Abstract
Objective
To determine the effects of late surfactant on respiratory outcomes determined at one-year corrected age In the Trial of Late Surfactant (TOLSURF), which randomized extremely low gestational age newborns (ELGAN, ≤28 weeks’ gestational age) ventilated at 7–14 days to late surfactant and inhaled nitric oxide versus inhaled nitric oxide-alone (control).
Study design
Caregivers were surveyed in a double-blinded manner at 3, 6, 9 and 12 months corrected age to collect information on respiratory resource utilization (infant medication use, home support and hospitalization). Infants were classified for composite outcomes of Pulmonary Morbidity (No PM, determined in infants with no reported respiratory resource utilization) and Persistent PM (determined in infants with any resource utilization in ≥3 surveys).
Results
Infants (n=450, late surfactant n=217, control n=233) were 25.3±1.2 weeks’ gestation and 713±164g at birth. In the late surfactant group, fewer infants received home respiratory support than in the control group [35.8% versus 52.9%, Relative Benefit (RB) 1.28 (1.07, 1.55)]. There was no benefit of late surfactant for No PM versus PM (RB 1.27; 95% CI 0.89, 1.81) or No Persistent PM versus Persistent PM (RB 1.01; 95% CI 0.87, 1.17). After adjustment for imbalances in baseline characteristics, relative benefit of late surfactant treatment increased: RB 1.40 (95% CI 0.89, 1.80) for No PM and RB 1.24 (95% CI 1.08, 1.42) for No Persistent PM.
Conclusion
Treatment of ELGAN with late surfactant in combination with inhaled nitric oxide decreased use of home respiratory support and may decrease persistent pulmonary morbidity.
Trial registration
ClinicalTrials.gov: NCT01022580
Keywords: bronchopulmonary dysplasia, prematurity, pulmonary morbidity, wheeze
Extreme prematurity carries a risk of ongoing pulmonary morbidity and resource utilization following hospital discharge (1–4). Interventional trials of both drugs and respiratory support strategies in extremely low gestational age newborns (ELGAN) focus on decreasing the rate of bronchopulmonary dysplasia (BPD) at 36 weeks’ post-menstrual age (PMA) (5–8). Although BPD is an imperfect predictor of later pulmonary morbidity (1, 4, 9), clinical trials have not reported broadly accepted later respiratory outcomes. Outcomes previously evaluated at 1–2 years of age include respiratory symptoms, medication use, respiratory exacerbations and hospitalizations due to respiratory disease (2–4, 10–12).
The Trial of Late Surfactant (TOLSURF) was a randomized, controlled, masked clinical trial in which ELGAN at high risk for BPD who remained intubated in the second week of life were randomized to late surfactant (up to 5 doses) and inhaled nitric oxide (iNO) versus iNO-alone (13). We found no difference in the primary outcome of survival without BPD at 36 weeks’ PMA. However, a potential benefit of treatment with late surfactant emerged with a later respiratory assessment at 40 weeks’ PMA (term). Data on respiratory resource utilization after hospital discharge were collected. We sought to determine if there were effects of late surfactant on several clinically-relevant respiratory outcomes determined through one-year corrected age. We hypothesized that late surfactant and iNO would improve respiratory outcomes compared to iNO-alone.
METHODS
The TOLSURF study (ClinicalTrials.gov: NCT01022580) has been described in detail (13). Parental informed consent for participation was obtained under institutional review board approval at 25 US hospitals. In brief, 511 infants ≤ 28 0/7 weeks’ gestational age (GA) underwent stratified randomization (< 26 weeks’ GA or ≥ 26 weeks’ GA) by site to late surfactant and iNO versus iNO-alone at 7–14 days (n = 252 and 259, respectively). Calfactant (Infasurf, ONY Inc, Amherst NY) was administered in standard doses every 1–3 days for up to 5 doses in the late surfactant group. Control (iNO-alone) infants had no intervention (sham procedure behind a screen to maintain blinding). All infants received iNO for a 25-day course, per the protocol of our prior study of Inhaled Nitric Oxide to Prevent Chronic Lung Disease (NO CLD) (14, 15). The primary outcome of TOLSURF was survival without BPD, determined by oxygen/flow reduction challenge at 36.0±1 weeks’ PMA. Infants on nasal cannula support with effective FiO2<0.30 who remained hospitalized at 40 weeks’ PMA had a repeat assessment. No statistically significant differences were identified in primary or secondary outcomes during the neonatal hospitalization (13). Clinical study personnel and families remained blinded to treatment group assignment through the follow-up period (completed February 2016). Unblinded outcomes were periodically reviewed by an NIH-appointed Data Safety Monitoring Board.
Parents/caregivers were surveyed at 3, 6, 9 and 12 months corrected age (for prematurity) for interval events since discharge or last contact. Responses to questions regarding respiratory medication prescription, hospitalization for respiratory illness and home respiratory support (supplemental oxygen by nasal cannula or tracheostomy with or without assisted ventilation/oxygen) were collated. Specific respiratory medication categories queried were inhaled bronchodilators (BD), inhaled corticosteroids (ICS), diuretics, systemic steroids and pulmonary vasodilators. We also asked caregivers if they had been told by a medical professional that their child had wheeze on auscultation. These questions were posed over the same time interval, since the last contact.
Respiratory outcomes at one-year corrected age
We focused the analysis of pulmonary morbidity following neonatal discharge on caregiver-reported health resource utilization for respiratory indications in three domains (medications, hospitalization, and home support), using a short recall interval. We pre-determined several outcomes to quantify the degree and type of morbidity experienced by these infants. Our primary outcomes were Pulmonary Morbidity (PM) and Persistent Pulmonary Morbidity. We assigned an outcome of No PM to infants whose caregivers reported no medications, hospitalizations or home respiratory support on any survey through 12 months corrected age.
