Abstract
Background
Autism spectrum disorders (ASDs) are a heterogeneous group of neurodevelopmental conditions that vary in both etiology and phenotypic expression. Expressions of ASD characterized by a more severe phenotype, including autism with intellectual disability (ASD+ID), autism with a history of developmental regression (ASD+R), and minimally verbal autism (ASD+MV) are understudied generally, and especially in the domain of neuroimaging. However, neuroimaging methods are a potentially powerful tool for understanding the etiology of these ASD subtypes.
Scope and Methodology
This review evaluates existing neuroimaging research on ASD+MV, ASD+ID, and ASD+R, identified by a search of the literature using the PubMed database, and discusses methodological, theoretical, and practical considerations for future research involving neuroimaging assessment of these populations.
Findings
There is a paucity of neuroimaging research on ASD+ID, ASD+MV, and ASD+R, and what findings do exist are often contradictory, or so sparse as to be ungeneralizable. We suggest that while greater sample sizes and more studies are necessary, more important would be a paradigm shift toward multimodal (e.g., imaging genetics) approaches that allow for the characterization of heterogeneity within etiologically diverse samples.
Keywords: Autism spectrum disorders, intellectual disability, neuroimaging
Introduction
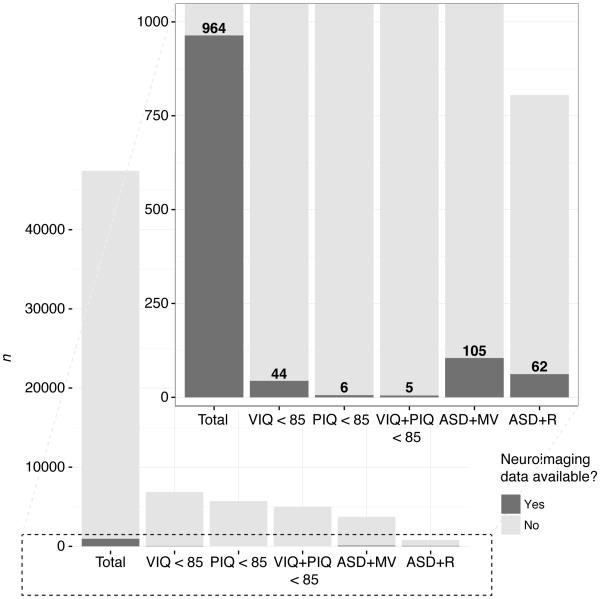
The majority of autism research has focused on individuals with relatively intact language and cognitive functioning. Those characterized as having ‘low-functioning autism,’ including minimally verbal individuals, children with regressive trajectories, and those with intellectual disability, have tended to go overlooked. Methodological and recruitment difficulties have played a role in this trend (e.g., Kasari, Brady, Lord, & Tager-Flusberg, 2013), but a desire to study a ‘pure’ form of autism, uncomplicated by potentially confounding factors, has likely contributed as well. This gap in the literature is exacerbated in the domain of human neuroimaging, where a high degree of behavioral compliance is often necessary to obtain usable data. One illustration of this tendency can be found in the National Database for Autism Research (Hall, Huerta, McAuliffe, & Farber, 2012), in which (as of late 2016) out of approximately 47,400 total participants with ASD, only about 11% have a verbal and performance IQ less than 85, and a little under 1% of these have any type of neuroimaging data available (see Figure 1). However, a growing appreciation of autism as a heterogeneous, multiply-determined disorder, exemplified by the adoption of the term ‘autism spectrum disorder’ (ASD) by the DSM-5 (American Psychiatric Association, 2013), has led to increased appreciation of the importance of characterizing and understanding the roots of variability in the phenotypic expression of this condition.
Figure 1.
Stacked bar graph representing the results of a general query to the National Database for Autism Research (NDAR) on Oct. 14, 2016 at https://ndar.nih.gov/query_data.html, aimed at determining the number of individuals with ASD in the database with and without neuroimaging data who had lower than average IQ, were of the minimally verbal subtype, or who had a history of regression in their development. The main figure demonstrates the overall number of participants with ASD in NDAR who fell into the following categories: verbal IQ less than 85 (VIQ < 85), performance IQ less than 85 (PIQ < 85), both VIQ and PIQ < 85, minimally verbal subtype (ASD+MV), and regression observed any time between 12 and 100 months (ASD+R). The inset zooms in on the lower portion of the figure to reveal the number of participants in each of these categories for whom any type (spectroscopy, MRI, fMRI, DTI, EEG, PET, or MEG) of neuroimaging data was available (darkened section of bars).
We suggest that the next frontier of brain research in ASD lies not so much in the development of new technologies or statistical methods as in increased attention to understudied sub-populations within the spectrum, particularly those with significant cognitive, language, or adaptive deficits. Neuroimaging approaches are a particularly powerful means to parse underlying differences (or similarities) in the biological bases of varying ASD phenotypes. Herein, we review extant brain research on several understudied areas of the autism spectrum: 1) minimally verbal individuals; 2) individuals with intellectual disability; and 3) individuals who experience regression in their developmental trajectory. In addition, we discuss methodological, theoretical, and practical considerations for neuroimaging assessment of these populations. Although there is overlap among all three of these categories, it is productive to consider the issues of minimal language, intellectual disability, and developmental regression separately in order to better understand unique features of their etiology and presentation.
Minimally verbal individuals
Minimally verbal (MV) individuals make up a significant minority of the ASD population (ASD+MV), with recent estimates suggesting that between 25 and 30% of children with ASD could be classified as MV (Anderson et al., 2007; Norrelgen et al., 2015; Rose, Trembath, Keen, & Paynter, 2016). As others have noted (Tager-Flusberg & Kasari, 2013), the literature on this population is not only thin, but the definitions of what constitutes ‘minimally verbal’ have also varied, and at times have included children with no speech whatsoever, children whose speech is extremely limited in context, frequency, or functionality, and/or children who rely on non-speech modalities to communicate. These children (for research has focused almost exclusively on children) have also often been included in ‘low-functioning autism’ groups for research purposes. This ‘lumping’ may mask underlying differences in verbal and nonverbal cognitive profiles (Munson et al., 2008), as well as in expressive versus receptive language capabilities (Rapin et al., 2009). In studies focused on young minimally verbal children, the problem of heterogeneity is magnified by the likelihood that some portion of the sample will go on to develop functional language (Tager-Flusberg & Kasari, 2013), and these preverbal children may have a different endophenotype than those who remain minimally verbal. Neuroimaging may be one means of parsing this heterogeneity and identifying meaningful subtypes of MV individuals with ASD. In particular, as we discuss in this section, neuroimaging approaches may prove a tool for predicting language outcomes in ASD, discovering subtle processing differences that could become targets for intervention, and understanding more about the etiology both of this manifestation of ASD and of language impairment more generally.
To evaluate extant literature on neuroimaging studies of ASD+MV we conducted a PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) search (Oct 13, 2016) using the following search terms: (nonverbal OR ‘minimally verbal’) AND (autism OR pervasive developmental disorder) AND (mri OR fmri OR eeg OR erp OR pet OR fnirs OR nirs OR meg OR dti OR dwi), or variants of these terms, with a species limiter of ‘humans’. (See Appendix S1, available online, for the full search syntax.) 49 results were returned, of which 4 were potentially relevant: 1 was an empirical study that used DTI to investigate ASD+MV, 1 was a literature review of work on ASD+MV, and 2 were empirical studies that used neuroimaging methods to investigate ASD with either current language impairment or a history of language delay. We reviewed the literature cited in these publications to identify another 3 relevant studies.
Neuroanatomy of language
Language processes are thought to be subserved by distinct dorsal and ventral pathways originating in primary auditory cortex in the superior temporal gyrus (Hickok & Poeppel, 2007). The dorsal stream, arcing up through inferior parietal regions and terminating in the pars opercularis of the inferior frontal gyrus (IFG) and dorsal premotor regions, appears to be associated with the ‘how’ of sound-to-articulation mappings; conversely, the ventral stream, passing through middle and inferior temporal regions to terminate in ventrolateral prefrontal cortex, appears to be associated with the ‘what’ of sound-to-meaning mappings (Saur et al., 2008). It would be reasonable to expect, therefore, that given their core difficulties with expressive language, ASD+MV would demonstrate atypicalities along the dorsal stream, which might include white matter deficits in the tract subserving this pathway—the arcuate fasciculus—as well as functional and/or structural abnormalities in key nodes of the pathway, i.e. superior temporal gyrus (STG), IFG, or dorsal premotor cortex. Given the typical left-lateralization of language function, especially expressive language function, we might also expect to see these deficits primarily in the left hemisphere.
What work currently exists suggests that disruption of dorsal pathway function may indeed be occurring in these children. Specifically, several themes arise that require more extensive investigation. Functionally, MV children appear to have atypical responses to spoken language, with enhanced responses to nonspeech sounds, and blunted responses to speech; these differences are left-lateralized and may be localized in IFG. Structurally, these children may show reduced white matter integrity along arcuate fasciculus and cortical atypicalities in IFG.
Functional atypicalities
Research on functional brain response to language in MV children is extremely limited, but concordant findings have been obtained across several modalities. A recent case study of a MV girl with ASD and cerebral palsy by Yau and colleages (2015) involved assessment of this child at eight years with magnetoencephalography (MEG) and ten years with electroencephalography (EEG). She and two comparison groups – typically developing (TD) controls and verbal children with ASD – listened to speech and nonspeech stimuli. Compared to both verbal comparison groups, in whom the preattentive/early attentive auditory response (M50/M100) was stronger to speech, the proband had an abnormally intense, left-lateralized response to non-speech sounds and a weak response to speech sounds. These findings were replicated two years later using EEG, demonstrating the reliability of the effect and the feasibility of both methods for interrogating early auditory response to speech stimuli. The M50 and M100 components have also been implicated in language impairment regardless of ASD status. Specifically, higher latency in the right hemispheric M50 (and to a lesser extent, the M100) as assessed via MEG appears to distinguish children with language deficits (both children with specific language impairment and children with ASD who, while not minimally verbal, do evidence some degree of language deficit) from those without (both TD children and children with ASD who have intact language) (Oram Cardy, Flagg, Roberts, & Roberts, 2008). Taken together, these findings suggest that abnormalities in preattentive auditory perceptual processing may influence language outcomes in ASD. In particular, they suggest that two types of processes might be at work in ASD+MV, one specific to autism, in which attention to socially-relevant (i.e., speech) sounds is disrupted, and another more broadly applicable to language impairment, in which early auditory processing is delayed. Such hypotheses can only be fully developed and tested using neuroimaging methods.
Additional evidence indicating atypical allocation of attention to speech has been reported in the domain of functional magnetic resonance imaging (fMRI). Specifically, 36 MV patients with ASD were scanned (1/3 while alert and the remainder under sedation) during presentation of recordings of their parents’ voices, versus preferred songs (Lai, Pantazatos, Schneider, & Hirsch, 2012). During spoken language, awake MV children with ASD did not demonstrate significant activation in left IFG, and showed reduced activity in secondary auditory cortices relative to TD controls; however, these children did show left IFG activation during song stimuli, as well as stronger activity in secondary auditory cortices than in the speech condition. ASD+MV (awake and sedated) also showed increases in functional connectivity between left IFG and superior temporal gyrus during song relative to speech.
This study was the only we could find that used magnetic resonance imaging (MRI) to understand the functional differences in speech perception in ASD+MV. It is notable for several features of the experimental design. For alert ASD+MV children undergoing scanning, a preferred video was played silently throughout the entirety of functional image acquisition to increase compliance. Secondly, stimuli, both in speech and song conditions, were individualized for each child. Given the sensitivity of fMRI to even small amounts of movement, preparing the environment to engage and appeal to the child is crucial. Further, ASD+MV children are most likely to demonstrate the full extent of their capabilities in naturalistic, familiar scenarios (Kasari et al., 2013). Previously, in the domain of face perception, Pierce and colleagues (2004) demonstrated that individuals with ASD may not exhibit classic deficits in fusiform face area activation when viewing the faces of familiar others such as parents. By presenting individualized speech stimuli--recordings of parents speaking to their child as they would normally--the researchers present a situation in which the MV child would presumably be most responsive if such a brain response were to occur at all.
Structural atypicalities
This study (Lai et al., 2012) also investigated structural differences characterizing MV children with ASD by using diffusion tensor imaging (DTI) to characterize white matter integrity along dorsal and ventral language pathways. Using probabilistic tractography to identify the dorsal and ventral tracts, the investigators found lower average fractional anisotropy (FA; a measure for which higher values are thought to reflect greater axonal diameter and/or myelination (Paus, 2010)) along the left dorsal tract as well as an association between FA in the dorsal and ventral pathways, and degree of speech-related activity in left IFG.
Other studies have yielded similar findings. In an investigation of five completely nonverbal children with ASD, Wan and colleagues (2012) observed that all but one child demonstrated an atypical pattern of asymmetry in the arcuate fasciculus, with greater tract volumes in the right versus left hemisphere. Billeci and colleagues (2012) scanned young children with ASD who possessed a wide range of expressive language abilities, including minimally verbal children, and found that streamline length in the indirect anterior arcuate fasciculus bilaterally was associated with greater expressive language abilities, while, surprisingly, mean and parallel diffusivity in the left indirect anterior segment of the arcuate fasciculus were associated with poorer expressive language function. In a surface-based morphometry study of low-versus high-functioning individuals with ASD, in which at least some of the individuals in the low-functioning group were minimally verbal, abnormalities characterizing the low-functioning group centered around the left pars opercularis of the IFG (Nordahl et al., 2007). Taken together, these findings suggest potential structural disruption to the dorsal language pathway in minimally verbal children with ASD; however, there are important limitations to this body of work.
Considerations for future work
Most notable, of course, is the paucity of neuroimaging research focused on minimally verbal individuals with ASD. This population is receiving increasing attention, as evidenced by recent NIH working group recommendations on evaluation of minimally verbal children with ASD (Kasari et al., 2013; Tager-Flusberg & Kasari, 2013). Neuroimaging considerations released by this group focused on the use of EEG/ERP or MEG methods. In particular, electroencephalography is an appealing method given its lower cost and greater availability relative to MEG or MRI. Moreover, net or cap systems allow for efficient electrode placement, and a (slightly) greater degree of motion during data collection can be tolerated than for MRI, minimizing demand on participants. This method allows for excellent capture of the temporal dynamics of speech perception across cortex, and would allow for testing of hypotheses regarding the latency, magnitude, and lateralization of, e.g., early auditory perceptual response; however, different approaches are required to address questions related to the localization of deficits and the contribution of subcortical structures.
