Abstract
Background
Numerous pre-clinical studies using bone marrow derived cells for the treatment of traumatic brain injury and stroke have demonstrated efficacy in terms of blood-brain barrier preservation, neurogenesis, and other functional outcomes. Phase 1 clinical trials using bone marrow mononuclear cells infused intravenously in children with severe TBI demonstrated safety and potentially a CNS structural preservation treatment effect. This study sought to confirm the safety, logistic feasibility, and potential treatment effect size of structural preservation/inflammatory biomarker mitigation in adults to guide Phase 2 clinical trial design.
Methods
Adults (aged 18-55) with severe traumatic brain injury (GCS 5-8) and without signs of serious other injury or irreversible brain injury (see Table 1) were evaluated for entry into the trial. A dose escalation format was performed in 25 patients: 5 controls, followed 5 patients in each dosing cohort (6,9,12 ×106 cells/kg body weight), then 5 more controls. Bone marrow harvest, cell processing to isolate the mononuclear fraction, and re-infusion occurred within 48 hours after injury. Patients were monitored for harvest/infusion related hemodynamic changes, infusional toxicity, and adverse events. Outcome measures included MRI based measurements of supratentorial and corpus callosal volumes as well as DTI based measurements of fractional anisotropy and mean diffusivity of the corpus callosum and the corticospinal tract at the level of the brainstem at 1 month and 6 months post-injury. Functional and neurocognitive outcomes were measured and correlated with imaging data. Inflammatory cytokine arrays were measured in the plasma pre-treatment, post-treatment, and at 1 and 6 month follow-up.
Results
There were no serious adverse events related to harvest/infusion. There was a mild pulmonary toxicity of the highest dose that was not clinically significant. Despite the treatment group having greater injury severity, there was structural preservation of critical regions of interest that correlated with functional outcomes. Key inflammatory cytokines were down-regulated after BMMNC infusion.
Conclusions
Treatment of severe, adult traumatic brain injury using an intravenously delivered autologous bone marrow mononuclear cell infusion is safe and logistically feasible. There appears to be a treatment signal as evidenced by CNS structural preservation, consistent with previous pediatric trial data. Inflammatory biomarkers are down-regulated after cell infusion. A Phase 2, prospective, randomized trial excluding the highest dose is warranted and can be powered based upon structural outcome variables.
Keywords: traumatic brain injury, bone marrow stromal cells, cellular therapy, clinical trials, diffusion tensor imaging, adult stem cells, adult human bone marrow
Graphical Abstract
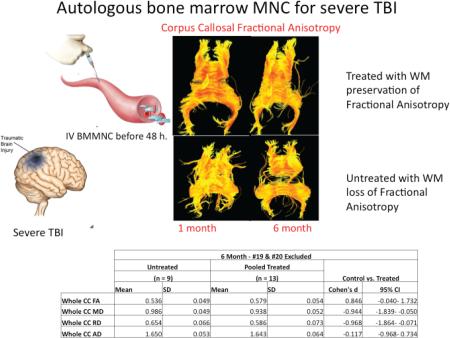
Autologous bone marrow mononuclear cells (BMMNC) in escalating doses of 6,9,12 ×106 cells/kg were injected intravenously to treat patients with severe traumatic brain injury within 48 hours of injury and compared to concurrent, placebo controlled controls in a Phase 1/2a clinical trial. Primary outcome measures included white matter volumetrics and corpus callosal (CC) fractional anisotropy (FA) as a marker of myelination/myelin loss over time. There was a strong treatment effect noted (Cohen's d) in corpus callosal FA and mean diffusivity (MD), providing powerful estimates for the follow-on trial sample size determination. The corpus callosal fiber tractography from individual patients imaged in the early period post-injury (1 month) and at 6 months, and those images demonstrate the fiber tract drop out over time in the untreated patient that is preserved with treatment. These data are summarized in the table below.
Introduction
Approximately 1.5 million people suffer traumatic brain injury (TBI) yearly in the United States. The annual mortality approaches 50,000 with the remaining patients suffering from varying levels of long term sequelae.1 Overall, 6.5 million patients are burdened by the physical, cognitive, and psychosocial deficits associated with TBI leading to a total economic impact of approximately 60 billion dollars.2
Numerous pre-clinical studies have shown that autologous bone marrow derived mononuclear cells (BMMNCs) improve outcomes after TBI with a positive effect on early outcomes (blood brain barrier permeability and cerebral edema) as well as late functional outcomes such as improved spatio-temporal memory. These positive effects on functional outcomes have been replicated in various animal stroke models as well.3 Similarly, in both injury models, BMMNCs dampen the secondary neuroinflammatory response to injury.4-6 Finally, there appears to be a consistent treatment window of 24-72 hours.7 In terms of translational feasibility, autologous bone marrow derived mononuclear cells have shown potential benefit in pre-clinical proof-of-concept studies in TBI and stroke, and have an excellent safety profiles in Phase 1 trials of pediatric TBI and adult stroke.8-10
We have pursued the use of autologous BMMNCs for a number of reasons: (1) well-developed pre-clinical proof of concept and toxicity data, (2) Phase 1 trial without observed serious adverse events or toxicity (3) no immune barrier considerations, (4) biologically sound route of delivery due to the 5-8 micron cell size (vs. 13-19 micron size for mesenchymal stromal cells) obviates pulmonary sequestration, or the “pulmonary first pass effect” making intravenous delivery more practical,11 (5) no in vitro culture/scaling issues for autologous application, (6) ready availability, (7) no issues with uncontrolled replication as with embryonic stem cells or fetal cells, (8) no ethically objectionable issues with cell type.
The purpose of this study was to evaluate the safety, logistical feasibility, and potential signals of a treatment effect in a prospective, single center, dose escalation trial in adult patients with acute TBI. Safety was assessed by organ injury related to infusional toxicity. The efficacy outcome measure was based upon serial imaging data obtained early post-injury and at 6 months, evaluating global white matter volume and metrics of white matter integrity using diffusion tensor magnetic resonance imaging. Exploratory analysis of circulating markers of the inflammatory response to injury were evaluated sequentially before and after injury as well as at 6 months (representing the patient's endogenous baseline pre-injury).
Materials and Methods
The Treatment of Severe Adult Traumatic Brain Injury Using Bone Marrow Mononuclear Cells (NCT 0157540) trial was an open label, non-randomized, single center clinical trial. This phase I/IIa trial was performed under the authority of the Federal Investigational New Drug Application BB 12620. The trial was approved by the University of Texas Health Sciences Center at Houston Committee for the Protection of Human Subjects and by the Memorial Hermann Office of Research. Patients were enrolled into the trial from March of 2012 through October of 2014. The trial was funded by the U.S. Army Medical Research and Materials Command grant number W81XWH-11-1-0460. Safety monitoring during the trial was provided by an independent medical monitor and a medical safety monitor in coordination with the data safety monitoring board. The study was planned to proceed until completing enrollment or after one of the following stopping rules: 4 deaths; PaO2:FiO2 < 70 with a PaCO2 > 90 mmHg within 48 hours of infusion; AST/ALT > 900 U/dL within 24 hours of infusion in any subject; Grade 4 – 5 CNS cerebrovascular ischemia event or Grade 4 – 5 seizure event as defined by version 4 of the NCI CTCAE within 12 hours of infusion; Any Grade 4 – 5 adverse event as defined by the NCI CTCAE that is determined to be temporally-related by the data safety monitoring board.
Study Population
Trauma patients between the ages of 18 and 55 years of age presenting to the trauma Emergency Department were screened for enrollment into the study. Eligible patients were those with non-penetrating head trauma, a post-resuscitation Glasgow Coma Scale12 of 5 to 8 and injury occurring less than 24 hours prior to consent. Patients were excluded on the basis of pre-existing serious medical comorbidities or infection as revealed by patient history: prior moderate-severe brain injury, major psychiatric disorders, or any neurologic impairment or seizure disorder. In addition, patients were excluded if there was evidence of hypoxic-ischemic insult, opening intracranial pressure > 40 mmHg, continued hemodynamic instability at the time of consent, uncorrected coagulopathy at the time of harvest, pelvic fracture requiring operative fixation, spinal cord injury, AAST grade IV solid or hollow visceral organ injury, prolonged hypoxia, weight ≥ 300 lbs. (MRI limitation), pregnancy, and unwillingness to return for follow-up visits (Table 1).
Table 1. Trial Inclusion and Exclusion Criteria.