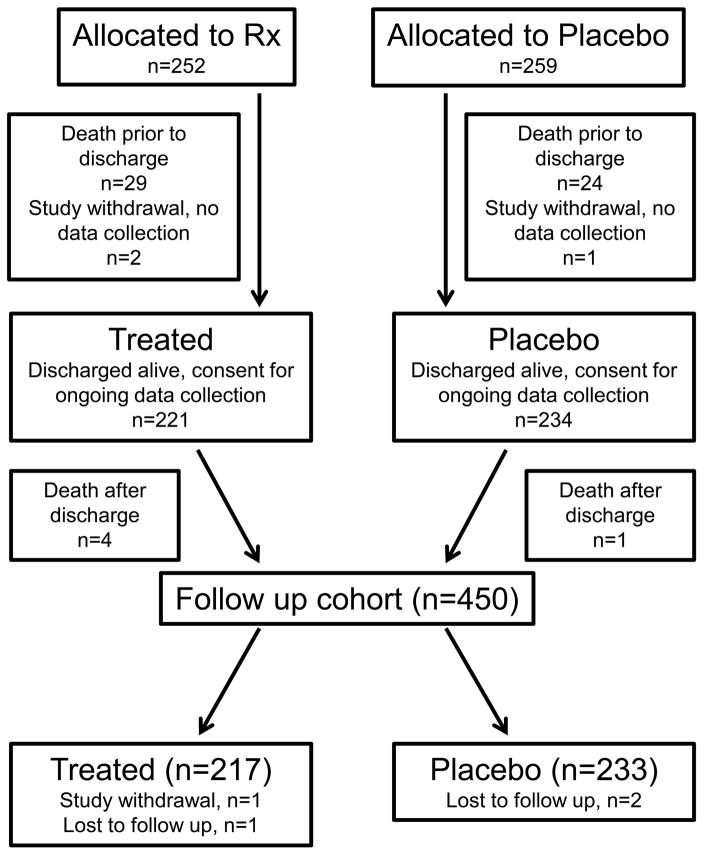
We assigned an outcome of Any PM to all other infants. We defined Persistent PM in infants with morbidity on any domain on at least 3 surveys. Infants with morbidity on two or fewer surveys were classified as No Persistent PM. A committee of investigators who remained blinded to treatment assignment (RLK, EER, RAB) evaluated 37 infants with incomplete survey data who were unclassified for one or both outcomes, for adjudication of missing outcomes. Using simple imputation when data were missing between two other time points (e.g., no resource utilization reported), and additional respiratory resource utilization data collected during follow up visits in the second year of life and among infants with prolonged neonatal hospitalizations beyond 3 months corrected age, we were able to impute either missing PM or PPM for eight infants, and both for one infant. Four infants had no follow up data, two had insufficient data for both outcomes (but contributed other data on resource utilization), and the remainder were unable to be classified for one missing outcome (Figure 1; available at www.jpeds.com). Infants were classified as a wheezing phenotype if caregivers reported any ICS or BD use, or wheeze (versus no wheezing phenotype). They were sub-classified into four ordered categories of wheezing phenotype: Likely (ICS with/without BD use), Probable (BD use with/without wheeze), Possible (Wheeze without BD/ICS use), or None (no ICS, BD or wheeze).
Figure 1.
(online only): Patient flow diagram. Deaths and study withdrawals prior to neonatal discharge detailed in Ballard et al, 2016 (13).
Statistical analyses
To estimate treatment effect, we used generalized estimating equations (GEE) to account for clustering of siblings. Analyses of baseline characteristics and potential modifiers of infant lung disease did not account for clustering. All analyses were by intent-to-treat, based on initial randomized allocation. Due to known impact of sociodemographic factors in post-discharge outcomes among extremely preterm infants, we planned a priori to adjust estimations of treatment effect for our primary outcomes (PM and Persistent PM) for differences (P<0.05) in baseline characteristics noted between groups.
RESULTS
Of 471 infants alive at 36 weeks’ PMA and enrolled January 2010 and September 2013, 455 who remained in the study were discharged alive and 5 infants died after discharge without further follow-up data collected (Figure 1). Consistent with characteristics of the original study participants, infants with one-year follow up were predominantly male and averaged 25.3±1.2 weeks’ gestation, with birth weight 713±164 grams (Table I) (13). The duration of mechanical ventilation was prolonged; 65% of infants had a diagnosis of BPD at 36 weeks’ PMA and 37% had a diagnosis of BPD at 40 weeks’ PMA. Eleven infants had undergone tracheostomy, six were receiving assisted ventilation at home and one was lost to follow up. There were significant baseline differences between late surfactant treated and control infants. Namely, infants in the late surfactant group had mothers who were 2 years younger (with a trend to lower educational attainment), and there were fewer products of multiple gestation.
Table 1.
