Abstract
Objective
To evaluate the effects of 12-week polestriding intervention on gait and disease severity in people with mild to moderate Parkinson’s disease
Design
A-B-A withdrawal study design
Setting
Outpatient movement disorder center and community facility.
Participants
Individuals (9 females and 8 males; mean age: 63.7 ± 4.9 years; range 53 – 72 years) with mild to moderate PD according to UK brain bank criteria with Hoehn and Yahr (H&Y) score from 2.5 to 3.0 with a stable medication regimen and ability to tolerate ‘off’ medication state.
Intervention
12-week polestriding intervention with 12-week follow-up.
Main Outcome Measures
Gait was evaluated using several quantitative temporal, spatial, and variability measures. In addition, disease severity was assessed using clinical scores such as Unified Parkinson’s Rating Scale (UPDRS), H&Y score, and Parkinson’s Disease Questionnaire-39 (PDQ-39).
Results
Step and stride lengths, gait speed, and step-time variability were improved significantly (p < 0.05) due to 12-week polestriding intervention. Also, the UPDRS motor score, the UPDRS axial score, and the UPDRS subscales on walking and balance improved significantly after the intervention.
Conclusions
Since increased step-time variability and decreased step/stride lengths are associated with PD severity and an increased risk of falls in PD, the observed improvements suggest that regular practice of polestriding may reduce the risk of falls and improve mobility in people with PD.
Keywords: Parkinson’s disease, polestriding, gait, axial symptoms, clinical scores
Individuals with Parkinson’s disease (PD) experience gait impairments that manifest as reduced walking speed, step and stride lengths, and swing phase1,2,3, and increased step-time and stride-time variabilities that correlate well with disease severity and falls4,5. These impairments can lead to decreased independence and associated increases in institutionalization and health care costs6-8. Importantly, pharmacological treatments do not fully remove these impairments9 and their benefits wane over time10,11. Therefore, the need for non-pharmacological interventions in PD has come to the forefront. Polestriding (or ‘polewalking’ or ‘Nordic walking’) is an outdoor noncompetitive fitness activity in which the practitioners perform brisk walking with specially designed poles. It involves walking upright and looking forward with the poles used bilaterally in a movement similar to cross-country skiing. Compared to normal walking, polestriding involves greater activation of arm and trunk muscles to produce larger arm swings and trunk rotation12. Placement of the poles provides additional points of support, thereby increasing stability. Proper polestriding involves deliberate arm swings, which may promote longer steps13 and it provides external cues from the landing of the poles for each step14, which may encourage greater regularity in step/stride times. In non-PD populations, polestriding has been shown to improve maximum oxygen uptake15,16, blood pressure17, and claudication pain18. It is of special interest to people with PD since it provides an increased base of support and is more aerobic than regular walking19.
In PD, polestriding interventions have been shown to improve quality of life20,14, walking speed14 and sit-to-stand transfer21. A study22 involving polestriding training resulted in improvements in many clinical scores on balance, lower limb muscles strength, 6-minute walking test, and Timed Up and Go test during medication-on state. But more detailed gait measures were not obtained. In contrast, a recent study23 that utilized a polestriding intervention did not find any improvements in UPDRS and gait measures during medication-off state when the entire cohort of subjects were considered. The purpose of this study was to investigate the effects of polestriding intervention on several quantitative gait indices calculated from hundreds of strides in the medication-off state. It was hypothesized that a 12-week intervention program of polestriding would increase step/stride lengths, decrease step/stride-time variability measures, and would alleviate disease symptoms in people with mild to moderate PD. Documentation of improvements in these gait measures could provide strong motivation for clinical exercise prescription because these measures are related to disease severity and falls in PD4,5 and do not respond well to pharmacological treatment9-11.
Methods
All study procedures were approved by the appropriate institutional review boards and all subjects voluntarily signed the informed consent form prior to participating in the screening process.
Subjects
Subjects were recruited by placement of flyers in several movement disorder clinics in the Phoenix metropolitan area. Individuals who expressed interest in participating were screened by a movement disorder neurologist (co-author) in an outpatient movement disorders center to determine eligibility for the study. Criteria for inclusion were: (1) the presence of idiopathic PD according to UK brain bank criteria24, (2) age between 50-75 years, (3) disease severity represented by Hoehn and Yahr (H&Y) score from 2.5 to 3 in the medication-on state, (4) stable medication regimen for the 4 weeks prior to the study, and (5) ability to tolerate the medication-off condition. Criteria for exclusion were: (1) presence of dementia as defined by Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV criteria, (2) significant hepatic, renal, cardiovascular, cardiopulmonary, or endocrine issues, (3) significant dyskinesia, (4) significant on/off fluctuation, (5) freezing of gait leading to falls, (6) other medical condition which, in the opinion of the movement disorder neurologist (co-author) and/or the subject’s treating physician would affect subject safety or ability to comply with the study procedures, or (7) recent or current participation in any aerobic exercise program or any exercise program (such as Tai Chi or treadmill training) to specifically improve gait or balance.
Twenty-two persons with PD were screened; 17 were enrolled (Table 1: 9 females and 8 males; mean age: 63.7 ± 4.9 years; range 53 – 72 years). Two were found ineligible (1 due to a cardiac issue; 1 due to an orthopedic issue) and 3 declined to enroll. Subjects were allowed to continue simple exercise routines such as regular walking or stretching over the course of the study.
Table 1.