The inclusion and exclusion criteria had the intent of including patients with acute, severe TBI without signs of irreversibility. Also, severe other organ injury was excluded as defined in the exclusions with most of these being excluded due to the presence of hemorrhagic shock.
| Inclusion Criteria |
| Between 18 and 55 years of age on the day of injury |
| Post-resuscitation GCS of 5 to 8 |
| Initial injury occurring less than 24 hours prior to consent |
| English speaking |
| Exclusion Criteria |
| Known history of: prior brain injury, psychiatric disorder, neurological impairment and/or deficit, seizure disorder requiring anti-convulsant therapy, recently treated infection, renal disease, hepatic disease, cancer, substance abuse or positive urine drug screen at admission, cancer, immunosuppression, HIV |
| Obliteration of perimesencephalic cistern on initial head CT suggesting prolonged hypoxic ischemic insult |
| Opening ICP >40 mm Hg |
| Hemodynamic instability at the time of consent with ongoing fluid resuscitation and/or inotropic support* |
| Uncorrected coagulopathy at the time of bone marrow harvest defined as INR > 1.6, PTT > 36s, PLT < 100k, Fibrinogen < 100 mg/dL |
| Unstable pelvic fracture requiring operative fixation |
| Pulmonary contusions defined as a chest x-ray with non-anatomic opacification and/or PaO2:FiO2 < 250 associated with mechanism of injury |
| Greater than AAST Grade III solid or hollow visceral injury of the abdomen and/or pelvis |
| Spinal cord injury |
| Weight ≥ 300 lbs |
| Any contraindication to MRI |
| Positive urine pregnancy test |
| Participation in a concurrent interventional study |
| Unwillingness to return for follow-up visits |
a GCS, Glasgow Coma Scale; HIV, Human Immunodeficiency Virus; CT, computed tomography; INR, international normalized ratio; PTT, partial thromboplastin time; PLT, platelet; PaO2, partial pressure arterial oxygen; FiO2, fraction of inspired oxygen; AAST, American Association for the Surgery of Trauma; MRI, magnetic resonance imaging
Does not include cerebral perfusion pressure based inotropic support
Study personnel approached the patient's legally authorized representative (LAR) with information about study participation, and the PI/Co-I obtained informed consent. A research intermediary interviewed each LAR to confirm consent and avoid therapeutic misconception. A total of 25 patients were enrolled into the study. Patients were enrolled sequentially into 1 of 5 different groups: control group 1(n=5; sham intervention), low dose treatment group (n=5; 6×106 cells/k), medium dose treatment group (n=5; 9×106 cells/kg), high dose treatment group (n=5; 12×106 cells/kg) and control group 2 (n=5; sham intervention). The additional controls were added to include patients across the time spectrum of the study.
Patient Management
Patients were admitted to the Shock Trauma or Neurotrauma Intensive Care Units (STICU and NTICU) at Memorial Hermann Hospital, which is an American College of Surgeons Verified/Texas Department of Health approved Level 1 Adult Trauma Center. Patients initially resuscitated according to Advanced Trauma Life Support guidelines, and subsequently in accordance with the Guidelines for the Management of Severe Traumatic Brain Injury, 3rd Edition.13 Central vascular catheter placement was performed via either the subclavian or femoral route, as well as radial or femoral arterial cannula placement according to standard techniques. Coagulopathy was corrected with fresh frozen plasma (FFP) titrated based on thromboelastography results on arrival to the hospital and to the ICU. All patients received an intraparenchymal pressure monitor or ventriculostomy for continuous monitoring of intracranial pressures (ICP) and drainage of cerebrospinal fluid (CSF). ICPs were managed in accordance with Memorial Hermann Trauma Guidelines with a goal ICP < 20. If ICP was refractory to mechanical measures and respiratory measures (minimal stimulation, head of bed elevation, PaCO2 35-40), hyperosmotic therapy was initiated with a goal sodium 145–155. Sedation was managed singly or in combination with infusions of midazolam and fentanyl or propofol and titrated according to the Richmond Agitation and Sedation Scale and maintaining ICP below 20. For refractory ICP spikes a 30 mL bolus of 23.4% NaCl was infused. Unexpected neurologic changes, ICP spikes, or refractory elevated ICP prompted repeat CT imaging of the brain. Each ICU treated patients with seven days of phenytoin for post-traumatic seizure prophylaxis.
Study Intervention
Bone Marrow Harvest
Patients enrolled into the low, medium and high dose treatment arms underwent bone marrow harvest within 36 hours of injury. The harvest was performed aseptically using an 11 or 15 gauge trocar from the anterior superior iliac crest of each patient. A total volume of 3 to 5 mL per kg of body weight was aspirated from each patient with a 30 mL syringe. Pain was controlled through the use of local anesthesia (1% lidocaine without epinephrine) and infusions of fentanyl, midazolam and/or propofol.
Blood pressure, heart rate, oxygen saturation, and intracranial pressure were measured at 5 minute intervals for the duration of the procedure. Recordings of these parameters were continued every 15 minutes post-harvest for 1 hour, then every 30 minutes for 2 hours and every hour until the time of cell product infusion. Hemoglobin and hematocrit were measured at 4 hour intervals for 12 hours after the harvest. Harvest related adverse events were defined as a 20% decrease in either cerebral perfusion pressure or mean arterial pressure that was sustained for at least 10 minutes.
Cell Processing
Following completion of the procedure, the bone marrow sample was transferred in an anticoagulant-containing blood collection bag at ambient temperatures to the processing facility in a validated cooler using a professional courier service in compliance with requirements dictated by the Code of Federal Regulations Titles 29 and 49 for both OSHA and HIPAA Medical Courier and Biohazard training. The cell processing facility was located at the University of Texas Health Science Center at Houston – Medical School, Evelyn H. Griffin Stem Cell Therapeutics Research Laboratory. The facility is FDA-registered, FACT accredited, and compliant with current Good Manufacturing Practice (cGMP).
Upon its arrival at the cGMP facility, the bone marrow was filtered through a 170 – 260 μm filter to remove aggregates and/or spicules. The bone marrow was sampled for aerobic, anaerobic and fungal sterility as well as nucleated cell count and multi-parameter flow cytometric analysis. The MNC-enriched fraction was obtained by density gradient centrifugation of the bone marrow on Ficoll-Paque PREMIUM (GE Healthcare Life Sciences, Pittsburgh, PA, USA) using the Biosafe Sepax 2 RM cell processor, the NeatCell protocol and the CS-900.2 kit for regenerative medicine applications (Biosafe America, Houston, TX, USA). Risk management methods used for the production of clinical-grade cells were performed, and all reagents used had the manufacturer's certificate of analysis on file and maintained according to applicable regulation. Any residual Ficoll-Paque PREMIUM was removed during the automated washing step of the Sepax 2 RM cell enrichment procedure and no other infusion-incompatible reagents were added during manufacturing.
Prior to product release, aliquots of the final product were taken for quality-control testing (nucleated cell count, viability, gram stain, 14 day aerobic and anaerobic bacterial and 28 day fungal cultures, endotoxin and mycoplasma content, colony forming unit assays and multi-parameter flow cytometry for an extended differential cell count and to assess cell population identity). Only products that passed the release acceptance criteria (negative Gram stain; >70% viability determined with the Trypan Blue exclusion method and endotoxin levels <5.0 EU/Kg measured using the Endosafe PTS system by Charles River Laboratories) were authorized by the quality team for infusion. Upon meeting the release criteria, the final dose consisting of 6×106 cells/ml/Kg, 9×106 cells/ml/Kg or 12×106 cells/ml/Kg in 0.9% saline containing 5% volume/volume human serum albumin was prepared in one or two sterile syringes and transported to the infusion site at ambient temperature in a validated cooler using a professional courier service.
Cell Product Infusion
The patients enrolled into the low dose, medium dose and high dose groups were to receive target doses of 6 × 106 BMMNC/kg, 9 × 106 BMMNC/kg and 12 × 106 BMMNC/kg, respectively. The dosing range was derived from our pre-clinical laboratory data6 and our prior phase I pediatric trial which used a similar protocol.8 The BMMNC infusion was performed through either peripheral or central venous catheters approximately 7 - 8 hours after harvest. Post-infusion monitoring of post-harvest hemodynamics was performed every 15 minutes for the 1st hour, every 30 minutes for hours 2 – 3 and 1 hour for hours 3 – 7.