Baseline characteristics and neonatal respiratory outcomes of infants discharged alive by treatment group (late surfactant versus control)
| Follow-up cohort (n=450) | Late surfactant (n=217) | Control (n=233) | P value | ||
|---|---|---|---|---|---|
| GA (weeks) | 25.3±1.2 | 25.3±1.2 | 25.3±1.2 | 0.90 | |
| < 26 0/7 weeks’ | 310 (68.9) | 148 (68.2) | 162 (69.5) | 0.76 | |
| Birth weight (g) | 713±164 | 715±174 | 711±154 | 0.81 | |
| Intrauterine growth restriction | 73 (16.2) | 40 (18.4) | 33 (14.2) | 0.22 | |
| Antenatal steroids | 388 (86.2) | 184 (84.8) | 204 (87.6) | 0.11 | |
| Male sex | 248 (55.1) | 124 (57.1) | 124 (53.2) | 0.40 | |
| Multiple gestation | 139 (30.9) | 56 (25.8) | 83 (35.6) | 0.02 | |
| Multiple siblings enrolled | 102 (22.7) | 41 (18.9) | 61 (26.2) | 0.07 | |
| Maternal characteristics | |||||
| Race/ethnicity | 0.47 | ||||
| White, non-Hispanic | 220 (48.9) | 101 (46.5) | 119 (51.1) | ||
| African American | 159 (35.3) | 82 (37.8) | 77 (33.0) | ||
| Hispanic | 50 (11.1) | 21 (9.7) | 29 (12.4) | ||
| Asian | 13 (2.9) | 5 (2.1) | 8 (3.7) | ||
| Other | 8 (1.8) | 5 (2.3) | 3 (1.3) | ||
| Age (y) | 28.8±6.4 | 27.8±6.1 | 29.8±6.6 | 0.0007 | |
| Education | 0.06 | ||||
| High school not complete | 56 (12.4) | 23 (10.6) | 33 (14.2) | ||
| High school graduate or some college | 227 (50.4) | 123 (56.7) | 104 (44.6) | ||
| College graduate ± graduate school | 166 (36.9) | 71 (32.7) | 95 (40.8) | ||
| Unknown | 1 (0.2) | 0 | 1 (0.4) | ||
| Neonatal respiratory outcomes | |||||
| BPD at 36 weeks’ PMA | 291 (64.7) | 140 (64.5) | 151 (64.8) | 0.95 | |
| BPD at 40 weeks’ PMA | 165 (36.7) | 71 (32.7) | 94 (40.3) | 0.09 | |
| Duration of mechanical ventilation (d) | 41.9±30.4 | 42.3±30.6 | 41.4±30.3 | 0.77 | |
Data are mean ± standard deviation or n (%)
P value by t-test or chi-square
Intrauterine growth restriction ≤ 10th percentile for gestational age per fetal growth curves derived from Fenton and Kim, 2013 (38).
We also evaluated for differences in potential modifiers of infant lung disease identified from the caregiver discharge survey. These included potential environmental tobacco smoke exposure, anticipated breast milk feeding, furry pet exposure, private insurance status and parental history of asthma. There were no significant differences by treatment group, although there was a trend toward a lower proportion of parents with asthma in the late surfactant group (14.5% versus 21.9%, P=0.05) (Table II; available at www.jpeds.com).
Table 2.
Potential modifiers of post-discharge pulmonary morbidity by treatment group (late surfactant versus control)
| Follow-up cohort (n=432)* | Late surfactant (n=211) | Control (n=221) | P value | |
|---|---|---|---|---|
| Breast milk (full or partial) anticipated | 195 (45.1) | 91 (43.1) | 104 (47.1) | 0.41 |
| Furry pet in home | 178 (41.2) | 85 (40.3) | 93 (42.1) | 0.70 |
| Young child exposure anticipated | 228 (52.8) | 115 (54.5) | 113 (51.1) | 0.48 |
| Potential ETS exposure | 105 (24.3) | 51 (24.2) | 54 (24.4) | 0.95 |
| Private insurance | 170/430 (39.5) | 78/210 (37.1) | 92/220 (41.8) | 0.32 |
| Asthma in parent | 77/422 (18.2) | 30/207 (14.5) | 47/215 (21.9) | 0.05 |
Data are n/N (%)
Missing data for infants not discharged to their biological parents’ care (family history), and those discharged from non-study hospitals
P value by chi-square
ETS, environmental tobacco smoke
“Young child exposure anticipated” was classified as Yes if caregiver reported another child < 5 years in the home or anticipated a young child at day care.
“Potential ETS exposure” was classified as Yes if caregiver reported 1) a parent smokes, 2) there is a smoker living in the home, 3) smoking is allowed in home, or 4) the child will travel in vehicle where smoking is permitted.
“Breast milk anticipated” was classified as Yes if caregiver reported either a breast milk only diet or combination of breast milk and formula at discharge.
Surveys were completed near the target dates (3.2±0.6, 6.2±0.7, 9.1±0.6, and 12.3±0.8 months corrected age), with 421, 423, 413 and 414 fully completed surveys at 3, 6, 9 and 12 months corrected age, respectively. We were able to classify 439 infants (97.6%) for Pulmonary Morbidity (No PM versus Any PM) and 426 infants for Persistent PM (No Persistent PM versus Persistent PM); 25% (110/439) of the infants had No PM, and 36% (153/426) had Persistent PM. Of infants who reported resource utilization, 96 had morbidity at only one survey, 80 at two surveys, 73 at three surveys, and 80 at four surveys. The distribution of respiratory outcomes of interest at one-year corrected age by treatment group is shown (Table III). No benefits of late surfactant on composite outcomes were seen in unadjusted analyses: Relative Benefit (RB) of treatment with late surfactant 1.27 (95% CI 0.89, 1.81; P=0.19) for No PM and 1.01 (95% CI 0.87, 1.17; P=0.91) for No Persistent PM. After adjustment for baseline imbalances (maternal age and multiple gestation status), the relative benefit of treatment with late surfactant increased to RB 1.40 (95% CI 0.96, 2.04; P=0.08) for No PM, and RB 1.24 (95% CI 1.08, 1.42; P=0.003) for No Persistent PM. With adjustment for parental history of asthma in sensitivity analyses, there were no significant benefits of treatment for either No PM nor No Persistent PM. There was also no difference between groups for our definition of wheezing phenotype.
Table 3.