Awake MRI may be possible for some individuals, particularly with advance training using a mock scanner (Greene, Black, & Schlaggar, 2015), and imaging protocols that minimize the time needed for data acquisition. Such protocols may involve use of newly-developed sequences that reduce acquisition time, such as simultaneous multislice imaging techniques (Barth, Breuer, Koopmans, Norris, & Poser, 2015), and experimental considerations such as use of blocked versus event-related designs with a single contrast of interest. Recommendations for scanning very young children (e.g., Raschle et al., 2012) are largely applicable to this population as well.
Scanning under sedation is another possibility in this population, via the addition of research sequences to medically indicated scans. High-quality structural images can be obtained under sedation via T1-weighted or diffusion tensor imaging sequences; however, useful functional data can also be acquired. Lai and colleagues (2012), for example, took advantage of the fact that some responses to language stimuli can be observed even in sedated subjects (Souweidane et al., 1999). While frontal language processes are impaired by sedation, perceptual response to language stimuli remains relatively intact in temporal regions, with preferential responding to speech versus nonspeech stimuli by healthy adults even under deep sedation (Davis et al., 2007). Thus, while useful information about, e.g., IFG activation is unlikely to be obtained under deep sedation, primary and secondary auditory cortex activity may be usefully interrogated. Comparison to TD comparison subjects also undergoing medically indicated sedated scans would be ideal, as meaningful comparisons cannot be made between awake and sedated subjects.
An as-of-yet unused method in this domain which may hold promise is functional near-infrared spectroscopy (fNIRS), which is highly motion-tolerant, easily applied via headband or cap, and represents a compromise between spatial (~ 2 cm) and temporal (~ 10 Hz) resolution (Aslin, Shukla, & Emberson, 2015). This method, which allows for assessment of changes in oxygenated or deoxygenated hemoglobin across the cortical surface, has been used successfully for assessment of language function in both healthy and clinical populations at a variety of ages (see for review Quaresima, Bisconti, & Ferrari, 2012). Given that this method is relatively lightweight and robust to motion, it could be used to capture brain response to speech over temporal and inferior frontal regions during naturalistic interactions with parents/siblings, and thus address recommendations that assessments occur in ecologically valid settings and capture natural language samples (Kasari et al., 2013).
Finally, more rigorous characterization of both the expressive and receptive language abilities of these samples, as well as longitudinal assessment, is needed. Group inclusion criteria generally depend upon productive language abilities, but additional group variance in results—particularly, we suspect, in terms of structure and function along the ventral language pathways—may be explicable in terms of dissociations between expressive and receptive abilities. Following up minimally verbal cohorts over time will be another powerful development in research in this area. This will allow investigators to distinguish between those who were preverbal at initial assessment versus those who remained minimally verbal, and thus to identify brain-based predictors of successful language acquisition. Longitudinal methods also allow for inferences about causality based on temporal precedence, which will be valuable in understanding which brain features may serve as predictive biomarkers that potentially influence language outcomes, and which features may instead reflect changes resulting from either a sustained lack of language or successful acquisition thereof. For example, it has been observed that ASD individuals with a history of delayed language acquisition but current verbal fluency show no significant differences in cortical volume, surface area, or thickness from those without a history of language delay (Balardin et al., 2015); however, these observations were made only at a single timepoint, making it impossible to distinguish whether these individuals displayed initial differences in cortical structure during early childhood that then resolved upon later language acquisition, or whether this lack of difference in cortical metrics could help to predict, in a cohort of young MV children, those who would be most likely to go on to develop functional speech. Along similar lines, it has previously been demonstrated that the efficacy of augmentative and alternative communication treatment appears to be associated with white matter integrity in uncinate fasciculus (Wan et al., 2012); however, this research was conducted retrospectively, limiting our ability to determine whether treatment induces these changes or whether children with higher baseline white matter integrity along key tracts demonstrate greater success. Thus, longitudinal assessment of language in this population will also allow for practical insights that may have the potential to impact treatment.
Intellectual disability
Recent surveillance data indicates that approximately 31% of children with ASD in the United States have IQ scores in the range of intellectual disability (ID; IQ ≤ 70) and a further 23% fall into the borderline (71 ≤ IQ ≤ 85) range (Centers for Disease Control and Prevention, 2014); global prevalence estimates vary widely (Elsabbagh et al., 2012). It is clear, however, that individuals with some degree of cognitive deficit make up a significant portion of the ASD population. There are indications that the neurobiological profile(s) of ASD with co-occurring ID (ASD+ID) may differ from that of ASD without ID (ASD−ID); syndromic forms of ASD often feature ID (Moss & Howlin, 2009), and rates of epilepsy are elevated in ASD+ID (Amiet et al., 2008; Woolfenden, Sarkozy, Ridley, Coory, & Williams, 2012). Attempts to parse the etiology of idiopathic ASD+ID have largely relied on genetic approaches, given (among other reasons), the seeming promise of genetic leads provided by syndromic forms of ASD, and the substantial overlap between ID- and ASD-associated genes (Betancur, 2011; Srivastava & Schwartz, 2014). However, extant research in the domain of genetics, far from helping narrow down our field of view, has revealed that the genetic risk factors involved in both ASD+ID and ASD−ID are many, and as a rule operate probabilistically and pleiotropically (e.g., Rutter & Thapar, 2014). Gaining a better understanding of the neuroendophenotype between genotype and phenotype via neuroimaging may help us to better understand the complex and nondeterministic results emerging at the genetic level, with implications for our understanding not only of ASD+ID, but of ASD−ID and ID-only as well. In this section, after reviewing the relevant extant literature, we will argue that neuroimaging applications in this domain have thus far fallen short of their full potential, and that information about brain structure in ASD+ID must be integrated with information about participants' phenotypic profile and, in particular, their genotype, in order to begin to make sense of this heterogeneous population.
Structural atypicalities
Neuroimaging research that includes individuals with ASD+ID is limited and difficult to interpret. As with minimally verbal individuals, challenges related to behavioral compliance and task comprehension constrain research in this population (e.g., Cox, Virues-Ortega, Julio, & Martin, 2016); consequently, most neuroimaging research with this population has focused on assessing structural, rather than functional, atypicalities. To evaluate extant literature on structural neuroimaging studies of ASD+ID we conducted a PubMed search (July 29, 2016) using the following search terms: (‘intellectual disability’ OR ‘mental retardation’ OR ‘low functioning’) AND (autism OR pervasive developmental disorder) AND (mri OR dti OR dwi OR voxel based morphometry OR volumetric), or variants of these terms, with a species limiter of ‘humans’. (See Appendix S1.) 170 results were returned, of which 19 were empirical studies that used either MRI or diffusion imaging methods and included participants with idiopathic ASD+ID. We then examined the literature cited by these reports to identify a further 7 relevant studies. Of these 26 reports, two reported on neuroradiologic abnormalities from an apparently identical cohort of children with ASD+ID, once with (Erbetta et al., 2015) and once without (Erbetta et al., 2014) a comparison group. Consequently, we only included the report with the comparison group in our review. Of the final collection of 25 studies, 15 analyze an ASD+ID group independently of participants with ASD−ID; we summarize these results in Table 1 and refer to this group of studies as ‘Set A.’ The remaining 10 studies analyze an ASD group that includes participants both with and without ID (ASD±ID, see Table 2, ‘Set B’).
Table 1.
Structural MRI and diffusion imaging studies of idiopathic ASD+ID.
| Study | Field strength |
ASD criteria |
Analysis | ASD − ID | ASD + ID | ID | TD | Age (y) | Main Findings |
|---|---|---|---|---|---|---|---|---|---|
| IQ: M(SD); Range |
IQ: M(SD); Range |
IQ: M(SD); Range |
IQ: M(SD); Range |
M(SD); Range |
|||||
| A. Whole brain analyses: MRI | |||||||||
| Erbetta et al., 2015 | ? | DSM-5 | Neuroradiologic abnormalities |
N/A |
n = 41a
IQ 50(12); 28-68 |
n = 41 IQ 52(12); 23-74 |
n = 41 IQ 106(7); 95-113 |
7.2(3.4) 1.5-15.4 |
Abnormalities in 53% of ID with hypoplastic corpus callosum most frequent (12% of ID); in 44% of ASD+ID with mega cisterna magna most frequent (12% of ASD+ID); and in 22% of TD. |
| Riva et al., 2013 | 1.5 T | DSM-IV | Voxel-based morphometry (DARTEL) |
N/A |
n = 26 IQ 52(9); 39-70 |
N/A | n = 21 | 5.8(2.5) 2.6-10.8 |
No WM findings. ASD+ID, relative to TD, showed GM volume reductions bilaterally in hippocampus & cerebellar lobules Crus II and vermis VIII-IX, as well as primarily left-lateralized reductions in frontal and occipito-temporal regions, including IFG & ITG. |
| Riva et al., 2011 | 1.5 T | DSM-IV | Voxel-based morphometry |
N/A |
n = 21 IQ 53(10); 32-66 |
N/A | n = 21 | 6.7(2.3) 3.2-10.8 |
No WM findings. Reduced GM volume in ASD+ID relative to TD in regions including bilateral basal forebrain, striatum, supplementary motor area, & cerebellar posterior lobe, as well as left-lateralized reductions in DLPFC, IFG, ITG, MTG, precuneus, and occipito-basal cortex, and in right postcentral cortex. |
| Ünal et al., 2009 | 1.5 T | DSM-IV | Neuroradiologic abnormalities |
Non-ID (IQ > 70): n = 12 |
Mild ID (IQ 50- 69): n = 23 Mod. ID (IQ 35- 49): n = 30 Sev. ID (IQ < 35): n = 16 |
N/A | N/A | 6.6(3.0) 2-15 |
11.6% of ASD+ID had MR abnormalities versus 16.7% of ASD−ID. ASD+ID abnormalities were only found in participants with moderate (n = 6) or severe (n = 2) ID. Most frequently observed abnormalities were mild cortical atrophy and bilateral periventricular leukomalacia. |
| Nordahl et al., 2007 | 1.5 T | DSM-IV | Surface-based morphometry |
HFA: n = 14 IQ 89(16) ASP: n = 15 IQ 97(17) |
n = 17 56(10) |
N/A |
n = 29 IQ 115(12) |
12.2(3.0) 7.5-18 |
ASD+ID showed a cortical folding abnormality in left pars opercularis of IFG, which resulted in greater sulcal depth in left frontal operculum & anterior insula. |
| Spencer et al., 2006 | 1.5 T | SCQb | Voxel-based morphometry |
N/A | ASD n = 15 IQ 60(9) PDD n = 16 IQ 60(7) |
n = 32 IQ 60 (8) |
n = 72c
IQ 101(16) |
16.4(2.0) 13-22 |
ID, regardless of diagnostic status, associated with lower brain volume & lower WM volumes (particularly in posterior corpus callosum) relative to controls, as well as reduced GM density in left temporo-parietal cortex. PDD+ID showed reduced GM density in thalamus relative to ID, & ASD+ID showed increased WM density in STG relative to ID. |
| Lotspeich et al., 2004 | 3.0 T or 1.5 Td |
DSM-IV | Volumetrice | HFA: n = 18 IQ 94(17) PIQ 105(19) ASP: n = 21 IQ 108(20) PIQ 104(20) |
n = 13 PIQ 46(10) |
N/A |
n = 21 IQ 114(10) PIQ 113(12) |
12.2(3.0) 7.8-17.9 |
ASD+ID and HFA had higher cerebral GM volume than TD. ASD groups did not differ from each other in cerebral GM. ASD+ID (versus all other groups) had high variance in total cerebral volume. ASP had positive correlation between cerebral WM and PIQ; HFA had negative correlation between cerebral GM and PIQ. |
| B. ROI-based analyses: MRI | |||||||||
| Scott et al., 2009 f | 1.5 T | DSM-IV | Volumetric: cerebellum |
HFA: n = 15 IQ 88(16) ASP: n = 15 IQ 97(17) |
n = 19 IQ 56(10) |
N/A |
n = 18 IQ 113(12) |
12.4(3.1) 7.5-18.5 |
Vermis area not reduced relative to TD either with all ASD analyzed as a group or for any subgroup. Total cerebellar volume did not differ. Vermis volume was lower in HFA than TD. Vermis volume was lower for combined ASD group than TD. |
| Schumann et al., 2004 f | 3.0 T or 1.5 T |
DSM-IV | Volumetric: total cerebrum, amygdala, & hippocampus |
HFA: n = 21 IQ 91(15) ASP: n = 24 IQ 106(120) |
n = 18 IQ 56(10) |
N/A |
n = 22 IQ 115(11) |
13.0(3.1) 7.5-18.5 |
No differences in total cerebral volume. ASD+ID had greater right amygdala and right hippocampus volumes than TD. HFA had greater bilateral hippocampus volumes than TD. Among 7.5-12.5y children only, ASD+ID and HFA had greater bilateral amygdala volume than TD, and this finding remained significant after covarying for PIQ. |
| Elia et al., 2000 | 0.5 T | DSM-IV | Midsagittal area: cerebrum, corpus callosum, midbrain, vermis, vermal lobules VI & VII |
N/A |
n = 22 Dev. age (mo) 20.0(10.4) |
N/A | n = 11 | 10.9(3.4) 4.7-16.6 |
No ROI area differences for ASD+ID vs. TD. Cerebral area negatively correlated with age in ASD+ID but not TD. Midbrain area positively correlated with PEP-R imitation, perception, fine motor, and eye-hand coordination in ASD+ID. |
| Manes et al., 1999 | 1.5 T | DSM-IV | Midsagittal area of corpus callosum & cerebellum |
N/A |
n = 27 Mental age (y) 4.6(5.6) |
n = 17 Mental age 4.5(2.7) |
N/A | 13.1(5.9) | ASD+ID showed smaller corpus callosum area than ID, but no differences in cerebellar area. |
| Hashimoto et al., 1992 | 0.5 T | DSM-III- R |
Midsagittal area of midbrain, pons, medulla oblongata, cerebellum, & posterior fossa |
N/A |
n = 12 IQ 49(15) 33-73 |
n = 15 IQ 60(14) 42-78 |
n = 14 | 7.0(1.7) 5.0-10.8 |
Midbrain and medulla oblongata area decreased in ASD+ID and ID relative to TD. Pons area reduced in ID (but not ASD+ID) versus TD. No differences in cerebellar vermis area. |
| C. Whole-brain analyses: Diffusion imaging | |||||||||
| Cascio et al., 2013 | 1.5 T | DSM-IV | Analysis of FA distributions |
N/A |
n = 33 IQ 57(17) |
n = 8 IQ 59(10) |
n = 17 111(18) |
4.1(1.3) 1.5-6.6 |
ASD+ID had lower/more variable FA across global WM, relative to TD and a combined TD + ID comparison group; the ASD+ID vs. ID comparison trended towards significance (p = 0.076). ASD+ID also had lower/more variable FA in cerebellum relative to TD and the combined TD+ID group, but not relative to ID. |
| D. ROI-based analyses: Diffusion imaging | |||||||||
| Jeong et al., 2012 | 3.0 T | ADI-R | Q-ball imaging: dentatorubro- thalamic tract |
n = 13 IQ 104(8) |
n = 11 IQ 55(5) |
N/A |
n = 14 IQ ≥ 85 |
5.4(2.9) 1.9-13.3 |
Whole-pathway analysis showed that relative to TD, both ASD+ID and ASD−ID had lower FA in all subregions of right dentate nucleus. FA in right DRDN positively correlated with daily living skills in ASD+ID. |
| Pardini et al., 2009 | 3.0 T | DSM-IV | Tractography: networks generated from OFC seed |
N/A |
n = 10 PIQ 49(7); 38-58 |
N/A | n = 10 | 19.8(2.7) 18-27 |
Relative to TD, ASD+ID had lower FA values & lower network volume for tracts generated from left OFC, & PIQ in ASD+ID was positively correlated with FA in left OFC tracts. |
Note: Unless otherwise noted, IQ indicates full-scale intelligence quotient. Diagnoses: ASD+ID: Autism spectrum disorder with accompanying intellectual disability. ASD−ID: Autism spectrum disorder without intellectual disability. ASP: Asperger Syndrome. HFA: ‘High-functioning autism.’ ID: Intellectual disability. PDD: Pervasive developmental disorder. TD: Typically developing. Measures: ADI-R: Autism Diagnostic Interview-Revised. DSM: Diagnostic and Statistical Manual of Mental Disorders. PEP-R: Psychoeducational Profile-Revised. PIQ: Performance IQ. SCQ: Social Communication Questionnaire. Neuroimaging metrics and methods: FA: Fractional anisotropy. GM: Grey matter. MRI: Magnetic resonance imaging. T: Tesla. WM: White matter. Brain sites: DRDN: dorso-rostral dentate nuclei. IFG: Inferior frontal gyrus. ITG: Inferior temporal gyrus. OFC: Orbitofrontal cortex. STS: Superior temporal sulcus. STG: Superior temporal gyrus.