Demographic and clinical (during baseline evaluation) characteristics of enrolled subjects. The daily levodopa equivalent dosage (LED) at the start and end of the study are also provided. Subject 14 did not complete the study for reasons unrelated to the study.
| S. No. | Age (years) |
Weight (kg) |
Height (cm) |
Gender | H&Y score |
UPDRS-I | UPDRS-II | UPDRS-III | LED (mg/d) Start |
LED (mg/d) End |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | 65.5 | 155 | F | 3 | 1 | 10 | 18 | 300 | 300 |
|
| ||||||||||
| 2 | 64 | 79.5 | 175 | M | 2.5 | 0 | 6 | 18 | 489 | 637 |
|
| ||||||||||
| 3 | 66 | 77.3 | 170 | M | 2.5 | 0 | 7 | 5 | 240 | 740 |
|
| ||||||||||
| 4 | 68 | 90.1 | 180 | M | 3 | 0 | 3 | 21 | 420 | 520 |
|
| ||||||||||
| 5 | 53 | 72.7 | 178 | M | 3 | 1 | 14 | 17 | 676 | 536 |
|
| ||||||||||
| 6 | 66 | 100 | 165 | F | 2.5 | 1 | 9 | 13 | 1000 | 900 |
|
| ||||||||||
| 7 | 66 | 94.5 | 171 | F | 3 | 3 | 9 | 17 | 600 | 800 |
|
| ||||||||||
| 8 | 63 | 88.6 | 175 | M | 3 | 2 | 9 | 23 | 400 | 400 |
|
| ||||||||||
| 9 | 72 | 68.5 | 163 | F | 2.5 | 2 | 8 | 16 | 250 | 250 |
|
| ||||||||||
| 10 | 60 | 66.4 | 173 | F | 2.5 | 0 | 4 | 12 | 375 | 375 |
|
| ||||||||||
| 11 | 60 | 59.73 | 155 | F | 2.5 | 1 | 2 | 9 | 325 | 325 |
|
| ||||||||||
| 12 | 69 | 95 | 175 | M | 3 | 0 | 8 | 19 | 525 | 650 |
|
| ||||||||||
| 13 | 69 | 88.1 | 173 | F | 2.5 | 0 | 2 | 8 | 400 | 400 |
|
| ||||||||||
| 14 | 60 | 107 | 165 | F | 3 | 1 | 5 | 21 | 400 | 199 |
|
| ||||||||||
| 15 | 58 | 86.4 | 170 | M | 3 | 0 | 4 | 9 | 750 | 750 |
|
| ||||||||||
| 16 | 64 | 62.3 | 160 | F | 3 | 2 | 7 | 11 | 75 | 200 |
|
| ||||||||||
| 17 | 58 | 130.1 | 178 | M | 3 | 1 | 12 | 39 | 158 | 132 |
Polestriding protocol
The 12-week polestriding intervention (three 1-hour sessions/week including 10-minute warm up and cool down periods involving stretching exercises) was conducted in an indoor track at a community facility. The polestriding intervention was conducted in two groups (9 subjects, 8 subjects) to facilitate effective supervision during the intervention sessions and completion of the gait and clinical evaluations in a timely manner before and after the intervention. Prior to the intervention, each subject attended a 2-hour class that included watching an instructional video provided by Exerstridera. After the video session, each subject was given telescopic Exerstridera poles adjusted such that their elbows were bent at about 90° when the poles were held at the grips and were placed vertically with the tips on the floor. Next, they were asked to practice for about an hour under the supervision of a recreation therapist to ensure that they learned proper polestriding technique. The duration of the practice session was selected based on studies that provided practice from one-half to two hours12,22,25,26. The instructional video CD and printed manual were given to each subject for optional home-viewing, but poles were not provided to ensure that they practiced polestriding only during the intervention sessions.
The polestriding intervention sessions were conducted at the same time of the day in the morning during subjects’ medication-on state to maximize motor capabilities. All sessions were conducted by a recreational therapist with extensive experience in teaching exercise classes in people with PD including polestriding. During each intervention session, subjects were asked to wear a pedometer (Omron HJ-720ITC)c and a heart rate monitor (Polar FT4)d to measure their average and maximum heart rates, calories burned, number of steps, and distance walked. For each subject, the step length obtained during the pre-intervention evaluation was used to program the pedometer for calculation of walking distance. During each intervention session, subjects were asked to polestride at a brisk pace for 40 minutes. For subjects who were unable to polestride continuously for 40 minutes, the protocol allowed for short breaks (up to 2 minutes) and included a plan to increase the exercise duration progressively as tolerated for each subject by up to 5 minutes per 2-week period until the subject was able to complete the full 40-minute session.
Gait and Clinical Evaluations
Each subject was evaluated in the same outpatient movement disorder center at four time points, each separated by 12-week intervals: (1) baseline evaluation, (2) pre-intervention evaluation, (3) post-intervention evaluation, and (4) follow-up evaluation. During the 12 weeks between baseline and pre-intervention evaluations and during the 12 weeks between the post-intervention evaluation and the follow-up evaluation, the subjects were asked not to participate in any aerobic exercise program or any exercise program to specifically improve gait or balance, including polestriding.
Quantitative gait measures were obtained using IDEEA (MiniSun, CA)b, a portable gait system that can provide reliable gait parameters27,28. Sensors approximately the size of a nickel (~18mm × 15mm × 3mm) and the weight of a penny (~ 2 grams) were attached to the sternum, the anterior surface of each thigh, and the sole of each foot; the sensors were connected with thin wires to the lightweight processor unit that was worn at the waist. This setup did not noticeably affect subjects’ walking patterns and the data were downloaded at the end of the gait trials. During each evaluation session, the subject walked overground at their comfortable speed for 2 trials of 160 meters each in a straight corridor, with a 180° turn at every 40 meters. In addition to the gait evaluation, at each evaluation time point each subject’s resting state heart rate was obtained while supine and his/her maximum heart rate was obtained during a treadmill-based stress test following the Balke-Ware protocol29. Moreover, disease severity was evaluated by a movement disorder neurologist (co-author) using the Unified Parkinson’s Disease Rating Scale (UPDRS), the H&Y scale, and the Parkinson’s Disease Questionnaire-39 (PDQ-39). The neurologist did not observe any of the polestriding intervention sessions and did not have access to prior evaluation scores during subsequent evaluation sessions. All gait and clinical evaluations including the stress test were performed in the subjects’ medication-off state (at least 12 hours after the last usual dosage of antiparkinson medication) and at the same time of the day.