Respiratory outcomes at one-year corrected age by treatment group.
| Follow-up cohort | Late surfactant | Control | Relative benefit (95% CI) | ||
|---|---|---|---|---|---|
| No pulmonary morbidity | 110/439 (25.1) | 59/210 (28.1) | 51/229 (22.3) | 1.27 (0.89, 1.81) | |
| Persistent pulmonary morbidity | 153/426 (35.9) | 74/208 (35.6) | 79/218 (36.2) | 1.01 (0.87, 1.17) | |
| Wheezing phenotype | |||||
| Dichotomous | Any | 291/436 (66.7) | 140/210 (66.7) | 151/226 (66.8) | 0.99 (0.74, 1.31) |
| Ordered* | Inhaled corticosteroid ± bronchodilator | 134/436 (30.7) | 66/210 (31.4) | 68/226 (30.1) | |
| Bronchodilator ± wheeze | 108/436 (24.8) | 51/210 (24.3) | 57/226 (25.2) | ||
| Wheeze only | 49/436 (11.2) | 23/210 (11.0) | 26/226 (11.5) | ||
| None | 145/436 (33.3) | 70/210 (33.3) | 75/226 (33.3) | ||
| Post-discharge morbidity domains | Respiratory hospitalization | 121/428 (28.3) | 60/205 (29.3) | 61/223 (27.4) | 0.97 (0.85, 1.10) |
| Home respiratory support | 198/431 (45.9) | 80/208 (38.5) | 118/223 (52.9) | 1.28 (1.07, 1.55) | |
| Any respiratory medication exposure | 277/438 (63.2) | 132/211 (62.6) | 145 (63.9) | 1.07 (0.82, 1.40) | |
| Post-discharge medication use | Diuretic | 89/430 (20.7) | 40/207 (19.3) | 49/223 (22.0) | 1.04 (0.94, 1.15) |
| Bronchodilator | 221/435 (50.8) | 110/210 (52.4) | 111/225 (49.3) | 0.95 (0.77, 1.16) | |
| Inhaled corticosteroid | 134/430 (31.2) | 66/208 (31.7) | 68/222 (30.6) | 0.99 (0.87, 1.13) | |
| Systemic steroid | 70/430 (16.3) | 37/208 (17.8) | 33/222 (16.3) | 0.97 (0.89, 1.06) |
Data are n/N (%) and Relative benefit (95% confidence interval)
Relative benefit by generalized estimating equation, accounting for clustering of siblings
P value 0.97 by chi-square
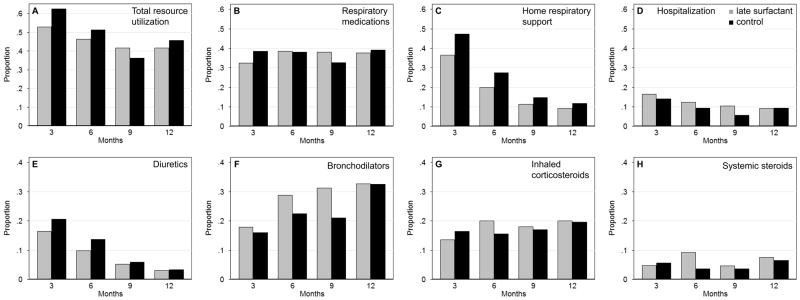
To further describe the relationship of treatment to post-discharge domains of pulmonary morbidity, we plotted overall resource utilization and utilization in each domain (medications, home support and hospitalizations) at each survey time point by treatment group (Figure 2, AD). Resource utilization was higher in the control group at all time points except 9 months. The treatment effect on these domains was limited. However, the overall proportion of infants in the late surfactant group that received home respiratory support (predominantly home oxygen use) over the follow-up period was lower (RB 1.28, 95% CI 1.07, 1.55; Table III), consistent with the observed pattern of less support at each time point. We also evaluated the relationship of reported exposures to respiratory medication classes (diuretics, bronchodilators, inhaled corticosteroids, systemic steroids) and treatment group, at each survey (Figure 2, E–H). Diuretic use tended to be higher in controls and bronchodilator use tended to be higher in the late surfactant group. Only 9 infants reported pulmonary vasodilator therapy, so this exposure was not evaluated further.
Figure 2.
Proportion of infants with respiratory resource utilization at each survey time point, 3, 6, 9 and 12 months corrected age, in late surfactant and control groups: A, Total resource utilization, B, Respiratory medication exposure, C, Home respiratory support, D, Hospitalization for respiratory cause, and E, Diuretic, F, Bronchodilator, G, Inhaled corticosteroid and H, Systemic steroid exposure.
DISCUSSION
We demonstrated no substantial benefit of late surfactant as administered in TOLSURF on novel respiratory outcomes determined at one-year corrected age. However, there was significantly less use of home respiratory support over the first year in infants in the late surfactant group, consistent with the trend previously shown toward decreased need for oxygen at 40 weeks’ PMA (13). As no substantial adverse effects of late surfactant were found during the neonatal hospitalization, the current data further support the safety of late surfactant therapy in high risk ELGAN.
Many randomized trials in ELGAN aim to improve infant respiratory status during the neonatal hospitalization. A number of these have failed to identify benefit in the neonatal period but have subsequently demonstrated advantage in at least one measure of respiratory status at follow-up (4–6, 10, 12, 16). Although Zivanovic et al found no difference in symptomatic lung disease at follow-up, measures of air flow obstruction and diffusing capacity were less compromised in children who were randomized to primary high frequency ventilation compared to conventional mechanical ventilation (16). Davis et al demonstrated a trend toward fewer respiratory exacerbations requiring asthma medications by one-year corrected age in preterm infants receiving antioxidant therapy with recombinant human superoxide dismutase compared to controls (10). Similarly, Stevens et al demonstrated fewer respiratory exacerbations by 22 months corrected age in children randomized at birth to primary nasal continuous positive airway pressure (CPAP) versus intubation with surfactant administration and mechanical ventilation (4). Akin to our findings, infants who were intubated and mechanically ventilated at 14 days of age and received a single dose of poractant alpha (Curosurf, Chiesi USA Inc., Cary NC) had lower rates of respiratory hospitalization by 9½ months corrected age, although there were no other significant differences in respiratory status during the neonatal hospitalization or among other follow-up outcomes (12).