This same ASD+ID cohort is also reported on in (Erbetta et al., 2014), but without comparison to an ID group.
ASD+ID group was split by Social Communication Questionnaire scores into those scoring above the ‘Autism’ threshold and those within the Pervasive Developmental disorder (PDD) range.
The comparison group consisted of ‘siblings and associates of subjects.’
n = 29 participants were scanned at 3.0 T at one site and n = 44 participants were scanned at 1.5 T at another site. All LFA participants were scanned at 1.5 T.
Cerebellar volumetrics were not assessed because cerebellar (but not cerebral) metrics showed significant intersite differences.
The same cohort was used by both Scott et al., 2009 and Schumann et al., 2004.
Table 2.
Structural MRI and diffusion imaging studies of idiopathic ASD±ID.
| Study | Field Strength |
ASD criteria |
Analysis | ASD±ID | ID | TD | Age (y) | Main Findings |
|---|---|---|---|---|---|---|---|---|
| IQ: M(SD); Range | IQ: M(SD); Range |
IQ: M(SD); Range |
||||||
| A. Whole-brain analyses: MRI | ||||||||
| Zeegers et al., 2009 | 1.5 T | DSM-IV | Volumetric |
n = 34 IQ 64.2(23)a |
DDb: n = 11 IQ 73.6(17.8)a |
N/A | 3.6(1.0) 1.8-6.4 |
No ASD vs. DD group difference in intracranial volume, total brain volume, cerebral WM or GM, ventricular volume, cerebellar volume, amygdala volume, or hippocampal volume. DD (but not ASD or TD) showed a positive correlation between total brain volume and IQ after controlling for intracranial volume. |
| Bonilha et al., 2008 | 2.0 T | DSM-IV & ICD- 10 |
Voxel-based morphometry |
n = 12 6 with ID |
N/A | n = 16 | 12.8(4.5) 5.8-23.3 |
Widespread increases in GM volume, & decreases in WM volume, in ASD relative to TD. |
| Hazlett et al., 2006 c | 1.5 T | DSM-III- R |
Volumetric |
n = 23 PIQ 90(22); 52-136 |
N/A |
n = 15 PIQ 102(11); 80-122 |
20.4(4.3) 13-29 |
ASD showed greater left hemisphere GM volume relative to TD; this effect was localized in frontal & temporal lobes. For both groups, lower PIQ predicted greater GM & WM volumes. |
| Boddaert et al., 2004 | 1.5 T | DSM-IV | Voxel-based morphometry |
n = 21 IQ & DQ 42(22) 12-91 |
N/A | n = 12 | 10.1(2.5) 7-15 |
Relative to TD, ASD showed GM decrease in STS bilaterally, & WM decreases in right temporal pole and left cerebellar hemisphere. |
| Piven et al., 1996 c | 1.5 T | DSM-III- R |
Volumetric |
n = 35 PIQ 91(20); 52-136 |
N/A |
n = 36 PIQ 102(13); 72-135 |
19.1(4.2) 12-29 |
ASD showed greater total brain volume, specifically with enlargement in temporal, parietal, & occipital lobes. |
| B. ROI-based analyses: MRI | ||||||||
| Trontel et al., 2015 | 3.0 T | DSM-IV | Volumetric: hippocampus, parahippocampal gyrus, entorhinal cortex, & amygdala |
VIQ ≥ 85 group: n = 38 IQ 107(12); 85-137 VIQ < 85 group: n = 18 IQ 80(9); 61-99 |
N/A |
n = 31 IQ 116(15); 93-152 |
11.2(4.2) 5.3-19.4 |
For VIQ < 85 group, but not VIQ ≥ 85 or TD groups, greater amygdala volume was correlated with lower memory performance. |
| Dager et al., 2007 | 1.5 T | DSM-IV | Hippocampal shape mapping |
AD: n = 29 NVIQ 59(17); VIQ 47(25) PDD: n =16 NVIQ 75(17); VIQ 65(21) |
N/A | n = 13 | 3.8(0.4) 3.0-4.6 |
Hippocampal shape (but not volume) differed between ASD & TD and between AD & PDD. Hippocampal shape composite scores were associated with IQ. |
| Haas et al., 1996 | 1.5 T | DSM-III-R | Midsagittal area: Vermal lobules VI & VII, corpus callosum clark areas 3 & 5 |
n = 10 VIQ or NVIQ > 70 n = 18 VIQ or NVIQ ≤ 70 |
N/A | n = 24 | 11.0(3.9) 6-19 |
Clark area 5 of corpus callosum smaller in ASD vs. TD. 24 of 28 participants with ASD showed hypoplasia of vermis VI & VII; 2 ASD participants showed hyperplasia. |
| Egaas et al., 1995 | 1.5 T | DSM-III- R |
Area of corpus callosum |
n = 51 16 participants had ID. |
N/A | n = 51 | 15.5(10.0) 3-42 |
ASD showed smaller corpus callosum area, particularly in posterior regions. |
| Whole-brain analyses: DTI | ||||||||
| Billeci et al., 2012 | 1.5 T | DSM-IV | Tract-based spatial statistics |
n = 22 IQ 71(23); 35-108 |
N/A |
n = 10 IQ 99(8); 85-108 |
5.4(2.2) 2.0-11.3 |
Relative to TD, ASD showed higher FA broadly across the WM skeleton, particularly in corpus callosum, cingulum, arcuate fasciculus, & internal capsule. |
Note: Unless otherwise noted, IQ indicates full-scale intelligence quotient. Diagnoses: ASD±ID: Autism spectrum disorder with and without accompanying intellectual disability. DD:Developmental delay. ID: Intellectual disability. PDD: Pervasive developmental disorder. TD: Typically developing. Measures: DSM: Diagnostic and Statistical Manual of Mental Disorders. ICD: International Statistical Classification of Diseases and Health Problems 10th Revision. NVIQ: Nonverbal IQ. PIQ: Performance IQ. VIQ: Verbal IQ. Neuroimaging metrics and methods: FA: Fractional anisotropy. GM: Grey matter. MRI: Magnetic resonance imaging. T: Tesla. WM: White matter. Brain sites: STS: Superior temporal sulcus.
‘Level of intellectual functioning.’ 31 total children had an FSIQ score; 20 children received the Mullen Scales of Early Learning; 10 children received the Psychoeducational Profile-Revised; 6 children received either the Griffith, the Dutch Snijders-Oomen Nonverbal Intelligence Test, or the Kaufman Assessment Battery for Children. 11 children could not complete any cognitive assessment. FSIQ in the full sample appears to range between ~35 and ~100 in Figure 1.
The developmental delay group was comprised of 5 children with ID and 8 children with language delay.
Hazlett et al. used a subset of the MRI images analyzed in Piven et al. with updated tissue segmentation methods.
At a glance (see Tables 1 and 2), a few broad conclusions can be drawn from this literature, despite its limited scope and its diversity in experimental design, imaging methodologies, and research questions. First, regions often implicated in ASD pathology more broadly (e.g., Ecker, Bookheimer, & Murphy, 2015; Stanfield et al., 2008) emerge in samples that include ASD+ID, with disruptions reported in frontal (Hazlett, Poe, Gerig, Smith, & Piven, 2006; Nordahl et al., 2007; Pardini et al., 2009; Riva et al., 2011, 2013), striatal (Billeci et al., 2012; Riva et al., 2011), temporal (Boddaert et al., 2004; Hazlett et al., 2006; Piven, Arndt, Bailey, & Andreasen, 1996; Spencer et al., 2006), and cerebellar (Boddaert et al., 2004; Cascio et al., 2013; Jeong, Chugani, Behen, Tiwari, & Chugani, 2012; Riva et al., 2011, 2013) regions (although for negative cerebellar results in ASD+ID, see Hashimoto, Murakawa, Miyazaki, Tayama, & Kuroda, 1992; Manes et al., 1999; Scott, Schumann, Goodlin-Jones, & Amaral, 2009; Zeegers et al., 2009), as well as in the corpus callosum (Billeci et al., 2012; Egaas, Courchesne, & Saitoh, 1995; Haas et al., 1996; Manes et al., 1999; Spencer et al., 2006) and the amygdala-hippocampal complex (Dager et al., 2007; Schumann et al., 2004; Trontel et al., 2015; although see for a negative result Zeegers et al., 2009). Second, it appears that grey matter volumes and white matter structure are impacted in ASD+ID relative to typically developing controls, but clear patterns regarding the directionality and localization of these effects are difficult to ascertain in such a diverse literature. Finally, a number of projects illustrate that harnessing the power of modern statistical techniques to characterize multidimensional data may allow us to recognize features relevant to the neurobiology of ASD+ID that might otherwise go overlooked (Cascio et al., 2013; Dager et al., 2007; Jeong et al., 2012). For example, Dager and colleagues demonstrate that while classic volumetric analysis of the hippocampus in ASD±ID indicates no differences among TD, ASD, and pervasive developmental disorder-not otherwise specified (PDD) groups, analysis of hippocampal shape reveals a perturbation that distinguishes ASD±ID from TD, and is associated with IQ. To attempt to make sense of these findings as regards their implications for our understanding of the neurobiology of ASD+ID, we will focus primarily on Set A, that is, studies that consider individuals with ASD+ID separately from those with ASD and no co-occurring cognitive deficit.
Given that the approach to comparison group composition fundamentally shapes the inferences we can make from a particular report, we begin by overviewing the variety of approaches employed in this set of studies. Of the 15 studies in Set A, only five included an ID-only comparison group (Cascio et al., 2013; Erbetta et al., 2015; Hashimoto et al., 1992; Manes et al., 1999; Spencer et al., 2006), and of these, two (Hashimoto et al., 1992; Manes et al., 1999) were early studies of the midsagittal area of a few specific regions of interest (ROIs). All but one (Manes et al., 1999) of these five studies with an ID-only comparison group also included a TD group, and none of these studies also included an ASD−ID comparison group. Six studies in Set A did include an ASD−ID group; four of these ran statistical tests that compared the ASD+ID to the ASD−ID group (Lotspeich et al., 2004; Schumann et al., 2004; Scott et al., 2009; Ünal et al., 2009) and two compared the ASD−ID and ASD+ID group(s) only to the TD group (Jeong et al., 2012; Nordahl et al., 2007). Finally, among Set A, four studies compared their ASD+ID sample only to a TD group (Elia et al., 2000; Pardini et al., 2009; Riva et al., 2011, 2013), with Riva and colleagues (2013) arguing that contrasting probands to an ID-only comparison group carries with it its own potential confounds given the etiological heterogeneity of non-specfic ID. While this is a valid point, using only a TD comparison group leaves open the question of whether observed neuroanatomical variance is attributable to autism, intellectual disability, or to a unique ASD+ID profile. Thus, of the 25 studies total that we identified, only three were designed in such a way as to be capable of potentially identifying neuroanatomical features unique to ASD+ID versus those shared across expressions of intellectual disability (by directly comparing an ASD+ID group to an ID-only group), and used relatively modern imaging techniques: (Cascio et al., 2013; Erbetta et al., 2015; Spencer et al., 2006). Similarly, only four studies were designed in such a way as to be able to potentially identify ID-unique neuroanatomical features from a group of individuals with ASD by contrasting ASD+ID with ASD−ID (Lotspeich et al., 2004; Schumann et al., 2004; Scott et al., 2009; Ünal et al., 2009). We discuss these studies below.
Erbetta and colleagues (2015) examined the rates and type of neuroradiologic abnormalities observed in MRI scans of children (~2-15y, mean ~7y) with ASD+ID, as well as age-matched comparison groups of children with ID and TD children. Rates of MRI abnormalities were elevated in both ASD+ID (44%) and ID (54%) relative to TD (22%). However, we note that these rates differ dramatically from those observed in another study of MRI abnormalities in ASD+ID (Ünal et al., 2009). Ünal and colleagues examined abnormalities in children with ASD (of approximately the same age of those in Erbetta and colleagues' study, see Table 1) with varying levels of ID: none, mild, moderate, or severe. Overall, the observed rate of MRI abnormalities across children with ASD+ID was much lower than that observed by Erbetta and colleagues (11.6%); the rate observed in ASD−ID was 16.7%. Moreover, the types of abnormalities observed most frequently also differed. Consequently, while we can conclude that MRI abnormalities may be evident in some children and adolescents with ASD+ID, and that these abnormalities may appear more frequently than in the neurotypical population, it is far from clear how prevalent such abnormalities may be in the overall ASD+ID population, or whether certain types of abnormalities occur more frequently than others.