Flow Cytometry
A 4-color direct immunofluorescent “lyse/no wash” labeling method was used in the evaluation of progenitor cells and lymphocyte subsets. Samples of bone marrow starting material and final product (MNCs) were stained with the following four panels: (1) 7AAD to assess overall viability; (2) CD45 / CD14 to identify lymphocytes, lymphoblasts, monocytes, and granulocytes; (3) CD45 / CD19 / CD3 / CD16+56 to identify T, B, NK, and NKT subsets; (4) Lin1 / CD34 / CD45 / CD133 to identify hematopoietic stem cells and other progenitor cells. The 4th panel was done in TruCount tubes (BD Biosciences, San Jose, CA) to allow calculation of absolute cell counts. The following antibodies were used at saturated concentrations: CD45 clone 2D1 conjugated to PerCP-Cy5.5 (BD Biosciences), CD14 clone M5E2 conjugated to FITC (BD Biosciences), MultiTest CD3/CD16+56/CD45/CD19 conjugated to FITC/PE/PerCP/APC (BD Biosciences), CD34 clone 561 conjugated to APC (BioLegend, San Diego, CA), CD133/1 clone AC133 conjugated to PE (Miltenyi Biotech, San Diego, CA), and Lineage 1 cocktail (“Lin-1” consisting of CD3, CD14, CD16, CD19, CD20 and CD56) conjugated to FITC (BD Biosciences). Immediately after lysing samples for 15 minutes with PharmLyse (BD Biosciences), data was acquired with an LSRII cytometer equipped with FACSDiva software (BD Biosciences). For the four-part differential panel 10,000 singlet cells were acquired, for the lymphocyte subsets 10,000 singlet CD45+ lymphs were acquired, and (where possible) 100,000 singlet CD45+ cells were acquired for progenitor cells. Analysis was performed with FlowJo (Tree Star Inc., Ashland, OR) and FCS Express 4 (De Novo Software, Glendale, CA). The differential was analyzed using CD45 and light scatter gates, with CD14 to confirm monocytes. Lymphocyte subsets were gated using the CD3, CD19, and CD16+56 markers within the low side-scatter CD45+ lymphocyte population. The progenitor cells were evaluated using ISHAGE gating strategy for total CD34+ progenitors, with additional gating to identify the Lin-1[neg]CD34+CD133+, Lin-1[neg]CD34+CD133[neg], and Lin-1[neg]CD34[neg]CD133+ subsets. Process controls CD-Chex Plus and CD-Chex CD34 (Streck, Omaha, NE) were used to ensure assay consistency.
Study Outcomes
The primary aims of this study were to evaluate safety of bone marrow harvest/infusional toxicity of BMMNC after severe traumatic brain injury, compare changes in white matter metrics longitudinally and investigate potential changes in the inflammatory cytokine response.
Harvest procedure safety was measured by measuring systemic and cerebral hemodynamic responses to bone marrow withdrawal as well as harvest site complications. To assess for multi-organ dysfunction the Sequential Organ Failure Assessment (SOFA) was calculated prospectively for each patient which has been validated for use in trauma patients.14 Evidence of pulmonary infusion related toxicity was determined by using the Murray score which is based upon PaO2:FiO2, chest radiograph, lung compliance and positive end expiratory pressure.15 A basic metabolic profile and complete blood count with differential were obtained each day in the intensive care unit to monitor for renal or hematologic insults. Hepatic transaminases were followed daily to assess for any potential hepatic injury as a result of microthrombosis. Finally, neurologic status was also assessed daily while in the ICU. Intracranial pressure was recorded hourly by the nursing staff and entered into the hospital's electronic medical record. With the exception of bone marrow harvest and BM-MNC infusion monitoring, all ICP data used for analysis was obtained from the hospital's electronic medical record.
Whole blood was obtained at the time of consent and in the acute period every 12 hours for 7 days following infusion by study staff. Chronic samples were obtained at 1 month and 6 months. Blood samples were collected in EDTA tubes and immediately centrifuged to obtain a platelet poor plasma sample. All samples were then stored at −80° C until biomarker analyses were performed. Cytokine levels for interleukin 1 beta, interleukin 4, interleukin 6, interleukin 10, interferon gamma and tumor necrosis factor alpha were then assessed by enzyme linked immunosorbent assay (Legend Max™, BioLegend, San Diego, CA, USA).
Imaging Protocols
All imaging data were acquired using a General Electric 3.0 Tesla Signa HD scanner with an 8 channel parallel imaging head coil. Imaging was performed at 1 month and 6 months from injury to acquire data for volumetric and diffusion tensor imaging comparisons. MRI acquisition parameters for the high-resolution anatomical and 30-direction DTI sequences quantitatively analyzed in this study are as follows:1) Sagittal isotropic 3D T1-weighted spoiled gradient echo (1×1×1 mm3); repetition time (TR) = 7.236 ms; echo time (TE)=2.968 ms; flip angle = 11. 2) Axial 30 direction single-shot spin-echo diffusion sensitized echo-planar (with additional 3 b0 volumes) (2.73 × 2.73 × 2.70 mm3): TR = 14900 ms; TE = 85.6 ms; flip angle = 90. The high resolution anatomical MRI sequence from each MRI session was processed using Freesurfer software version 5.3.0 (www.surfer.nmr.mgh.harvard.edu) to obtain global measures of supra-tentorial and total white matter volumes. The isotropic 30 direction DTI sequence from each imaging session was processed with FMRIB's Software Library version 5.0.7 (FSL, Oxford, U.K.) to correct for eddy currents and motion before using DTIFIT to estimate the diffusion tensor at each voxel. Based on prior neuropathological and imaging studies reporting major impact of TBI on corpus callosum and corticospinal tract,16 we selected these regions for analysis. To obtain DTI metrics of white matter integrity from the corpus callosum (CC), a binary mask was manually created on three adjacent midsagittal slices. Additionally, DTI metrics from five subregions of the corpus callosum were obtained by creating individual binary masks for each of five equally-spaced segments of the corpus callosum. To obtain DTI metrics from a region of interest at the level of the brainstem, a single axial slice was selected for probing the cortical spinal tract (CST) at the mid-pons level where the pontine crossing tract was distinctly visible.17
Neurocognitive Outcome Testing
A licensed neuropsychologist blinded to treatment groups performed neurobehavioral testing as well as functional outcome assessments at 1 month and 6 months following injury. Assessments were determined by direct assessment of each patient and patient or caregiver interviews. The measures are consistent with recommendations supported by the NIH, NIDRR, NIMH, and DoD for the use of common outcome measures in TBI research in adults18 as well as recommendations for assessment of outcome in clinical trials of TBI.19 Functional outcomes included ratings of the global level of functioning, independence in daily functioning, adjustment, and community participation. Neuropsychological outcome measures targeted psychological adjustment, declarative memory, working memory, attention, fine motor skills, processing speed, and verbal fluency. The neurocognitive testing matrix in the supplementary figures provides descriptive information and lists dependent variables of interest for each measure in italics.
Data Analysis
Data are presented as mean±SEM. Neuroimaging metrics were evaluated using repeated measures ANOVA used to evaluate within subject change over time in each group and test for group*time interaction. Correlations of fractional anisotropy of the corpus callosum and neurocognitive outcomes were assessed using Spearman's rho. Biomarker data were analyzed in both a pooled and dose-dependent format using ANOVA and post-hoc non-parametric analysis with Wilcoxon Rank-Sum after confirming non-normal distribution of data. Cohen's d was calculated to estimate treatment effect size between control and treated groups.
Results
Enrollment and Patient Characteristics
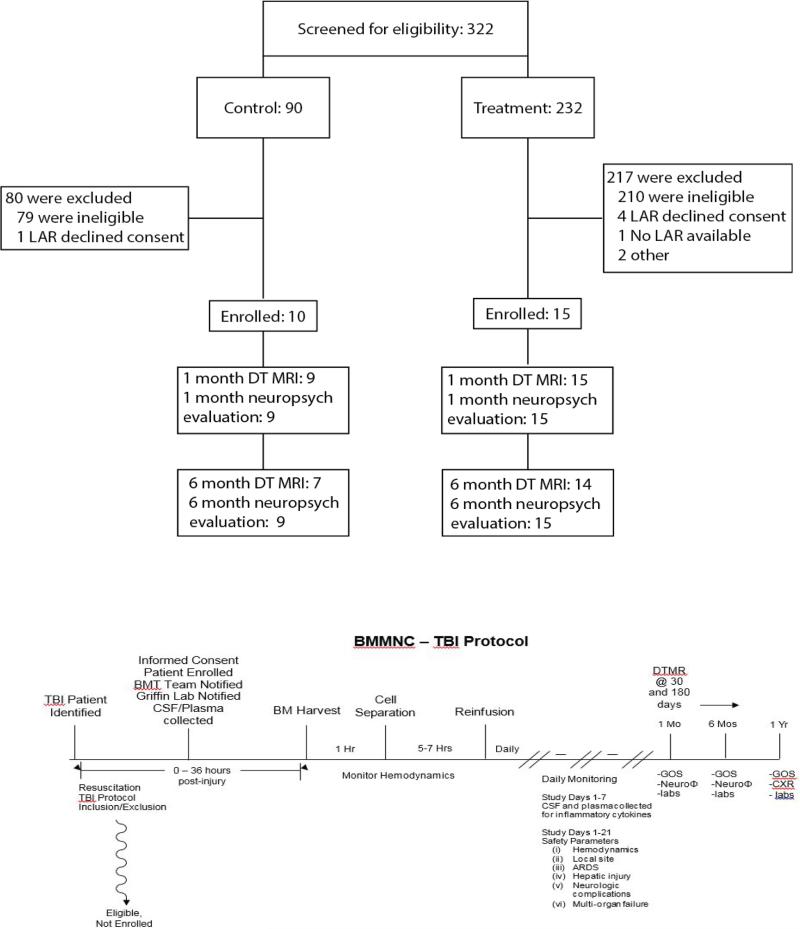
Through the course of the study 320 patients were screened for enrollment with 90 in the control phase and 230 in the treatment phase (Figure 1). During the control phase, 11 patients were found to meet eligibility requirements with the legally authorized representative (LAR) agreeing to consent in 10 cases. Twenty-two out of 230 patients screened during the treatment phase met eligibility requirements. In 4 cases, the patient's LAR declined enrollment, 1 patient had no available LAR and patients were unable to be enrolled due to a safety review in 2 cases. The most frequent reason for not meeting study eligibility was a post resuscitation GCS outside of the 5 – 8 range.