Data Analysis
Data from the gait trials during the evaluation sessions were used to calculate several quantitative gait indices. Only steady-state walking segments were considered by excluding 6 steps immediately after gait initiation, before gait termination, and before and after the turns. Step length, stride length, gait speed, cadence, single leg support duration, double leg support duration, and swing power (defined as the maximum deceleration during mid and terminal swing phases30) were calculated. Also, coefficients of variation (CV) of step time and stride time were obtained to characterize variability. All gait indices were calculated using about 500 steps from gait trials during each session to improve reliability31.
For each intervention session, the total distance walked was calculated using the number of steps recorded on the pedometer during that session and the step length that was measured during the pre-intervention session. Exercise intensity was calculated according to the formula of Karvonen32,33 using the values of resting heart rate (obtained while supine) and maximum heart rate (obtained during the treadmill-based stress test).
The effects of the polestriding intervention on axial symptoms were evaluated by calculating the sum of the UPDRS-Part II subscores for speech (item 5), swallowing (7), turning in bed (12), falling (13), freezing (14), walking (15) and of the UPDRS-Part III subscores for speech (18), neck rigidity (22), rising from a chair (27), posture (28), gait (29), and postural instability (30)34. In addition, the effects of the polestriding intervention on gait and balance (termed as UPDRS– GB) were assessed by calculating the sum of the scores of the UPDRS-Part II items for falling (13), freezing (14), walking (15) and the UPDRS-Part III items for rising from a chair (27), posture (28), gait (29), and postural instability (30).
A repeated measures ANOVA (SPSS, IBM) was performed for each measure on the data obtained at baseline, pre-intervention, post-intervention, and follow-up evaluations. Following the omnibus test, post-hoc pairwise comparisons were performed with the Bonferroni correction (for 6 pairwise comparisons); changes were considered significant between two conditions at p < 0.05.
Results
Sixteen of 17 subjects successfully completed the protocol; 1 subject could not complete the protocol for reasons unrelated to the study. There were no adverse effects reported. Of the subjects who completed the protocol, subjects completed 32 ± 4 sessions out of a total of 36 sessions (Table 2) and all the subjects except one (subject #4) completed more than 75% of the sessions. Across the intervention sessions, subjects expended 178 ± 44 calories/session with a heart rate of 112 ± 13 beats/minute and maximum heart rate of 136 ± 15 beats/minute. Exercise intensity values calculated for each subject across all of the sessions ranged from 20% to 90% with most values in the range from 40% to 70% and no consistent increasing/decreasing trends across sessions were observed. Subjects took an average 5194 ± 899 steps/session and covered a distance of 2 ± 0.6 miles/session (Table 2). Although participation in a minimum of 27 sessions (75% of the sessions) was specified for inclusion in the statistical analysis, the data from subject #4 (who attended only 19 of the 36 intervention sessions) were included in the analyses presented here using the intention-to-treat approach. However, inclusion/exclusion of the data from subject #4 did not change the significance of the results. All subjects were able to complete 40 minutes of polestriding in each session attended, but three subjects took a couple of short breaks (< 2 minutes) during the first few intervention sessions.
Table 2.
Subjects’ performances during 12-week polestriding intervention
| S. No. | Number of Training Sessions Attended |
Energy Expenditure (calories) Mean (Std) |
Mean Heart Rate (beats/min) Mean (Std) |
Peak Heart Rate (beats/min) Mean (Std) |
Number of Steps Walked Mean (Std) |
Distance Walked (kilometer) Mean (Std) |
|---|---|---|---|---|---|---|
| 1 | 35 | 162 (9) | 141 (6) | 160 (18) | 6661 (407) | 3.70 (0.2) |
|
| ||||||
| 2 | 31 | 208 (30) | 97 (6) | 117 (21) | 5588 (754) | 3.96 (0.5) |
|
| ||||||
| 3 | 34 | 186 (13) | 95 (5) | 116 (32) | 5666 (310) | 3.46 (0.2) |
|
| ||||||
| 4 | 19 | 141 (28) | 113 (5) | 123 (5) | 4229 (911) | 2.37 (0.5) |
|
| ||||||
| 5 | 32 | 159 (6) | 104 (19) | 134 (39) | 5186 (230) | 3.32 (0.3) |
|
| ||||||
| 6 | 29 | 164 (37) | 121 (14) | 144 (26) | 4499 (1110) | 2.24 (0.5) |
|
| ||||||
| 7 | 32 | 104 (20) | 122 (4) | 143 (18) | 3574 (756) | 1.35 (0.2) |
|
| ||||||
| 8 | 36 | 275 (23) | 117 (7) | 133 (16) | 6305 (539) | 4.62 (0.5) |
|
| ||||||
| 9 | 36 | 168 (13) | 105 (9) | 152 (41) | 6570 (461) | 3.67 (0.3) |
|
| ||||||
| 10 | 32 | 266 (24) | 102 (9) | 121 (27) | 5666 (267) | 5.18 (0.3) |
|
| ||||||
| 11 | 33 | 146 (25) | 105 (10) | 166 (47) | 5423 (934) | 3.70 (0.6) |
|
| ||||||
| 12 | 34 | 146 (18) | 105 (7) | 126 (25) | 4289 (680) | 2.32 (0.5) |
|
| ||||||
| 13 | 34 | 160 (6) | 121 (6) | 142 (23) | 4870 (225) | 2.72 (0.2) |
|
| ||||||
| 14 | 30 | 191 (20) | 124 (5) | 139 (9) | 5611 (609) | 3.35 (0.5) |
|
| ||||||
| 15 | 33 | 162 (36) | 124 (15) | 146 (19) | 5411 (275) | 3.85 (0.2) |
|
| ||||||
| 16 | 36 | 210 (17) | 93 (5) | 124 (37) | 3848 (323) | 2.24 (0.2) |
|
| ||||||
| Mean (Std) | 32 (4) | 178 (44) | 112 (13) | 136 (15) | 5194 (899) | 3.22 (1.0) |
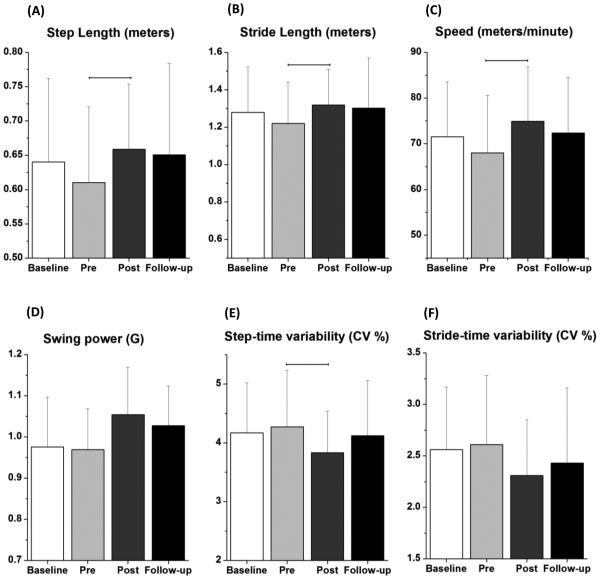
The repeated measures ANOVA with post-hoc pairwise comparisons using the Bonferroni correction demonstrated improvements due to the polestriding intervention as indicated by significant differences between the pre-intervention and post-intervention values for step length (p = 0.008), stride length (p = 0.008), gait speed (p = 0.023) and step time variability (p = 0.049) (Figure 1, Table 3). None of these variables showed a statistically significant difference between the baseline and pre-intervention values and the associated effect sizes35 were opposite in sign and much smaller in magnitude than the effect sizes of the pre-intervention to post-intervention comparisons (Table 4). These results indicate that the effects of the polestriding intervention were statistically significant and that they more than offset any changes that occurred due to detraining between the baseline and pre-intervention time points.