Using DTI, Cascio and colleagues (2013) assessed FA distribution scores (where lower scores are thought to represent lower and/or more variable anisotropy) in young (1.5-6.6 y) children with ASD+ID (n = 33), TD children (n = 17) and children with idiopathic developmental delay including ID (n =8). They found that the ASD+ID group exhibited lower FA distribution scores across global white matter (WM) and cerebellum relative to the TD group, and to both comparison groups (TD + ID) combined; however, there was no significant difference between the ASD+ID and ID group in cerebellar scores, and only a trend toward significance (p = 0.076) between these groups in global WM scores. When the FA distribution score was broken down into its component parts, one representing the mean and the other the standard deviation, it was revealed that the standard deviation component primarily drove their findings of group differences, and that the ASD+ID group showed significantly greater FA variability than both the TD and the ID groups. Cascio and colleagues suggest that this result is reflective of diverse etiologies in ASD+ID. While this is a reasonable assessment, it is worthwhile to note that ID is itself etiologically heterogeneous (e.g., Betancur, 2011; Vissers, Gilissen, & Veltman, 2015), and that the greater variability in their ASD+ID sample may well be related more to the small size of the ID comparison group than to a true difference at the population level.
Spencer and colleagues (2006) studied a cohort of adolescents and young adults (13-22y) with ID and varying degrees of autistic features (non-ASD, PDD, and ASD) according to Social Communication Questionnaire screening cutoffs, and compared these individuals to a group of non-ID participants recruited from probands' ‘siblings and associates.’ Compared to controls, the ID participants, regardless of their reported degree of autistic features, showed lower global WM volume; however, there were no differences among the ID groups (non-ASD, PDD, and ASD) in global GM or WM volume. Voxel-based morphometry analyses indicated that this overall ID group also demonstrated greater WM density and lower GM density in right cerebellum, as well as lower GM density in left temporo-parietal cortex, and lower WM density in posterior corpus callosum, suggesting that these features may be associated with cognitive deficit in adolescence and young adulthood regardless of autistic symptomology. Participants with ID who scored within the ‘PDD’ range on the SCQ showed lower thalamic GM density than ID participants who scored in the non-ASD range, and participants with ID who scored within the ‘ASD’ range on the SCQ demonstrated greater WM density in superior temporal gyrus, relative to ID participants who scored in the non-ASD range. Given that participants in this study did not necessarily have clinical diagnoses of ASD, we hesitate to draw strong conclusions from these findings; moreover, the inclusion of siblings in the comparison group may have confounded the results, given that siblings of individuals with ASD may have a different neuroendophenotype from individuals without a familial risk for ASD (e.g., Kaiser et al., 2010).
Lotspeich et al. (2004), Schumann et al. (2004), and Scott et al. (2009) all report on results from a study of brain volumetrics, assessed via MRI, in a cohort of children and teens aged 7.5-18.5 y (mean ~12.5y) with either ‘high functioning autism’ (HFA), Asperger syndrome, or ASD+ID, compared to TD youth. Both Lotspeich et al. and Schumann et al. present some challenges to interpretation given that neuroimaging assessments were conducted across sites with magnets of differing field strength (1.5 T vs. 3.0 T, see Table 1), with ASD+ID participants all scanned at the site with lower field strength. One striking finding from these reports in fact emerges from their analysis of inter-site reliability, which indicated high variability in estimates of cerebellar (but not cerebral) volumetrics across sites (Lotspeich et al., 2004). This suggests that one possible explanation for variability in reports regarding cerebellar neuroanatomy in ASD+ID across the literature (Boddaert et al., 2004; Cascio et al., 2013; Hashimoto et al., 1992; Jeong et al., 2012; Manes et al., 1999; Riva et al., 2011, 2013; Scott et al., 2009; Zeegers et al., 2009) may be related to site-specific differences in factors such as field strength and signal-to-noise-ratio. Given this finding, Lotspeich et al. and Schumann et al. do not analyze cerebellar volumetrics in their reports, and Scott examines cerebellum in a subset of the cohort obtained at the same site.
Across these three studies of whole-brain (Lotspeich et al., 2004), amygdala-hippocampal (Schumann et al., 2004), and cerebellar (Scott et al., 2009) volumetrics, few, if any, marked differences between the ASD+ID participants and either of the ASD−ID groups were identified. Lotspeich and colleagues (2004) observed that the ASD+ID group was primarily differentiated from the ASD−ID groups by high variance in total cerebral volume; however, it does not appear that this difference was verified statistically. We note that this finding of high variability in ASD+ID (versus ASD−ID and TD) echoes that of Cascio and colleagues (2013) in comparing ASD+ID to TD and ID.
Overall, our review of the sparse literature in ASD+ID suggests that this population is characterized by its diversity more than by any clearly definable neuroanatomical pattern. The difficulty in extracting clear themes from this body of research may be due in part to variability in the composition of proband and comparison groups, as well as to differences in the degree to which known sources of variation, such as age and IQ, are accounted for in statistical modeling. We found no studies, for example, that used a full 2 × 2 design (ASD vs no ASD × ID vs no ID) to attempt to dissociate between structural correlates of ASD vs. ID. When comparing an ASD+ID group to a TD group, it is difficult to determine whether differences are better explained by autism or cognitive deficit. In a number of studies, IQ was not formally assessed in the TD group. However, particularly when the ASD group represents a broad distribution of IQ scores, including individuals both with and without ID, adding IQ as a regressor in neuroimaging analyses may prove valuable. Hazlett and colleagues (2006), for example, found that their estimates of ASD vs. TD differences in tissue volume changed notably depending on whether or not performance IQ (PIQ) was included in their models. Specifically, when PIQ was removed from the model, they found that the contribution of ASD group membership was inflated for grey matter volumes, and decreased for white matter volumes. Ultimately, in their full model, higher grey and white matter volumes were associated with lower PIQ, and this relationship between volume and PIQ did not significantly differ across groups. This suggests that failing to account for variability in IQ may lead to mis-estimation of the unique contribution of ASD diagnostic status.
All this being said, even careful group matching on phenotypic parameters such as age and IQ is unlikely to fully elucidate matters. Moreover, while we also clearly need more studies with larger sample sizes, we suggest that the essential problem underlying this research domain is not (or not merely) an issue of numbers. Until now the literature has been primarily concerned with detecting and describing differences between groups, but in and of itself this approach to an etiologically diverse population is flawed. To meaningfully address questions relevant to the etiology of ASD, ID, and their co-manifestation in ASD+ID, we need to drill down into individual differences in a manner that parses and separates, rather than clumps. Given that current evidence suggests there is considerable within-group variability in ASD+ID at the genomic level (e.g., Srivastava & Schwartz, 2014), we propose that imaging genetics approaches may help to clarify our understanding of overlap and divergence between ASD and ID pathology moving forward.
Considerations for future work
Imaging genetics approaches
Hundreds of genes have been identified as potentially implicated in ASD, and likewise have hundreds of genes been implicated as potential contributors to ID (Betancur, 2011). Only a small proportion of ASD and ID cases are related to variation to a single gene, and it appears likely that multigenic processes are more frequently responsible for the development of these conditions (Abrahams & Geschwind, 2008; Robinson, Neale, & Hyman, 2015; Srivastava & Schwartz, 2014). Many of the genes implicated in ASD and in ID overlap, such as NRXN1, FMR1 (which is disturbed in fragile X syndrome), SHANK3, UBE3A (which is affected by copy number variations to 15q11-13), NLGN3, and CNTNAP2; moreover, they often appear to converge upon common biological pathways, notably those supporting synaptic structure and function (for a comprehensive review, see Srivastava & Schwartz, 2014). Imaging genetics work in mouse models and in neurotypical adults suggests that characterizing samples not just by phenotypic characteristics (such as diagnostic category or IQ) but also by genotype will allow us to better parse etiological heterogeneity.
A recent MRI investigation of mouse models of ASD (Ellegood et al., 2015) demonstrated how different genotypes can be associated with differing brain endophenotypes despite broadly similar behavioral phenotypes, and provided indications as to the structural features we ought to expect to see in MRI of individuals with specific genetic variants. Upon scanning 26 different mouse models of ASD, Ellegood and colleagues found that these models clustered into three groups, all of which contained some models of variants that have also been associated with ID. The first group, which included among others Fmr1 (−/Y),(−/y); Nrxn1α (−/−),(−/+); and Shank3 (−/+),(−/−), demonstrated volumetric increases in key structures including corpus callosum, cerebellum, frontal lobes, and parieto-temporal lobes. The second group, which included among others 15q11-13 (patDp/+); and Nlgn3 knock-in, demonstrated volumetric decreases in regions including striatum, hippocampus, and corpus callosum. The third group, which included among others 16p11.2 (df/dp),(dp/+),(df/+); Cntnap2 (−/−), and Mecp2 (−/y), showed volumetric increases in cerebellum but decreases in parieto-temporal and frontal lobes. The grey and white matter decreases observed in the Nlgn3 knock-in mouse have also been reported by a separate research group (Kumar et al., 2014). These differing patterns suggest that inconsistent volumetric findings in the extant ASD+ID literature may be partially related to variability in underlying genotype; moreover, they provide us with some initial hypotheses as to the direction of volumetric effects we might expect in humans with these genetic variants.
Imaging genetics work in healthy adults has probed structural and functional correlates of ASD− and ID-related genetic variants, notably NRXN1 and CNTNAP2. NRXN1 codes for neurexins 1α and 1β, presynaptic cell adhesion molecules important for synaptic function. Voineskos and colleagues (2011) investigated associations between white matter volumes and genetic variation in NRXN1 among TD adults. They found that a polymorphism in the 3' untranslated region of this gene was related to lower frontal white matter volumes, with CC homozygotes at the single nucleotide polymorphism (SNP) rs1045881 expressing this variation. CNTNAP2 is a large gene that codes for another member of the neurexin superfamily, contactin associated protein-like 2. In healthy adults, homozygosity for the risk allele (T) at SNP rs7794745 of CNTNAP2 has been associated with lower grey matter volumes in occipital, frontal, and cerebellar regions, and reduced white matter volume and FA in thalamic radiation and inferior fronto-occipital fasciculus (Tan, Doke, Ashburner, Wood, & Frackowiak, 2010). Functional imaging of healthy adults indicates that carriers of the CNTNAP2 SNP rs2710102 risk allele (C) demonstrate a differing pattern of functional connectivity with medial prefrontal cortex from noncarriers, showing increased and more diffuse local frontal connectivity, but less long-range connectivity to occipito-temporal regions (Scott-Van Zeeland et al., 2010). Those homozygous for this risk allele also show differences in structural connectivity as measured via graph theory metrics calculated on DTI images (Dennis et al., 2011), including higher global efficiency, a potential indicator of greater randomness in connections.
These studies suggest that rare and common variations to genes implicated in both ASD and ID are associated with stuctural brain differences, even when phenotypic outcomes may be similar. Genotyping participants to determine which, if any, ASD/ID-associated variants they carry may allow investigators to better explain variability in their neuroimaging data.
Multivariate approaches
Multivariate approaches that allow for identification of previously unidentified subgroups or patterns in the data may be another powerful approach to understanding ASD+ID. An example of the potential utility of this approach can be found in a recent investigation that used topological data analysis, a form of unsupervised (i.e., data-driven) multivariate pattern analysis, to detect subgroups within a sample of young boys with fragile X syndrome (Romano et al., 2014). Even within this etiologically relatively homogenous sample (all participants had a confirmed full mutation of FMR1), topological data analysis of structural brain images identified two large subgroups within the data. One group showed widespread volumetric increases in white and grey matter relative to the other group; these increases were found in regions such as amygdala, insula, orbitofrontal cortex, posterior temporal lobe, and cerebellar hemispheres and vermis. When this subgroup’s clinical profile was evaluated, it was evident that they expressed a more severe phenotype, both cognitively and in terms of ASD symptom load. Thus, unsupervised multivariate approaches may allow us to parse heterogeneity by finding patterns in the data that hypothesis-driven analyses would not have detected.
Regression
Most forms of ASD are thought to result from the cumulative effects of an early derailment of the normal developmental trajectory. The earlier this occurs, the more severe the clinical consequences are expected to be (W. Jones & Klin, 2009). However, a substantial proportion of children develop ASD after a period of typical development and are said to ‘regress’ when they exhibit a loss of language and/or other skills. The definition of autism with regression (ASD+R) has varied across publications, and while language loss is frequently the central feature of interest, in some reports loss of other important developmental skills (e.g., social, adaptive, motor) may also be sufficient for or included in the operational definition of ‘regression’ (see Barger, Campbell, & McDonough, 2013 for extended discussion of this topic). Regression is frequently described in ASD. A meta-analysis of 85 studies representing over 29,000 people with ASD determined that the overall prevalence rate of regression was 32%, with an average onset of regression at 1.78 years (Barger et al., 2013). We note, however, that rates vary depending on how regression is defined and on the sampling method. Barger and colleagues (2013), for example, found that the lowest prevalence estimates are derived from regression defined by langauge loss and from population-based samples, while the highest rates are found from parent survey and when a definition that uses a mix of language and/or social skills loss is used. Further, studies that prospectively track the development of infant siblings of children with ASD detect much lower rates of regression (Rogers, 2009), which may indicate either that probands within multiplex families are less likely to exhibit this particular developmental trajectory, and/or that retrospective techniques for identifying regression are more prone to error. The causes of regressive ASD in the vast majority of cases are unclear; no consistent genetic or environmental risk factors have been identified (Ozonoff, Heung, Byrd, Hansen, & Hertz-Picciotto, 2008; Stefanatos, 2008). Thus, while regression has a profound impact on the functioning of those affected, it is one of the least-understood aspects of ASD. Given that these children are often perceived by caregivers as following a typical trajectory prior to the regression, methods that allow for assay of features beyond observable behavior are necessary for us to learn to predict and potentially intervene in regressive trajectories. The window that neuroimaging provides into the living brain is an ideal tool for better understanding the etiology of ASD+R.
