Abstract
In this study, we reexamined the effect of CART peptide on psychostimulant (PS)-induced locomotor activity (LMA) in individual rats. The Methods utilized were as previously published. The PS-induced LMA was defined as the distance traveled after PS administration (intraperitoneal), and the CART peptide effect was defined as the change in the PS-induced activity after bilateral intra-NAc administration of CART peptide. The experiments included both male and female Sprague-Dawley rats, and varying the CART peptide dose and the PS dose. While the average effect of CART peptide was to inhibit PS-induced LMA, the effect of CART peptide on individual PS treated animals was not always inhibitory and sometimes even produced an increase or no change in PS-induced LMA. Upon further analysis, we observed a linear correlation, reported for the first time, between the magnitude of PS-induced LMA and the CART peptide effect. Because CART peptide inhibits PS-induced LMA when it is large, and increases PS-induced LMA when it is small, the peptide can be considered a homeostatic regulator of dopamine (DA)-induced LMA, which supports our earlier homeostatic hypothesis.
Key words/Phrases: CART peptide, nucleus accumbens, psychostimulant, dopamine, locomotor activity, homeostasis
INTRODUCTION
Cocaine-and-Amphetamine-Regulated-Transcript peptide (CART 55-102, CART peptide) is an active substance/neurotransmitter with a variety of effects throughout the body (Rogge et al., 2008; Zhang et al., 2012; Subhedar et al., 2014; Kuhar, 2016). In the brain, CART mRNA and peptides are found in many discrete nuclei including the nucleus accumbens (NAc), a region with a strong dopamine (DA) input (Douglass et al., 1995; Koylu et al., 1998), and exposure to cocaine (COC) or amphetamine (AMPH) increases the levels of CART mRNA in the NAc (Douglass et al., 1995; Albertson et al., 2004). Electron microscopic immunohistochemical data and confocal microscopic immunofluorescence data show that tyrosine hydroxylase-positive nerve terminals synapse on CART peptide-containing neurons in the NAc (Smith et al., 1999; Upadhya et al., 2012). Injection of COC increases the number of CART peptide positive cells that co-stain for c-Fos in the NAc (Hubert and Kuhar, 2008).
There are additional data that support a CART peptide-DA interaction. For example, several studies have shown that intra-NAc injections of CART peptide inhibit psychostimulant (PS)- and DA-induced behavioral effects, notably locomotor activity (LMA), and COC self-administration (Jaworski et al., 2003; Kim et al., 2003; Jaworski et al., 2008; Job and Kuhar, 2012; Job et al., 2012; Job et al., 2013; Job et al., 2014; Job, 2016). The bulk of these previous studies on CART peptide-DA interactions have involved an intra-NAc injection of CART peptide followed by other drug treatments and behavioral analyses. The mechanism of this inhibitory effect by CART peptide appears to require extracellular DA release (Job, 2016) and simultaneous activation of at least D1 and D2 dopamine receptors (Moffett et al., 2011). Also, PS-induced effects were altered in CART knockout mice (Couceyro et al., 2005), though not all studies agree (Moffett et al., 2006). More recent findings have been reviewed (Kuhar, 2016).
Intra-NAc CART peptide effect on DA-mediated activity is not always inhibitory. For example, the inhibition is lost after repeated doses of COC (Job et al., 2013). Also, intra-NAc CART peptide has no effect on LMA evoked by selective activation of DA D2 receptors in the NAc (Moffett et al., 2011). Interestingly, CART peptide may exert excitatory/facilitatory effects on DA-mediated activity. For example, in one study (Upadhya et al., 2012), administration of CART peptide antibody into the NAc shell suppressed food self-administration induced by systemic injection of a DA D2/D3 agonist implying that blockade of CART peptide action may result in inhibition of specific DA action. In another study, intra-NAc CART peptide potentiated LMA evoked by intra-NAc injections of a selective DA D1 agonist (Moffett et al., 2011). These studies suggest that, under certain conditions, intra-NAc CART peptide may have no effect or enhance/facilitate some DA-induced behavioral effects.
The mechanism of CART peptide effect is not very clear but it has been suggested that it is a homeostatic regulator of DA-mediated activity (Rogge et al., 2008). This implies that the effect of CART peptide should depend on the DA-mediated activity: when DA activity is high, CART peptide effect should be more inhibitory; when DA activity is low, CART peptide should be less inhibitory or even excitatory. In line with this idea, a previous report showed that COC-induced LMA was related to endogenous CART peptide levels in the NAc when individual subjects were considered (Job et al., 2012). In characterizing CART peptide function, it is important to determine, in individual subjects, whether this relationship occurs after exogenous administration of CART peptide into the NAc.
This study was undertaken to determine if the CART peptide effect is related to the PS effect. In this study, we again examine the effects of bilateral intra-NAc injections of CART peptide on COC- and AMPH-induced LMA. A major difference between this and earlier studies is that earlier studies looked at average changes in PS-induced activity after intra-NAc CART peptide administration, but the approach here was to consider the response of each individual animal. Thus, the relationship of CART peptide to the action of psychostimulants was reexamined.
EXPERIMENTAL PROCEDURES
Animals
Animal care was provided in accordance with the Emory University Institute of Animal Care and Use Committee and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. For this study, we used a total of fifty-eight Sprague Dawley rats: thirty-two male and twenty-six female rats (Charles River Inc, Wilmington, MA). The rats had access to chow and water ad libitum, and were maintained on a 12-hour light: dark cycle (lights on at 7am). The males and females used in this study were age-matched: the male and female rats were aged 3.4–7.8 months and 4–6 months, respectively. The male and female rats weighed 400 – 700 g and 250 – 400 g, respectively, at the time of the experiments.
Stereotaxic surgery
The rats were surgically implanted with bilateral stainless steel guide cannulae (22 gauge; Plastics One, Roanoke, VA) to target the NAc, under isoflurane anesthesia, as previously described (Job et al., 2013; Job et al., 2014). The implantation was done with the aid of a stereotaxic instrument (David Kopf, Tujunga, CA). The coordinate targets used were according to the rat brain atlas by Paxinos and Watson (1988) and were as follows: anterior-posterior (A/P) 1.6 mm, medial-lateral (M/L) ± 1.5 mm and dorsal-ventral (D/V) −5.7 mm to place the tip of the guide cannulae at a point 2 mm above the NAc. The placement of the guide cannulae was done in this way to minimize damage to the NAc. When placed into the guide, the injection cannulae extended 2 mm beyond the end of the guide to access the NAc. After implantation, the bilateral guide assembly was secured to the skull via acrylic dental cement and 2–4 stainless steel screws. Bilateral dummy cannulae (obturators) that did not extend beyond the guide cannulae were inserted into the bilateral guide cannulae to prevent occlusion. After surgery, the rats were allowed to recover for at least 1 week before experimentation.