Figure 1.
The changes in (A) step length, (B) stride length, (C) speed, (D) swing power, and variability in (E) step-time and (F) stride-time due to polestriding training averaged across 16 subjects (± 1 std. dev.) with mild to moderate Parkinson’s disease are shown. The variability in step-time and stride-time are provided in terms of coefficient of variation in percentage. Values are plotted for measurements at each of 4 time points: baseline, pre-intervention, post-intervention, and follow-up. The horizontal lines connecting different conditions denote that the improvements in corresponding gait indices were statistically significant between those conditions at p < 0.05 after Bonferroni correction for pairwise comparisons.
Table 3.
Subjects’ gait characteristics at different evaluation time points and results of statistical analyses. CV: coefficient of variation; g = 9.8 m/s2.
| Clinical scores | Clinical scores: mean (standard deviation) | Omnibus F value; p value |
Pairwise comparison (p < 0.05) |
|||
|---|---|---|---|---|---|---|
|
|
|
|||||
| baseline | pre- intervention |
post- intervention |
follow-up | pre vs. post | ||
| Step length (meters) | 0.64 (0.12) | 0.61 (0.11) | 0.66 (0.10) | 0.65 (0.13) | 4.24; 0.01 | p = 0.008 |
|
| ||||||
| Stride length (meters) | 1.28 (0.24) | 1.22 (0.22) | 1.32 (0.19) | 1.30 (0.27) | 4.34; 0.009 | p = 0.008 |
|
| ||||||
| Speed (meters/min) | 71.5 (12.1) | 68.0 (12.5) | 74.9 (11.9) | 72.4 (12.1) | 4.32; 0.009 | p = 0.023 |
|
| ||||||
| Cadence (steps/min) | 113.2 (7.8) | 112.3 (8.2) | 114.5 (9.6) | 113.7 (7.4) | 0.90; 0.44 | |
|
| ||||||
| Single leg support (% gait cycle) | 37.12 (2.16) | 36.78 (2.42) | 37.32 (1.84) | 36.61 (2.47) | 1.64; 0.19 | |
|
| ||||||
| Double leg support (% gait cycle) | 12.81 (2.29) | 13.07 (2.42) | 12.72 (1.85) | 13.06 (2.02) | 0.78; 0.51 | |
|
| ||||||
| Swing Power (g) | 0.98 (0.12) | 0.97 (0.10) | 1.05 (1.1) | 1.02 (0.97) | 3.65; 0.019 | |
|
| ||||||
| Step-time variability (CV in %) | 4.17 (0.85) | 4.27 (0.96) | 3.83 (0.71) | 4.12 (0.94) | 3.31; 0.02 | p = 0.049 |
|
| ||||||
| Stride-time variability (CV in %) | 2.56 (0.61) | 2.61 (0.67) | 2.31 (0.54) | 2.43 (0.73) | 2.51; 0.07 | |
Table 4.
The effect sizes for several gait indices are listed for pairwise comparisons between different time points. A negative effect size indicates that the measure was lower at the later time point; a positive value indicates that it was higher at the later time point.
| Gait indices | Baseline vs. Pre |
Baseline vs. Post |
Pre vs. Post | Post vs. Follow-up |
|---|---|---|---|---|
| Step length | −0.25 | 0.16 | 0.45 | −0.05 |
|
| ||||
| Stride length | −0.25 | 0.16 | 0.46 | −0.05 |
|
| ||||
| Speed | −0.28 | 0.28 | 0.56 | −0.21 |
|
| ||||
| Cadence | −0.12 | 0.15 | 0.24 | −0.09 |
|
| ||||
| Single leg support | −0.14 | 0.10 | 0.22 | −0.30 |
|
| ||||
| Double leg support | 0.11 | −0.05 | −0.15 | 0.17 |
|
| ||||
| Swing Power | −0.06 | 0.67 | 0.79 | −0.25 |
|
| ||||
| Step-time variability CV | 0.03 | −0.34 | −0.40 | 0.25 |
|
| ||||
| Stride-time variability CV | 0.08 | −0.42 | −0.47 | 0.18 |
The polestriding intervention also decreased disease severity as indicated (Table 5) by significant reductions in H&Y score (p < 0.001), UPDRS motor score (p = 0.028), UPDRS-axial score (p = 0.045), and UPDRS-GB score (p = 0.022). The significant reductions were sustained at follow-up for the H&Y score (p = 0.009) and UPDRS-GB score (p = 0.034). There were no significant changes in the UPDRS – Part I, Part II, total UPDRS or in the PDQ-39 total score or its sub-components.