EEG abnormalities and seizures
The most frequent neuroimaging method applied to questions of autistic regression has been clinical EEG, in the context of determining rates of epilepsy and/or epileptiform EEG abnormalities. Rates of epilepsy and EEG abnormalities are already elevated in ASD relative to the general population (Jeste & Tuchman, 2015; Tuchman, Hirtz, & Mamounas, 2013); some work suggests they may be higher still in ASD+R. In Table 3, we summarize the characteristics of studies that have examined rates of epilepsy and EEG abnormalities in individuals with ASD+R, and in Table 4 we summarize reported frequencies of different types of EEG abnormalities among those with a history of regression, where available. These tables were generated based on a PubMed search (Aug. 8, 2016) for (eeg OR erp OR seizure OR epilepsy) AND (autism OR ‘pervasive developmental disorder’) AND (regression OR regressive OR ‘childhood disintegrative disorder’), or variants of these terms, which yielded 193 results, of which 13 were relevant empirical studies; 4 additional studies of interest were identified by reviewing work cited by these publications (see Appendix S1). We excluded case studies from our review. Among individuals with ASD+R, reported rates of paroxysmal abnormalities (PA) with or without epilepsy range between 29.2 and 64.6%; rates of epilepsy in this population are reported between 6.7 and 56.3% (see Table 4). The few studies that examined rates of non-epileptiform EEG abnormalities in addition to rates of PA and epilepsy have yielded overall rates of any type of EEG abnormality (PA, non-epileptiform abnormality, and/or epilepsy) between 35.4-79.3% (Table 4).
Table 3.
Studies of EEG abnormalities and epilepsy in children with ASD, either with or without regression.
| Study | ASD dx | Epilepsy criteria | Regression assessment | EEG recording | EPI−/PA− |
EPI−/PA+
n with regression/ Group n; % with regression |
EPI+(/PA+) | Sig.? |
|---|---|---|---|---|---|---|---|---|
| Shubrata et al., 2015 | DSM-IV; PDD Assessment Scale, CARS |
≥2 seizures | ‘detailed history’ | 5 awake, 1 sleep, 19 medicated sleep |
2/25; 8% | N/A | 9/25; 26% | + |
| Valvo et al., 2016 | DSM-IV; ADOS-G in 79% cases |
N/A; Focused on interictal EEG abnorm. |
Clinical interview with caregiver and chart review |
Awake + sleep interictal EEG |
17/64; 26.6% |
PA+focal slowing: 65/150; 43.3% |
N/A | + |
| Mulligan & Trauner, 2014 | DSM-IV | N/A; Focused on EEG abnorm. |
Chart review | 24h EEG awake + natural sleep |
Overall N = 101 with n = 60 demonstrating PA. Regression hx was not associated with presence of PA. No regression n reported. |
− | ||
| Viscidi et al., 2013 | ADOS, ADI-R | ADI-R parent report (item 85) |
ADI-R all loss items (11-28) | N/A | 1210/4305; 28.1% |
N/A | 87/201; 43.3% |
+/−a |
| Bolton et al., 2011 | ICD-10; ADOS- G, ADI-R |
≥1 non-FC seizure after 5y of age. |
ADI-R item 11 (loss of language) |
EEG reports available for n = 18; other determinations made via seizure interview + clinical records |
13/95; 13.7% | N/A | 4/31; 12.9% | − |
| Jones & Campbell, 2010 b | DSM-IV; ADI-R, ADOS |
‘presence of seizures’ in chart review |
ADI-R items 11-19 (items on language regression) |
N/A | 26/98; 26.5% | N/A | 6/10; 60% | − c |
| Parmeggiani et al., 2010 | DSM-IV; CARS | Included focal EPI, generalized EPI, FC, & epileptic encephalopathy |
Chart review | Awake + sleep EEG | 28/178; 15.7% |
22/81; 27.2% |
29/86; 33.7% |
+ |
| Baird et al., 2008 | ICD-10; ADI-R, ADOS-G |
ADI-R item 85; medical hx; medical notes |
ADI-R item 11 (definite loss of language) |
N/A | 24/132; 18.2% |
N/A | 2/17; 11.8% |
− |
| Giannotti et al., 2008 | DSM-IV; ADOS-G, ADI- R |
≥2 unprovoked seizures |
ADI-R items 11 (loss of language), and/or 20 (loss of general skills), and/or 25 (loss of social interest/ engagement) |
Prolonged awake + sleep EEG w/ photic stimulation & hyperventilation when possible |
12/42; 28.6% |
14/42; 33.3% Freq. PA+: 6/13; 46.2% |
8/20; 40% |
+ |
| Baird et al., 2006 | ICD-10; ADI-R (+ADOS-G for ~50%) |
N/A; excluded children with clinical seizures or epilepsy |
ADI-R ‘regression of babbling, any words, and social behaviour’ |
Daytime sleep EEG ≥1 hr (natural or medicated) |
24/44; 54.5% | 15/20; 75.0% | N/A | − |
| Chez et al., 2006 | DSM-IV | N/A; excluded children with seizures |
Regression after 12 mos. | 24 hour ambulatory EEG | 71/349; 20.3% |
106/540;19.6% | N/A | − |
| Canitano et al., 2005 | DSM-IV | ILAE (1989) classifications |
Parent report: Loss of language + loss in social-emotional domain |
Awake + sedated sleep EEG |
15/30; 50% | EPI+/PA+ or EPI−/ 9/16; 56.3% |
PA+: | − |
| Hrdlicka et al., 2004 | ICD-10; ADI-R, CARS |
Not specified | Parent report: ‘severe developmental downturn’ in social, communication, play, & sometimes cognitive domains |
Some awake, some overnight sleep (both natural and medicated) EEGs |
EPI−: 7/45 15.6% Rates of EEG abnorm. by regression hx not reported. |
EPI+: 9/17 52.9% |
+ for EPI; − for EEG abnorm. |
|
| Kobayashi & Murata, 1998 | DSM-III-R | Not specified | Parent report: loss of language + loss of social interest |
No information | EPI−: 33/135; 24.4% |
N/A | EPI+: 15/33; 45.5% |
+ |
| Tuchman & Rapin, 1997 | DSM-IV | ≥2 unprovoked seizures |
Parent report: loss of language | Total sample: EEG & no EEG (N = 585) |
EPI−: 155/519; 29.9% |
EPI+: 21/66; 31.8% |
− | |
| Sleep EEG available (n =392) | 84/268; 29.6% |
21/43; 48.8% |
17/57; 29.8% |
|||||
| Rossi et al., 1995 | DSM-III-R | ‘Epilepsy including FC and EEG PA’ |
Report of regression in language and/or behavior |
Awake + sleep interictal EEG |
25/61; 41% |
5/20; 25% |
9/25; 36% |
− |
Note: EPI +/−: Epilepsy present/not present. PA+/−: Paroxysmal EEG abnormalities present/not present. Sig. ? + or − indicates whether or not the study reported a significant difference in rates of either EEG abnormalities or epilepsy between those with or without a history of regression. Abnorm.: Abnormalities. ADOS-G: Autism Diagnostic Observation Schedule-Generic. ADI−R: Autism Diagnostic Interview-Revised. CARS: Childhood Autism Rating Scale. DSM: Diagnostic and Statistical Manual of Mental Disorders. FC: Febrile convulsions. Hx: history. ICD-10: International Statistical Classification of Diseases and Related Health Problems 10th Revision. ILAE: International League Against Epilepsy.
Difference no longer significant after adjusting for IQ.
Only children with ASD and either language regression, language plateau, or language delay were included.
Statistical tests compared three groups; here we collapse the language plateau and language delay groups together to comprise the ADI-R group.
Table 4.
Reported rates of EEG abnormalities in children with ASD, with and without a history of regression.
| Sample n | Any EEG abnormality (%) | PA and/or epilepsy (%) | Epilepsy (%) | |||||
|---|---|---|---|---|---|---|---|---|
| ASD−R | ASD+R | ASD−R | ASD+R | ASD−R | ASD+R | ASD−R | ASD+R | |
| (Valvo et al., 2016) | 132 | 82 | 64.4 | 79.3 | 56.8 | 64.6 | 19.7a | 26.8a |
| (Viscidi et al., 2013) | 3209 | 1297 | -- | -- | -- | -- | 3.6 | 6.7 |
| (Bolton et al., 2011) | 109 | 17 | -- | -- | -- | -- | 24.8 | 23.5 |
| (L. A. Jones & Campbell, 2010) | 76 | 32 | -- | -- | -- | -- | 5.3 | 18.8 |
| (Parmeggiani et al., 2010) | 266 | 79 | -- | -- | 43.6 | 64.6 | 21.4b | 36.7b |
| (Baird et al., 2008) | 123 | 26 | -- | -- | -- | -- | 12.0 | 7.0 |
| (Giannotti et al., 2008) | 70 | 34 | -- | -- | 39.9c | 41.2c | 14.7 | 24.2 |
| (Baird, Robinson, Boyd, & Charman, 2006) | 25 | 39 | -- | -- | 25.0d | 62.5d | -- | -- |
| (Chez et al., 2006) | 712 | 177 | -- | -- | 60.9d | 59.9d | -- | -- |
| (Canitano, Luchetti, & Zappella, 2005) | 22 | 24 | -- | -- | 31.8 | 37.5 | 0.0 | 4.2 |
| (McVicar, Ballaban-Gil, Rapin, Mosh, & Shinnar, 2005) | -- | 103 | -- | 39.8 | -- | 28.2 | -- | 7.8a |
| (Hrdlicka et al., 2004) | 46 | 16 | -- | -- | -- | -- | 17.4 | 56.3 |
| (Kobayashi & Murata, 1998) | 120 | 48 | -- | -- | -- | -- | 15.0 | 31.3 |
| (Tuchman & Rapin, 1997) | 409 | 176 | 20.3e | 28.4e | 16.4e | 23.9e | 11.0 | 11.9 |
| (Rossi, Parmeggiani, Bach, Santucci, & Visconti, 1995) | 67 | 39 | -- | -- | 46.3 | 35.9 | 23.9b | 23.1b |
Note. Percentages are the % of the indicated subsample with the feature. ASD−R: ASD without regression. ASD+R: ASD with regression. PA: Paroxysmal abnormality. Any EEG abnormality includes epilepsy, paroxysmal abnormalities, and/or nonepileptiform abnormalities.
Positive seizure history or clinical seizures observed, but epilepsy diagnosis not specified.
Included febrile convulsions.
It is not clear from the original report if this group contains children with epilepsy and PA, or only children with PA.
Epilepsy specifically excluded.
Tuchman and Rapin calculated their proportions based on their entire sample (N = 585) rather than the total number of participants who had an EEG (N = 392), based on the presumption that children without an EEG would be ‘more likely than not’ to have a normal EEG.
Findings are mixed regarding whether these rates differ from those in the broader ASD population, likely in no small part due to the wide variety among these studies in the quality and detail of raw EEG or EEG reports available and the diagnostic criteria used to make determinations regarding ASD and epilepsy status (see Table 3). However, as others have noted (El Achkar & Spence, 2015), this issue is also confounded by the overlap between ASD+ID and ASD+R; individuals who experience developmental regression are likely to also demonstrate cognitive deficits (Kobayashi & Murata, 1998; Tuchman & Rapin, 1997), and elevated rates of epilepsy in ASD+ID versus ASD without ID are well-established (Amiet et al., 2008; Woolfenden et al., 2012). A recent large-scale study found elevated rates of epilepsy in ASD+R (Viscidi et al., 2013), but after controlling for IQ in their models, this difference was no longer significant. This effect demonstrates the importance of assessing and accounting for cognitive function when addressing questions regarding epilepsy and EEG abnormality rates in ASD+R.
The meaning of epilepsy and/or PA in autistic regression is also unclear. There has been speculation regarding whether some of these cases might be linked to epileptic encephalopathy (that is, loss of cognitive and/or behavioral function due to epileptic activity, beyond what would be expected given the underlying pathology (Berg et al., 2010)), similar to what is observed in Landau-Kleffner syndrome; however, there is no evidence that such an etiology is widespread in ASD+R. For example, despite finding elevated rates of PA and epilepsy in ADI+R, Parmeggiani and colleagues (2010) failed to find that this activity temporally preceded the onset of regression. It seems likely that in many cases EEG abnormalities may be best considered as one expression of a particular neural endophenotype associated with ASD+R, rather than a causal factor. A full treatment of this issue is beyond the scope of this article; however, interested readers may find extensive reviews in Jeste & Tuchman (2015) and Rapin (1995).
A promising avenue for investigation may lie in greater attention to the nature of all types of EEG abnormalities in ASD+R, not just epileptiform ones. Valvo and colleagues (2016) conducted an investigation of interictal EEG abnormalities in 220 individuals with ASD who either did or did not evidence a history of developmental regression. They characterized observed EEG abnormalities by type (PA or focal slowing), localization (focal or multifocal/diffuse), and site (anterior, posterior, or temporal). They found elevated rates of temporal abnormalities in ASD+R. These consisted of both PA and focal slowing, but were more frequently of the focal slowing type. Individuals without a regression history, on the other hand, were much more likely to exhibit posterior abnormalities. Given the temporal lobe’s role in social perception (see for reviews Allison, Puce, & McCarthy, 2000; Pelphrey & Morris, 2006) and language (e.g., Hickok & Poeppel, 2007; Saur et al., 2008), as well as robust evidence of functional and structural abnormalities in temporal lobe in ASD (e.g., Hadjikhani, Joseph, Snyder, & Tager-Flusberg, 2006; Pelphrey, Shultz, Hudac, & Vander Wyk, 2011; Zilbovicius et al., 2006), further investigation of this phenomenon seems warranted.
Structural atypicalities
The use of structural MRI may allow us to better understand endophenotypes associated with both regression and EEG abnormalities, particularly when used as part of multimodal investigations; however, very few such MRI studies have been conducted in ASD+R. Indeed, a PubMed search (Oct. 17, 2016) for (((autism OR ‘pervasive developmental disorder’) AND (regressive OR regression)) OR (‘childhood disintegrative disorder’)) AND (mri OR dti OR dwi), or variants of these terms (see Appendix S1) returned 84 hits but only 1 relevant result. We then searched literature reviews and consulted with experts in the area to identify the remaining work cited in the following sections. We were able to locate two empirical studies that used structural imaging methods to examine brain characteristics of ASD+R.
Valvo and colleagues (2016) extended their EEG work by conducting an MRI investigation of brain morphometry in a subsample (n = 11) of the individuals they had identified as showing temporal EEG abnormalities. In this small sample, they found that individuals who displayed both macrocephaly and regression (n = 3) were unique in respect to all other members of the sample (regression alone, macrocephaly alone, or neither) in demonstrating reduced cortical volume in right temporal lobe. This suggests it may be worthwhile to investigate whether a regressive subtype characterized by enlarged head circumference and temporal abnormalities (both electrophysiological and structural) may exist. However, it is not clear from this report the degree (if any) to which regressive history in their sample overlapped with ID, a potentially confounding factor given that macrocephaly in ASD tends to be associated with greater functional impairment (Sacco, Gabriele, & Persico, 2015).