Drug administration
CART peptide (CART 55-102, American peptide Co, Sunnyvale CA) (or saline control) was bilaterally administered directly into the NAc. Both hemispheres were injected simultaneously. The dose of CART peptide injected into each hemisphere was 1.0 or 2.5 μg, depending on the experiment. Intra-accumbal infusions were done through stainless steel bilateral injector cannulae (28 gauge, Plastics One) inserted into the implanted bilateral guide cannulae. The injector cannulae were connected to two 25 μL microsyringes (Hamilton Co, Reno, NV) via polyethylene-50 (PE-50) tubing. The two microsyringes were driven by micropumps connected to Micro4 Microsyringe Pump Controller (World Precision Instruments, Sarasota, FL). To inject, the rats were restrained gently, the obturator was removed and the injector cannulae were inserted in its place. Drug was bilaterally injected for 30 seconds, with an additional 30 seconds to allow the injected fluid to diffuse before removal of the injector cannulae. After removal of the injector cannulae, the obturator was placed back into the guide cannulae. Afterwards, animals were immediately given systemic administration of PS, either COC (cocaine hydrochloride, NIDA) or AMPH (D-Amphetamine hemisulfate, Sigma-Aldrich, St Louis, MO) or saline control. The systemic administration of PS (or saline control) was done via the intraperitoneal (i.p.) route. In accordance with previous studies (Jaworski et al., 2003; Kim et al., 2003; Jaworski et al., 2008; Job and Kuhar, 2012; Job et al., 2012; Job et al., 2013; Job et al., 2014; Job, 2016), all bilateral intra-NAc and systemic drug and vehicle control injections were given in volumes of 0.5 μL/side and 1 mL/kg, respectively.
Locomotor activity testing
Locomotor activity testing was done in locomotor chambers. The locomotor chambers (Omnitech Electronics, Columbus, OH, USA) were 40 × 40 × 30 cm in dimension and made of transparent plexiglass walls and contained 32 photobeams located 5 cm above the floor. The locomotor chambers were connected to a computer equipped with software (Digipro, Omnitech Electronics) to measure LMA. On an experimental day, rats were placed into the locomotor chambers for 30 min to habituate to their surroundings before the recording of basal LMA. After 30 min of basal LMA recording, rats were removed from the chambers and pretreated with bilateral intra-NAc saline or CART peptides and systemic PS and returned to the chamber. PS was administered immediately after bilateral intra-NAc administration of CART peptide or saline. The total distance traveled was the parameter used to assess LMA.
Experiments were conducted using a counterbalanced design with at least three days between experiments. Every animal received bilateral intra-NAc saline or CART peptide pretreatment on another experiment day, immediately before an i.p. injection of PS. The pretreatments were not always in the same order: some subjects received intra-NAc saline before intra-NAc CART peptide, whereas other subjects received intra-NAc CART peptide before intra-NAc saline.
Animal use details
As mentioned previously a total of 58 rats were used: 32 males and 26 females. As these data and analyses were being considered, some data were added from a previous publication (Job et al., 2014) to increase the number of subjects and to further test if a correlation existed; at the COC dose of 10 mg/kg, 6 males and 4 females were added, and at the COC dose of 15 mg/kg, 3 males and 5 females were added. These additions supplemented the number of animals, but they were fewer in number than the animals examined here for the first time.
Thirteen and ten male rats, respectively, were used for the effect of CART peptide (2.5 μg/side) on i.p. COC 10 mg/kg and 15 mg/kg. In males, six out of the thirteen animals that were used for the COC 10 dose were re-used for COC 0 dose. In females, for C0C 10 and 15 mg/kg i.p., fifteen and eleven rats were used, respectively. In females, five rats out of the fifteen rats used for the COC 10 dose and six rats out of the eleven rats used for the COC 15 dose were reused for COC 0 dose. Nine male rats were used for the effect of CART peptide (1.0 μg/side) on i.p. COC 10 dose. Of these, six animals were from the ten rats that were used for the effect of CART peptide (2.5 μg/side) on i.p. COC 15 mg/kg. The animals that were re-used were randomly selected.
Six male rats were used for the study investigating the effect of CART peptide (2.5 μg/side) on AMPH-mediated LMA. These same six animals were used for all the treatments (saline and CART peptide) and all the AMPH doses (0, 0.3 and 2 mg/kg i.p.).
Histology
The details for perfusions are as previously described (Job et al., 2014). Briefly, after experimentation, the animals were given an overdose of a cocktail of Ketaset (Ketamine HCl, Fort Dodge Animal Health, IA, USA; 70 mg/kg i.p.) and Dexdomitor (dexmedetomidine HCl, Orion Corporation, Espoo, Finland; 0.5 mg/kg i.p.) and were perfused with fixative (4% paraformaldehyde, intra-cardiac route) and the brains removed from the skulls. As soon as the brains were extracted, they were immersed in fixative overnight at 4°C. The next day, the brains were transferred into a solution containing 4% w/v paraformaldehyde and 30% w/v sucrose and kept herein for several days. Afterwards, the brains were sliced to a thickness of 60 microns using a cryostat (Leica Microsystems, Germany) and processed for Nissl staining. The sections were used to identify the locations of the injection sites and these were recorded on templates from Paxinos and Watson (Paxinos and Watson, 1998).
Statistical analyses
Statistical analyses were performed using GraphPad Prism v5 (GraphPad Software Inc, La Jolla, CA) and SigmaPlot for Windows v13.0 (Systat Software Inc, San Jose, CA). Data were expressed as mean ± SEM, and significance was set at P < 0.05. We tested for and found normal distributions (using Shapiro-Wilk test) and equal variances (Bartlett’s test) in our sampled distributions.
