Abstract
Objective
To test the hypothesis that feeding and antibiotic exposures affect intestinal barrier maturation in preterm infants, we serially measured intestinal permeability (IP) biomarkers in infants <33 wks gestation (GA) during the first two weeks of life.
Study design
Eligible infants <33 wks GA were enrolled within 4 days of birth in a prospective study of IP biomarkers (NCT01756040). Study participants received the non-metabolized sugars lactulose/rhamnose (La/Rh) enterally on study days 1, 8 and 15 and La/Rh were measured in urine by HPLC. Serum zonulin and fecal alpha-1 antitrypsin, two other IP markers, were measured by semi-quantitative western blot and ELISA, respectively.
Results
In a cohort of 43 subjects, the La/Rh ratio was elevated on day 1 and decreased over 2 weeks, but remained higher in infants ≤28 wk GA compared with IP in infants >28 wk GA. Exclusive breastmilk feeding was associated with more rapid maturation in intestinal barrier function. A cluster analysis of 35 subjects who had urine samples from all time points revealed three IP patterns (Cluster 1, normal maturation [N=20 (57%)]; Cluster 2, decreased IP during the first week and subsequent substantial increase [N=5 (14%)]; and Cluster 3, delayed maturation [N=10 (29%)]). There were trends towards more prolonged antibiotic exposure (p=0.092) and delayed initiation of feeding ≥4 days (p=0. 0.064) in infants with abnormal IP patterns.
Conclusions
Intestinal barrier maturation in preterm infants is GA and postnatal age-dependent and is influenced by feeding with a maturational effect of breastmilk feeding and may be by antibiotic exposures.
Keywords: Intestinal permeability, prematurity, zonulin, alpha-1-anti-trypsin
Introduction
Necrotizing enterocolitis (NEC), a life-threatening, gastrointestinal emergency affects approximately 7 to 10% of very low birthweight preterm neonates 1, 2 with mortality as high as 30-50% 3. Although breast milk has been shown to be protective against NEC 4, 5, postnatal antibiotic exposure may increase the risk for NEC 6, 7. Intestinal barrier immaturity is the proximate cause of susceptibility to NEC in preterm neonates 8, 9, but few preterm infants <30 wk gestation have been included in prior studies of intestinal permeability (IP) 10-15 and the impact of current feeding practices and antibiotic exposures on intestinal barrier maturation in the extremely preterm population is unknown.
The percent urinary excretion of orally administered isotonic solutions of the non-metabolized sugars lactulose and rhamnose as markers of the intestinal paracellular and transcellular pathways, respectively, is the gold standard to assess IP. These tests have been used extensively for over 30 years to assess IP in adults 16-18 and in preterm 10-15 and term infants 13, 19, 20 as well as older children. The sugar probes have been used safely to assess IP in newborns with birth asphyxia 10, necrotizing enterocolitis (NEC) 11, and congenital heart disease20, 21. Other markers of impaired intestinal barrier function include zonulin in adults22 and alpha 1-antrypsin (A1AT) in children23 . Zonulin is a 47 kDA eukaryotic cellular protein that regulates the intestinal epithelial paracellular pathway by reversibly opening mature tight junctions 24 and is up-regulated in several autoimmune diseases, including celiac disease and type 1 diabetes 22. Whether zonulin is involved in tight junction maturation in preterm infants is unknown. Determination of fecal A1AT is routinely used in clinical practice as an indicator of substantial increased IP and protein-losing enteropathy 23.
To test the hypothesis that feeding and antibiotic exposures modulate intestinal barrier function in preterm infants, we conducted a prospective study to measure IP biomarkers (urinary La/Rh ratio, serum zonulin, and fecal A1AT) serially in infants <33 weeks gestation (GA) during the first two weeks of life.
Materials and Methods
Participants
All admissions to the University of Maryland Medical Center and Mercy Medical Center NICUs who were 240-326 weeks gestation <4 d age were screened for study eligibility and parental consent of eligible subjects was obtained (ClinicalTrials.gov: NCT01756040). Institutional review boards of both institutions approved the study. Exclusion criteria included non-viability or planned withdrawal of life support; triplet or higher order multiples; severe asphyxia (Apgar <3 at 5 minutes and cord pH <7.0); lethal chromosomal abnormalities; cyanotic congenital heart disease; intestinal atresia or perforation; abdominal wall defects; significant GI dysfunction (e.g. heme-positive stools, abdominal distension (girth >2 cm baseline) or bilious emesis/aspirates) and infants with galactosemia or other forms of galactose intolerance. Prior to study procedures, a complete physical exam including vital signs, weight, height, and head circumference was performed. Demographic, clinical and adverse events data were collected from the medical record.