Table 5.
Clinical characteristics and results of statistical analyses.
| Clinical scores |
Clinical scores: mean (std. dev.) |
Omnibus F value; p value |
Pairwise comparisons (p < 0.05) |
|||||
|---|---|---|---|---|---|---|---|---|
| baseline | pre- intervention |
post- intervention |
follow-up | baseline vs. post |
pre vs. post |
pre vs. follow-up |
||
| UPDRS – I | 0.9 (1.0) | 0.5 (0.6) | 0.5 (1.36) | 0.8 (1.0) | 0.72; 0.55 | |||
|
| ||||||||
| UPDRS – II | 7.1 (3.6) | 7.1 (3.8) | 6.9 (3.2) | 6.6 (3.7) | 1.14; 0.79 | |||
|
| ||||||||
| UPDRS – III | 15.9 (8.0) | 14.5 (7.4) | 11.8 (9.6) | 11.9 (8.2) | 5.92; 0.01 | p=0.002 | p = 0.028 | |
|
| ||||||||
| UPDRS Total | 23.9 (10.6) | 22.1 (9.9) | 19.1 (12.1) | 19.1 (11.8) | 3.66; 0.06 | p=0.002 | ||
|
| ||||||||
| UPDRS Axial | 6.4 (3.8) | 6.0 (4.1) | 4.6 (3.4) | 5.2 (4.0) | 8.06; < 0.001 | p = 0.045 | ||
|
| ||||||||
| UPDRS – GB | 4.4 (2.3) | 3.9 (2.5) | 2.5 (2) | 2.9 (2.2) | 10.44; < 0.001 | p = 0.022 | p = 0.034 | |
|
| ||||||||
| H&Y | 2.8 (0.3) | 2.8 (0.3) | 2.3 (0.5) | 2.4 (0.4) | 19.28; < 0.001 | p=0.001 | p < 0.001 | p = 0.009 |
|
| ||||||||
| PDQ-39 | 36.8 (23.7) | 30.0 (18.7) | 32.6 (19.8) | 25.1 (13.0) | 2.76; 0.05 | |||
Discussion
This study documented the effects of a polestriding intervention in 17 subjects with mild to moderate PD. Fifteen of the 16 subjects who completed the study displayed excellent compliance with the intervention and evaluation procedures for the entire 9-month duration. The high compliance may be due to comfort with the activity, perceived benefits of polestriding and/or social interactions that the subjects received through group intervention.
The polestriding intervention significantly improved many indices of gait that are specifically affected in PD. People with PD walk with reduced step length, stride length and speed36-38 and polestriding significantly increased these indices (by 9%, 9% and 11%, respectively when comparing post-intervention with pre-intervention values). The increase in gait speed was 11.5 cm/sec, which is greater than the 10 cm/sec required to be considered as a meaningful change in older adults with mild to moderate mobility deficits39. This increase can be attributed to the increase in stride length, since there was no significant change in cadence. Gait rhythmicity is also affected in people with PD as observed by increases in step-to-step time and stride-to-stride time variabilities4,5,40. The polestriding intervention improved gait rhythmicity as indicated by reductions in step time variability (8.9%) and stride time variability (11.7%). The increases in these variability measures have been associated with disease severity and increased risk of falls in PD4,5,41. Therefore, the improvement in these indices due to polestriding suggests that this intervention may reduce fall risk, which would have a strong clinical impact on this population.
Regarding disease severity, polestriding significantly reduced H&Y (by 18%), reduced UPDRS motor score (by 25%), and UPDRS-GB (by 36%), thus indicating its clinical impact. This is supported by the observation that 7 subjects did not require any changes in their antiparkinsonian medication for the 9-month study period and 4 subjects reduced their medication across the study period. There were no significant changes in subscores of UPDRS motor score such as tremor and rigidity and therefore significant improvement in UPDRS motor score might be due to significant improvements in the UPDRS axial score. A decrease in the UPDRS total score of 3.5 or greater has been suggested to indicate clinically important differences due to medical treatment in early PD42. In this study, a significant mean decrease of 3 in UPDRS total score was observed due to the intervention. Importantly, the improvements in H&Y score and UPDRS-GB score were sustained at the follow-up evaluation (Table 5).
The subjects were asked to refrain from participating in any exercise program that could improve aerobic capacity, gait, or balance, during the first 12 weeks of the study (between the baseline and pre-intervention evaluations). This detraining period, which was intended to remove the effects of any on-going regular exercise prior to administering the intervention, may have had a detrimental effect on some of the gait and clinical indices that were measured at the pre-intervention time point. However, none of the gait or clinical indices changed significantly due to the detraining period and the effect size for each of the measures that changed significantly due to the intervention was much larger because of the intervention than because of the detraining (Table 4). This indicates that the positive effects of the intervention were much stronger than any negative effects of withdrawing regular participation in other exercise programs.
Prior studies that investigated the effects of polestriding in PD have demonstrated improvements in quality of life14,20, UPDRS total score20 and motor score22, H&Y score22, objective balance and gait parameters22,13 including stride length, sit-to-stand performance21, lower limb muscle strength22, and non-motor symptoms22. As in some of these studies, the current study also improved UPDRS motor score, H&Y, and stride length. There was high variability across these prior studies with respect to number of training sessions (from 12 to 78 sessions), medication state during evaluation (medication-on state22, medication-off state21,22, no information on medication state13,14,20), and the design of the intervention (only polestriding14,20,21,22; polestriding in combination with another form of exercise13). Unlike our study, only one of these studies conducted a follow-up evaluation but it was performed only in a subset of the subjects14. In contrast to these prior studies, two studies involving polestriding in PD did not find any significant improvements in UPDRS or gait23,43. One of these studies23 was similar to the study reported here with respect to the number and frequency of polestriding intervention sessions and evaluation at medication-off state. The fact that they did not observe improvements in the UPDRS or gait may have been due to the decision to perform one of the weekly sessions without the supervision of a trainer. Furthermore, the selection of the gait evaluation task (a short, 6-meter walking trial) might have contributed to lack of observed improvements in gait. The other study43, which compared polestriding with BIG training, did not observe improvements in UPDRS motor score, gait, or quality of life due to polestriding. This may have been due to the lower frequency and total number of polestriding training sessions (2 sessions per week for 8 weeks). Unlike the current study, none of the studies discussed above examined the effects of the polestriding intervention on step-time or stride-time variability, which are related to PD severity and falls4,5. The differences in results between the prior reports that did not find improvements due to polestriding and the work presented here underscore the value of utilizing more detailed and quantitative gait measures and suggest that a successful intervention may require 3 or more sessions per week or a longer training period with a trainer or with some other means of ensuring sufficient intensity throughout each session.