Three variables were considered: (1) the PS-induced LMA (PS effect/activity) with bilateral intra-NAc saline (no CART peptide), (2) the PS-induced LMA with bilateral intra-NAc CART peptide (PS effect + CART peptide effect) and (3) the difference between the two (CART peptide effect). The PS-induced LMA was defined as the total distance traveled within 10 min after PS administration minus total distance traveled within 10 min before PS administration. The CART peptide effect was defined as the change in PS-induced LMA due to CART peptide administration and was calculated as PS-induced activity with CART peptide minus PS-induced activity without CART peptide. A positive CART peptide effect indicates CART peptide increased PS-induced LMA, whereas a negative CART peptide effect indicates that CART peptide decreased PS-induced LMA.
We performed linear regression analyses to see if there were any correlations between the PS effect and the CART peptide effect after ensuring that normality and equality of variance criteria were not violated. The slopes of all the regression lines were calculated and slope comparisons were done using one-way ANOVA.
We also checked to see if the CART peptide mediated effect on PS-induced activity depended on the intra-NAc injection sites. Because bilateral injection sites were obtained per animal, there were two sets of coordinates per rat. These values were averaged and compared to the CART peptide effect. For this assessment, we performed multiple linear regression analysis with CART peptide effect as the dependent variable and the average coordinates of the injection sites (A/P, M/L and D/V) as the independent variables.
RESULTS
Animals were prepared as described in the Experimental Procedures section, and CART peptide or saline was injected bilaterally into the NAc, followed by varying doses (i.p.) of COC in males and females (Table 1A), varying CART peptide dose for same COC dose in males (Table 1B) or different PS (AMPH) in males (Table 1C) (various combinations shown in Table 1). The PS-induced LMA and the CART peptide effect (change in PS-induced LMA after CART peptide injection) were determined for each animal. The locations of the injectors tips in the NAc were histologically identified (Fig 1). All the animals in the study showed correct placements of the guide cannulae and injections in the NAc. As consistently observed previously, injection of CART peptide into the NAc without systemic PS injection produced no change in LMA. Again, as expected, systemic PS treatments (i.p.) increased LMA. The increases varied, with some animals exhibiting very low PS-induced LMA and with other animals exhibiting much greater levels of PS-induced LMA. On the average, the CART peptide effect was inhibitory, in agreement with previous studies.
Table 1. Experimental Groups.
A. When measuring the COC-induced LMA and the CART peptide effect in this group, only a CART peptide dose of 2.5 μg/side (and saline as a control) was used, but the COC dose varied (0, 10, 15 mg/kg). In addition, the gender was also varied. The experimental details are given in the text. The number of rats used are shown above.
B. When measuring the COC-induced LMA and the CART peptide effect in this group, the CART peptide dose varied (0, 1.0, 2.5 μg/side) while the COC dose was held constant (10 mg/kg). The sex (males) is held constant. Note that the CART peptide dose (2.5 μg/side) is the same as in experiment A above for males. The experimental details are given in the text. The number of rats used are shown above.
C. Similar to A above, but for a different PS (AMPH). When measuring the AMPH-induced LMA and the CART peptide effect in this group, only a CART peptide dose of 2.5 μg/side (and saline as a control) was used, but the AMPH dose varied (0, 0.3 and 2.0 mg/kg). The sex (males) is held constant. The experimental details are given in the text. The number of rats used are shown above.
| A. | ||||
|---|---|---|---|---|
| Sex | Intra-NAc CART peptide dose (μg/side) | Cocaine dose (mg/kg i.p.) | ||
| 0 | 10 | 15 | ||
| Males | 0, 2.5 | (n = 6) | (n = 13) | (n = 10) |
| Females | 0, 2.5 | (n = 11) | (n = 15) | (n = 11) |
| B. | |
|---|---|
| Intra-NAc CART peptide dose (μg/side) | Cocaine (10 mg/kg i.p.) |
| 0, 1.0 | (n = 9) |
| 0, 2.5 | (n = 13) |
| C. | |||
|---|---|---|---|
| Intra-NAc CART peptide dose (μg/side) | Amphetamine dose (mg/kg i.p.) | ||
| 0 | 0.3 | 2.0 | |
| 0, 2.5 | (n = 6) | (n = 6) | (n = 6) |
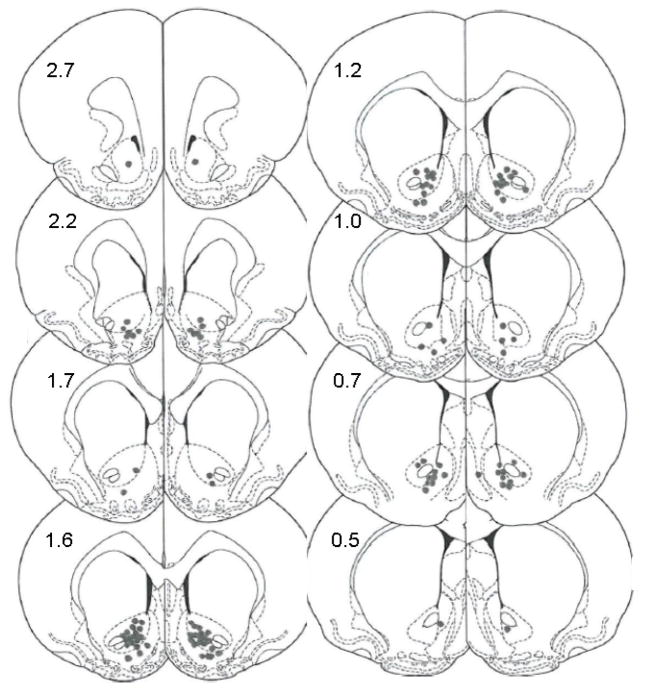
Fig 1. Summary of injector tip placements in the NAc.
The numbers on the histological diagrams show the distances from Bregma (in mm) as described in Methods and according to Paxinos and Watson (Paxinos and Watson, 1998). Each data point represents the location of an injector site for one hemisphere for one animal. Each animal has two injector sites. The number of data points on each side equal the number of animals used. The total number of animals is 58.