Both participating clinical centers used the same standardized feeding protocol. Feeds were initiated between the first and fourth day of life depending on clinical stability. After initial feeds of 10 mL/kg expressed breast milk (EBM) or 20 kcal/oz preterm formula daily for 3-5 days, feedings were advanced by 20 mL/kg/d until 100 mL/kg/d was reached. Subsequently, caloric density was advanced to 24 kcal/oz prior to increasing feeding volume by 20 mL/kg/d to 150 mL/kg/d. Feedings were held or discontinued for signs of feeding intolerance such as abdominal distension, gastric residuals, or hematochezia, or for clinical deterioration.
Lactulose/Rhamnose sugar absorption test and sample collection
On each of the three study days (days 1, 8 ± 2 and 15 ± 2), participants received 1 mL/kg La/Rh solution [8.6 g of lactulose (Cumberland Pharmaceuticals, Nashville, TN)+140 mg of rhamnose (Saccharides, Inc., Calgary, Alberta, Canada)/100 mL] by nipple or by gavage via a clinically indicated orogastric tube 12. A minimum of 2 mL of urine was collected over a 4-hour period after the La/Rh dose was administered, with cotton balls placed in the infants' diaper. The total urine volume recorded included the volume extracted from the cotton balls in addition to the estimated volume of urine that leaked into the diaper determined by the diaper weight. The test was repeated the following day if < 2 mL urine was collected. If there were signs of feeding intolerance (increased abdominal girth >2 cm, heme-positive stools, or gastric residuals), the sugar solution administration was either delayed until resolution of symptoms within the study four day window for each timepoint or not done. Serum (total 0.5 mL) was collected by heel stick 90-120 min post La/Rh dosing to measure La/Rh 25 and serum zonulin. A stool sample (∼1 g) was collected within 8 hours of the sugar probe dosing for A1AT analysis. Urine, serum and stool samples were stored at -80°C until analysis. The amount of lactulose and rhamnose in each sample was measured using high-performance liquid chromatography and adjusted for urine volume 26. The fractional urinary excretion of lactulose and rhamnose was calculated as the ratio of the total urinary excretion of each sugar probe to the total oral dose of the probe. For each subject, the lactulose/rhamnose ratio (La/Rh ratio) was calculated as the fractional excretion of lactulose divided by that of rhamnose 27. A La/Rh ratio >0.05 was considered indicative of increased intestinal permeability 10.
Serum zonulin western blot
Serum samples (70 μg per well) were run under non-denaturing conditions on 4-20% Tris-Glycine gels (Invitrogen, Waltham, MA). Protein was transferred onto a PVDF membrane (Millipore, Bilerica, MA) and probed with 1.5 μg/ml mouse monoclonal zonulin antibody (Bio-Rad, Hercules, CA). Bands were detected with Alexa Flor 680 conjugated goat anti-mouse IgG antibodies (ThermoFisher, Waltham, MA). Bands were visualized and densitometry was measured using Image Studio software (LI-COR Biosciences, Lincoln, NE). All samples were normalized to a healthy term control reference sample run separately on each gel.
Stool A1AT ELISA
Stool samples diluted 1:250 according to the manufacturer's protocol were analyzed by double sandwich enzyme-linked immunosorbent assay (ELISA) (Eagle Biosciences, Nashua, NH) and results expressed as μg/g stool.
Statistical analysis
La/Rh ratio, serum zonulin, and stool A1 AT data are represented as the mean and standard deviation, at each of the three time points. Categorical data were compared using the χ2 test and continuous data were compared with Student t test. To quantify the association between urine La/Rh ratio, serum zonulin and stool A1 AT, Pearson correlation coefficients between the gold standard urinary La/Rh ratio and each of the other IP measures were calculated. Intestinal permeability patterns were differentiated using cluster analysis based on Ward's minimum variance method, as implemented in SAS 9.3.