The choice of respiratory outcomes after neonatal discharge in these and other studies has varied broadly, and has included symptoms, respiratory resource utilization (medications, hospitalization and home respiratory support) and measures of pulmonary function. Early and persistent decreases in pulmonary function are likely to have repercussions throughout life, even in the absence of symptoms (17, 18). Thus, pulmonary function testing can yield data that are relevant to clinicians and families. However, there is also a burden on children and families associated with persistent clinically symptomatic lung disease, related to repeated hospitalizations, exposures to multiple medications with potentially adverse effects, cost and other effects (1–4). For some clinical trials, multiple single areas (domains) of symptoms or resource utilization have been evaluated (2, 4). The NO CLD trial, for instance, showed benefit of iNO therapy in high-risk preterm infants both with neonatal respiratory outcomes and in multiple domains of post-discharge respiratory resource utilization at one-year corrected age, including medication use and home respiratory support (2). However, the SUPPORT trial, which investigated primary delivery room management with CPAP, did not show an effect on diagnosis of BPD at 36 weeks’ PMA, but did demonstrate lower rates of wheeze and respiratory exacerbations at 6–22 months corrected age (4). Composite outcomes of respiratory status have had limited application in neonatal clinical trials, and prospective data collection toward outcomes as we have developed and analyzed in the current study have not been employed (9). Although both resource utilization and symptom reporting can be affected by socioeconomic status, biological variables also demonstrate associations with these later outcomes, making them plausible primary or secondary trial outcomes (19–24). This is analogous to the use of neurodevelopmental impairment as an outcome, which is often assessed at 18–24 months corrected age, and is also influenced by socioeconomic status (25).
Given the young age of our cohort at the time of analysis, we explored a limited “wheezing phenotype” in this study, derived only from reported inhaled medication exposure and wheeze. We did not see a signal for treatment effect on this outcome, nor for the medication components of the outcome. This observation suggests that any effect of late surfactant with iNO on persistent lung disease may be distinct from wheezing illness commonly present in former preterm infants (which occur across the spectrum of preterm birth) (26). Earlier studies evaluating childhood respiratory outcomes prior to and following the advent of surfactant replacement therapy showed mild improvements or no change in prevalence of wheezing illness or airway obstruction in the surfactant era, despite an increase in survival of more immature infants (27, 28). These findings are in contrast to our prior findings with iNO-alone, wherein beneficial effects of iNO in the NO CLD study were seen on both classes of inhaled medications (2). Finally, although data supporting the association of parental asthma and preterm respiratory outcomes are variable, we did plan a sensitivity analysis as this characteristic is of interest with respect to childhood wheezing illness (29, 30).
With lack of effect on our wheezing phenotype, it is of interest that the treatment effect we did demonstrate was on the use of home respiratory support. In contrast to wheezing illness, the use of home oxygen is more specific to extremely preterm infants than to the broader population of preterm infants as a whole (31). Thus, the effect of late surfactant may be more specific to the perturbed lung development of ELGAN. Diuretics similarly are more common is this population, although we failed to show a significant benefit of late surfactant on diuretic exposure (32). Notably, the results of the current study represent the effects of late surfactant in infants receiving iNO, which, when administered by this protocol in the NO CLD study, decreased home oxygen use and respiratory medication exposure from all drug classes. Thus, the effects of late surfactant administration alone (without iNO), as administered by Hascoet et al, may differ from the effects seen in the current study (12).
The data for the current study were collected by caregiver recall, which may raise concerns regarding its accuracy. However, prior studies have shown that parents can report important events from the last 12 months (such as hospitalizations and medical visits for asthma and bronchitis), with moderate-to-substantial (85–95%) agreement with medical records (33, 34). In addition, test-retest reliability for questions related to respiratory illness administered to parents of preterm infants at 6 months corrected age revealed substantial-to-perfect agreement over the two tests, and strong internal consistency (35). Thus, within the short time frame of surveys for the current study, we would expect good accuracy and reliability of parent responses.
In conclusion, compared to iNO-alone, late surfactant decreases use of home respiratory support following initial hospital discharge. Unfortunately, we did not demonstrate a substantial benefit of late surfactant on our novel, composite respiratory outcomes. One possible explanation for the lack of benefit is that we didn’t treat with late surfactant frequently enough to achieve a persistent effect on lung function (13, 36). Regardless, these novel respiratory outcomes are clinically relevant and should be considered for assessment of the effects of interventions in neonatal clinical trials. Neurodevelopmental outcomes for this trial will be reported separately, however, from the current data, late surfactant with iNO appears to be a safe intervention, with no adverse effects demonstrated at one-year corrected age. These data are reassuring as we consider using surfactant as a vehicle for instillation of local corticosteroids to the lung, which may prove an effective therapy to prevent BPD in high risk ELGAN (37).
Acknowledgments
TOLSURF was funded by National Heart, Lung, and Blood Institute (NHLBI; U01 HL094338, U01 HL094355, and UL1 TR000004 [to K.W.]). The NHLBI Scientific Officer, Carol Blaisdell, MD, was present and participated in all Steering Committee meetings as a nonvoting member. Ikaria Inc and ONY Inc provided drugs for the conduct of the trial, but had no input into study design, data analysis, data interpretation or manuscript preparation. R.S. serves as Associate Editor of The Journal of Pediatrics.
We thank Nancy Newton, MS, RN, CCRP (NHLBI U01 HL094338), the project director for the first 4 years of the trial; Karin L. Knowles (NHLBI U01 HL094338), for managing the administrative and regulatory aspects of the study; Carol Blaisdell, MD (funded by the National Institutes of Health), and the Data Safety Monitoring Board for their service; the intensive care nursery and high risk infant follow-up clinic staffs who made this study possible; and the children and families who participated in the study.