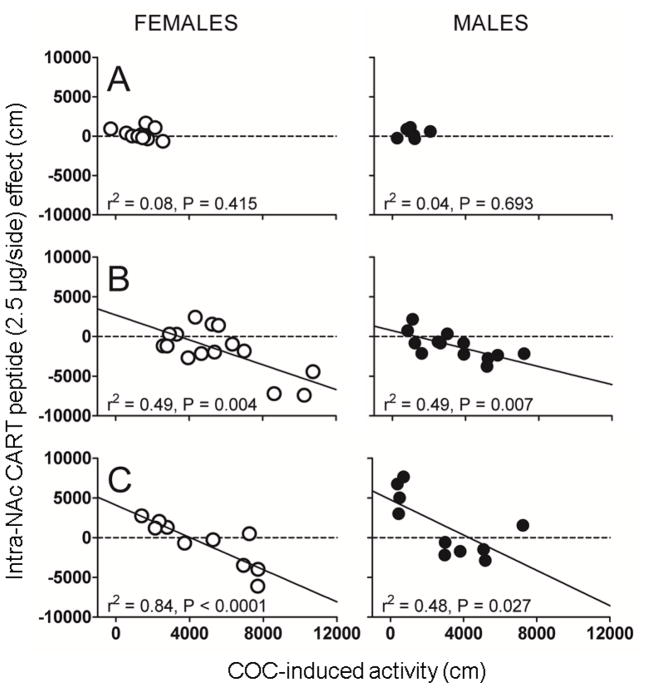
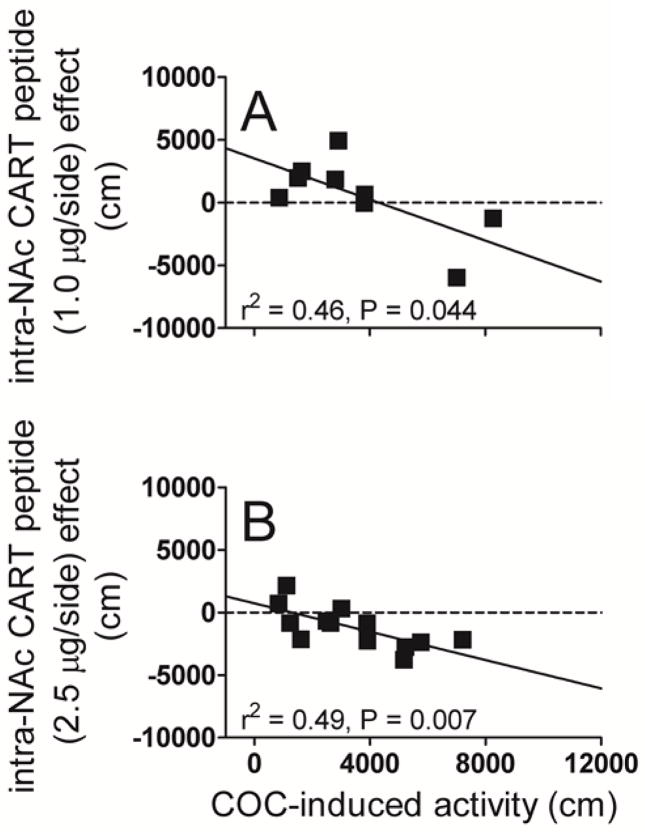
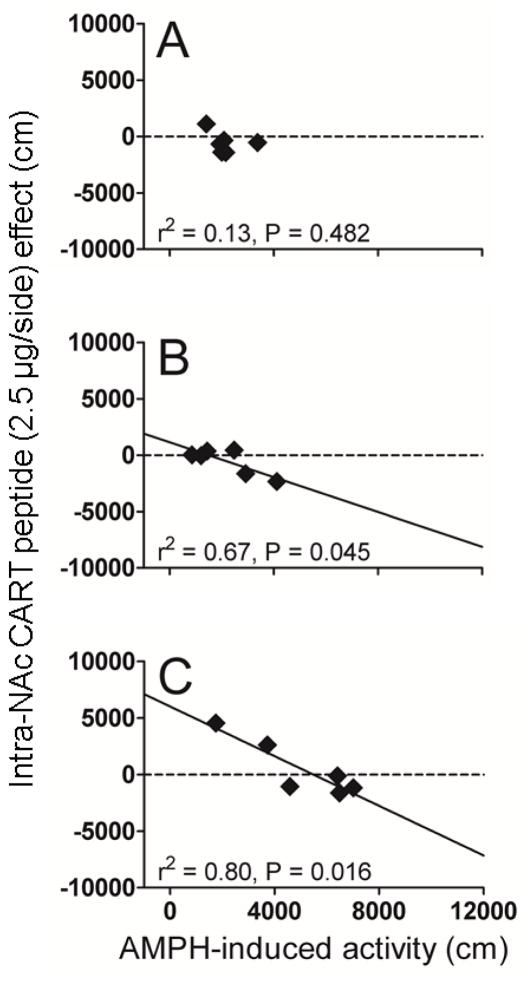
Considering the data from the individual animals, the CART peptide effect was variable, from inhibiting PS-induced LMA, to having no effect, to increasing PS-induced LMA (Fig 2, 3 and 4). Further examination of the data revealed a relationship not previously known or appreciated: there was a correlation between the intra-NAc CART peptide effect and the magnitude of the PS effect (slopes are significantly different from zero). This correlation is specific to PS administration, as it is not observed after saline injections (Fig 2, 4). For the COC studies, the correlation was true for both males and females (Fig 2). The correlation was also evident for different COC doses (10 and 15 mg/kg) (Fig 2) when the dose of CART peptide was held the same, i.e., 2.5 μg. The correlations were still evident when CART peptide doses were varied from 2.5 μg/side to 1.0 μg/side and the COC dose was held the same at 10 mg/kg (Fig 3):note that the data in Fig. 3B are the same as in Fig. 2B (males). We also observed a correlation with another PS: AMPH (Fig 4). Again, CART peptide injection alone, without AMPH, had no effect on LMA. For all conditions, the correlation was negative in that CART peptide was inhibitory when the PS-induced LMA was high, and the CART peptide effect was absent or even stimulatory when the PS-induced LMA was low. The summary of the data is that there exists a correlation between CART peptide effect and PS effect regardless of PS (AMPH and COC), gender, PS dose or CART peptide dose (Fig 2, 3, 4).
Fig 2. Correlations between the intra-NAc CART peptide (2.5 μg/side) effect and COC-induced LMA in male and female rats: Effect of sex and varying the dose of COC.