Results
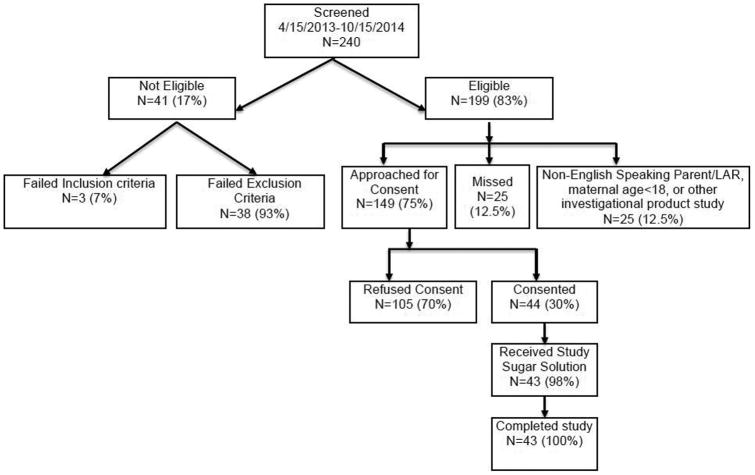
Forty-four subjects were enrolled over an 18 month period from April 15, 2013 to October 15, 2014 and 43 subjects received at least 1 dose of sugar solution (Figure 1; available at www.jpeds.com) (ClinicalTrials.gov: NCT01756040). Demographic characteristics of the participants are represented in the Table. Because we were interested in the maturation of intestinal barrier function in the extremely low gestational age infants, we analyzed the data for the entire cohort and stratified by gestational age ≤28 weeks (N=12) and >28 weeks (N=31). The gestational age strata were similar in sex and race composition and obstetrical factors. However, feeding was delayed and antibiotic exposure more common and for longer durations in the less mature infants (gestational age ≤28 weeks). There was a trend towards higher exclusive breast milk feedings in the less mature infants. No subject developed NEC during their NICU stay.
Figure 1.
Study cohort recruitment. Forty-four of 199 eligible <33 weeks gestation infants were enrolled during the 18 month enrollment period April 15, 2013 to October 15, 2014. One infant died due to acute pulmonary hemorrhage after consent was obtained, but before the study solution was administered. At least one dose of study solution was administered to 43 subjects.
Table. Study cohort clinical variables stratified by gestational age*.
| Variables | Total cohort (n=43) | GA≤28 wk (n=12) | GA>28 wk (n=31) | P value |
|---|---|---|---|---|
|
| ||||
| Sex (Male) | 23 (53%) | 8 (67%) | 15 (52%) | 0.33 |
|
| ||||
| Race (African-American) | 23 (55%) | 8 (67%) | 15 (48%) | 0.33 |
|
| ||||
| Gestational age (wk) | 30 ± 2.3 | 26.6 ± 1.0 | 31.3 ± 1.0 | <0.0001 |
|
| ||||
| Birth weight (g) | 1336 ± 421 | 862 ± 94 | 1519 ± 348 | <0.0001 |
|
| ||||
| POL | 17 (40%) | 7 (58%) | 10 (32%) | 0.17 |
|
| ||||
| Duration ROM | 1.0 | |||
| <1 hr | 19 (44%) | 6 (50%) | 13 (42%) | |
| 1-72 hrs | 18 (42%) | 5 (42%) | 13 (42%) | |
| >72 hrs | 5 (12%) | 1 (8%) | 4 (13%) | |
| Unknown | 1 (2%) | 0 | 1 (3%) | |
|
| ||||
| PPROM | 13 (30%) | 4 (33%) | 9 (29%) | 1.0 |
|
| ||||
| Pre-eclampsia | 9 (21%) | 4 (33%) | 5 (16%) | 0.24 |
|
| ||||
| Antenatal carticosteroids | 36 (84%) | 11 (92%) | 25 (81%) | 0.65 |
|
| ||||
| Clinical chorioamnionitis | 2 (5%) | 0 | 2 (6%) | 1.0 |
|
| ||||
| Maternal antibiotics | 29 (67%) | 6 (50%) | 23 (74%) | 0.16 |
|
| ||||
| Cesarean delivery | 31 (72%) | 8 (67%) | 23 (74%) | 0.71 |
|
| ||||
| Day first enteral feeding | 0.17 | |||
| Day 1 | 19 (44%) | 5 (42%) | 14 (45%) | |
| Day 2-3 | 17 (40%) | 3 (25%) | 14 (45%) | |
| Day ≥4 | 7 (16%) | 4 (33%) | 3 (10%) | |
|
| ||||
| Day First Full Feeding | 0.0002 | |||
| Day 1-7 | 8 (19%) | 0 | 8 (26%) | |
| Day 8-14 | 20 (47%) | 2 (17%) | 18 (58%) | |
| Day ≥15 | 15 (35%) | 10 (83%) | 16 (5%) | |
|
| ||||
| Exclusive breast milk | 25 (58%) | 10 (83%) | 15 (48%) | 0.08 |
|
| ||||
| Days on antibiotics | 0.0019 | |||
| None | 8 (19%) | 0 | 8 (26%) | |
| 1-3 | 14 (33%) | 1 (8%) | 13 (42%) | |
| ≥4 | 21 (49%) | 11 (92%) | 10 (32%) | |
Data are expressed as N (%) or mean ± SD.