Figure 1. CONSORT Diagram for Trial Enrollment/Participation.
Patients were screened with a 10-15X ratio of screened to enrolled. This has logistical implications for trial design. Long term follow-up was exceptionally strong for an acute trauma study (normally approximately30-50%). The Flow diagram shows how patients are identified, enrolled and treated within 48 hours, with a measured mean of approximately 36 hours.
Seven of 10 patients in the control group and 14 of 15 in the treatment group were able to complete all phases of study follow-up. One patient in the control group was lost to follow-up prior to the 1 month post-injury assessment. Additionally, 3 control patients and 1 treated patient were unable to complete the 6 month time point imaging study. No patient fatalities were recorded during the 6 month study follow-up period.
Sex distribution was similar between control and treatment groups with approximately 70% of patients enrolled male. Injury severity scores and the head component of the abbreviated injury scale (AIS-head) were calculated and provided by the hospital trauma registry. These scores in addition to post-resuscitation GCS were similar across all patient cohorts. With the exception of 1 patient in the control arm of the study, all AIS-head scores were greater or equal to 3 and thus consistent with alternative measures of severe traumatic brain injury.
Table 2 describes the patient characteristics. In the control arm, 1 out of 10 patients underwent surgical decompression on presentation for their injury-not after or a consequence of treatment. Four out of 15 patients in the treated arm (1 low dose, 1 medium dose, 2 high dose) required decompressive craniectomy for ICP management on presentation. No patients in the control arm had ventriculostomy placement on initial presentation or for management of refractory ICP. In the treated arm of the study, 2 patients underwent ventriculostomy placement for initial management of ICP and 3 patients underwent ventriculostomy placement after several days of admission for refractory ICP issues. Additionally, the peak ICP and Therapeutic Intensity Level recorded prior to treatment and over the first 24 hours of injury was significantly higher in treated patients than controls (Supplemental Figure 1).
Table 2. Patient Characteristics.
There were no differences in the injury demographics. However, treated patients had a greater therapeutic intensity with decompressive craniectomy and ventricular drain placement for ICP management compared to control patients. This is shown in subsequent Figures as well.
| Variable | Control | Low Dose | Medium Dose | High Dose | Treated Combined |
|---|---|---|---|---|---|
| Males, % | 70(7) | 80(4) | 80(4) | 60(3) | 73(11) |
| Mean Age | 34 ± 5 | 25 ± 4 | 31 ± 4 | 34 ± 3 | 30 ± 2 |
| Injury Severity Score | 28 ± 3 | 28 ± 2 | 27 ± 4 | 27 ± 5 | 28 ± 2 |
| Post-resuscitation GCS | 7 ± 0 | 7 ± 1 | 7 ± 0 | 7 ± 0 | 7 ± 0 |
| Craniectomies (prior to infusion), % | 10(1) | 20(1) | 20(1) | 40(2) | 27(4) |
| External Ventricular Drain, % | 0(0) | 0(0) | 40(2) | 60(3) | 33(5) |
| AIS Head | 4 ± 0 | 4 ± 0 | 4 ± 0 | 4 ± 0 | 4 ± 0 |
| 1 month GOS-E | 3 ± 0.1 | 3 ± 0.2 | 3 ± 0 | 3 ± 0 | 3 ± 0 |
| 6 month GOS-E | 5 ± 1 | 4 ± 1 | 5 ± 1 | 3 ± 0 | 4 ± 0 |
| Time of infusion (hrs) | - | 33 ± 3 | 39 ± 2 | 36 ± 2 | 36 ± 1 |
| Time of harvest (hrs) | - | 25 ± 3 | 30 ± 2 | 28 ±1 | 28 ± 1 |
| Time of enrollment (hrs) | 16 ± 2 | 13 ± 1 | 15 ± 0 | 19 ± 1 | 16 ± 1 |
Bone Marrow Harvest
Bone marrow harvest was performed at an average of 28 hours following admission. Harvest was performed at bedside in 14 of 15 cases and in the operating room following a decompressive craniectomy in 1 case. No major adverse events were recorded during bone marrow harvest for any of the patients and there were no significant hemodynamic changes during post-harvest monitoring. These data are graphically displayed in Supplemental Figure 3.
Cell Dose Characterization
The immunophenotypic characterization of the infused cell product by dose group is shown in Supplemental Table 1 and Supplemental Figure 2, as well as the underlying graphics regarding progenitor cell dose infused per kilogram body weight.
Infusion-Related Toxicity/Safety Outcomes
Adverse Events
The adverse events are tabulated in Supplemental Table 2 and grouped according to type and control vs. treatment groups. There were no events that triggered the stopping rules and no serious adverse events related to the study protocol.
Hemodynamics: Central and Cerebrovascular
Intra-harvest and post-harvest hemodynamic monitoring are shown in Supplemental Figure 3. There was no drop in the mean arterial pressure (MAP) or the cerebral perfusion pressure (CPP) or increase in intracranial pressure (ICP).
SOFA Scoring
No statistically significant difference in SOFA scores was present over a 21-day follow-up period when comparing pooled or individual [Supplemental Figure 4A] treatment groups against control patients. There was a trend toward increased organ dysfunction in the high dose treatment group.
Pulmonary Function-Murray Scoring
Treated patients were found to have a significant increase in Murray scores in the pooled treated arm of the study when compared to control patients (p<0.05) [Supplemental Figure 4B]. On subgroup analysis, the high dose treatment group remained with a significant increase (p<0.01) in Murray scores [Supplemental Figure 4C].
Imaging Data and Functional Outcome Correlation
Global measures of volumetric changes over time
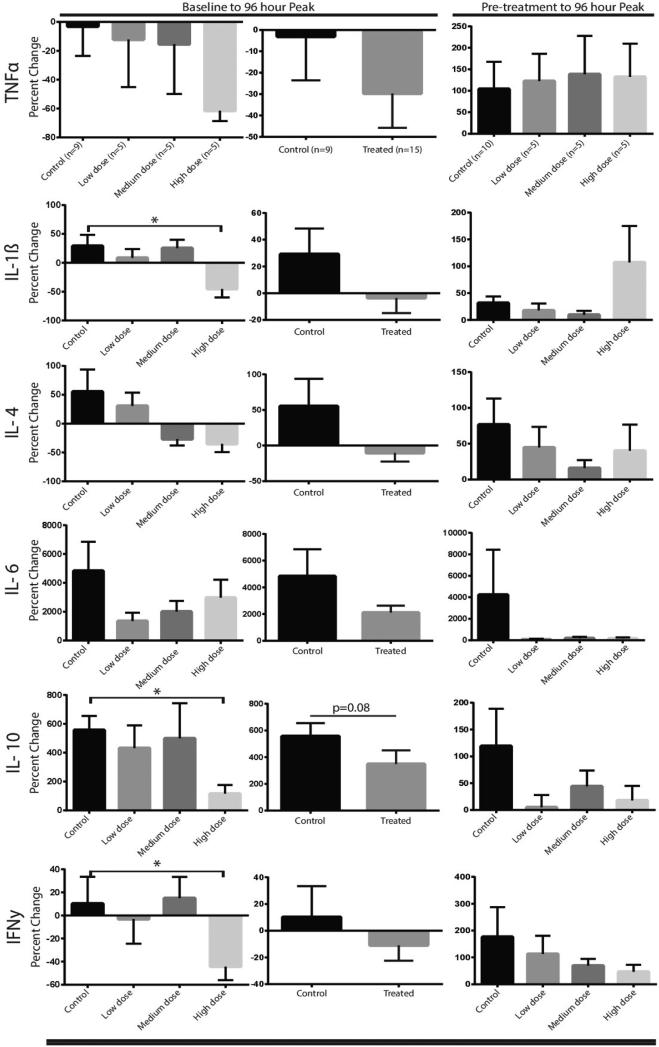
Quantitative analyses of the high-resolution 3D T1-weighted images yielded longitudinal volumetric measures of the supra-tentorium and cerebral white matter at 1 month and 6 months post-injury in 20 of 25 patients. These data are graphically summarized in Figure 2. In the treated group of patients, these data were available for all but 1 of 15 subjects; one subject who was in the high dose group of treated patients did not complete the 6 month imaging session. In the untreated group of patients, these data were not available for 4 of 10 subjects; 3 subjects did not complete the 6 month imaging session and 1 subject had significant artifacts distorting the images at the 6 month time-point.
Figure 2. Global Change in White Matter Volume.