Abbreviations
- BD
bronchodilator
- BPD
bronchopulmonary dysplasia
- ELGAN
extremely low gestational age newborn
- GA
gestational age
- ICS
inhaled corticosteroids
- iNO
inhaled nitric oxide
- PM
pulmonary morbidity
- PMA
post-menstrual age
- TOLSURF
Trial of Late Surfactant
Appendix
Additional members of the TOLSURF Study Group include: UCSF Benioff Children’s Hospital, San Francisco, CA: Suzanne Hamilton Strong, RN, Jill Immamura-Ching, RN, Margaret Orfanos-Villalobos, RN, Cassandra Williams, RN; Alta Bates Summit Medical Center, Berkeley, CA, and UCSF Benioff Children’s Hospital Oakland, Oakland, CA: Dolia Horton, RRT, Loretta Pacello, RCP, April Willard, RN; Children’s Mercy Hospital, Kansas City, MO: Cheryl Gauldin, RN, Anne Holmes, RN, Patrice Johnson, RRT, Kerrie Meinert, RRT; Women and Children’s Hospital of Buffalo, Buffalo, NY: Anne Marie Reynolds, MD, Janine Lucie, NNP, Patrick Conway, Michael Sacilowski, Michael Leadersdorff, RRT, Pam Orbank, RRT, Karen Wynn, NNP; Anne and Robert H. Lurie Children’s Hospital/Northwestern University, Chicago, IL: Maria deUngria, MD, Janine Yasmin Khan, MD, Karin Hamann, RN, Molly Schau, RN, Brad Hopkins, RRT, James Jenson, RRT; Texas Children’s Hospital, Houston, TX: Carmen Garcia, RN; Stony Brook University Hospital, Stony Brook, NY: Aruna Parekh, MD, Jila Shariff, MD, Rose McGovern, RN, Jeff Adelman, RRT, Adrienne Combs, RN, Mary Tjersland, RRT; University of Washington, Seattle, WA: Elizabeth Howland, Susan Walker, RN, Jim Longoria, RRT, Holly Meo, RRT; University of Texas Health Science Center, Houston, TX: Amir Khan, MD, Georgia McDavid, RN, Katrina Burson, RN, BSN, Richard Hinojosa, BSRT, RRT, Christopher Johnson, MBA, RRT, Karen Martin, RN, BSN, Sarah Martin, RN, BSN, Shawna Rogers, RN, BSN, Sharon Wright, MT; University of Florida College of Medicine, Jacksonville, UF Health Shands Hospital, and Wolfson Children’s Hospital, Jacksonville, FL: Kimberly Barnette, RRT, Amanda Kellum, RRT, Michelle Burcke, RN, Christie Hayes, RRT, Stephanie Chadwick, RN, Danielle Howard, RN, Carla Kennedy, RRT, Renee Prince, RN; Wake Forest School of Medicine and Forsyth Medical Center, Winston Salem, NC: Michael O’Shea MD, Beatrice Stefanescu, MD, Kelly Warden, RN, Patty Brown, RN, Jennifer Griffin, RRT, Laura Conley, RRT; University of Minnesota Amplatz Children’s Hospital, Minneapolis, MN: Michael Georgieff, MD, Bridget Davern, Marla Mills, RN, Sharon Ritter, RRT; Medical University of South Carolina, Charleston, SC: Carol Wagner, MD, Deanna Fanning, RN, Jimmy Roberson, RRT; Children’s Hospitals and Clinics of Minnesota, St. Paul, MN: Andrea Lampland, MD, Pat Meyers, RRT, Angela Brey, RRT; Children’s Hospitals and Clinics of Minnesota, Minneapolis, MN: Neil Mulrooney MD, Cathy Worwa, RRT, Pam Dixon, RN, ANM, Gerald Ebert, RRT-NPS, Cathy Hejl, RRT, Molly Maxwell, RT, Kristin McCullough, RN; University of Tennessee Health Science Center, Memphis, TN: Mohammed T. El Abiad, MD, Ajay Talati, MD, Sheila Dempsey, RN, Kathy Gammage, RRT, MBA, Gayle Gower, RN, Kathy James, RRT, Pam LeNoue, RN; All Children’s Hospital, St. Petersburg, FL: Suzi Bell, DNP, Dawn Bruton, RN, BSN, CCRP, Michelle Beaulieu, DNP, Richard Williams, RRT; Florida Hospital for Children, Orlando, FL: Robin Barron-Nelson, RN, Shane Taylor, RRT; Arkansas Children’s Hospital and University of Arkansas Medical Sciences, Little Rock, AK: Carol Sikes, RN, Gary Lowe, RRT, Betty Proffitt, RRT.
Clinical Coordinating Center--University of California San Francisco, San Francisco, CA: Cheryl Chapin, Hart Horneman, Karin Hamann, RN, Susan Kelley, RRT, Karin Knowles, Nancy Newton, RN, MS. Data Coordinating Center--University of California San Francisco, San Francisco, CA: Eric Vittinghoff, PhD, Jean Hietpas, Laurie Denton, Lucy Wu.
Data Safety Monitoring Board--Cincinnati Children’s Hospital Medical Center, Cincinnati, OH: Allan Jobe, MD, (Chair 2009–2010); UH Rainbow Babies and Children’s Hospital, Cleveland, OH: Avroy Fanaroff, MD (Chair 2010–2016); EMMES Corporation, Rockville, MD: Clemons; Boston University School of Public Health, Boston, MA: Leonard Glantz, JD; Wake Forest School of Medicine, Winston-Salem, NC: David Reboussin, PhD; Stanford University, Stanford, CA: Krisa Van Meurs MD (2009–2010); Johns Hopkins University, Baltimore, MD: Marilee Allen, MD (2010–2016); Women and Infants Hospital, Providence, RI: Betty Vohr, MD.
