryopreservation of neonatal rat cardiomyocytes. J Vis Exp 2015; DOI: 10.3791/52726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vandergriff A.C., Hensley M.T., and Cheng K. Cryopreservation of neonatal cardiomyocytes. Methods Mol Biol 1299, 153, 2015 [DOI] [PubMed] [Google Scholar]
- 10.Andrade J.N., Tang J., Hensley M.T., Vandergriff A., Cores J., et al. . Rapid and efficient production of coronary artery ligation and myocardial infarction in mice using surgical clips. PLoS One 10, e0143221, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng K., Li T.S., Malliaras K., Davis D.R., Zhang Y., and Marbán E. Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiospherederived cells in myocardial infarction. Circ Res 106, 1570, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam C.K., Yoo T., Hiner B., Liu Z., and Grutzendler J. Embolus extravasation is an alternative mechanism for cerebral microvascular recanalization. Nature 465, 478, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng K., Malliaras K., Shen D., Tseliou E., Ionta V., et al. . Intramyocardial injection of platelet gel promotes endogenous repair and augments cardiac function in rats with myocardial infarction. J Am Coll Cardiol 59, 256, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostadal B. The past, the present and the future of experimental research on myocardial ischemia and protection. Pharmacol Rep 61, 3, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Wei K., Serpooshan V., Hurtado C., Diez-Cuñado M., Zhao M., et al. . Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 525, 479, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freytes D.O., Santambrogio L., and Vunjak-Novakovic G. Optimizing dynamic interactions between a cardiac patch and inflammatory host cells. Cells Tissues Organs 195, 171, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radisic M., and Christman K.L. Materials science and tissue engineering: repairing the heart. Mayo Clin Proc 88, 884, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laflamme M.A., and Murry C.E. Regenerating the heart. Nat Biotechnol 23, 845, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Vunjak-Novakovic G., Tandon N., Godier A., Maidhof R., and Marsano A. Challenges in cardiac tissue engineering. Tissue Eng Part B Rev 16, 169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radosevich M., Goubran H.I., and Burnouf T. Fibrin sealant: scientific rationale, production methods, properties, and current clinical use. Vox Sang 72, 133, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Christman K.L., Vardanian A.J., Fang Q., Sievers R.E., and Fok H.H. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol 44, 654, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Nakamuta J.S., Danoviz M.E., Marques F.L., dos Santos L., Becker C., et al. . Cell therapy attenuates cardiac dysfunction post myocardial infarction: effect of timing, routes of injection and a fibrin scaffold. PLoS One 4, 6005, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo H.D., Wang H.J., Tan Y.Z., and Wu J.H. Transplantation of marrow-derived cardiac stem cells carried in fibrin improves cardiac function after myocardial infarction. Tissue Eng Part A 17, 45, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Danoviz M.E., Nakamuta J.S., Marques F.L., dos Santos L., Alvarenga E.C., et al. . Rat adipose tissue-derived stem cells transplantation attenuates cardiac dysfunction post infarction and biopolymers enhance cell retention. PLoS One 5, 12077, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang X., Wang H., Ma X., Adila A., Wang B., et al. . Preservation of the cardiac function in infarcted rat hearts by the transplantation of adipose-derived stem cells with injectable fibrin scaffolds. Exp Biol Med (Maywood) 235, 1505, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Wendel J.S., Ye L., Tao R., Zhang J., Zhang J., et al. . Functional effects of a tissue-engineered cardiac patch from human induced pluripotent stem cell-derived cardiomyocytes in a rat infarct model. Stem Cells Transl Med 4, 1324, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christman K.L., Fok H.H., Sievers R.E., Fang Q., and Lee R.J. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng 10, 403 2004 [DOI] [PubMed] [Google Scholar]
- 28.Li T.S., Cheng K., Malliaras K., Smith R.R., Zhang Y., et al. . Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol 59, 942, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shweiki D., Itin A., Soffer D., and Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359, 843, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Angoulvant D., Ivanes. F., Ferrera R., Matthews P.G., Nataf S., and Ovize M. Mesenchymal stem cell conditioned media attenuates in vitro and ex vivo myocardial reperfusion injury. J Heart Lung Transplant 30, 95, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Yan L., Zhu T.B., Wang L.S., Pan S.Y., Tao Z.X., et al. . Inhibitory effect of hepatocyte growth factor on cardiomyocytes apoptosis is partly related to reduced calcium sensing receptor expression during a model of simulated ischemia/reperfusion. Mol Biol Rep 38, 2695, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Davis M.E., Hsieh P.C., Takahashi T., Song Q., Zhang S., et al. . Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A 103, 8155, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan L., Zhu T.B., Wang L.S., Pan S.Y., Tao Z.X., et al. . Inhibitory effect of hepatocyte growth factor on cardiomyocytes apoptosis is partly related to reduced calcium sensing receptor expression during a model of simulated ischemia/reperfusion. Mol Biol Rep 38, 2695, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Shweiki D., Itin A., Soffer D., and Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359, 843, 1992 [DOI] [PubMed] [Google Scholar]
- 35.Hynes B., Kumar A.H., O'Sullivan J., Klein , Buneker C., Leblond A.L., et al. . Potent endothelial progenitor cell-conditioned media-related anti-apoptotic, cardiotrophic, and pro-angiogenic effects post-myocardial infarction are mediated by insulin-like growth factor-1. Eur Heart J 34, 782, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Hodgkinson C.P., Bareja A., Gomez J.A., and Dzau V.J. Emerging concepts in paracrine mechanisms in regenerative cardiovascular medicine and biology. Circ Res 118, 95, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.