CART 55-102 (2.5 μg) and saline were administered bilaterally intra-NAc immediately before an i.p. injection of different doses of COC in males and females and total distance traveled in 10 min (COC-induced LMA) was measured. Panels A, B and C correspond to COC doses of 0, 10 and 15 mg/kg, respectively. The x-axis represents COC-induced LMA (cm), and the y-axis represents the CART peptide effect (cm). COC-induced activity (independent variable) and CART peptide effect (dependent variable) data were analyzed using linear regression analysis. All linear regression analyses passed the normality and equality of variance tests (P > 0.05). Each data point in the figure represents data from one animal. The correlation coefficients (Pearson’s r2) and P values are shown on the graphs. Note: For the females, one data point corresponding to a CART peptide effect of −9161 and COC-15-induced LMA of 12716 is just outside the axis, but is included in the analysis. Linear regression revealed the following values: F1, 13 = 12.627, P = 0.004, r = −0.702, n = 15 (Fig 2B, females, slope = −0.7820 ± 0.2201) and F1, 9 = 46.914, P < 0.001, r = −0.916, n = 11 (Fig 2C, females, slope = −1.015 ± 0.1481); F1, 11 = 10.703, P = 0.007, r = −0.702, n = 13 (Fig 2B, males, slope = −0.5664 ± 0.1731); F1, 8 = 7.318, P = 0.027, r = −0.691, n = 10 (Fig 2C, males, slope = −1.112 ± 0.4110). The data shows that there was no significant effect of CART peptide on LMA when no COC was administered (A, males and females), but there was a significant relationship between COC-induced LMA and the CART peptide effect (B, C, males and females).
Fig 3. Correlations between the intra-NAc CART peptide (1.0 or 2.5 μg/side.) effect and COC (10 mg/kg i.p.)-induced LMA in male rats: Effect of varying the dose of intra-NAc CART peptide.
All animals (males only in this comparison) were used for all treatments (counterbalanced design) and served as their own controls for both psychostimulant and CART peptide. CART 55-102 (1.0 or 2.5 μg) saline alone were administered bilaterally intra-NAc immediately before an i.p. injection of COC (10 mg/kg) and total distance traveled in 10 min (COC-induced LMA) was measured. The x-axis represents COC-induced LMA (cm), and the y-axis represents the CART peptide effect (cm). The points on the graphs represent individual subjects. COC-induced activity and CART peptide effect data were analyzed using linear regression. The correlation coefficients (Pearson’s r2) and P values are shown on the graphs. The linear regression analysis (Fig 3A) passed the normality and equality of variance tests (P = 0.846). Linear regression revealed the following values for the relationship between CART peptide effect (1.0 μg/side) and COC-induced activity: F1, 7 = 6.032, P = 0.044, r = −0.680, n = 9 (Fig 3A, slope = −0.8174 ± 0.3328). The F value, degrees of freedom, P value, r and slope for CART peptide 2.5 μg/side dose shown above has been previously reported in Fig 2B (males). There was a correlation between COC-induced LMA and intra-NAc CART peptide (both 1.0 μg and 2.5 μg) effect for COC (10 mg/kg i.p.). Note that the data in Fig. 3B are the same as in Fig. 2B (males).
Fig 4. Correlation between the intra-NAc CART peptide (2.5 μg/side) effect and the AMPH-induced LMA in male rats: Effect of different PS (compare to COC).
The same rats (n = 6) were used for all treatments (counterbalanced design) and served as their own controls. CART 55-102 (2.5 μg) and/or saline were administered bilaterally intra-NAc immediately before an i.p. injection of different doses of AMPH (in the order shown in panels A, B and C which correspond to AMPH 0, 0.3, 2 mg/kg, respectively) and total distance traveled in 10 min (AMPH-induced LMA) was measured. The x-axis represents AMPH-induced activity (cm). The y-axis represents CART peptide effect (cm). The points on the graphs represent individual subjects. AMPH-induced activity and CART peptide effect data were analyzed using linear regression analysis. All linear regression analyses passed the normality and equality of variance tests (P > 0.05). The correlation coefficients (Pearson’s r2) and P values are shown on the graph. Linear regression revealed the following values: F1, 4 = 8.258, P = 0.045, r = −0.821 (Fig 4B, slope = −0.7723 ± 0.2687) and F1, 4 = 16.032, P = 0.016, r = −0.895 (Fig 4C, slope = −1.096 ± 0.2737). The analysis reveals that there was no relationship between the CART peptide effect and saline injections (A), but there was a relationship between AMPH-induced effect and CART peptide effect for AMPH (0.3 and 2 mg/kg i.p., B and C).
We determined and compared the slopes of the regression lines. There were no differences between the slopes of all the regression lines shown in Fig 2B, C (males and females), Fig 3A, Fig 4 B, C (F6, 63 = 0.587, P = 0.740). Thus, the slope between CART peptide effect and PS effect are similar for different PS (AMPH and COC), different gender, different PS doses or CART peptide doses (Fig 2, 3, 4). CART peptide effect is related to PS activity [CART peptide effect = K * (PS activity), where K is a constant].
We wanted to know if the injection sites in the NAc were related with the CART peptide effect. There was no relationship between the NAc injection site coordinates and CART peptide (2.5 μg/side) effect for males, COC 10 (Fig 2B, F3, 9 = 1.335, P = 0.323), females, COC 10 (Fig 2B, F3, 11 = 0.344, P = 0.794), males, COC 15 (Fig 2C, F3, 6 = 1.321, P = 0.352), females, COC 15 (Fig 2C, F3, 7 = 0.825, P = 0.521). There was no relationship between NAc coordinates and CART peptide (1.0 μg/side) effect (Fig 3A, P >0.05). For the AMPH study, the A/P coordinate was the same for all six animals used (A/P = 1.6 mm) and there was no relationship between the NAc injection site coordinates (M/L and D/V) and CART peptide (2.5 μg/side) effect for AMPH 0.3 mg/kg (Fig 4B, F2, 3 = 1.633, P = 0.331) and AMPH 2 mg/kg (Fig 4C, F2, 3 = 0.133, P = 0.880). Thus, while there was a correlation between the intra-NAc CART peptide effect and the magnitude of the PS effect (Fig 2, 3, 4), there was no correlation between intra-NAc CART peptide effect and the injection site in the NAc.
We also performed additional linear regressions to see if there were any relationships between body weights or age versus PS activity and CART peptide effects, but none were found (not shown).