Clinical Steering Committee--UCSF Benioff Children’s Hospital San Francisco, San Francisco CA: Roberta Ballard, MD, Philip Ballard, MD, PhD, Roberta Keller, MD, Elizabeth Rogers, MD, Nancy Newton, MS, RN; University of California, San Francisco, San Francisco, CA: Dennis Black, PhD; National Heart, Lung and Blood Institute: Carol Blaisdell, MD; UCSF Benioff Children’s Hospital Oakland, Oakland, CA: David Durand, MD, Jeffrey Merrill, MD, Jeanette Asselin, MS, RRT; University of Texas Health Science Center, Houston, TX: Eric Eichenwald, MD; Children’s Hospital and Clinics of Minnesota, St. Paul, MN: Mark Mammel, MD; Medical University of South Carolina, Charleston, SC: Rita Ryan, MD; Children’s Mercy Hospital, Kansas City, MO: William Truog, MD.
Footnotes
The other authors declare no conflicts of interest.
Portions of the study were presented as an abstract at the meeting of the Pediatric Academic Societies, April 30–May 3, 2016, Baltimore, MD
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–60. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 2.Hibbs AM, Walsh MC, Martin RJ, Truog WE, Lorch SA, Alessandrini E, et al. One-year respiratory outcomes of preterm infants enrolled in the Nitric Oxide (to prevent) Chronic Lung Disease trial. J Pediatr. 2008;153:525–9. doi: 10.1016/j.jpeds.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson RS, Clermont G, Kinsella JP, Kong L, Arendt RE, Cutter G, et al. Clinical and economic effects of iNO in premature newborns with respiratory failure at 1 year. Pediatrics. 2009;124:1333–43. doi: 10.1542/peds.2009-0114. [DOI] [PubMed] [Google Scholar]
- 4.Stevens TP, Finer NN, Carlo WA, Szilagyi PG, Phelps DL, Walsh MC, et al. Respiratory outcomes of the surfactant positive pressure and oximetry randomized trial (SUPPORT) J Pediatr. 2014;165:240–9. e4. doi: 10.1016/j.jpeds.2014.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson AH, Peacock JL, Greenough A, Marlow N, Limb ES, Marston L, et al. High-frequency oscillatory ventilation for the prevention of chronic lung disease of prematurity. N Engl J Med. 2002;347:633–42. doi: 10.1056/NEJMoa020432. [DOI] [PubMed] [Google Scholar]
- 6.SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362:1970–9. doi: 10.1056/NEJMoa0911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirpalani H, Millar D, Lemyre B, Yoder BA, Chiu A, Roberts RS, et al. A trial comparing noninvasive ventilation strategies in preterm infants. N Engl J Med. 2013;369:611–20. doi: 10.1056/NEJMoa1214533. [DOI] [PubMed] [Google Scholar]
- 8.Beam KS, Aliaga S, Ahlfeld SK, Cohen-Wolkowiez M, Smith PB, Laughon MM. A systematic review of randomized controlled trials for the prevention of bronchopulmonary dysplasia in infants. J Perinatol. 2014;34:705–10. doi: 10.1038/jp.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis PG, Thorpe K, Roberts R, Schmidt B, Doyle LW, Kirpalani H, et al. Evaluating “old” definitions for the “new” bronchopulmonary dysplasia. J Pediatr. 2002;140:555–60. doi: 10.1067/mpd.2002.123291. [DOI] [PubMed] [Google Scholar]
- 10.Davis JM, Parad RB, Michele T, Allred E, Price A, Rosenfeld W, et al. Pulmonary outcome at 1 year corrected age in premature infants treated at birth with recombinant human CuZn superoxide dismutase. Pediatrics. 2003;111:469–76. doi: 10.1542/peds.111.3.469. [DOI] [PubMed] [Google Scholar]
- 11.Manley BJ, Makrides M, Collins CT, McPhee AJ, Gibson RA, Ryan P, et al. High-dose docosahexaenoic acid supplementation of preterm infants: respiratory and allergy outcomes. Pediatrics. 2011;128:e71–7. doi: 10.1542/peds.2010-2405. [DOI] [PubMed] [Google Scholar]
- 12.Hascoët JM, Picaud JC, Ligi I, Blanc T, Moreau F, Pinturier MF, et al. Late surfactant administration in very preterm neonates with prolonged respiratory distress and pulmonary outcome at 1 year of age: a randomized clinical trial. JAMA Pediatr. 2016;170:365–72. doi: 10.1001/jamapediatrics.2015.4617. [DOI] [PubMed] [Google Scholar]
- 13.Ballard RA, Keller RL, Black DM, Ballard PL, Merrill JD, Eichenwald EC, et al. Randomized trial of late surfactant treatment in ventilated preterm infants receiving inhaled nitric oxide. J Pediatr. 2016;168:23–9. e4. doi: 10.1016/j.jpeds.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, et al. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med. 2006;355:343–53. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 15.Ballard RA. Inhaled nitric oxide in preterm infants--correction. N Engl J Med. 2007;357:1444–5. doi: 10.1056/NEJMc076350. [DOI] [PubMed] [Google Scholar]