These data graphically represent the changes in WM volume by treatment group (Panel A), the individual patients in the high dose group with their corresponding computed tomography images explaining their outlier status (Panel B) and the pooled comparisons of treated vs. controls (Panel C). Supra-tentorial White Matter Volume is demonstrated with and without the outlier patients 19 and 20. Treated patients show greater supra-tentorial WM volume preservation compared to Controls.
As indicated in figure 2, pooled comparisons of all treated vs. untreated patients showed similar supratentorial volumes at 1 month. Whereas the treated group demonstrated a 1.87% decrease in supra-tentorial volume, the untreated group showed a 3.9% reduction. When treated patients #19 and #20 were excluded from the analyses, there was a significant interaction between group and time (p=0.0294) as the treated group demonstrated well-preserved supra-tentorial volume (<0.15% decrease) at the 6 month time-point. In Figure 2, similar cerebral white matter volumes are evident in comparisons of treated vs. untreated patients at the 1 month time-point. At the 6 month time-point, the treated group demonstrated a 2% decrease in cerebral white matter volume while the untreated group showed a 3.6% reduction. After reviewing unique aspects of patients #19 and 20, we decided to analyze the data with and without these patients. One had a massive intracerebral contusion/hematoma and the other had a MCA distribution stroke as imaging confounders (Figure 2B). Representative CT scans are shown in Figure 5B. When treated patients #19 and #20 were excluded, there was a significant group*time interaction (p=0.0049) as the treated group exhibited well-preserved cerebral white matter volume (<0.65% decrease) at the 6 month time-point. Although statistical comparisons across dose levels did not reach significance, a trend for greater white matter volume preservation is evident in the low and middle dose treated groups relative to the untreated group of patients (see Figure 2A).
Figure 5. Inflammatory Cytokine Array.
A panel of pro and anti-inflammatory cytokines were measured in plasma. Peak levels within the first 96 hours post-injury were considered the physiological maximal response, and the 6 month follow-up levels were considered “baseline”. True un-injured baseline/pre-treatment cytokine data cannot be obtained. Pre-treatment and peak levels were also analyzed and presented. The data show a significant decrease in IL-1β, and IFN-γ in the high dose group and an apparent dose-dependent downward trend in those cytokines as well as TNF-α. The anti-inflammatory cytokine IL-10 was decreased in the high dose group with a dose dependent downward trend in IL-4.
ROI-based measures of white matter integrity changes over time
Quantitative analyses of the DTI data yielded measures of white matter integrity which included fractional anisotropy (FA), and mean diffusivity (MD). These data were available longitudinally in 21 of 25 patients as 4 subjects (1 treated and 3 untreated) did not complete the 6 month neuroimaging session.
Corpus callosum
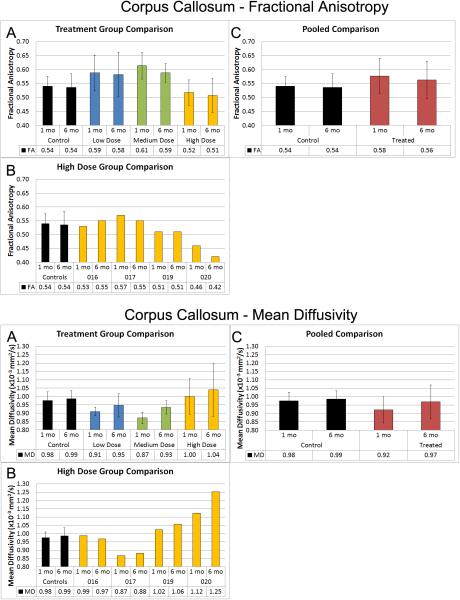
As shown in figure 3, FA values of the CC in the untreated group were lower at both imaging time-points relative to the low and middle dose groups of treated patients. The high dose group of treated patients exhibited the lowest FA values of the CC at both imaging time-points (CC-FA Figure 3A). Inspection of individual subject's data in the high dose treated group of patients (CC-FA Figure 3B) revealed exceptionally low FA values of the CC at both imaging time-points for patients #19 and #20. Pooled comparisons of all treated vs. untreated patients demonstrated a trend for better FA values at both imaging time-points in the treated group (CC-FA Figure 3C). When patients #19 and #20 were excluded, group differences approached significance (p=0.055) with the treated patients exhibiting higher FA values (mean=0.59 and 0.57 at 1 month and 6 months respectively) relative to the untreated patients (mean=0.54 and 0.53).
Figure 3. Corpus Callosum Fractional Anisotropy and Mean Diffusivity.
These data graphically display the changes in FA and MD in the Control and Treated groups in a dose dependent manner (Panels A), High dose individual patients (Panel B) and pooled comparisons (Panel C). FA is a summary measure of microstructural integrity. Oversimplified, High FA values are a surrogate measure of coherent, tightly packed and myelinated fibers. Mean diffusivity is an inverse measure of cell membrane density and is sensitive to edema and necrosis (higher FA is “good”, and lower MD is “good”). FA is higher in the treated groups except in the outliers as demonstrated in Panel B. MD is lower in the low and medium dose groups, and again the higher dose outliers skew those results.
As shown in figure 3, MD values of the corpus callosum in the untreated group were higher at both imaging time-points relative to the low and middle dose groups of treated patients. The high dose group of treated patients showed the highest MD values of the corpus callosum at both imaging time-points (CC-MD Figure 3A). Evaluation of individual subject's data in the high dose treated group of patients identified exceptionally high MD values of the CC for patients #19 and #20 (CC-MD Figure 3B). Pooled comparisons of all treated vs. untreated patients indicated lower MD values of the CC at both imaging time-points (CC-MD Figure 3C). When patients #19 and #20 were excluded, group differences were significant (p=0.008) with the treated patients exhibiting lower MD values (mean=0.897 ×10−3 mm2/s and 0.938 ×10−3mm2/s) at 1 month and 6 months respectively, relative to the untreated patients (mean=0.975 ×10−3mm2/s and 0.986 ×10−3mm2/s).
Corticospinal tract (CST) at the mid-pons level of the brainstem
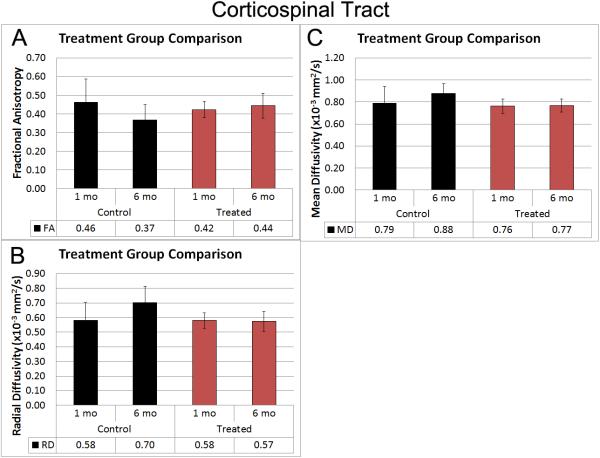
As summarized in figure 4, DTI metrics extracted from a region of interest of the CST at the level of the brainstem exhibited characteristics of better white matter integrity in the pooled group of treated patients relative to the untreated group of patients. A significant interaction between group and time was evident for FA (p=0.0137). While FA values decreased over time in the untreated group, FA values increased over time in the treated group of patients (Figure 4A). Additionally, a significant group*time interaction for analyses of radial diffusivity (RD) (p=0.033) indicated well-preserved RD values over time in the treated group of patients whereas RD values increased over time in the untreated group of patients (Figure 4B). Analyses of MD showed a significant group difference in MD values (p=0.0137). The treated group of patients demonstrated stable MD values over time, which was lower than the untreated group at both imaging time-points (Figure 4C). These data include patients 19-20 as the imaging was below the anatomic level of the injury.
Figure 4. Corticospinal tract Fractional Anisotropy, Mean Diffusivity and Radial Diffusivity.
These data demonstrate the increase in FA over time in the treated group compared to the decrease over time in the control group. Conversely, the diffusivity measurements show progression in the control group and improvement in the treated group.
Correlation of Callosal and Neurobehavioral Outcomes
Callosal fibers are generally topographically organized relative to the cortical regions that they connect. Our parcellation system is based on dividing the callosal fibers into 5 equal segments roughly corresponding to the following cortical termination sites: CC1- rostrum and genu, CC2-rostral and anterior mid-body, CC3-posterior mid-body, CC4-isthmus, and CC5-splenium. Based on our prior studies, FA was selected as the most sensitive DTI metric in relation to post-traumatic changes in cognitive and motor abilities.20 Consequently, neuropsychological outcomes for the total sample from the 6 month follow-up were examined in relation to FA values from the regional and total CC using Spearman rho or Pearson r depending on the variable distribution (Supplemental Table 3). Due to the small sample size, control and treated cases were pooled (n=21). FA of the splenium (CC5) and/or the whole CC were significantly related to all major functional outcomes, confirming that more favorable outcomes were seen in patients with greater tissue integrity. Similarly, all neuropsychological outcomes were significantly correlated with the total CC FA. Tasks with a major motor speed component (Trail Making B, Processing Speed, Grooved Pegboard), were significantly related to FA of CC2-5, reflecting the adverse impact of generalized white matter injury on tasks requiring speed and efficiency. In addition, some test scores were significantly related to specific CC regions; verbal fluency and working memory were related to CC2 and CC3. Memory of a word list was related to CC1 and CC2; delayed recall of the same list was related to CC4 and CC5.the same list was related to CC4 and CC5.