Another structural MRI study available also suggests a relationship between brain enlargement and autistic regression. Nordahl and colleagues (2011) examined the relationship between total brain volume and the presence or absence of regression in a large sample (n = 53 with regression; n = 61 without regression) of 2- to 4-y-old boys and girls with ASD and a comparison group of age-matched TD controls. They found that abnormal brain enlargement was most commonly found in boys with regressive ASD. Brain size in boys without regression did not differ from controls. Retrospective head circumference measurements indicated that head circumference in boys with regressive ASD was normal at birth but diverged from the other groups around 4–6 months of age, and that these results held after controlling for participants' developmental quotient. There were no differences in brain size in girls with ASD. These results suggest that there may be distinct neural phenotypes associated with different onset patterns of ASD, and these neural phenotypes may differ by sex of the child. Among the boys with regressive ASD examined by Nordahl and colleagues, divergence in brain size occurred before the age at which loss of skills is commonly reported (~ 2 years of age). Thus, rapid head growth may be a risk factor for regressive ASD in boys.
Considerations for future work
Regression should receive increased attention in future neuroimaging work. As studies involve increasingly larger samples, analyses can readily be conducted to compare brain structure and function in those with and without regression, as well as to examine the potentially key question of age of regression. In particular, we believe that future work should focus on breaking down the broad categorization, ‘with regression,’ into more manageable units of analysis. It seems likely that ASD+R, like other types of ASD, is driven by multiple etiologies that converge upon a similar behavioral outcome. Identifying and describing subtypes of ASD+R can be facilitated in a variety of ways, including more rigorous, objective characterization of the regression history, consideration of molecular and cellular indicators, and examination of the most rare, extreme manifestations of regression.
Characterization
The validity of ASD+R research hinges upon reliable, precise operationalization of regression. However, criteria for determining regression status have greatly varied in extant research (see, e.g., Table 2). The Autism Diagnostic Interview-Revised (ADI-R) includes a handful of items concerning regression (Lord, Rutter, & Le Couteur, 1994), which can be supplemented by follow-up questions via measures such as the Regression Validation Interview (Heung, 2008; Luyster et al., 2005) or the Regression Supplement Form (Goldberg et al., 2003). These supplements, however, are not in wide distribution, nor have they been frequently used. Further, the ADI-R, even with additional regression supplements, is less than ideal as a sole measure of regression because it relies on a parent’s memory of the subject’s early developmental history. Preferably, parental report would be supplemented by an objective data source, such as early videos illustrating the child’s abilities before and after the regression. Investigators that have used this method have found discrepancies between parent report and behavioral data obtained from home video (Goldberg, Thorsen, Osann, & Spence, 2008; Ozonoff et al., 2011); parents may, for example, miss subtle indicators of true regression or, conversely, report as a regression what may be more accurately be characterized as a plateauing or slowing of development. Thus, it is important to obtain information about regression onset, duration, and characteristics from multiple sources.
Molecular and cellular work
Integration of molecular and cellular insights into neuroimaging research into ASD+R may be useful as well. Recent work has found reduced levels of protein kinases A (Ji, Chauhan, Flory, & Chauhan, 2011) and C (Ji, Chauhan, & Chauhan, 2012) in frontal cortex tissue from donors with ASD+R versus those with ASD but no regression history; no such reduction was found in tissue from other lobes of the brain. The protein kinases are involved in a wide array of crucial functions related to neurodevelopment and cell signalling (among others), and disruption to protein kinases A and C specifically in frontal lobe suggests that attention to frontal cortex properties in in vivo MRI studies of ASD+R may be warranted.
Coupling MRI investigations with blood assays that are able to index cellular or molecular properties of interest may also provide insight. One recent study (Breece et al., 2013) investigated circulating levels of dendritic cells thought to be associated with innate immune response in young children with ASD (both with and without regression) and TD children, and coupled this assessment with a structural MRI scan obtained during natural sleep. They found that in children with ASD, plasmacytoid dendritic cell frequency was positively associated with amygdala volume bilaterally, and that frequencies of these cells were also associated with developmental regression or later ASD symptom onset. Thus, innate immune response may be an area that requires further research in terms of its role in ASD+R; in addition, attention to amygdala volume in future studies may be warranted.
Childhood Distintegrative Disorder
A potentially productive approach involves examining an extreme cohort to maximize our ability to identify the neurobiology of regression. Richard Lifton and colleagues, who identified genetic and physiological mechanisms of blood pressure regulation in the general population by studying extreme cases of blood pressure variation, pioneered this kind of approach (Choi et al., 2011). Regression, or the loss of skills, has been associated with autism since Kanner (1943) first used the word ‘autistic’ to describe a group of children with abnormal sociability and repetitive patterns of behavior. Kanner described one of his patients as having ‘gone backward mentally’ for two years. Today, any of the ASDs may be associated with regression, but one disorder, childhood disintegrative disorder (CDD)1, is defined by a late-onset, catastrophic form of regression. Despite significant changes in classification systems since Theodor Heller described the disorder as ‘dementia infantilis’ at the turn of the century, the clinical features Heller described (1908) remain the backbone of today’s definition of childhood disintegrative disorder, which was introduced in the DSM-IV in 1994. CDD is defined in the ICD-10 (World Health Organization, 1990) and the DSM-IV-TR (American Psychiatric Association, 2000) by normal development for at least the first two years of life followed by regression before age 10 years in at least two of the following areas: 1) expressive or receptive language, 2) social skills or adaptive behavior, 3) bowel or bladder control, 4) play, and 5) motor skills. This diagnostic category has been subsumed by the diagnosis ASD in the DSM-5 (American Psychiatric Association, 2013), but may yet have research utility, particularly in terms of better characterizing potential subtypes of ASD+R. While regression is most commonly reported in the first two years of life, children with CDD have later regressions and generally have the poorest outcome among individuals with ASD. While the prevalence of ASD is estimated to be as high as 1/68 (Centers for Disease Control and Prevention, 2014), CDD is rare, with a prevalence of 1-2/100,000 (Fombonne, 2009).
We recommend multimodal investigation of patients with CDD, involving structural and functional neuroimaging as well as careful behavioral phenotyping and comprehensive (whole genome) genetic analyses. Such work could examine points of convergence and divergence between this extreme form of regression and ASDs with and without regression. It would provide for the identification of genetic mutations associated with regression, characterize the systems-level brain mechanisms supporting regression, and quantify the behavioral sequela of regression. We note that in preliminary work from our team, children with CDD have been observed to have a distinctive phenotype from ASD+ID, one which is characterized by a fear prodrome, preserved attention to the eye region of the face, and recruitment of atypical regions for the processing of negative facial expressions (Westphal, 2013); further work to distinguish whether CDD’s severe, late-onset regression is driven by a unique etiology, or a similar etiology whose distinct phenotype is driven by a difference in developmental timing, is warranted. One team has proposed that synaptic over-pruning can broadly explain early-onset, late-onset, and regressive ASD (Thomas, Davis, Karmiloff-Smith, Knowland, & Charman, 2015; Thomas, Knowland, & Karmiloff-Smith, 2011), using artificial neural network population modeling to demonstrate that varying pruning thresholds and pruning onsets can produce simulated approximations of all three developmental trajectories. In this model, regression onset over two years of age would have have the same underlying cause as regression onset under two years, but would have differing developmental sequelae. Further application of in vivo neuroimaging techniques, coupled with genetic, molecular, and behavioral assays, may allow us to test whether the etiology of CDD can be considered as separate from that of other ASD+R cases or not.
Conclusions
What has often been termed ‘low-functioning autism’ deserves much greater research attention, particularly in the neuroimaging domain, than it has thus far received. Lumping these individuals under a broad umbrella term has led to a contradictory and diffuse literature. Even when considered separately, as minimally verbal individuals, individuals with intellectual disability, and those with a history of regression, it becomes clear that these categories, too, likely contain multiple subtypes of cases, differing in their behavioral, genetic, developmental, and brain profiles. Despite these challenges, the available literature allows us to identify several trends and promising avenues for future research as regards ASD+MV, ASD+ID, and ASD+R, which we summarize below.
Available neuroimaging work on minimally verbal children with ASD suggests that abnormalities in preattentive auditory perceptual processing, which may include both delays in processing and atypical allocation of attention to nonspeech versus speech stimuli, may be associated with the phenotype. Further, MRI and DTI evidence indicates that the ASD+MV phenotype may be related to underlying deficits in brain structures, such as IFG and arcuate fasciculus, that contribute to expressive language function. However, these conclusions are based on only a handful of studies, and larger sample sizes are necessary to replicate these findings. Longitudinal assessments and more detailed phenotyping of language profiles are particularly needed in neuroimaging work with ASD+MV.
Individuals with ASD+ID have been studied somewhat more frequently than ASD+MV, but the resultant literature has yielded few consistent patterns. Review of available structural neuroimaging studies indicates that regions often implicated in ASD−ID are also impacted in ASD+ID, that grey matter volumes and white matter structure are disturbed in ASD+ID, and that MRI abnormalities may be elevated in this population. Structural imaging metrics assessed within this population may also show higher variability than those of comparison groups. Recent imaging genetics work on genetic variants associated with both ASD and ID (e.g. FMR1, NRXN1, CNTNAP2), suggests that these variants may shape brain structure in predictable but often opposing ways. Thus, sampling ASD+ID without respect to genotype may be one factor that has led to the often contradictory findings evident in the current literature, and imaging genetics approaches are likely critical for the field to progress.
Neuroimaging of ASD+R has primarily focused on assessment of EEG abnormalities in this population. While some studies suggest that rates of epilepsy are elevated in ASD+R versus ASD−R, this is by no means a consistent finding, and covarying out IQ may eliminate significant differences in epilepsy rates between these populations. Further, available evidence does not indicate that epileptic encephalopathy is a widespread causal factor in autistic regression. Recent work, however, suggests the possibility that non-paroxysmal EEG abnormalities may be a worthwhile avenue of investigation as regards the ASD+R endophenotype. Finally, there are indications of a possible relationship between brain enlargement and ASD+R, particularly among boys. Neuroimaging investigations that target severe manifestations of this phenotype (i.e., CDD) and/or that incorporate molecular assays may be possible pathways to greater insight into regressive trajectories.
Overall, findings on these understudied populations within the autism spectrum have been mixed and often contradictory. It is evident that rigorous, detailed phenotypic assessment, clearly operationalized definitions, and multimodal approaches that integrate information from microscopic to macroscopic levels of analysis are necessary for the field to progress. Moreover, we must be willing to embrace the challenges inherent in assessing individuals whose symptom presentation makes comprehending and complying with research protocols difficult. By using multiple de-sensitization measures and brief but statistically powerful experimental paradigms, it is possible to obtain both functional and structural information even from individuals with severe to profound ID accompanied by serious deficits in adaptive behavior. Extensive planning with the family, experienced staff, and thoughtful experimental design are required. However, we believe that the effort expended is worthwhile, as this kind of research could lead to individualized and more effective interventions based on genetic and neural systems profiles.
Supplementary Material
Key points.
Expressions of ASD characterized by a more severe phenotype, including autism with intellectual disability (ASD+ID), autism with a history of developmental regression (ASD+R), and minimally verbal autism (ASD+MV) are understudied generally, and especially in the domain of neuroimaging. However, neuroimaging methods are a potentially powerful tool for understanding the etiology of these ASD subtypes.
In a literature review of neuroimaging work on ASD+MV, ASD+ID, and ASD+R, we find several promising trends in the existing research and suggest directions for future work.
ASD+MV may display abnormalities in preattentive auditory perceptual processing and structural deficits in brain structures that support expressive language function. In future, longitudinal assessments and more detailed phenotyping of language profiles are particularly needed with this population.
ASD+ID brain research findings have been highly variable. This may be related, in part, to underlying genetic heterogeneity in ASD+ID. Recent developments in imaging genetics of variants linked to ASD+ID (e.g., FMR1, NRXN1, CNTNAP2) suggests that these variants may shape brain structure in predictable but often opposing ways, indicating the need to take genotype into account in future ASD+ID work.
ASD+R neuroimaging findings have focused mostly on EEG abnormalities and epilepsy, but do not support for the hypothesis that epileptic encephalopathy contributes to the phenotype in the majority of cases; however, there is some suggestion that further exploration of non-epileptiform abnormalities may be worth investigating in some ASD+R cases. In the few MRI studies of ASD+R, there is some suggestion that brain enlargement may also be a worthwhile avenue of exploration. Investigating more extreme expressions of the phenotype may be a productive direction for future research.
Acknowledgments
This work was supported, in part, by an institutional training award from the US National Institute of Mental Health (T32 MH018268) to trainee A.J. All authors have declared that they have no competing or potential conflicts of interest pertaining to the present article. This review was invited by the Editors of JCPP (for which the first author has been offered a small honorarium towards expenses) and has been subject to full external peer review. The authors would like to thank Shafali Jeste, for her insight into EEG abnormalities and regression, Abha Gupta, for her helpful feedback regarding the neurobiology and genetics of CDD, and to the anonymous reviewers for comments that significantly improved the manuscript.
Footnotes
This condition was also often referred to as disintegrative psychosis or Heller’s syndrome, particularly in early literature on the topic.
Conflict of interest statement: No conflicts declared.
Supporting Information
Additional Supporting Information may be found on the online version of this article.