- 16.Zivanovic S, Peacock J, Alcazar-Paris M, Lo JW, Lunt A, Marlow N, et al. Late outcomes of a randomized trial of high-frequency oscillation in neonates. N Engl J Med. 2014;370:1121–30. doi: 10.1056/NEJMoa1309220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370:758–64. doi: 10.1016/S0140-6736(07)61379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Islam JY, Keller RL, Aschner JL, Hartert TV, Moore PE. Understanding the short and long-term respiratory outcomes of prematurity and bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;192:134–56. doi: 10.1164/rccm.201412-2142PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wynne J, Hull D. Why are children admitted to the hospital? Br Med J. 1977;2:1140–2. doi: 10.1136/bmj.2.6095.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenough A, Limb E, Marston L, Marlow N, Calvert S, Peacock J. Risk factors for respiratory morbidity in infancy after very premature birth. Arch Dis Child Fetal Neonatal Ed. 2005;90:F320–F3. doi: 10.1136/adc.2004.062018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alpern ER, Stanley RM, Gorelick MH, Donaldson A, Knight S, Teach SJ, et al. Epidemiology of a pediatric emergency medicine research network: the PECARN Core Data Project. Pediatr Emerg Care. 2006;22:689–99. doi: 10.1097/01.pec.0000236830.39194.c0. [DOI] [PubMed] [Google Scholar]
- 22.Michel G, Silverman M, Strippoli MP, Zwahlen M, Brooke AM, Grigg J, et al. Parental understanding of wheeze and its impact on asthma prevalence estimates. Eur Respir J. 2006;28:1124–30. doi: 10.1183/09031936.06.00008406. [DOI] [PubMed] [Google Scholar]
- 23.Mohangoo AD, de Koning HJ, Hafkamp-de Groen E, van der Wouden JC, Jaddoe VW, Moll HA, et al. A comparison of parent-reported wheezing or shortness of breath among infants as assessed by questionnaire and physician-interview: The Generation R study. Pediatr Pulmonol. 2010;45:500–7. doi: 10.1002/ppul.21208. [DOI] [PubMed] [Google Scholar]
- 24.Pryhuber GS, Maitre NL, Ballard RA, Cifelli D, Davis SD, Ellenberg JH, et al. Prematurity and Respiratory Outcomes Program (PROP): study protocol of a prospective multicenter study of respiratory outcomes of preterm infants in the United States. BMC Pediatr. 2015;15:37. doi: 10.1186/s12887-015-0346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doyle LW, Cheong JL, Burnett A, Roberts G, Lee KJ, Anderson PJ, et al. Biological and social influences on outcomes of extreme-preterm/low-birth weight adolescents. Pediatrics. 2015;136:e1513–20. doi: 10.1542/peds.2015-2006. [DOI] [PubMed] [Google Scholar]
- 26.Been JV, Lugtenberg MJ, Smets E, van Schayck CP, Kramer BW, Mommers M, et al. Preterm birth and childhood wheezing disorders: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001596. doi: 10.1371/journal.pmed.1001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palta M, Sadek-Badawi M, Sheehy M, Albanese A, Weinstein M, McGuinness G, et al. Respiratory symptoms at age 8 years in a cohort of very low birth weight children. Am J Epidemiol. 2001;154:521–9. doi: 10.1093/aje/154.6.521. [DOI] [PubMed] [Google Scholar]
- 28.Doyle LW the Victorian Infant Collaborative Study Group. Respiratory function at age 8–9 years in extremely low birth weight/very preterm children born in Victoria in 1991–1992. Pediatr Pulmonol. 2006;41:570–6. doi: 10.1002/ppul.20412. [DOI] [PubMed] [Google Scholar]
- 29.Halterman JS, Lynch KA, Conn KM, Hernandez TE, Perry TT, Stevens TP. Environmental exposures and respiratory morbidity among very low birth weight infants at 1 year of life. Arch Dis Child. 2009;94:28–32. doi: 10.1136/adc.2008.137349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teune MJ, van Wassenaer AG, van Buuren S, Mol BW, Opmeer BC Dutch POPS Collaborative Study Group. Perinatal risk-indicators for long-term respiratory morbidity among preterm or very low birth weight neonates. Eur J Obstet Gynecol Reprod Biol. 2012;163:134–41. doi: 10.1016/j.ejogrb.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 31.Lagatta JM, Clark RH, Brousseau DC, Hoffmann RG, Spitzer AR. Varying patterns of home oxygen use in infants at 23–43 weeks’ gestation discharged from United States neonatal intensive care units. J Pediatr. 2013;163:976–82. e2. doi: 10.1016/j.jpeds.2013.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laughon MM, Chantala K, Aliaga S, Herring AH, Hornik CP, Hughes R, et al. Diuretic exposure in premature infants from 1997 to 2011. Am J Perinatol. 2015;32:49–56. doi: 10.1055/s-0034-1373845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pless CE, Pless IB. How well they remember. The accuracy of parent reports. Arch Pediatr Adolesc Med. 1995;149:553–8. doi: 10.1001/archpedi.1995.02170180083016. [DOI] [PubMed] [Google Scholar]
- 34.D’Souza-Vazirani D, Minkovitz CS, Strobino DM. Validity of maternal report of acute health care use for children younger than 3 years. Arch Pediatr Adolesc Med. 2005;159:167–72. doi: 10.1001/archpedi.159.2.167. [DOI] [PubMed] [Google Scholar]
- 35.Boggs E, Minich N, Hibbs AM. Performance of commonly used respiratory questionnaire items in a cohort of infants born preterm. Open J Pediatr. 2013;3:260–5. doi: 10.4236/ojped.2013.33045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keller RL, Merrill JD, Black DM, Steinhorn RH, Eichenwald EC, Durand DJ, et al. Late administration of surfactant replacement therapy increases surfactant protein-B content: a randomized pilot study. Pediatr Res. 2012;72:613–9. doi: 10.1038/pr.2012.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeh TF, Chen CM, Wu SY, Husan Z, Li TC, Hsieh WS, et al. Intratracheal administration of budesonide/surfactant to prevent bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2016;193:86–95. doi: 10.1164/rccm.201505-0861OC. [DOI] [PubMed] [Google Scholar]
- 38.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]