Study Limitations
Although the intervention sessions were performed during the medication-on state, the evaluations were carried out during the medication-off state in order to minimize the confounding effects of medication that may vary during an experimental session and to compare the results across different time points, since some subjects reduced their medication over the course of the study. However, this limited our ability to investigate the synergistic effects of polestriding and medication. The clinical tests that might have indicated improvements in balance such as Timed Up and Go test and Berg Balance Test were not included in the protocol. Therefore, we extracted axial measures from UPDRS to investigate the effects of polestriding on balance and gait. The increased base of support offered by poles and practice of proper polestriding might have facilitated taking steps with greater step length and arm swing, which was not measured.
The total distance walked during each intervention session was calculated using the number of steps recorded during that session and the step length that was recorded in the pre-intervention session. Any improvement (increase) in step length over the course of the intervention would have introduced errors into this calculation. Based on the magnitude of the increase in step length between the post- and pre-intervention time points, the error in distance walked due to the use of the smaller step length was about 9% across the subjects. Such errors would underestimate the total distance walked in each session and the errors might have been the largest during the final weeks of the intervention. The IDEEA sensor system, which was used to calculate the spatiotemporal gait measures during the evaluation session, has been demonstrated to provide reliable measurements27,28. Although, there have been reports of poor reliability 44-46, each of these studies with poor reliability either included subjects with a high degree of asymmetry or subjects that used a walking aid, neither of which was true for the subjects in this study. Moreover, the values of spatiotemporal gait indices obtained in this study are comparable to those reported for people with PD in other studies4,47-49.
In this study, the fact that the subjects and the clinical rater were not blinded could have influenced the impressions of the subjects or the clinical ratings. However, the objective and quantitative gait scores provide strong evidence of improvements and these were highly consistent with the recorded clinical scores. A follow-up to this study should use a randomized controlled trial with blinded clinical assessments in order to compare polestriding to one or more exercise alternatives and to more thoroughly characterize the relationship between the quantitative measures and the clinical ratings.
Conclusions
In this study, most subjects found polestriding to be easy to learn and they liked performing it on a regular basis. Results indicated that the 12-week intervention produced significant improvement in a number of quantitative gait measures and some important clinical measures of disease severity. The clinical impact of the documented improvements in step/stride lengths and step-time variability measures may be very high because these gait indices have been shown to be strongly related to disease severity and falls in the PD population. Although this study included only subjects with mild to moderate PD, it seems likely that polestriding may also provide benefits to subjects with higher levels of disease severity and more pronounced deficits in posture and gait.
Highlights.
Gait impairments in Parkinson’s disease severely affect quality of life
Traditional treatments do not fully alleviate gait difficulties
Increased gait variability and decreased step length are risk factors for falls
Polestriding improved gait rhythmicity and step/stride amplitude
Regular polestriding may reduce the risk of falls in Parkinson’s disease
Acknowledgments
Funding Sources:
The study was supported by a research grant from National Institute of Child Health and Human Development, National Institutes of Health (1R21HD060315).
Abbreviations
- ANOVA
Analysis of Variance
- CV
Coefficient of variation
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders – IV
- BG
Gait and balance
- H&Y
Hoehn & Yahr
- PD
Parkinson’s disease
- PDQ-39
Parkinson’s Disease Questionnaire-39
- UPDRS
Unified Parkinson’s Disease Rating Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Places where study was conducted:
Center for Adaptive Neural Systems, Arizona State University, Tempe, AZ 85281 Banner Sun Health Research Institute, Sun City, AZ 85351
Financial Disclosure:
The authors have no conflicts of interest/financial disclosures to report
List of Suppliers:
Exerstrider Products Inc.
MiniSun Inc.
Omron Healthcare Inc.
Polar Electro Inc.