Biomarker Analysis
The plasma concentrations of cytokines were analyzed in both a pooled and dose dependent format. These are graphically represented in Figure 5. The data are shown as baseline (we used the 6 month value that should represent the patient's endogenous level remote from injury/trauma/operation/infection/stress) to the peak value obtained within the first 96 hours as well as a post-injury/pre-treatment to 96 hour peak. There is a dose-dependent trend for TNF-α suppression and a statistically significant, high-dose reduction in IL-1β, IL-10, and IFN-γ interferon gamma (IFN).
Discussion
This is the first controlled trial to test BMMNC as a treatment for traumatic brain injury, confirming safety in a prior trial in pediatric TBI, and now providing signals of a treatment effect on structural preservation and the global neuroinflammatory response8. Specifically, (1) early, autologous BMMNC harvest and infusion are safe and logistically feasible within a 36 hour time window of treatment, (2) there is a treatment signal of brain tissue preservation measureable on DT-MRI in a clinically relevant setting, (3) functional outcomes correlate with brain tissue preservation, and (4) BMMNC infusion may be altering the global inflammatory response to injury as measured by cytokine profiles. As a Phase 1/2a trial in TBI with small patient numbers, this study suffers from numerous limitations: (1) injury heterogeneity as exemplified by the higher severity of injury in the treatment group as measured by initial ICPs, and statistically greater numbers of patients requiring decompressive craniectomy prior to BMMNC infusion, (2) “early” DT-MRI may actually be too late to capture much of the FA/MD effects, (3) a non-randomized/un-blinded design impacting patient injury severity heterogeneity and potential for observer bias.
Rationale for Cell Type/Dosing and Route
Numerous cell types have been proposed for the treatment of TBI: BMMNC, MSC/MAPC, as well as neural stem cells from either fetal or embryonic derivation; pre-clinical studies have used all of these, as well as a variety of routes of administration (intra-cranial, intra-thecal, intra-arterial, and intravenous). At the pathophysiologic level the majority of traumatic brain injuries are multi-focal, and even focal injuries have areas exacerbated by secondary neuroinflammatory processes. Thus, localized cell replacement is impractical for a diffuse process. Additionally, we have not been in favor of localized injection strategies since these strategies require a craniotomy or multiple burr-holes (possibly causing more damage), and also depend on stereotactic implantation and cell migration followed by differentiation and engraftment. Our pre-clinical data in both stroke and TBI demonstrate efficacy with intravenous delivery, thus we chose this low risk approach with equivalent efficacy.6,9 Previously, we and others attempted to track numerous cell types post IV infusion after TBI in pre-clinical models.11,21 Not surprisingly, most cell types lodge at least transiently in the pulmonary circulation but later reside in the reticuloendothelelial system, specifically the spleen.22 We did not track engraftment location in our current clinical study, though this could be attempted in future studies using super-paramagnetic iron particles.
Numerous pre-clinical studies demonstrate the efficacy of MSC in TBI.23-36 When infused intravenously, bone marrow derived cells (either allogeneic or autologous) seem to have similar mechanism of action and similar efficacy dampening the innate immune response to injury. We and others have shown that infusion of bone marrow derived cells preserves the blood-brain barrier and improves long term outcomes by dampening the microglial activation that occurs in response to the primary injury.3 Of course, the logistical constraints of the current paradigm for infusion of autologous bone marrow mononuclear cells limits the application of this technique to major centers. The advantages of an allogeneic MSC treatment would include an “off-the-shelf” availability and a product that has already been thoroughly characterized and tested for release criteria and potency. This would obviate the need for an invasive bone marrow harvest in the acute period as well as cGMP cell processing. However, there are potential risks with MSC administration and careful expansion is required to maintain potency. Moll, et al., have described an “instant blood-mediated inflammatory reaction” to MSC infusion.37 This could be of greater theoretical concern in acutely injured trauma patients in whom the endothelial/coagulation cascade are up-regulated to promote thrombosis.38 Further, Moira, et al. reported loss of immune regulatory potency of MSCs after freeze-thaw cycles, but this has not been universally replicated.39 With this knowledge in mind, we proceeded with the safest and most readily available treatment strategy: intravenous autologous BMMNC administration.
Safety
This study demonstrates that early BMMNC harvest and infusion is safe. There were no episodes of hypotension, hypoxia, or exacerbation of ICP/CPP parameters associated with harvest of the bone marrow at up to 5 ml/kg body weight or infusion of the cell product. These parameters were prospectively collected and evaluated as a safety read-out. There were no serious events in terms of organ failure. However, there did appear to be a dose-dependent pulmonary toxicity with an increase in the Murray score suggesting a low-level lung injury. While a Murray score of less than 1.5 is not a clinically critical/serious event, it highlights that patients with underlying lung disease or concomitant lung injury may need to be excluded from these protocols/treatments. No patients developed hypoxia related to the infusion, however since hypoxia adversely affects TBI outcomes, it may be prudent to avoid any potential intervention that exacerbates pulmonary function.
Logistical Feasibility
There are a number of critical elements required to execute this research or future treatment strategy. There must be a robust clinical neurotrauma program that is expert in critical care of the patient with severe traumatic brain injury. The clinical neurotrauma critical care team must have extensive experience in care of patients with severe TBI. Standard placement of intracranial pressure monitors/ventricular drainage catheters must occur routinely without delay. The clinical trial infrastructure must support the ability to rapidly identify and screen all TBI patients, thus a research “on call” team is mandatory. The call team includes the ability to harvest and process/infuse cells every day and night including week-ends and holidays. This requires a significant increase in activity and personnel that goes beyond what is available at centers with pure oncologic stem cell laboratories. The future may allow closed system, point-of-care processing, which is not currently available. Allogeneic cell infusions using a pharmacy type “off the shelf” model would obviate the burden of the allogeneic harvest and processing, with each approach having pros and cons. A substantial amount of coordination with the clinical care team is mandatory to obtain data and to harmonize safe and timely imaging acquisition. Imaging infrastructure is another important component of the logistical requirements as these imaging data require a 3T magnet that has flexible availability. Finally, there must be a strong follow-up program with engaged social services to facilitate transport of patients back to the hospital for long-term neurocognitive and imaging evaluation. Failure of any of these points in the protocol can undermine the progress of the program.
Imaging outcomes
Our study sought to examine whether there were any potential structural changes associated with BMMNC treatment for TBI. The rationale that this treatment may result in structural preservation is based upon the proposed pleiotropic mechanisms of action described in pre-clinical studies and our own Phase 1 trial in children.40-42 Pre-clinical studies in rodents demonstrate that there was a reduction in the degree of microglial activation with autologous BMMNC infusion in rodents and microglial activation after TBI is associated with white matter loss over time 3,43. Adult and pediatric TBI longitudinal imaging studies have established a chronic-phase volumetric reduction in grey and white matter.18,44-47 An important finding noted in this study is the treatment effect size determination (Supplemental Table 3) that has allowed us to make the best possible sample size estimate for an upcoming Phase 2b trial. Our imaging data show that there is a protective effect that may have a dose-dependent relationship. Despite two confounding patients (19, 20) with higher injury severity requiring decompressive surgery prior to cell infusion that altered the imaging read-out, there were significant differences and trends in structural preservation in global volumetric measures of the supratentorium and cerebral white matter. Further corpus callosal tract preservation as measured by fractional anisotropy and mean diffusivity demonstrated preservation with treatment and correlated strongly with neurocognitive outcomes. We estimated the effect size of BMMNC treatment on DTI metrics of corpus callosal integrity which is important in planning a Phase 2b trial with CC DTI metrics as an outcome measure. BMMNC treatment has a moderate to strong treatment effect on FA in the CC (Cohen's d 0.5-0.8). These data would allow a sample size estimation for a study powered at 0.8 with a Cohen's d between 26 (0.8) and 64. Adding an exclusion in future trials for hemispheric stroke/contusion volume threshold value that invalidates volumetric/DTI metrics would also make mean diffusivity a powerful outcome measure. These data and the outcome correlates (Supplemental Table 3) also suggest that CC DTI metrics are useful primary outcome measures.
Neurocognitive outcomes
Despite substantial patient heterogeneity, small sample size, and the restricted range of TBI severity, callosal FA values were strongly correlated with functional and neuropsychological outcomes. The strength of this relationship provides additional rationale for using imaging variables as a primary clinical endpoint. FA of the whole CC was significantly correlated with nearly all of the functional status, motor, and cognitive measures. Moreover, neuropsychological variables were significantly related to the integrity of fibers coursing through different callosal segments. Motor and processing speed scores, which are sensitive markers of generalized injury, were correlated with FA in 4 of 5 segments. Memory and verbal fluency scores were more strongly related to FA from anterior and mid-callosal regions carrying fibers terminating in frontal, parietal, and temporal cortical areas. The integration of sensitive metrics from neuroimaging and neuropsychological outcome domains will enhance the detection of intervention effects in larger samples.