Appendix S1: PubMed search details for review
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nature Reviews. Genetics. 2008;9(5):341–55. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: Role of the STS region. Trends Cogn Sci. 2000;4(7):267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th American Psychiatric Association; Arlington, VA: 2000. [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM-5. American Psychiatric Publishing; Washington, D.C.: 2013. [Google Scholar]
- Amiet C, Gourfinkel-An I, Bouzamondo A, Tordjman S, Baulac M, Lechat P, Cohen D. Epilepsy in autism is associated with intellectual disability and gender: evidence from a meta-analysis. Biological Psychiatry. 2008;64(7):577–82. doi: 10.1016/j.biopsych.2008.04.030. [DOI] [PubMed] [Google Scholar]
- Anderson DK, Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Patterns of growth in verbal abilities among children with autism spectrum disorder. Journal of Consulting and Clinical Psychology. 2007;75(4):594–604. doi: 10.1037/0022-006X.75.4.594. [DOI] [PubMed] [Google Scholar]
- Aslin RN, Shukla M, Emberson LL. Hemodynamic correlates of cognition in human infants. Annual Review of Psychology. 2015;66:349–79. doi: 10.1146/annurev-psych-010213-115108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird G, Charman T, Pickles A, Chandler S, Loucas T, Meldrum D, Simonoff E. Regression, developmental trajectory and associated problems in disorders in the autism spectrum: the SNAP study. Journal of Autism and Developmental Disorders. 2008;38(10):1827–36. doi: 10.1007/s10803-008-0571-9. [DOI] [PubMed] [Google Scholar]
- Baird G, Robinson RO, Boyd S, Charman T. Sleep electroencephalograms in young children with autism with and without regression. Developmental Medicine and Child Neurology. 2006;48(7):604–8. doi: 10.1017/S0012162206001265. [DOI] [PubMed] [Google Scholar]
- Balardin JB, Sato JR, Vieira G, Feng Y, Daly E, Murphy C, Ecker Relationship Between Surface-Based Brain Morphometric Measures and Intelligence in Autism Spectrum Disorders: Influence of History of Language Delay. Autism Research. 2015;8(5):556–66. doi: 10.1002/aur.1470. [DOI] [PubMed] [Google Scholar]
- Barger BD, Campbell JM, McDonough JD. Prevalence and onset of regression within autism spectrum disorders: a meta-analytic review. Journal of Autism and Developmental Disorders. 2013;43(4):817–28. doi: 10.1007/s10803-012-1621-x. [DOI] [PubMed] [Google Scholar]
- Barth M, Breuer F, Koopmans PJ, Norris DG, Poser BA. Simultaneous multislice (SMS) imaging techniques. Magnetic Resonance in Medicine. 2015;75(1):63–81. doi: 10.1002/mrm.25897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Scheffer IE. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010;51(4):676–85. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Betancur C. Etiological heterogeneity in autism spectrum disorders: More than 100 genetic and genomic disorders and still counting. Brain Research. 2011;1380(1):42–77. doi: 10.1016/j.brainres.2010.11.078. [DOI] [PubMed] [Google Scholar]
- Billeci L, Calderoni S, Tosetti M, Catani M, Muratori F. White matter connectivity in children with autism spectrum disorders: a tract-based spatial statistics study. BMC Neurology. 2012;12(1):148. doi: 10.1186/1471-2377-12-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet M-H, Zilbovicius M. Superior temporal sulcus anatomical abnormalities in childhood autism: A voxel-based morphometry MRI study. NeuroImage. 2004;23(1):364–9. doi: 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Bolton PF, Carcani-Rathwell I, Hutton J, Goode S, Howlin P, Rutter M. Epilepsy in autism: features and correlates. The British Journal of Psychiatry. 2011;198(4):289–94. doi: 10.1192/bjp.bp.109.076877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breece E, Paciotti B, Nordahl CW, Ozonoff S, Van de Water JA, Rogers SJ, Ashwood P. Myeloid dendritic cells frequencies are increased in children with autism spectrum disorder and associated with amygdala volume and repetitive behaviors. Brain, Behavior, and Immunity. 2013;31:69–75. doi: 10.1016/j.bbi.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canitano R, Luchetti A, Zappella M. Epilepsy, electroencephalographic abnormalities, and regression in children with autism. Journal of Child Neurology. 2005;20(1):27–31. doi: 10.1177/08830738050200010401. [DOI] [PubMed] [Google Scholar]
- Cascio C, Gribbin M, Gouttard S, Smith RG, Jomier M, Field S, Piven J. Fractional anisotropy distributions in 2- to 6-year-old children with autism. Journal of Intellectual Disability Research. 2013;57(11):1037–49. doi: 10.1111/j.1365-2788.2012.01599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Prevalence of autism spectrum disorder among children aged 8 years -- Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2010. 2014 [PubMed] [Google Scholar]
- Chez M, Chang M, Krasne V, Coughlan C, Kominsky M, Schwartz A. Frequency of epileptiform EEG abnormalities in a sequential screening of autistic patients with no known clinical epilepsy from 1996 to 2005. Epilepsy & Behavior. 2006;8(1):267–71. doi: 10.1016/j.yebeh.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, Lifton RP. K+ Channel Mutations in Adrenal Aldosterone-Producing Adenomas and Hereditary Hypertension. Science. 2011;331(6018):768–772. doi: 10.1126/science.1198785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox AD, Virues-Ortega J, Julio F, Martin TL. Establishing motion control in children with autism and intellectual disability: Applications for anatomical and functional MRI. Journal of Applied Behavior Analysis. 2016 doi: 10.1002/jaba.351. [DOI] [PubMed] [Google Scholar]
- Dager SR, Wang L, Friedman SD, Shaw DW, Constantino JN, Artru AA, Csernansky JG. Shape mapping of the hippocampus in young children with autism spectrum disorder. American Journal of Neuroradiology. 2007;28(4):672–7. [PMC free article] [PubMed] [Google Scholar]
- Davis MH, Coleman MR, Absalom AR, Rodd JM, Johnsrude IS, Matta BF, Menon DK. Dissociating speech perception and comprehension at reduced levels of awareness. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(41):16032–7. doi: 10.1073/pnas.0701309104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Jahanshad N, Rudie JD, Brown JA, Johnson K, McMahon KL, Thompson PM. Altered structural brain connectivity in healthy carriers of the autism risk gene, CNTNAP2. Brain Connectivity. 2011;1(6):447–59. doi: 10.1089/brain.2011.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C, Bookheimer SY, Murphy DGM. Neuroimaging in autism spectrum disorder: brain structure and function across the lifespan. The Lancet Neurology. 2015;14(11):1121–34. doi: 10.1016/S1474-4422(15)00050-2. [DOI] [PubMed] [Google Scholar]
- Egaas B, Courchesne E, Saitoh O. Reduced size of corpus callosum in autism. Archives of Neurology. 1995;52(8):794–801. doi: 10.1001/archneur.1995.00540320070014. [DOI] [PubMed] [Google Scholar]
- El Achkar CM, Spence SJ. Clinical characteristics of children and young adults with co-occurring autism spectrum disorder and epilepsy. Epilepsy & Behavior. 2015;47:183–90. doi: 10.1016/j.yebeh.2014.12.022. [DOI] [PubMed] [Google Scholar]
- Elia M, Ferri R, Musumeci SA, Panerai S, Bottitta M, Scuderi C. Clinical correlates of brain morphometric features of subjects with low-functioning autistic disorder. Journal of Child Neurology. 2000;15(8):504–8. doi: 10.1177/088307380001500802. [DOI] [PubMed] [Google Scholar]
- Ellegood J, Anagnostou E, Babineau BA, Crawley JN, Lin L, Genestine M, Lerch JP. Clustering autism: using neuroanatomical differences in 26 mouse models to gain insight into the heterogeneity. Molecular Psychiatry. 2015;20(1):118–25. doi: 10.1038/mp.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh M, Divan G, Koh Y-J, Kim YS, Kauchali S, Marcín C, Fombonne E. Global prevalence of autism and other pervasive developmental disorders. Autism Research. 2012;5(3):160–79. doi: 10.1002/aur.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbetta A, Bulgheroni S, Contarino VE, Chiapparini L, Esposito S, Annunziata S, Riva D. Low-Functioning Autism and Nonsyndromic Intellectual Disability: Magnetic Resonance Imaging (MRI) Findings. Journal of Child Neurology. 2015;30(12):1658–63. doi: 10.1177/0883073815578523. [DOI] [PubMed] [Google Scholar]
- Erbetta A, Bulgheroni S, Contarino V, Chiapparini L, Esposito S, Vago C, Riva D. Neuroimaging findings in 41 low-functioning children with autism spectrum disorder: a single-center experience. Journal of Child Neurology. 2014;29(12):1626–31. doi: 10.1177/0883073813511856. [DOI] [PubMed] [Google Scholar]
- Fombonne Epidemiology of pervasive developmental disorders. Pediatric Research. 2009;65(6):591–8. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- Giannotti F, Cortesi F, Cerquiglini A, Miraglia D, Vagnoni C, Sebastiani T, Bernabei P. An investigation of sleep characteristics, EEG abnormalities and epilepsy in developmentally regressed and non-regressed children with autism. Journal of Autism and Developmental Disorders. 2008;38(10):1888–97. doi: 10.1007/s10803-008-0584-4. [DOI] [PubMed] [Google Scholar]
- Goldberg WA, Osann K, Filipek PA, Laulhere T, Jarvis K, Modahl C, Spence MA. Language and other regression: assessment and timing. Journal of Autism and Developmental Disorders. 2003;33(6):607–16. doi: 10.1023/b:jadd.0000005998.47370.ef. [DOI] [PubMed] [Google Scholar]
- Goldberg WA, Thorsen KL, Osann K, Spence MA. Use of home videotapes to confirm parental reports of regression in autism. Journal of Autism and Developmental Disorders. 2008;38(6):1136–46. doi: 10.1007/s10803-007-0498-6. [DOI] [PubMed] [Google Scholar]
- Greene DJ, Black KJ, Schlaggar BL. Considerations for MRI study design and implementation in pediatric and clinical populations. Developmental Cognitive Neuroscience. 2015 doi: 10.1016/j.dcn.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas RH, Townsend J, Courchesne E, Lincoln AJ, Schreibman L, Yeung-Courchesne R. Neurologic abnormalities in infantile autism. Journal of Child Neurology. 1996;11(2):84–92. doi: 10.1177/088307389601100204. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Anatomical differences in the mirror neuron system and social cognition network in autism. Cerebral Cortex. 2006;16(9):1276–82. doi: 10.1093/cercor/bhj069. [DOI] [PubMed] [Google Scholar]
- Hall D, Huerta MF, McAuliffe MJ, Farber GK. Sharing heterogeneous data: the national database for autism research. Neuroinformatics. 2012;10(4):331–9. doi: 10.1007/s12021-012-9151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Murakawa K, Miyazaki M, Tayama M, Kuroda Y. Magnetic resonance imaging of the brain structures in the posterior fossa in retarded autistic children. Acta Paediatrica. 1992;81(12):1030–4. doi: 10.1111/j.1651-2227.1992.tb12169.x. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Gerig G, Smith RG, Piven J. Cortical gray and white brain tissue volume in adolescents and adults with autism. Biological Psychiatry. 2006;59(1):1–6. doi: 10.1016/j.biopsych.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Heller T. Dementia infantilis. Zeitschrift Fur Die Erforschung Und Behandlung Des Jugenlichen Schwachsinns. 1908;2:141–165. [Google Scholar]
- Heung K. Is autistic regression too narrowly defined? 2008 (Doctoral dissertation). Available from ProQuest Dissertations and Theses database. (UMI No. 3317935) [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hrdlicka M, Komarek V, Propper L, Kulisek R, Zumrova A, Faladova L, Urbanek T. Not EEG abnormalities but epilepsy is associated with autistic regression and mental functioning in childhood autism. European Child & Adolescent Psychiatry. 2004;13(4):209–13. doi: 10.1007/s00787-004-0353-7. [DOI] [PubMed] [Google Scholar]
- Jeong J-W, Chugani DC, Behen ME, Tiwari VN, Chugani HT. Altered white matter structure of the dentatorubrothalamic pathway in children with autistic spectrum disorders. Cerebellum. 2012;11(4):957–71. doi: 10.1007/s12311-012-0369-3. [DOI] [PubMed] [Google Scholar]
- Jeste SS, Tuchman R. Autism Spectrum Disorder and Epilepsy: Two Sides of the Same Coin? Journal of Child Neurology. 2015;30(14):1963–71. doi: 10.1177/0883073815601501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L, Chauhan A, Chauhan V. Reduced activity of protein kinase C in the frontal cortex of subjects with regressive autism: relationship with developmental abnormalities. International Journal of Biological Sciences. 2012;8(7):1075–84. doi: 10.7150/ijbs.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L, Chauhan V, Flory MJ, Chauhan A. Brain region-specific decrease in the activity and expression of protein kinase A in the frontal cortex of regressive autism. PloS One. 2011;6(8):e23751. doi: 10.1371/journal.pone.0023751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LA, Campbell JM. Clinical characteristics associated with language regression for children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2010;40(1):54–62. doi: 10.1007/s10803-009-0823-3. [DOI] [PubMed] [Google Scholar]
- Jones W, Klin A. Heterogeneity and homogeneity across the autism spectrum: The role of development. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(5):471–473. doi: 10.1097/CHI.0b013e31819f6c0d. [DOI] [PubMed] [Google Scholar]
- Kaiser MD, Hudac CM, Shultz S, Lee SM, Cheung C, Berken AM, Pelphrey KA. Neural signatures of autism. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(49):21223–8. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2(2):217–250. [PubMed] [Google Scholar]
- Kasari C, Brady N, Lord C, Tager-Flusberg H. Assessing the minimally verbal school-aged child with autism spectrum disorder. Autism Research. 2013;6(6):479–93. doi: 10.1002/aur.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi R, Murata T. Setback phenomenon in autism and long-term prognosis. Acta Psychiatrica Scandinavica. 1998;98(4):296–303. doi: 10.1111/j.1600-0447.1998.tb10087.x. [DOI] [PubMed] [Google Scholar]
- Kumar M, Duda JT, Hwang W-T, Kenworthy C, Ittyerah R, Pickup S, Poptani H. High resolution magnetic resonance imaging for characterization of the neuroligin-3 knock-in mouse model associated with autism spectrum disorder. PloS One. 2014;9(10):e109872. doi: 10.1371/journal.pone.0109872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai G, Pantazatos SP, Schneider H, Hirsch J. Neural systems for speech and song in autism. Brain. 2012;135:961–75. doi: 10.1093/brain/awr335. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24(5):659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lotspeich LJ, Kwon H, Schumann CM, Fryer SL, Goodlin-Jones BL, Buonocore MH, Reiss AL. Investigation of neuroanatomical differences between autism and Asperger syndrome. Archives of General Psychiatry. 2004;61(3):291–8. doi: 10.1001/archpsyc.61.3.291. [DOI] [PubMed] [Google Scholar]
- Luyster R, Richler J, Risi S, Hsu W-L, Dawson G, Bernier R, Lord C. Early regression in social communication in autism spectrum disorders: a CPEA Study. Developmental Neuropsychology. 2005;27(3):311–36. doi: 10.1207/s15326942dn2703_2. [DOI] [PubMed] [Google Scholar]