Names and mailing address of each supplier:
Exerstrider Products Inc., P. O. Box 6714, Madison, WI 53713-6714
MiniSun LLC, 935 E MillCreek Drive, Fresno, CA 93720
Omron Healthcare Inc., 1200 Lakeside Drive, Bannockburn, Illinois 60015
Polar Electro Inc., 1111 Marcus Avenue, Lake Success, NY 11042-1034
References
- 1.Knutsson E. An analysis of Parkinsonian gait. Brain. 1972;95(3):475–486. doi: 10.1093/brain/95.3.475. [DOI] [PubMed] [Google Scholar]
- 2.Murray MP, Sepic SB, Gardner GM, Downs WJ. Walking patterns of men with parkinsonism. Am J Phys Med. 1978;57(6):278–294. [PubMed] [Google Scholar]
- 3.Stern GM, Franklyn SE, Imms FJ, Prestidge SP. Quantitative assessments of gait and mobility in Parkinson's disease. J Neural Transm Suppl. 1983;19:201–214. [PubMed] [Google Scholar]
- 4.Hausdorff JM, Cudkowicz ME, Firtion R, Wei JY, Goldberger AL. Gait variability and basal ganglia disorders: stride-to-stride variations of gait cycle timing in Parkinson's disease and Huntington's disease. Mov Disord. 1998;13(3):428–437. doi: 10.1002/mds.870130310. [DOI] [PubMed] [Google Scholar]
- 5.Schaafsma JD, Giladi N, Balash Y, Bartels AL, Gurevich T, Hausdorff JM. Gait dynamics in Parkinson's disease: relationship to Parkinsonian features, falls and response to levodopa. J Neurol Sci. 2003;212(1-2):47–53. doi: 10.1016/s0022-510x(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 6.Ashburn A, Stack E, Pickering RM, Ward CD. A community-dwelling sample of people with Parkinson's disease: characteristics of fallers and non-fallers. Age Ageing. 2001;30(1):47–52. doi: 10.1093/ageing/30.1.47. [DOI] [PubMed] [Google Scholar]
- 7.Gray P, Hildebrand K. Fall risk factors in Parkinson's disease. J Neurosci Nurs. 2000;32(4):222–228. doi: 10.1097/01376517-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Koller WC, Glatt S, Vetere-Overfield B, Hassanein R. Falls and Parkinson's disease. Clin Neuropharmacol. 1989;12(2):98–105. doi: 10.1097/00002826-198904000-00003. [DOI] [PubMed] [Google Scholar]
- 9.McNeely ME, Duncan RP, Earhart GM. Medication improves balance and complex gait performance in Parkinson disease. Gait Posture. 2012;36(1):144–148. doi: 10.1016/j.gaitpost.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olanow CW, Agid Y, Mizuno Y, et al. Levodopa in the treatment of Parkinson's disease: Current controversies. Mov Disord. 2004;19(9):997. doi: 10.1002/mds.20243. [DOI] [PubMed] [Google Scholar]
- 11.Kostic V, Przedborski S, Flaster E, Sternic N. Early development of levodopa-induced dyskinesias and response fluctuations in young-onset Parkinson's disease. Neurology. 1991;41(2):202–205. doi: 10.1212/wnl.41.2_part_1.202. Pt 1. [DOI] [PubMed] [Google Scholar]
- 12.Shim JM, Kwon HY, Kim HR, Kim BI, Jung JH. Comparison of the Effects of Walking with and without Nordic Pole on Upper Extremity and Lower Extremity Muscle Activation. J Phys Ther Sci. 2013;25(12):1553–1556. doi: 10.1589/jpts.25.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reuter I, Mehnert S, Leone P, Kaps M, Oechsner M, Engelhardt M. Effects of a flexibility and relaxation programme, walking, and nordic walking on Parkinson's disease. Journal of aging research. 2011;2011:232473. doi: 10.4061/2011/232473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Eikeren FJM, Reijmers RSJ, Kleinveld HJ, Minten A, Ter Bruggen JP, Bloem BR. Nordic walking improves mobility in Parkinson's disease. Parkinsonism & Related Disorders. 2008;14(Supplement 1):S67. doi: 10.1002/mds.22293. [DOI] [PubMed] [Google Scholar]
- 15.Figard-Fabre H, Fabre N, Leonardi A, Schena F. Physiological and perceptual responses to Nordic walking in obese middle-aged women in comparison with the normal walk. Eur J Appl Physiol. 2010;108(6):1141–1151. doi: 10.1007/s00421-009-1315-z. [DOI] [PubMed] [Google Scholar]
- 16.Figard-Fabre H, Fabre N, Leonardi A, Schena F. Efficacy of Nordic walking in obesity management. Int J Sports Med. 2011;32(6):407–414. doi: 10.1055/s-0030-1268461. [DOI] [PubMed] [Google Scholar]
- 17.Collins EG, Langbein WE, Orebaugh C, et al. Cardiovascular training effect associated with polestriding exercise in patients with peripheral arterial disease. J Cardiovasc Nurs. 2005;20(3):177–185. doi: 10.1097/00005082-200505000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Langbein WE, Collins EG, Orebaugh C, et al. Increasing exercise tolerance of persons limited by claudication pain using polestriding. J Vasc Surg. 2002;35(5):887–893. doi: 10.1067/mva.2002.123756. [DOI] [PubMed] [Google Scholar]
- 19.Schiffer T, Knicker A, Hoffman U, Harwig B, Hollmann W, Struder HK. Physiological responses to nordic walking, walking and jogging. Eur J Appl Physiol. 2006;98(1):56–61. doi: 10.1007/s00421-006-0242-5. [DOI] [PubMed] [Google Scholar]
- 20.Baatile J, Langbein WE, Weaver F, Maloney C, Jost MB. Effect of exercise on perceived quality of life of individuals with Parkinson's disease. J Rehabil Res Dev. 2000;37(5):529–534. [PubMed] [Google Scholar]
- 21.Fritz B, Rombach S, Godau J, Berg D, Horstmann T, Grau S. The influence of Nordic Walking training on sit-to-stand transfer in Parkinson patients. Gait Posture. 2011;34(2):234–238. doi: 10.1016/j.gaitpost.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Cugusi L, Solla P, Serpe R, et al. Effects of a Nordic Walking program on motor and non-motor symptoms, functional performance and body composition in patients with Parkinson's disease. NeuroRehabilitation. 2015;37(2):245–254. doi: 10.3233/NRE-151257. [DOI] [PubMed] [Google Scholar]