Inflammatory Markers
The cytokine/biomarker data are interesting in that there appears to be a dose-dependent down-regulation of the pro-inflammatory innate immune response to injury. Specifically, the IL-1β and IFN-γ signals show a statistically significant reduction in systemic concentrations relative to the patient's biological baseline (chronic follow-up level used as “baseline”). TNF-α demonstrates a similar trend. These data parallel pre-clinical data in cell therapy models of TBI (and stroke), in terms of dampening of the global inflammatory response to injury.3 However, most of the pre-clinical models use some other assays such as T-cell responsiveness to stimuli, modified mixed lymphocyte reactions, etc. that are impractical for many clinical trials. These types of assays typically require processing of fresh specimens for flow cytometry based interrogation of the cellular response to stimuli, thus requiring a constantly staffed immune function facility.
Conclusions
Autologous BMMNC infusion for adults with severe TBI is safe and logistically feasible. There is a potential signal of treatment effects in terms of structural preservation of critical CNS architecture, mimicking pre-clinical data in rodents.48 These structural data correlate with a dampening of the pro-inflammatory signaling. A Phase 2b trial has been planned to evaluate structural outcomes as the primary endpoint, eliminating the high-dose regimen.
Supplementary Material
Table 3. Treatment Effect Size Estimation (Cohen's d)-Corpus Callosal DTI Metrics.
We estimated the effect size of BMMNC treatment on DTI metrics of corpus callosal integrity as a region of interest. These data are important in planning a Phase 2b trial with CC DTI metrics as an outcome measure. BMMNC has a moderate to strong treatment effect on FA in the CC (Cohen's d 0.5-0.8). These data would allow a sample size estimation for a study powered at 0.8 with a Cohen's d between 26 (0.8) and 64. Adding an exclusion in future trials for hemispheric stroke/contusion volume threshold value that invalidates volumetric/DTI metrics would also make MD a powerful outcome measure. These data and the outcome correlates (Supplemental Table 3) also suggest that CC DTI metrics are useful primary outcome measures.
| 6 Month - All patients | ||||||
|---|---|---|---|---|---|---|
| Untreated | Pooled Treated | Untreated vs. Treated | ||||
| (n = 9) | (n = 15) | |||||
| Mean | SD | Mean | SD | Cohen's d | 95% CI | |
| Whole CC FA | 0.536 | 0.049 | 0.563 | 0.067 | 0.442 | −0.394- 1.278 |
| Whole CC MD | 0.986 | 0.049 | 0.969 | 0.100 | −0.200 | −1.028- 0.629 |
| Whole CC RD | 0.654 | 0.066 | 0.623 | 0.124 | −0.291 | −1.121- 0.540 |
| Whole CC AD | 1.650 | 0.053 | 1.661 | 0.080 | 0.154 | −0.673- 0.982 |
| GOS-E | 4.444 | 1.509 | 4.200 | 1.320 | −0.175 | −1.003- 0.653 |
| DRS | 4.889 | 4.285 | 5.267 | 2.738 | 0.112 | −0.715- 0.939 |
| 6 Month - #19 & #20 Excluded | ||||||
|---|---|---|---|---|---|---|
| Untreated | Pooled Treated | Control vs. Treated | ||||
| (n = 9) | (n = 13) | |||||
| Mean | SD | Mean | SD | Cohen's d | 95% CI | |
| Whole CC FA | 0.536 | 0.049 | 0.579 | 0.054 | 0.846 | −0.040- 1.732 |
| Whole CC MD | 0.986 | 0.049 | 0.938 | 0.052 | −0.944 | −1.839- −0.050 |
| Whole CC RD | 0.654 | 0.066 | 0.586 | 0.073 | −0.968 | −1.864- −0.071 |
| Whole CC AD | 1.650 | 0.053 | 1.643 | 0.064 | −0.117 | −0.968- 0.734 |
| GOS-E | 4.444 | 1.509 | 4.308 | 1.377 | −0.095 | −0.945- 0.755 |
| DRS | 4.889 | 4.285 | 4.923 | 2.783 | 0.010 | −0.840- 0.860 |
Acknowledgements
Research Coordinators: Steven Kosmach, RN., MSN, Mary-Clare Day, RN, BSN; Fernando Jimenez, RN, MS.
Cell Processing Team: Andrew Havens, Sufira Kiran, James Roye, Philippa Smith, Suchit Sahai, Ph.D., Marysuna Wilkerson.
Technical Support: Anthony Moore10
All Cellular Processing was performed at UTHealth-Medical School, The Evelyn H. Griffin Stem Cell Therapeutics Research Laboratory, FDA Establishment Identifier 3009561521.
Funding Sources: DOD Grant: W81XWH-11-1-0460 (Cox, PI); NIH 2T32 GM 0879201-11 (Holcomb, PI); Glassell Foundation Stem Cell Research Program (Cox, PI). Brown Foundation (Cox, PI).
Footnotes
Author Contributions:
Charles S Cox, Jr.1 Conception and design, financial support, administrative support, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Robert A Hetz2 Collection and assembly of data, data analysis and interpretation.
George P Liao2 Collection and assembly of data, data analysis and interpretation.
Benjamin M Aertker2 Collection and assembly of data, data analysis and interpretation.
Linda Ewing-Cobbs4,5 Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing.
Jennifer Juranek4 Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing.
Sean I. Savitz3 Conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing.
Margaret Jackson1 Collection and assembly of data, data analysis and interpretation, manuscript writing.
Anna M Romanowska-Pawliczek4 Collection and assembly of data, data analysis and interpretation.
Fabio Triolo1 Provision of study material; collection and assembly of data.
Pramod K.Dash6 Collection and assembly of data, data analysis and interpretation.
Claudia Pedroza7 Conception and design data analysis and interpretation.
Dean Lee9 Provision of study material or patients.
Laura Worth10 Provision of study material or patients
Imoigele Aisiku11 Provision of study material or patients.
HuiMahn Choi3,8 Provision of study material or patients, administrative report, manuscript writing.
John Holcomb2 Provision of study material or patients, administrative support.
Ryan Kitagawa8 Provision of study material or patients, administrative support, manuscript writing.