Abbreviations: POL, preterm onset of labor; ROM, rupture of membranes; PPROM, preterm premature rupture of membranes.
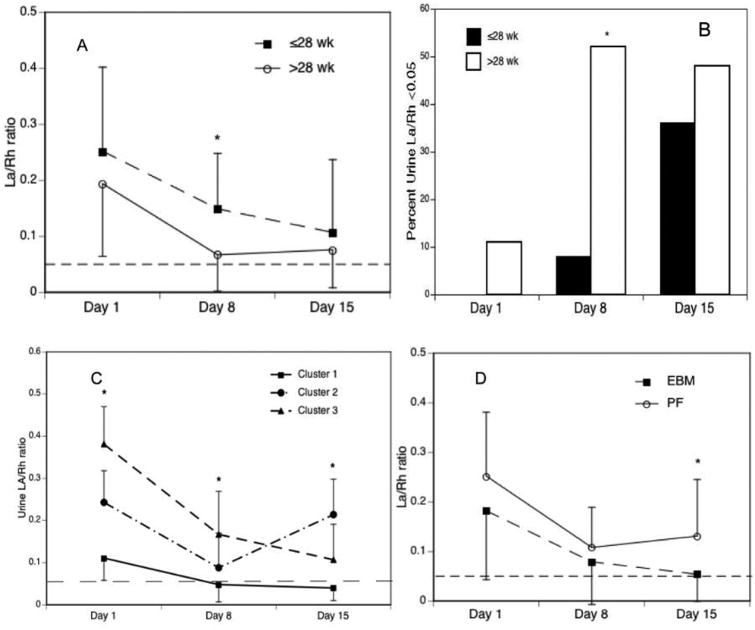
As shown in Figure 2, A, on average, intestinal permeability was elevated on study day 1, decreased over 2 weeks, but remained higher in infants ≤28 wk gestation compared with intestinal permeability in infants >28 wk gestation (p=0.015, study day 8). Only one third of infants ≤28 wk GA developed normal intestinal barrier function (La/Rh <0.05) by study day 15 (Figure 2, B). A cluster analysis of 35 subjects who had urine samples successfully collected at all 3 time points revealed that there were 3 distinct patterns of IP during the first 2 weeks of life (Cluster 1, normal maturation [N=20 (57%)]; Cluster 2, decrease IP during the first week and subsequent substantial increase [N=5 (14%)]; and Cluster 3, delayed maturation [N=10 (29%)] (Figure 2, C). Further analysis of factors associated with abnormal IP patterns (clusters 2 and 3) revealed trends toward more prolonged duration of antibiotic exposure ≥4 days [10/15 (67%) vs 7 (35%), p=0.092] and delayed initiation of feeding ≥4 days [5 (33%) vs 1 (5%), (p=0.064] in infants with abnormal maturation patterns compared with infants with normal maturation. However, it is difficult to tease out which may be the important factor. There was a trend towards greater co-exposure to prolonged antibiotics and delayed feeding ≥4d in infants with abnormal maturation patterns than in infants with normal maturation [4/15 (27%) vs 1/20 (5%), p=0.141]. Compared with infants fed preterm formula with or without EBM (N=18), infants fed exclusively with EBM (N=25) demonstrated more rapid improvement in intestinal barrier function on study day 15 (P=0.0088) (Figure 2, D).
Figure 2.