- Manes F, Piven J, Vrancic D, Nanclares V, Plebst C, Starkstein SE. An MRI study of the corpus callosum and cerebellum in mentally retarded autistic individuals. The Journal of Neuropsychiatry and Clinical Neurosciences. 1999;11(4):470–4. doi: 10.1176/jnp.11.4.470. [DOI] [PubMed] [Google Scholar]
- McVicar KA, Ballaban-Gil K, Rapin I, Moshé SL, Shinnar S. Epileptiform EEG abnormalities in children with language regression. Neurology. 2005;65(1):129–31. doi: 10.1212/01.wnl.0000167193.53817.0f. [DOI] [PubMed] [Google Scholar]
- Moss J, Howlin P. Autism spectrum disorders in genetic syndromes: implications for diagnosis, intervention and understanding the wider autism spectrum disorder population. Journal of Intellectual Disability Research. 2009;53(10):852–73. doi: 10.1111/j.1365-2788.2009.01197.x. [DOI] [PubMed] [Google Scholar]
- Mulligan CK, Trauner DA. Incidence and behavioral correlates of epileptiform abnormalities in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2014;44(2):452–8. doi: 10.1007/s10803-013-1888-6. [DOI] [PubMed] [Google Scholar]
- Munson J, Dawson G, Sterling L, Beauchaine T, Zhou A, Elizabeth K, Abbott R. Evidence for latent classes of IQ in young children with autism spectrum disorder. American Journal of Mental Retardation. 2008;113(6):439–52. doi: 10.1352/2008.113:439-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Dierker D, Mostafavi I, Schumann CM, Rivera SM, Amaral DG, Van Essen DC. Cortical folding abnormalities in autism revealed by surface-based morphometry. Journal of Neuroscience. 2007;27(43):11725–35. doi: 10.1523/JNEUROSCI.0777-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordahl CW, Lange N, Li DD, Barnett LA, Lee A, Buonocore MH, Amaral DG. Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(50):20195–200. doi: 10.1073/pnas.1107560108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrelgen F, Fernell E, Eriksson M, Hedvall Å, Persson C, Sjölin M, Kjellmer L. Children with autism spectrum disorders who do not develop phrase speech in the preschool years. Autism. 2015;19(8):934–43. doi: 10.1177/1362361314556782. [DOI] [PubMed] [Google Scholar]
- Oram Cardy JE, Flagg EJ, Roberts W, Roberts TPL. Auditory evoked fields predict language ability and impairment in children. International Journal of Psychophysiology. 2008;68(2):170–5. doi: 10.1016/j.ijpsycho.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Heung K, Byrd R, Hansen R, Hertz-Picciotto I. The onset of autism: patterns of symptom emergence in the first years of life. Autism Research. 2008;1(6):320–8. doi: 10.1002/aur.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Iosif A-M, Young GS, Hepburn S, Thompson M, Colombi C, Rogers SJ. Onset patterns in autism: correspondence between home video and parent report. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(8):796–806. doi: 10.1016/j.jaac.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini M, Garaci FG, Bonzano L, Roccatagliata L, Palmieri MG, Pompili E, Emberti Gialloreti L. White matter reduced streamline coherence in young men with autism and mental retardation. European Journal of Neurology. 2009;16(11):1185–90. doi: 10.1111/j.1468-1331.2009.02699.x. [DOI] [PubMed] [Google Scholar]
- Parmeggiani A, Barcia G, Posar A, Raimondi E, Santucci M, Scaduto MC. Epilepsy and EEG paroxysmal abnormalities in autism spectrum disorders. Brain & Development. 2010;32(9):783–9. doi: 10.1016/j.braindev.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Paus T. Growth of white matter in the adolescent brain: myelin or axon? Brain & Cognition. 2010;72(1):26–35. doi: 10.1016/j.bandc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP. Brain mechanisms for interpreting the actions of others from biological-motion cues. Current Directions in Psychological Science. 2006;15(3):136–140. doi: 10.1111/j.0963-7214.2006.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Shultz S, Hudac CM, Vander Wyk BC. Constraining heterogeneity: The social brain and its development in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2011;52(6):631–44. doi: 10.1111/j.1469-7610.2010.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Haist F, Sedaghat F, Courchesne E. The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 2004;127:2703–16. doi: 10.1093/brain/awh289. Pt 12. [DOI] [PubMed] [Google Scholar]
- Piven J, Arndt S, Bailey J, Andreasen N. Regional brain enlargement in autism: a magnetic resonance imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35(4):530–6. doi: 10.1097/00004583-199604000-00020. [DOI] [PubMed] [Google Scholar]
- Quaresima V, Bisconti S, Ferrari M. A brief review on the use of functional near-infrared spectroscopy (fNIRS) for language imaging studies in human newborns and adults. Brain and Language. 2012;121(2):79–89. doi: 10.1016/j.bandl.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Rapin I. Autistic regression and disintegrative disorder: how important the role of epilepsy? Seminars in Pediatric Neurology. 1995;2(4):278–85. doi: 10.1016/s1071-9091(95)80007-7. [DOI] [PubMed] [Google Scholar]
- Rapin I, Dunn MA, Dunn MA, Allen DA, Stevens MC, Fein D. Subtypes of language disorders in school-age children with autism. Developmental Neuropsychology. 2009;34(1):66–84. doi: 10.1080/87565640802564648. [DOI] [PubMed] [Google Scholar]
- Raschle N, Zuk J, Ortiz-Mantilla S, Sliva DD, Franceschi A, Grant PE, Gaab N. Pediatric neuroimaging in early childhood and infancy: challenges and practical guidelines. Annals of the New York Academy of Sciences. 2012;1252:43–50. doi: 10.1111/j.1749-6632.2012.06457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva D, Annunziata S, Contarino V, Erbetta A, Aquino D, Bulgheroni S. Gray matter reduction in the vermis and CRUS-II is associated with social and interaction deficits in low-functioning children with autistic spectrum disorders: a VBM-DARTEL Study. Cerebellum. 2013;12(5):676–85. doi: 10.1007/s12311-013-0469-8. [DOI] [PubMed] [Google Scholar]
- Riva D, Bulgheroni S, Aquino D, Di Salle F, Savoiardo M, Erbetta A. Basal forebrain involvement in low-functioning autistic children: a voxel-based morphometry study. American Journal of Neuroradiology. 2011;32(8):1430–5. doi: 10.3174/ajnr.A2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EB, Neale BM, Hyman SE. Genetic research in autism spectrum disorders. Current Opinion in Pediatrics. 2015;27(6):1. doi: 10.1097/MOP.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ. What are infant siblings teaching us about autism in infancy? Autism Research. 2009;2(3):125–37. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano D, Nicolau M, Quintin E-M, Mazaika PK, Lightbody AA, Cody Hazlett H, Reiss AL. Topological methods reveal high and low functioning neuro-phenotypes within fragile X syndrome. Human Brain Mapping. 2014;35(9):4904–15. doi: 10.1002/hbm.22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose V, Trembath D, Keen D, Paynter J. The proportion of minimally verbal children with autism spectrum disorder in a community-based early intervention programme. Journal of Intellectual Disability Research. 2016;60(5):464–77. doi: 10.1111/jir.12284. [DOI] [PubMed] [Google Scholar]
- Rossi PG, Parmeggiani A, Bach V, Santucci M, Visconti P. EEG features and epilepsy in patients with autism. Brain & Development. 1995;17(3):169–74. doi: 10.1016/0387-7604(95)00019-8. [DOI] [PubMed] [Google Scholar]
- Rutter M, Thapar A. Genetics of autism spectrum disorders. In: Volkmar FR, Rogers SJ, Paul R, Pelphrey KA, editors. Handbook of autism and pervasive developmental disorders. 4th John Wiley & Sons, Inc.; Hoboken, NJ: 2014. pp. 411–423. [Google Scholar]
- Sacco R, Gabriele S, Persico AM. Head circumference and brain size in autism spectrum disorder: A systematic review and meta-analysis. Psychiatry Research. 2015;234(2):239–51. doi: 10.1016/j.pscychresns.2015.08.016. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kümmerer D, Kellmeyer P, Vry M-S, Weiller C. Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(46):18035–40. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Amaral DG. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. Journal of Neuroscience. 2004;24(28):6392–401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JA, Schumann CM, Goodlin-Jones BL, Amaral DG. A comprehensive volumetric analysis of the cerebellum in children and adolescents with autism spectrum disorder. Autism Research. 2009;2(5):246–57. doi: 10.1002/aur.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Abrahams BS, Alvarez-Retuerto AI, Sonnenblick LI, Rudie JD, Ghahremani D, Bookheimer SY. Altered Functional Connectivity in Frontal Lobe Circuits Is Associated with Variation in the Autism Risk Gene CNTNAP2. Science Translational Medicine. 2010;2(56):56ra80–56ra80. doi: 10.1126/scitranslmed.3001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubrata KS, Sinha S, Seshadri SP, Girimaji S, Subbakrishna DK, Srinath S. Childhood autism spectrum disorders with and without epilepsy: clinical implications. Journal of Child Neurology. 2015;30(4):476–82. doi: 10.1177/0883073814540521. [DOI] [PubMed] [Google Scholar]
- Souweidane MM, Kim KH, McDowall R, Ruge MI, Lis E, Krol G, Hirsch J. Brain mapping in sedated infants and young children with passive-functional magnetic resonance imaging. Pediatric Neurosurgery. 1999;30(2):86–92. doi: 10.1159/000028768. [DOI] [PubMed] [Google Scholar]
- Spencer MD, Moorhead TWJ, Lymer GKS, Job DE, Muir WJ, Hoare P, Johnstone EC. Structural correlates of intellectual impairment and autistic features in adolescents. NeuroImage. 2006;33(4):1136–44. doi: 10.1016/j.neuroimage.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Srivastava AK, Schwartz CE. Intellectual disability and autism spectrum disorders: causal genes and molecular mechanisms. Neuroscience and Biobehavioral Reviews. 2014;(46):161–74. doi: 10.1016/j.neubiorev.2014.02.015. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield AC, McIntosh AM, Spencer MD, Philip R, Gaur S, Lawrie SM. Towards a neuroanatomy of autism: A systematic review and meta-analysis of structural magnetic resonance imaging studies. European Psychiatry. 2008;23(4):289–99. doi: 10.1016/j.eurpsy.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Stefanatos GA, editor. Regression in autistic spectrum disorders. Neuropsychology Review. 2008;18(4):305–19. doi: 10.1007/s11065-008-9073-y. [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Kasari C. Minimally verbal school-aged children with autism spectrum disorder: the neglected end of the spectrum. Autism Research. 2013;6(6):468–78. doi: 10.1002/aur.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan GCY, Doke TF, Ashburner J, Wood NW, Frackowiak RSJ. Normal variation in fronto-occipital circuitry and cerebellar structure with an autism-associated polymorphism of CNTNAP2. NeuroImage. 2010;53(3):1030–42. doi: 10.1016/j.neuroimage.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MSC, Davis R, Karmiloff-Smith A, Knowland VCP, Charman T. The over-pruning hypothesis of autism. Developmental Science. 2015 doi: 10.1111/desc.12303. [DOI] [PubMed] [Google Scholar]
- Thomas MSC, Knowland VCP, Karmiloff-Smith A. Mechanisms of developmental regression in autism and the broader phenotype: a neural network modeling approach. Psychological Review. 2011;118(4):637–54. doi: 10.1037/a0025234. [DOI] [PubMed] [Google Scholar]
- Trontel HG, Duffield TC, Bigler ED, Abildskov TJ, Froehlich A, Prigge MBD, Lainhart JE. Mesial temporal lobe and memory function in autism spectrum disorder: an exploration of volumetric findings. Journal of Clinical and Experimental Neuropsychology. 2015;37(2):178–92. doi: 10.1080/13803395.2014.997677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchman R, Hirtz D, Mamounas LA. NINDS epilepsy and autism spectrum disorders workshop report. Neurology. 2013;81(18):1630–6. doi: 10.1212/WNL.0b013e3182a9f482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuchman R, Rapin I. Regression in pervasive developmental disorders: seizures and epileptiform electroencephalogram correlates. Pediatrics. 1997;99(4):560–6. doi: 10.1542/peds.99.4.560. [DOI] [PubMed] [Google Scholar]
- Ünal Ö, Özcan Ö, Özner Ö, Akcakin M, Aysev A, Deda G. EEG and MRI findings and their relation with intellectual disability in pervasive developmental disorders. World Journal of Pediatrics. 2009;5(3):196–200. doi: 10.1007/s12519-009-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valvo G, Baldini S, Retico A, Rossi G, Tancredi R, Ferrari AR, Sicca F. Temporal lobe connects regression and macrocephaly to autism spectrum disorders. European Child & Adolescent Psychiatry. 2016;25(4):421–429. doi: 10.1007/s00787-015-0746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscidi EW, Triche EW, Pescosolido MF, McLean RL, Joseph RM, Spence SJ, Morrow EM. Clinical characteristics of children with autism spectrum disorder and co-occurring epilepsy. PloS One. 2013;8(7):e67797. doi: 10.1371/journal.pone.0067797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers LELM, Gilissen C, Veltman JA. Genetic studies in intellectual disability and related disorders. Nature Reviews Genetics. 2015;17(1):9–18. doi: 10.1038/nrg3999. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Lett TAP, Lerch JP, Tiwari AK, Ameis SH, Rajji TK, Kennedy JL. Neurexin-1 and frontal lobe white matter: an overlapping intermediate phenotype for schizophrenia and autism spectrum disorders. PloS One. 2011;6(6):e20982. doi: 10.1371/journal.pone.0020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan CY, Marchina S, Norton A, Schlaug G. Atypical hemispheric asymmetry in the arcuate fasciculus of completely nonverbal children with autism. Annals of the New York Academy of Sciences. 2012;1252:332–7. doi: 10.1111/j.1749-6632.2012.06446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal A, editor. The Place of Childhood Disintegrative Disorder on the Autism Spectrum. 2013 (Doctoral dissertation). Available from ProQuest Dissertations and Theses database. (UMI No. 3578470) [Google Scholar]
- Woolfenden S, Sarkozy V, Ridley G, Coory M, Williams K. A systematic review of two outcomes in autism spectrum disorder - epilepsy and mortality. Developmental Medicine and Child Neurology. 2012;54(4):306–12. doi: 10.1111/j.1469-8749.2012.04223.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization . The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. World Health Organization; Geneva: 1990. [Google Scholar]
- Yau SH, McArthur G, Badcock NA, Brock J. Case study: auditory brain responses in a minimally verbal child with autism and cerebral palsy. Frontiers in Neuroscience. 2015;9:208. doi: 10.3389/fnins.2015.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeegers M, Pol HH, Durston S, Nederveen H, Schnack H, van Daalen E, Buitelaar J. No differences in MR-based volumetry between 2- and 7-year-old children with autism spectrum disorder and developmental delay. Brain and Development. 2009;31(10):725–730. doi: 10.1016/j.braindev.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Zilbovicius M, Meresse I, Chabane N, Brunelle F, Samson Y, Boddaert N. Autism, the superior temporal sulcus and social perception. Trends in Neurosciences. 2006;29(7):359–66. doi: 10.1016/j.tins.2006.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.