DISCUSSION
The major finding of this study is the correlation between PS-induced LMA and the CART peptide effect. Previous work in the NAc indicated that CART peptide had an average inhibitory effect, and that this inhibition tended to regulate the maximal activity of the PS- or DA-induced behavioral effects; for reviews see (Rogge et al., 2008; Zhang et al., 2012; Subhedar et al., 2014; Kuhar, 2016). But in this study, we considered and included the individual responses in the analysis. CART peptide produced inhibition, or had no effect, or was excitatory in the various individual animals. The precise effect of CART peptide tended to depend on the magnitude of the effect of the PS; when the PS-induced LMA was low, the CART peptide effect was zero or excitatory and when the PS effect was high the CART peptide effect was inhibitory. Because CART peptide inhibits larger PS-induced LMA, and increases very small PS-induced LMA, it can be considered a homeostatic regulator of DA-induced LMA. Thus, this hypothesis which was stated in our earlier work has not changed (Rogge et al., 2008). Because CART peptide can exert several effects (excitation, inhibition, no effect) on PS-induced activity, therefore only considering the average of the effects as has been done earlier can now be considered inadequate in understanding how CART peptide functions. At the least, the effect of CART peptide in the NAc on PS-stimulated LMA is now more fully characterized.
There are several differences between this study and earlier ones. As already mentioned, only averages of CART peptide’s effects were considered earlier, whereas the effect in each individual animal is considered here. Also, in previous studies, the locations of the injectors were more restricted, from 1.2 to 1.7 mm bregma (Jaworski et al., 2003; Jaworski et al., 2008; Moffett et al., 2011), while the entire extent of the NAc (0.5 to 2.7 mm from bregma) was examined here. Additionally, most previous studies utilized male rats while both sexes were included here. There were also differences in the anatomical focus; Moffett et al (2011) focused on the shell of the NAc while Jaworski et al (2003) had injections mainly in the core of the NAc, but both regions were studied here. Also, in earlier studies, animals with i.p. injections that produced no increase in LMA may have been discarded as possible missed injections; in that case, excitatory effects would have been missed. All animals were included here. For all of the reasons mentioned above, this study may be considered more comprehensive than earlier ones.
The slopes of the regression lines obtained were not different. This implies that CART peptide is regulating PS-induced activity similarly for males and females (Job et al., 2014), for COC and AMPH, for different doses of PS and for different doses of CART peptide. Since PS drugs act by potentiating DA activity at the synapse, it is likely that the mechanism of the CART peptide effect involves the level of DA-induced activity in the NAc. Indeed, in earlier studies, it was shown that CART peptide inhibited DA-induced LMA as well as PS-induced LMA (Rogge et al., 2008; Kuhar, 2016). This level of DA activity may involve extracellular DA release. This is likely the case because CART peptide had no effect on caffeine-induced LMA which is characterized by a lack of extracellular DA release in the NAc (Job, 2016).
This study introduces the idea that intra-NAc CART peptide is not always inhibitory but depends on additional variables, mainly the PS response, and perhaps the state of the animal. Others have found that the LMA effects of CART peptide are somewhat different in different brain regions. Kimmel et al. (2000) reported that injections of CART peptide alone into the ventral tegmental area (VTA) increased LMA and induced conditioned place preference; thus CART peptide by itself was PS-like in the VTA (Kimmel et al., 2000). By contrast, injection of CART peptide by itself into a different region, the NAc, had no effect. Later Jaworski et al (2007) showed that injection of CART peptide into the VTA did inhibit COC-induced LMA which has supported the traditional idea that CART peptide inhibits the LMA due to COC (Jaworski et al., 2007). Similarly, injections of CART peptide into the ventral pallidum inhibit COC-induced LMA (Hubert et al., 2010). Even injections into the peritoneum can have effects on PS-induced activity (Job and Kuhar, 2012). Without a detailed mechanistic understanding of the CART peptide effect, it is difficult to predict the effect of CART peptide in any given brain region.
Does the effect of exogenously injected CART peptide mimic the role of endogenously released CART peptide? There are several studies that suggest the answer is yes, and most of these studies address the role of CART peptide in feeding and body weight. On the one hand, injection of CART peptide fragments causes an inhibition of feeding (Kristensen et al., 1998; Lambert et al., 1998; Thim et al., 1998), but injection of CART peptide antibodies can have the opposite effect (Lambert et al., 1998). Also, CART knockout mice gain weight (Asnicar et al., 2001; Wierup et al., 2005) and a human family with a CART gene mutation exhibits obesity (del Giudice et al., 2001). Overall, there is abundant evidence that CART peptides control feeding (Kristensen et al., 1998; Rogge et al., 2008; Bharne et al., 2015; Abels et al., 2016). Further, in other studies of PS effects, rats given shRNA injections to reduce levels of CART peptide (Job et al., 2012) exhibited an increased response to COC injections, and a gain in weight. Thus, there is evidence that endogenous CART has the same effect as exogenously applied CART peptide. Importantly, for both endogenous (Job et al., 2012) and exogenous (shown in this study) CART peptide, the magnitude of COC-mediated activity seems to be directly related to the CART peptide effect.
It has been reported by others that the effects of other peptides such as neurotensin or cholecystokinin (CCK) can depend on the dose/magnitude of effect of PS (Skoog et al., 1986; Higgins et al., 1994). In this study, we show that CART peptide effects depend on the magnitude of PS activity. Further studies are needed to fully understand how CART peptide actually functions. While the CART peptide receptor has been identified by binding (Yermolaieva et al., 2001; Lakatos et al., 2005; Vicentic et al., 2005; Keller et al., 2006; Maletinska et al., 2007; Rogge et al., 2008; Chiu et al., 2009; Lin et al., 2011; Nagelova et al., 2014), the receptor gene needs to be identified and studied. Further, the connectivity of CART peptide-containing neurons with other neurons needs to be elucidated in more detail as well. A review of recent studies shows that several different drug classes interact with CART peptide systems in brain (Kuhar, 2016). CART peptide remains an interesting peptide that is involved with and likely regulates the actions of many drugs of abuse.
Highlights.
CART peptide regulates psychostimulant (PS)-induced activity.
Intra-nucleus accumbens CART peptide effect is related to PS-induced activity.
CART peptide can excite, have no effect, or inhibit PS-induced activity.
The above CART peptide effects keep PS-induced activity within a certain range.