Urinary La/Rh ratio by (A) gestational age strata and study time points; (B) percent with normal intestinal barrier function (La/Rh ratio <0.05); and (C) cluster IP patterns and (D) exclusive breast milk feeding. Data are expressed as mean ± SD or percent. *p<0.05
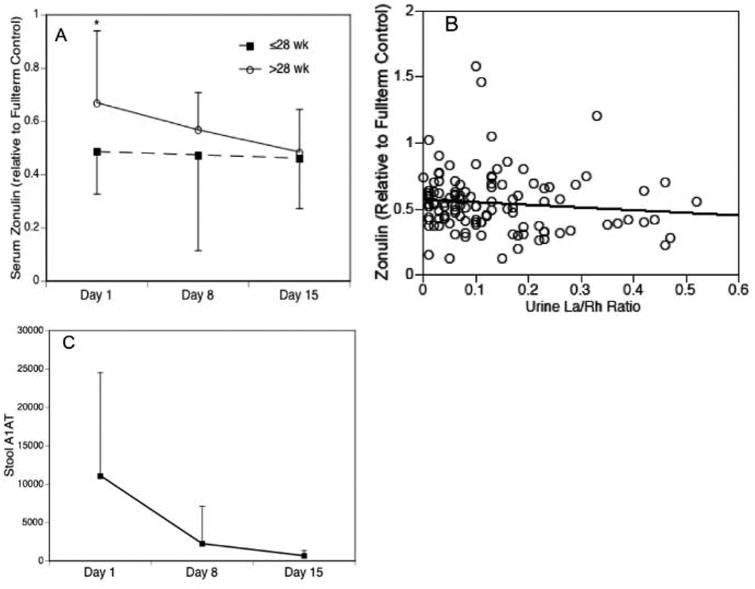
Gestational and postnatal age dependent changes in serum zonulin relative to a healthy full term control infant are represented in Figure 3, A. Although serum zonulin levels were low relative to a healthy full-term control, levels were significantly higher in infants >28 wk gestation compared with infants ≤28 wk gestation on day 1 (p=0.012). Serum zonulin did not correlate with urinary La/Rh ratios (Figure 3, B). Although there was considerable variability in stool A1 AT concentrations on study day 1, stool A1 AT decreased over time similar to urinary La/Rh, indicating maturation of the intestinal barrier (Figure 3, C). Although serum lactulose/rhamnose has been measured in serum of infants >4 months of age 25, rhamnose was undetectable in serum samples from our cohort collected 90-120 minutes post sugar solution dosing, so the La/Rh ratio could not be calculated (data not shown).
Figure 3.
Alternative measures of intestinal permeability. (A) Serum zonulin expressed relative to a healthy term control by gestational age strata and study time points; (B) Serum zonulin/urinary La/Rh ratio correlation; and (C) Fecal A1AT concentration (μg/g stool). Data are expressed as mean ± SD or percent. *p<0.05
Discussion
Our data suggests that intestinal barrier function is impaired in preterm infants and maturation is dependent on gestational and postnatal age and may be altered by feeding and antibiotic exposures, with a maturational effect of breast milk feeding. Although intestinal barrier function improved over time in 57% of subjects, intestinal permeability increased during the second week or maturation was delayed in the remaining infants. Almostone- half of all study subjects and >90% of subjects ≤28 weeks gestation were treated with antibiotics for more than 4 days, a known risk factor for NEC and death 6, 28, suggesting this is a modifiable risk factor. Study limitations included the small sample size, inability to distinguish the effects of delayed initiation of feeding and prolonged antibiotics because antibiotic use and delayed feeding commonly occurred together, and the challenges of adequate urine collection for the dual sugar permeability testing.
As confirmed in the current study, epithelial barrier integrity is itself dynamic and matures over time, starting soon after birth, although the mechanisms regulating dynamic permeability are poorly understood. Although intestinal permeability is higher at birth in preterm than term infants, previous studies observed that usually there is rapid maturation of the intestinal barrier over the first few days of life in both populations 29, suggesting that postnatal introduction of feeding accelerates maturation in both term and preterm infants. Whether intestinal permeability is related to gestational age among preterm infants has remained controversial. In agreement with the current study, Rouwet et al12 observed that intestinal permeability was higher at one week of age in infants <28 wk GA compared with more mature infants. However, in contrast to our study, all infants in the previous study received exclusive parenteral nutrition for the first week of life. In our study, feeding was were initiated prior to 4 days of age in 86% of the subjects. Other studies did not find an association of gestational age with intestinal permeability in preterm infants <34 wk gestational age, in the first few weeks of life, but feeding practices were not noted in several studies 10,29 and may have differed significantly from current practice. Despite similar cohort characteristics and feeding protocol with the current study, the study by Taylor and co-workers 30 did not find an association of gestational age with intestinal permeability measured by urinary lactulose/mannitol ratio. However, they did not evaluate intestinal permeability at <7 days of age and did not report antibiotic exposures.
There is accumulating evidence that timing of the initiation of feeds, feeding advancement, and feeding type impact intestinal permeability during the vulnerable first few weeks of life. Aberrant patterns of late increase in intestinal permeability and delayed maturation were associated with delayed initiation of feeding. Exclusive breast milk feeding was associated with more rapid improvement in intestinal barrier function, consistent with prior studies in term 31 and preterm infants14, 30, 32. Multiple retrospective and prospective randomized trials have demonstrated the beneficial effect of breast milk feeding on reducing the risk for NEC in preterm infants 33-38. In a secondary analysis of the NICHD glutamine trial, Colaizy et al5 determined a 12-fold increased risk of NEC associated with exclusive formula feeding compared with exclusive breast milk feeding in preterm neonates.