- 23.Herfurth M, Godau J, Kattner B, et al. Gait velocity and step length at baseline predict outcome of Nordic walking training in patients with Parkinson's disease. Parkinsonism Relat Disord. 2015;21(4):413–416. doi: 10.1016/j.parkreldis.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breyer MK, Breyer-Kohansal R, Funk GC, et al. Nordic walking improves daily physical activities in COPD: a randomised controlled trial. Respir Res. 2010;11:112. doi: 10.1186/1465-9921-11-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oakley C, Zwierska I, Tew G, Beard JD, Saxton JM. Nordic poles immediately improve walking distance in patients with intermittent claudication. Eur J Vasc Endovasc Surg. 2008;36(6):689–694. doi: 10.1016/j.ejvs.2008.06.036. discussion 695-686. [DOI] [PubMed] [Google Scholar]
- 27.Gardner MJ, Barker JU, Briggs SM, et al. An evaluation of accuracy and repeatability of a novel gait analysis device. Arch Orthop Trauma Surg. 2007;127(3):223–227. doi: 10.1007/s00402-006-0279-2. [DOI] [PubMed] [Google Scholar]
- 28.Gorelick ML, Bizzini M, Maffiuletti NA, Munzinger JP, Munzinger U. Test-retest reliability of the IDEEA system in the quantification of step parameters during walking and stair climbing. Clin Physiol Funct Imaging. 2009 doi: 10.1111/j.1475-097X.2009.00864.x. [DOI] [PubMed] [Google Scholar]
- 29.Ashley EA, Myers J, Froelicher V. Exercise testing in clinical medicine. Lancet. 3562000:1592–1597. doi: 10.1016/S0140-6736(00)03138-X. [DOI] [PubMed] [Google Scholar]
- 30.Zhang K, Gorjian A, Lester DK. Gait change after local anesthetic of chronically arthritic knee. J Long Term Eff Med Implants. 2006;16(3):223–234. doi: 10.1615/jlongtermeffmedimplants.v16.i3.30. [DOI] [PubMed] [Google Scholar]
- 31.Galna B, Lord S, Rochester L. Is gait variability reliable in older adults and Parkinson's disease? Towards an optimal testing protocol. Gait Posture. 2013;37(4):580–585. doi: 10.1016/j.gaitpost.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 32.Pollock ML, Graves JE, Swart DL, Lowenthal DT. Exercise training and prescription for the elderly. South Med J. 1994;87(5):S88–95. [PubMed] [Google Scholar]
- 33.Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35(3):307–315. [PubMed] [Google Scholar]
- 34.Bejjani BP, Gervais D, Arnulf I, et al. Axial parkinsonian symptoms can be improved: the role of levodopa and bilateral subthalamic stimulation. J Neurol Neurosurg Psychiatry. 2000;68(5):595–600. doi: 10.1136/jnnp.68.5.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunlap WP, Cortina JM, Vaslow JB, Burke MJ. Meta-analysis of experiments with matched groups or repeated measures design. Psychological Methods. 1996;1(2):170–177. [Google Scholar]
- 36.Morris ME, Iansek R, Matyas TA, Summers JJ. Stride length regulation in Parkinson's disease. Normalization strategies and underlying mechanisms. Brain. 1996;119:551–568. doi: 10.1093/brain/119.2.551. Pt 2. [DOI] [PubMed] [Google Scholar]
- 37.Rogers MW. Disorders of posture, balance, and gait in Parkinson's disease. Clin Geriatr Med. 1996;12(4):825–845. [PubMed] [Google Scholar]
- 38.Stolze H, Kuhtz-Buschbeck JP, Drucke H, Johnk K, Illert M, Deuschl G. Comparative analysis of the gait disorder of normal pressure hydrocephalus and Parkinson's disease. J Neurol Neurosurg Psychiatry. 2001;70(3):289–297. doi: 10.1136/jnnp.70.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 40.Cole MH, Silburn PA, Wood JM, Worringham CJ, Kerr GK. Falls in Parkinson's disease: kinematic evidence for impaired head and trunk control. Mov Disord. 2010;25(14):2369–2378. doi: 10.1002/mds.23292. [DOI] [PubMed] [Google Scholar]
- 41.Smulders K, Esselink RA, Weiss A, Kessels RP, Geurts AC, Bloem BR. Assessment of dual tasking has no clinical value for fall prediction in Parkinson's disease. J Neurol. 2012;259(9):1840–1847. doi: 10.1007/s00415-012-6419-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hauser RA, Auinger P. Determination of minimal clinically important change in early and advanced Parkinson's disease. Mov Disord. 2011;26(5):813–818. doi: 10.1002/mds.23638. [DOI] [PubMed] [Google Scholar]
- 43.Ebersbach G, Ebersbach A, Edler D, et al. Comparing exercise in Parkinson's disease--the Berlin LSVT(R)BIG study. Mov Disord. 2010;25(12):1902–1908. doi: 10.1002/mds.23212. [DOI] [PubMed] [Google Scholar]
- 44.Item-Glatthorn JF, Casartelli NC, Petrich-Munzinger J, Munzinger UK, Maffiuletti NA. Validity of the intelligent device for energy expenditure and activity accelerometry system for quantitative gait analysis in patients with hip osteoarthritis. Arch Phys Med Rehabil. 2012;93(11):2090–2093. doi: 10.1016/j.apmr.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 45.Backhouse MR, Hensor EM, White D, Keenan AM, Helliwell PS, Redmond AC. Concurrent validation of activity monitors in patients with rheumatoid arthritis. Clin Biomech (Bristol, Avon) 2013;28(4):473–479. doi: 10.1016/j.clinbiomech.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mackey AH, Stott NS, Walt SE. Reliability and validity of an activity monitor (IDEEA) in the determination of temporal-spatial gait parameters in individuals with cerebral palsy. Gait Posture. 2008;28(4):634–639. doi: 10.1016/j.gaitpost.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 47.Hausdorff JM, Gruendlinger L, Scollins L, O'Herron S, Tarsy D. Deep brain stimulation effects on gait variability in Parkinson's disease. Mov Disord. 2009;24(11):1688–1692. doi: 10.1002/mds.22554. [DOI] [PubMed] [Google Scholar]
- 48.Hausdorff JM, Lowenthal J, Herman T, Gruendlinger L, Peretz C, Giladi N. Rhythmic auditory stimulation modulates gait variability in Parkinson's disease. Eur J Neurosci. 2007;26(8):2369–2375. doi: 10.1111/j.1460-9568.2007.05810.x. [DOI] [PubMed] [Google Scholar]
- 49.Yogev-Seligmann G, Rotem-Galili Y, Dickstein R, Giladi N, Hausdorff JM. Effects of explicit prioritization on dual task walking in patients with Parkinson's disease. Gait Posture. 2012;35(4):641–646. doi: 10.1016/j.gaitpost.2011.12.016. [DOI] [PubMed] [Google Scholar]