This finding confirms the homeostatic hypothesis of CART peptide function.
Acknowledgments
The authors acknowledge the support of the Yerkes National Primate Research Center of Emory University. This project was funded by the National Center for Research Resources P51RR165 and supported by the Office of Research Infrastructure Programs/OD P51OD11132. It was also supported by DA15040 and the Georgia Research Alliance. These funding sources had no role in the direction of this research or publication.
The authors have no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abels M, Riva M, Bennet H, Ahlqvist E, Dyachok O, Nagaraj V, Shcherbina L, Fred RG, Poon W, Sorhede-Winzell M, Fadista J, Lindqvist A, Kask L, Sathanoori R, Dekker-Nitert M, Kuhar MJ, Ahren B, Wollheim CB, Hansson O, Tengholm A, Fex M, Renstrom E, Groop L, Lyssenko V, Wierup N. CART is overexpressed in human type 2 diabetic islets and inhibits glucagon secretion and increases insulin secretion. Diabetologia. 2016;59:1928–1937. doi: 10.1007/s00125-016-4020-6. [DOI] [PubMed] [Google Scholar]
- Albertson DN, Pruetz B, Schmidt CJ, Kuhn DM, Kapatos G, Bannon MJ. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem. 2004;88:1211–1219. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnicar MA, Smith DP, Yang DD, Heiman ML, Fox N, Chen YF, Hsiung HM, Koster A. Absence of cocaine- and amphetamine-regulated transcript results in obesity in mice fed a high caloric diet. Endocrinology. 2001;142:4394–4400. doi: 10.1210/endo.142.10.8416. [DOI] [PubMed] [Google Scholar]
- Bharne AP, Borkar CD, Subhedar NK, Kokare DM. Differential expression of CART in feeding and reward circuits in binge eating rat model. Behav Brain Res. 2015;291:219–231. doi: 10.1016/j.bbr.2015.05.030. [DOI] [PubMed] [Google Scholar]
- Chiu HY, Lin HH, Lai CC. Potentiation of spinal NMDA-mediated nociception by cocaine- and amphetamine-regulated transcript peptide via PKA and PKC signaling pathways in rats. Regul Pept. 2009;158:77–85. doi: 10.1016/j.regpep.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Couceyro PR, Evans C, McKinzie A, Mitchell D, Dube M, Hagshenas L, White FJ, Douglass J, Richards WG, Bannon AW. Cocaine- and amphetamine-regulated transcript (CART) peptides modulate the locomotor and motivational properties of psychostimulants. J Pharmacol Exp Ther. 2005;315:1091–1100. doi: 10.1124/jpet.105.091678. [DOI] [PubMed] [Google Scholar]
- del Giudice EM, Santoro N, Cirillo G, D’Urso L, Di Toro R, Perrone L. Mutational screening of the CART gene in obese children: identifying a mutation (Leu34Phe) associated with reduced resting energy expenditure and cosegregating with obesity phenotype in a large family. Diabetes. 2001;50:2157–2160. doi: 10.2337/diabetes.50.9.2157. [DOI] [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Sills TL, Tomkins DM, Sellers EM, Vaccarino FJ. Evidence for the contribution of CCKB receptor mechanisms to individual differences in amphetamine-induced locomotion. Pharmacol Biochem Behav. 1994;48:1019–1024. doi: 10.1016/0091-3057(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Hubert GW, Kuhar MJ. Cocaine administration increases the fraction of CART cells in the rat nucleus accumbens that co-immunostain for c-Fos. Neuropeptides. 2008;42:339–343. doi: 10.1016/j.npep.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert GW, Manvich DF, Kuhar MJ. Cocaine and amphetamine-regulated transcript-containing neurons in the nucleus accumbens project to the ventral pallidum in the rat and may inhibit cocaine-induced locomotion. Neuroscience. 2010;165:179–187. doi: 10.1016/j.neuroscience.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski JN, Hansen ST, Kuhar MJ, Mark GP. Injection of CART (cocaine- and amphetamine-regulated transcript) peptide into the nucleus accumbens reduces cocaine self-administration in rats. Behav Brain Res. 2008;191:266–271. doi: 10.1016/j.bbr.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski JN, Kimmel HL, Mitrano DA, Tallarida RJ, Kuhar MJ. Intra-VTA CART 55-102 reduces the locomotor effect of systemic cocaine in rats: an isobolographic analysis. Neuropeptides. 2007;41:65–72. doi: 10.1016/j.npep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski JN, Kozel MA, Philpot KB, Kuhar MJ. Intra-accumbal injection of CART (cocaine-amphetamine regulated transcript) peptide reduces cocaine-induced locomotor activity. J Pharmacol Exp Ther. 2003;307:1038–1044. doi: 10.1124/jpet.103.052332. [DOI] [PubMed] [Google Scholar]
- Job MO. Injection of Cocaine-Amphetamine Regulated Transcript (CART) peptide into the nucleus accumbens does not inhibit caffeine induced locomotor activity: Implications for CART peptide mechanism. Pharmacol Biochem Behav. 2016 doi: 10.1016/j.pbb.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job MO, Kuhar MJ. Intraperitoneal Administration of CART 55-102 Inhibits Psychostimulant-Induced Locomotion. J Drug Alcohol Res. 2012:1. doi: 10.4303/jdar/235601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job MO, Licata J, Hubert GW, Kuhar MJ. Intra-accumbal administration of shRNAs against CART peptides cause increases in body weight and cocaine-induced locomotor activity in rats. Brain Res. 2012;1482:47–54. doi: 10.1016/j.brainres.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job MO, Perry J, Shen LL, Kuhar MJ. Cocaine-and-Amphetamine Regulated Transcript (CART) peptide attenuates dopamine- and cocaine-mediated locomotor activity in both male and female rats: lack of sex differences. Neuropeptides. 2014;48:75–81. doi: 10.1016/j.npep.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job MO, Shen LL, Kuhar MJ. The inhibition of cocaine-induced locomotor activity by CART 55-102 is lost after repeated cocaine administration. Neurosci Lett. 2013;550:179–183. doi: 10.1016/j.neulet.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller PA, Compan V, Bockaert J, Giacobino JP, Charnay Y, Bouras C, Assimacopoulos-Jeannet F. Characterization and localization of cocaine- and amphetamine-regulated transcript (CART) binding sites. Peptides. 2006;27:1328–1334. doi: 10.1016/j.peptides.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Kim JH, Creekmore E, Vezina P. Microinjection of CART peptide 55-102 into the nucleus accumbens blocks amphetamine-induced locomotion. Neuropeptides. 2003;37:369–373. doi: 10.1016/j.npep.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Gong W, Vechia SD, Hunter RG, Kuhar MJ. Intra-ventral tegmental area injection of rat cocaine and amphetamine-regulated transcript peptide 55-102 induces locomotor activity and promotes conditioned place preference. J Pharmacol Exp Ther. 2000;294:784–792. [PubMed] [Google Scholar]