Although the mechanism(s) by which breast milk improves the intestinal barrier is not known, it is likely to involve breast milk components such as the oligosaccharides that influence the intestinal microbiota, and other immunomodulatory factors such as lactoferrin and human milk peptides 39. However, supplementing breast milk or preterm formula with a pre-biotic mixture of non-human milk neutral oligosaccharides did not improve intestinal barrier function compared with placebo in a randomized trial of infants <32 weeks, but supplementing preterm formula with the probiotic strain Bifidobacterium lactis improved barrier function at study day 30 40. Future studies of interventions to prevent NEC should include measures of intestinal permeability to identify high-risk infants and the impact of the intervention on intestinal barrier function.
Because adequate urine collection in preterm infants is challenging and analysis of the sugar probes by HPLC is time-consuming, we evaluated alternative biomarkers for intestinal barrier function in this study. Because it was previously demonstrated that zonulin is involved in intestinal epithelial paracellular pathway regulation41,42, intestinal innate immunity and is up-regulated in several autoimmune diseases22, we evaluated whether serum zonulin would be a relatively easy to measure biomarker to monitor intestinal permeability maturation in preterm infants and identify those at risk for NEC. However, serum zonulin measured by immunoblotting did not correlate with the urinary La/Rh ratios in this study, suggesting that zonulin is not involved in tight junction maturation. Although fecal A1AT is used routinely as a biomarker of severe protein-losing enteropathy in older children, there was considerable variability in stool A1AT concentrations in stool samples from preterm infants in the current study. Our analysis of multiple measures of intestinal permeability indicates that the administration of the non-metabolized sugar probes lactulose and rhamnose as markers of transcellular and paracellular pathways, respectively, remains the gold standard for assessment of intestinal permeability in newborns.
Acknowledgments
Supported by the NIH National Center for Complementary and Integrative Health (NCCIH) (R34AT006945).
We thank Dr Jonathan Meddings, University of Calgary, Calgary, Alberta, Canada for the HPLC analysis of serum and urine samples and Ashley Bathgate, Kirsty L. Chesko, and Elise Janofsky for research assistance (NCCIH R34AT006945).
List of abbreviations
- A1AT
alpha 1 anti-trypsin
- GA
gestational age
- IP
intestinal permeability
- NEC
necrotizing enterocolitis
- La/Rh
lactulose/rhamnose
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Claud EC, Walker WA. Bacterial colonization, probiotics, and necrotizing enterocolitis. J Clin Gastroenterol. 2008;42(2):S46–52. doi: 10.1097/MCG.0b013e31815a57a8. [DOI] [PubMed] [Google Scholar]
- 2.Berman L, Moss RL. Necrotizing enterocolitis: an update. Semin Fetal Neonatal Med. 2011;16:145–50. doi: 10.1016/j.siny.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Guner YS, Friedlich P, Wee CP, Dorey F, Camerini V, Upperman JS. State-based analysis of necrotizing enterocolitis outcomes. J Surg Res. 2009;157:21–9. doi: 10.1016/j.jss.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawoger R, Kiechl-Kohlendorfer U, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. 2010;156:562–7 e1. doi: 10.1016/j.jpeds.2009.10.040. [DOI] [PubMed] [Google Scholar]
- 5.Colaizy TT, Bartick MC, Jegier BJ, Green BD, Reinhold AG, Schaefer AJ, et al. Impact of Optimized Breastfeeding on the Costs of Necrotizing Enterocolitis in Extremely Low Birthweight Infants. J Pediatr. 2016;175:100–5 e2. doi: 10.1016/j.jpeds.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123:58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. 2011;159:392–7. doi: 10.1016/j.jpeds.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anand RJ, Leaphart CL, Mollen KP, Hackam DJ. The role of the intestinal barrier in the pathogenesis of necrotizing enterocolitis. Shock. 2007;27:124–33. doi: 10.1097/01.shk.0000239774.02904.65. [DOI] [PubMed] [Google Scholar]