References
- 1.Centers for Disease Control and Prevention . Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation. National Center for Injury Prevention and Control; Division of Unintentional Injury Prevention; Atlanta, GA.: 2015. [Google Scholar]
- 2.Kraus JF, McArthur DL. Epidemiologic aspects of brain injury. NEUROL CLIN. 1996;14:435–50. doi: 10.1016/s0733-8619(05)70266-8. [DOI] [PubMed] [Google Scholar]
- 3.Savitz SI, Cox CS., Jr. Concise review: Cell therapies for stroke and traumatic brain injury: Targeting microglia. Stem Cells. 2016;34:537–42. doi: 10.1002/stem.2253. [DOI] [PubMed] [Google Scholar]
- 4.Sharma S, Yang B, Strong R, et al. Bone marrow mononuclear cells protect neurons and modulate microglia in cell culture models of ischemic stroke. J Neurosci Res. 2010;88:2869–76. doi: 10.1002/jnr.22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suda S, Yang B, Schaar K, et al. Autologous Bone Marrow Mononuclear Cells Exert Broad Effects on Short- and Long-Term Biological and Functional Outcomes in Rodents with Intracerebral Hemorrhage. Stem Cells Dev. 2015;24:2756–66. doi: 10.1089/scd.2015.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedi SS, Walker PA, Shah SK, et al. Autologous bone marrow mononuclear cells therapy attenuates activated microglial/macrophage response and improves spatial learning after traumatic brain injury. J Trauma Acute Care Surg. 2013;75:410–6. doi: 10.1097/TA.0b013e31829617c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang B, Strong R, Sharma S, et al. Therapeutic time window and dose response of autologous bone marrow mononuclear cells for ischemic stroke. J Neurosci Res. 2011;89:833–9. doi: 10.1002/jnr.22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox CS, Jr, Baumgartner JE, Harting MT, et al. Autologous bone marrow mononuclear cell therapy for severe traumatic brain injury in children. Neurosurgery. 2011;68:588–600. doi: 10.1227/NEU.0b013e318207734c. [DOI] [PubMed] [Google Scholar]
- 9.Brenneman M, Sharma S, Harting M, et al. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 2010;30:140–9. doi: 10.1038/jcbfm.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savitz SI, Misra V, Kasam M, et al. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011;70:59–69. doi: 10.1002/ana.22458. [DOI] [PubMed] [Google Scholar]
- 11.Fischer UM, Harting MT, Jimenez F, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–91. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teasdale G, Jennett B. ASSESSMENT OF COMA AND IMPAIRED CONSCIOUSNESS. A Practical Scale. Lancet. 1974;304:81–4. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 13.Brain Trauma Foundation B, AANS, CNS, Care ACJSoNaC Guidelines for the management of severe traumatic brain injury. Journal of Neurotrauma. (3rd edition.) 2007;24:S1–S106. doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- 14.Antonelli M, Moreno R, Vincent JL, et al. Application of SOFA score to trauma patients. Intensive Care Med. 1999;25:389–94. doi: 10.1007/s001340050863. [DOI] [PubMed] [Google Scholar]
- 15.Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. AM REV RESPIR DIS. 1988;138:720–3. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 16.Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR. Diffuse axonal injury in head injury: Definition, diagnosis and grading. Histopathology. 1989;15:49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 17.Salamon N, Sicotte N, Alger J, et al. Analysis of the brain-stem white-matter tracts with diffusion tensor imaging. Neuroradiology. 2005;47:895–902. doi: 10.1007/s00234-005-1439-8. [DOI] [PubMed] [Google Scholar]
- 18.Wilde EA, Hunter JV, Newsome MR, et al. Frontal and temporal morphometric findings on MRI in children after moderate to severe traumatic brain injury. J Neurotrauma. 2005;22:333–44. doi: 10.1089/neu.2005.22.333. [DOI] [PubMed] [Google Scholar]
- 19.Bagiella E, Novack TA, Ansel B, et al. Measuring outcome in traumatic brain injury treatment trials: Recommendations from the traumatic brain injury clinical trials network. J Head Trauma Rehabil. 2010;25:375–82. doi: 10.1097/HTR.0b013e3181d27fe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ewing-Cobbs L, Prasad MR, Swank P, et al. Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: Relation to neurobehavioral outcomes. NeuroImage. 2008;42:1305–15. doi: 10.1016/j.neuroimage.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harting MT, Jimenez F, Xue H, et al. Intravenous mesenchymal stem cell therapy for traumatic brain injury: Laboratory investigation. J Neurosurg. 2009;110:1189–97. doi: 10.3171/2008.9.JNS08158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker PA, Shah SK, Jimenez F, et al. Intravenous multipotent adult progenitor cell therapy for traumatic brain injury: Preserving the blood brain barrier via an interaction with splenocytes. Exp Neurol. 2010;225:341–52. doi: 10.1016/j.expneurol.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anbari F, Khalili MA, Bahrami AR, et al. Intravenous transplantation of bone marrow mesenchymal stem cells promotes neural regeneration after traumatic brain injury. Neural Regen Res. 2014;9:919–23. doi: 10.4103/1673-5374.133133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arien-Zakay H, Gincberg G, Nagler A, et al. Neurotherapeutic effect of cord blood derived CD45+ hematopoietic cells in mice after traumatic brain injury. J Neurotrauma. 2014;31:1405–16. doi: 10.1089/neu.2013.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang J, Bu X, Liu M, Cheng P. Transplantation of autologous bone marrow-derived mesenchymal stem cells for traumatic brain injury. Neural Regen Res. 2012;7:46–53. doi: 10.3969/j.issn.1673-5374.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HJ, Lee JH, Kim SH. Therapeutic effects of human mesenchymal stem cells on traumatic brain injury in rats: secretion of neurotrophic factors and inhibition of apoptosis. J Neurotrauma. 2010;27:131–8. doi: 10.1089/neu.2008.0818. [DOI] [PubMed] [Google Scholar]
- 27.Li L, Jiang Q, Qu CS, et al. Transplantation of marrow stromal cells restores cerebral blood flow and reduces cerebral atrophy in rats with traumatic brain injury: in vivo MRI study. J Neurotrauma. 2011;28:535–45. doi: 10.1089/neu.2010.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu D, Sanberg PR, Mahmood A, et al. Intravenous administration of human umbilical cord blood reduces neurological deficit in the rat after traumatic brain injury. Cell Transplant. 2002;11:275–81. [PubMed] [Google Scholar]
- 29.Mahmood A, Lu D, Chopp M. Marrow stromal cell transplantation after traumatic brain injury promotes cellular proliferation within the brain. Neurosurgery. 2004;55:1185–93. doi: 10.1227/01.neu.0000141042.14476.3c. [DOI] [PubMed] [Google Scholar]
- 30.Mahmood A, Lu D, Lu M, Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697–702. doi: 10.1227/01.neu.0000079333.61863.aa. discussion -3. [DOI] [PubMed] [Google Scholar]
- 31.Osanai T, Kuroda S, Sugiyama T, et al. Therapeutic effects of intra-arterial delivery of bone marrow stromal cells in traumatic brain injury of rats--in vivo cell tracking study by near-infrared fluorescence imaging. Neurosurgery. 2012;70:435–44. doi: 10.1227/NEU.0b013e318230a795. discussion 44. [DOI] [PubMed] [Google Scholar]
- 32.Pischiutta F, D'Amico G, Dander E, et al. Immunosuppression does not affect human bone marrow mesenchymal stromal cell efficacy after transplantation in traumatized mice brain. Neuropharmacology. 2014;79:119–26. doi: 10.1016/j.neuropharm.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Turtzo LC, Budde MD, Dean DD, et al. Failure of intravenous or intracardiac delivery of mesenchymal stromal cells to improve outcomes after focal traumatic brain injury in the female rat. PLoS One. 2015;10:e0126551. doi: 10.1371/journal.pone.0126551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe J, Shetty AK, Hattiangady B, et al. Administration of TSG-6 improves memory after traumatic brain injury in mice. Neurobiol Dis. 2013;59:86–99. doi: 10.1016/j.nbd.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zanier ER, Montinaro M, Vigano M, et al. Human umbilical cord blood mesenchymal stem cells protect mice brain after trauma. Crit Care Med. 2011;39:2501–10. doi: 10.1097/CCM.0b013e31822629ba. [DOI] [PubMed] [Google Scholar]
- 36.Zhang R, Liu Y, Yan K, et al. Anti-inflammatory and immunomodulatory mechanisms of mesenchymal stem cell transplantation in experimental traumatic brain injury. J Neuroinflammation. 2013;10:106. doi: 10.1186/1742-2094-10-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moll G, Rasmusson-Duprez I, Von Bahr L, et al. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells. 2012;30:1565–74. doi: 10.1002/stem.1111. [DOI] [PubMed] [Google Scholar]
- 38.Cotton BA, Minei KM, Radwan ZA, et al. Admission rapid thrombelastography predicts development of pulmonary embolism in trauma patients. J Trauma Acute Care Surg. 2012;72:1470–7. doi: 10.1097/TA.0b013e31824d56ad. [DOI] [PubMed] [Google Scholar]
- 39.François M, Copland IB, Yuan S, Romieu-Mourez R, Waller EK, Galipeau J. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-γ licensing. Cytotherapy. 2012;14:147–52. doi: 10.3109/14653249.2011.623691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Y, McArthur DL, Alger JR, et al. Early nonischemic oxidative metabolic dysfunction leads to chronic brain atrophy in traumatic brain injury. J Cereb Blood Flow Metab. 2010;30:883–94. doi: 10.1038/jcbfm.2009.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohtaki H, Ylostalo JH, Foraker JE, et al. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci U S A. 2008;105:14638–43. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanberg PR, Park D, Kuzmin-Nichols N, et al. Monocyte transplantation for neural and cardiovascular ischemia repair. J Cell Mol Med. 2010;14:553–63. doi: 10.1111/j.1582-4934.2009.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loane DJ, Kumar A, Stoica BA, Cabatbat R, Faden AI. Progressive neurodegeneration after experimental brain trauma: Association with chronic microglial activation. J Neuropathol Exp Neurol. 2014;73:14–29. doi: 10.1097/NEN.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sidaros A, Skimminge A, Liptrot MG, et al. Long-term global and regional brain volume changes following severe traumatic brain injury: A longitudinal study with clinical correlates. NeuroImage. 2009;44:1–8. doi: 10.1016/j.neuroimage.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 45.Brezova V, Gøran Moen K, Skandsen T, et al. Prospective longitudinal MRI study of brain volumes and diffusion changes during the first year after moderate to severe traumatic brain injury. NeuroImage Clin. 2014;5:128–40. doi: 10.1016/j.nicl.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trivedi MA, Ward MA, Hess TM, et al. Longitudinal changes in global brain volume between 79 and 409 days after traumatic brain injury: Relationship with duration of coma. J Neurotrauma. 2007;24:766–71. doi: 10.1089/neu.2006.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ding K, De La Plata CM, Wang JY, et al. Cerebral atrophy after traumatic white matter injury: Correlation with acute neuroimaging and outcome. J Neurotrauma. 2008;25:1433–40. doi: 10.1089/neu.2008.0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vahidy FS, Rahbar MH, Zhu H, Rowan PJ, Bambhroliya AB, Savitz SI. Systematic Review and Meta- Analysis of Bone Marrow-Derived Mononuclear Cells in Animal Models of Ischemic Stroke. Stroke. 2016;47:1632–9. doi: 10.1161/STROKEAHA.116.012701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.