- Koylu EO, Couceyro PR, Lambert PD, Kuhar MJ. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol. 1998;391:115–132. [PubMed] [Google Scholar]
- Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, Larsen PJ, Hastrup S. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–76. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ. CART Peptides and Drugs of Abuse: A Review of Recent Progress. Journal of Drug and Alcohol Research. 2016:5. doi: 10.4303/jdar/235984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos A, Prinster S, Vicentic A, Hall RA, Kuhar MJ. Cocaine- and amphetamine-regulated transcript (CART) peptide activates the extracellular signal-regulated kinase (ERK) pathway in AtT20 cells via putative G-protein coupled receptors. Neurosci Lett. 2005;384:198–202. doi: 10.1016/j.neulet.2005.04.072. [DOI] [PubMed] [Google Scholar]
- Lambert PD, Couceyro PR, McGirr KM, Dall Vechia SE, Smith Y, Kuhar MJ. CART peptides in the central control of feeding and interactions with neuropeptide Y. Synapse. 1998;29:293–298. doi: 10.1002/(SICI)1098-2396(199808)29:4<293::AID-SYN1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Lin Y, Hall RA, Kuhar MJ. CART peptide stimulation of G protein-mediated signaling in differentiated PC12 cells: identification of PACAP 6-38 as a CART receptor antagonist. Neuropeptides. 2011;45:351–358. doi: 10.1016/j.npep.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maletinska L, Maixnerova J, Matyskova R, Haugvicova R, Sloncova E, Elbert T, Slaninova J, Zelezna B. Cocaine- and amphetamine-regulated transcript (CART) peptide specific binding in pheochromocytoma cells PC12. Eur J Pharmacol. 2007;559:109–114. doi: 10.1016/j.ejphar.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Moffett M, Stanek L, Harley J, Rogge G, Asnicar M, Hsiung H, Kuhar M. Studies of cocaine- and amphetamine-regulated transcript (CART) knockout mice. Peptides. 2006;27:2037–2045. doi: 10.1016/j.peptides.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Moffett MC, Song J, Kuhar MJ. CART peptide inhibits locomotor activity induced by simultaneous stimulation of D1 and D2 receptors, but not by stimulation of individual dopamine receptors. Synapse. 2011;65:1–7. doi: 10.1002/syn.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelova V, Pirnik Z, Zelezna B, Maletinska L. CART (cocaine- and amphetamine-regulated transcript) peptide specific binding sites in PC12 cells have characteristics of CART peptide receptors. Brain Res. 2014;1547:16–24. doi: 10.1016/j.brainres.2013.12.024. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; Burlington, MA: 1998. [Google Scholar]
- Rogge G, Jones D, Hubert GW, Lin Y, Kuhar MJ. CART peptides: regulators of body weight, reward and other functions. Nat Rev Neurosci. 2008;9:747–758. doi: 10.1038/nrn2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog KM, Cain ST, Nemeroff CB. Centrally administered neurotensin suppresses locomotor hyperactivity induced by d-amphetamine but not by scopolamine or caffeine. Neuropharmacology. 1986;25:777–782. doi: 10.1016/0028-3908(86)90095-x. [DOI] [PubMed] [Google Scholar]
- Smith Y, Kieval J, Couceyro PR, Kuhar MJ. CART peptide-immunoreactive neurones in the nucleus accumbens in monkeys: ultrastructural analysis, colocalization studies, and synaptic interactions with dopaminergic afferents. J Comp Neurol. 1999;407:491–511. doi: 10.1002/(sici)1096-9861(19990517)407:4<491::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Subhedar NK, Nakhate KT, Upadhya MA, Kokare DM. CART in the brain of vertebrates: circuits, functions and evolution. Peptides. 2014;54:108–130. doi: 10.1016/j.peptides.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Thim L, Nielsen PF, Judge ME, Andersen AS, Diers I, Egel-Mitani M, Hastrup S. Purification and characterisation of a new hypothalamic satiety peptide, cocaine and amphetamine regulated transcript (CART), produced in yeast. FEBS Lett. 1998;428:263–268. doi: 10.1016/s0014-5793(98)00543-2. [DOI] [PubMed] [Google Scholar]
- Upadhya MA, Nakhate KT, Kokare DM, Singh U, Singru PS, Subhedar NK. CART peptide in the nucleus accumbens shell acts downstream to dopamine and mediates the reward and reinforcement actions of morphine. Neuropharmacology. 2012;62:1823–1833. doi: 10.1016/j.neuropharm.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Vicentic A, Lakatos A, Kuhar MJ. CART (cocaine- and amphetamine-regulated transcript) peptide receptors: specific binding in AtT20 cells. Eur J Pharmacol. 2005;528:188–189. doi: 10.1016/j.ejphar.2005.11.041. [DOI] [PubMed] [Google Scholar]
- Wierup N, Richards WG, Bannon AW, Kuhar MJ, Ahren B, Sundler F. CART knock out mice have impaired insulin secretion and glucose intolerance, altered beta cell morphology and increased body weight. Regul Pept. 2005;129:203–211. doi: 10.1016/j.regpep.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Yermolaieva O, Chen J, Couceyro PR, Hoshi T. Cocaine- and amphetamine-regulated transcript peptide modulation of voltage-gated Ca2+ signaling in hippocampal neurons. J Neurosci. 2001;21:7474–7480. doi: 10.1523/JNEUROSCI.21-19-07474.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Han L, Xu Y. Roles of cocaine- and amphetamine-regulated transcript in the central nervous system. Clin Exp Pharmacol Physiol. 2012;39:586–592. doi: 10.1111/j.1440-1681.2011.05642.x. [DOI] [PubMed] [Google Scholar]