- 9.Bergmann KR, Liu SX, Tian R, Kushnir A, Turner JR, Li HL, et al. Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am J Pathol. 2013;182:1595–606. doi: 10.1016/j.ajpath.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beach RC, Menzies IS, Clayden GS, Scopes JW. Gastrointestinal permeability changes in the preterm neonate. Arch Dis Child. 1982;57:141–5. doi: 10.1136/adc.57.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piena-Spoel M, Albers MJ, ten Kate J, Tibboel D. Intestinal permeability in newborns with necrotizing enterocolitis and controls: Does the sugar absorption test provide guidelines for the time to (re-)introduce enteral nutrition? J Pediatr Surg. 2001;36:587–92. doi: 10.1053/jpsu.2001.22288. [DOI] [PubMed] [Google Scholar]
- 12.Rouwet EV, Heineman E, Buurman WA, ter Riet G, Ramsay G, Blanco CE. Intestinal permeability and carrier-mediated monosaccharide absorption in preterm neonates during the early postnatal period. Pediatr Res. 2002;51:64–70. doi: 10.1203/00006450-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Albers MJ, Steyerberg EW, Hazebroek FW, Mourik M, Borsboom GJ, Rietveld T, et al. Glutamine supplementation of parenteral nutrition does not improve intestinal permeability, nitrogen balance, or outcome in newborns and infants undergoing digestive-tract surgery: results from a double-blind, randomized, controlled trial. Ann Surg. 2005;241:599–606. doi: 10.1097/01.sla.0000157270.24991.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westerbeek EA, van den Berg A, Lafeber HN, Fetter WP, van Elburg RM. The effect of enteral supplementation of a prebiotic mixture of non-human milk galacto-, fructo- and acidic oligosaccharides on intestinal permeability in preterm infants. Br J Nutr. 2011;105:268–74. doi: 10.1017/S0007114510003405. [DOI] [PubMed] [Google Scholar]
- 15.Corpeleijn WE, van Elburg RM, Kema IP, van Goudoever JB. Assessment of intestinal permeability in (premature) neonates by sugar absorption tests. Methods Mol Biol. 2011;763:95–104. doi: 10.1007/978-1-61779-191-8_6. [DOI] [PubMed] [Google Scholar]
- 16.Marchbank T, Limdi JK, Mahmood A, Elia G, Playford RJ. Clinical trial: protective effect of a commercial fish protein hydrolysate against indomethacin (NSAID)-induced small intestinal injury. Aliment Pharmacol Ther. 2008;28:799–804. doi: 10.1111/j.1365-2036.2008.03783.x. [DOI] [PubMed] [Google Scholar]
- 17.van Wijck K, Bessems BA, van Eijk HM, Buurman WA, Dejong CH, Lenaerts K. Polyethylene glycol versus dual sugar assay for gastrointestinal permeability analysis: is it time to choose? Clin Exp Gastroenterol. 2012;5:139–50. doi: 10.2147/CEG.S31799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Wijck K, Verlinden TJ, van Eijk HM, Dekker J, Buurman WA, Dejong CH, et al. Novel multi-sugar assay for site-specific gastrointestinal permeability analysis: a randomized controlled crossover trial. Clin Nutr. 2013;32:245–51. doi: 10.1016/j.clnu.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Piena M, Albers MJ, Van Haard PM, Gischler S, Tibboel D. Introduction of enteral feeding in neonates on extracorporeal membrane oxygenation after evaluation of intestinal permeability changes. J Pediatr Surg. 1998;33:30–4. doi: 10.1016/s0022-3468(98)90355-4. [DOI] [PubMed] [Google Scholar]
- 20.Malagon I, Onkenhout W, Klok M, van der Poel PF, Bovill JG, Hazekamp MG. Gut permeability in neonates after a stage 1 Norwood procedure. Pediatr Crit Care Med. 2005;6:547–9. doi: 10.1097/01.pcc.0000175990.72753.97. [DOI] [PubMed] [Google Scholar]
- 21.Malagon I, Onkenhout W, Klok G, van der Poel PF, Bovill JG, Hazekamp MG. Gut permeability in paediatric cardiac surgery. Br J Anaesth. 2005;94:181–5. doi: 10.1093/bja/aei014. [DOI] [PubMed] [Google Scholar]
- 22.Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55:1443–9. doi: 10.2337/db05-1593. [DOI] [PubMed] [Google Scholar]
- 23.Braamskamp MJ, Dolman KM, Tabbers MM. Clinical practice. Protein-losing enteropathy in children. Eur J Pediatr. 2010;169:1179–85. doi: 10.1007/s00431-010-1235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fasano A. Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin Gastroenterol Hepatol. 2012;10:1096–100. doi: 10.1016/j.cgh.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haase AM, Kukuruzovic RH, Dunn K, Bright A, Brewster DR. Dual sugar permeability testing in diarrheal disease. J Pediatr. 2000;136:232–7. doi: 10.1016/s0022-3476(00)70107-7. [DOI] [PubMed] [Google Scholar]
- 26.Hilsden RJ, Meddings JB, Sutherland LR. Intestinal permeability changes in response to acetylsalicylic acid in relatives of patients with Crohn's disease. Gastroenterology. 1996;110:1395–403. doi: 10.1053/gast.1996.v110.pm8613043. [DOI] [PubMed] [Google Scholar]
- 27.Mishra A, Makharia GK. Techniques of functional and motility test: how to perform and interpret intestinal permeability. J Neurogastroenterol Motil. 2012;18:443–7. doi: 10.5056/jnm.2012.18.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cotten CM, Smith PB. Duration of empirical antibiotic therapy for infants suspected of early-onset sepsis. Curr Opin Pediatr. 2013;25:167–71. doi: 10.1097/MOP.0b013e32835e01f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Elburg RM, Fetter WP, Bunkers CM, Heymans HS. Intestinal permeability in relation to birth weight and gestational and postnatal age. Arch Dis Child Fetal Neonatal Ed. 2003;88:F52–5. doi: 10.1136/fn.88.1.F52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor SN, Basile LA, Ebeling M, Wagner CL. Intestinal permeability in preterm infants by feeding type: mother's milk versus formula. Breastfeed Med. 2009;4:11–5. doi: 10.1089/bfm.2008.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Catassi C, Bonucci A, Coppa GV, Carlucci A, Giorgi PL. Intestinal permeability changes during the first month: effect of natural versus artificial feeding. J Pediatr Gastroenterol Nutr. 1995;21:383–6. doi: 10.1097/00005176-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Shulman RJ, Schanler RJ, Lau C, Heitkemper M, Ou CN, Smith EO. Early feeding, antenatal glucocorticoids, and human milk decrease intestinal permeability in preterm infants. Pediatr Res. 1998;44:519–23. doi: 10.1203/00006450-199810000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Schanler RJ, Shulman RJ, Lau C. Feeding strategies for premature infants: beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics. 1999;103:1150–7. doi: 10.1542/peds.103.6.1150. [DOI] [PubMed] [Google Scholar]
- 34.Schanler RJ, Lau C, Hurst NM, Smith EO. Randomized trial of donor human milk versus preterm formula as substitutes for mothers' own milk in the feeding of extremely premature infants. Pediatrics. 2005;116:400–6. doi: 10.1542/peds.2004-1974. [DOI] [PubMed] [Google Scholar]
- 35.Sisk PM, Lovelady CA, Dillard RG, Gruber KJ, O'Shea TM. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J Perinatol. 2007;27:428–33. doi: 10.1038/sj.jp.7211758. [DOI] [PubMed] [Google Scholar]
- 36.Meinzen-Derr J, Poindexter B, Wrage L, Morrow AL, Stoll B, Donovan EF. Role of human milk in extremely low birth weight infants' risk of necrotizing enterocolitis or death. J Perinatol. 2009;29:57–62. doi: 10.1038/jp.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maayan-Metzger A, Avivi S, Schushan-Eisen I, Kuint J. Human milk versus formula feeding among preterm infants: short-term outcomes. Am J Perinatol. 2012;29:121–6. doi: 10.1055/s-0031-1295652. [DOI] [PubMed] [Google Scholar]
- 38.Cristofalo EA, Schanler RJ, Blanco CL, Sullivan S, Trawoeger R, Kiechl-Kohlendorfer U, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr. 2013;163:1592–5 e1. doi: 10.1016/j.jpeds.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Underwood MA. Missed Opportunities: The Cost of Suboptimal Breast Milk Feeding in the Neonatal Intensive Care Unit. J Pediatr. 2016;175:12–4. doi: 10.1016/j.jpeds.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 40.Stratiki Z, Costalos C, Sevastiadou S, Kastanidou O, Skouroliakou M, Giakoumatou A, et al. The effect of a bifidobacter supplemented bovine milk on intestinal permeability of preterm infants. Early Hum Dev. 2007;83:575–9. doi: 10.1016/j.earlhumdev.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Fasano A, Baudry B, Pumplin DW, Wasserman SS, Tall BD, Ketley JM, et al. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc Natl Acad Sci U S A. 1991;88:5242–6. doi: 10.1073/pnas.88.12.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang W, Uzzau S, Goldblum SE, Fasano A. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci. 2000;113 Pt 24:4435–40. doi: 10.1242/jcs.113.24.4435. [DOI] [PubMed] [Google Scholar]