Abstract
Objective
To examine associations of demographic, perinatal, and infant feeding characteristics with offspring body composition at ~5 months.
Study design
We collected data on 640 mother/offspring pairs from early pregnancy through ~5 months of age. We assessed offspring body composition with air displacement plethysmography at birth and ~5 months. Linear regression analyses examined associations between predictors and fat-free mass (FFM), fat mass (FM), and percent fat mass (adiposity) at ~5 months. Secondary models further adjusted for body composition at birth and rapid infant growth.
Results
Greater pre-pregnant BMI and gestational weight gain were associated with greater FFM at ~5 months, but not after adjustment for FFM at birth. Greater gestational weight gain was also associated with greater FM at ~5 months, independent of FM at birth and rapid infant growth, although this did not translate into increased adiposity. Greater percent time of exclusive breastfeeding was associated with lower FFM (−311g, p<0.001), greater FM (+224g, p<0.001), and greater adiposity (+3.51%, p<0.001). Compared with offspring of non-Hispanic white mothers, offspring of Hispanic mothers had greater adiposity (+2.72%, p<0.001) and offspring of non-Hispanic black mothers had lower adiposity (−1.93%, p<0.001). Greater adiposity at birth predicted greater adiposity at ~5 months, independent of infant feeding and rapid infant growth.
Conclusions
There are clear differences in infant body composition by demographic, perinatal, and infant feeding characteristics, although our data also show that increased adiposity at birth persists through ~5 months of life. Our findings warrant further research into implications of differences in infant body composition.
Keywords: Breastfeeding, maternal obesity, race, ethnicity, body composition
The developmental origins of obesity theory posits that early life exposures, including intrauterine exposures, influence obesity risk across the lifespan.1 There is compelling evidence that intrauterine exposure to maternal obesity is associated with increased obesity in offspring across the lifespan.2 We and others have previously reported that greater maternal pre-pregnant body mass index (BMI) and gestational weight gain are each associated with greater offspring body size and adiposity at birth3, 4, 5. However, the degree to which these associations persist into the postnatal period is not well understood.
There is evidence that intrauterine exposure to maternal obesity is associated with greater offspring fat mass and/or adiposity in the first few months of life.6, 7 Other studies report no associations,8–10 or evidence of slowed “catch-down” growth, in fat mass and/or adiposity during infancy among offspring exposed to maternal obesity.11, 12 Some of the discrepancy between studies may be explained by differences in methodology, including the age at which infant body composition is measured. Other important considerations that vary across studies include adjustment for size at birth or speed of growth, as well as evaluation of the effect of infant feeding (breastmilk, formula, complementary foods). Infant feeding is particularly important to examine, as it has been shown to modify the effect of adverse intrauterine exposures on offspring obesity risk.13
The main purpose of this analysis was to evaluate whether maternal pre-pregnant BMI, gestational weight gain and breastfeeding exclusivity are independently associated with offspring body size and composition at 5 months. In addition, we sought to determine whether these effects are independent of body composition at birth and early infant growth status. We also explored interactions between pre-pregnant BMI, gestational weight gain, and breastfeeding to understand whether infant feeding modified the effect of intrauterine exposure to maternal obesity on offspring body composition.
Methods
The Healthy Start study is an ongoing pre-birth cohort in Denver, Colorado. From 2009–2014, we recruited women in early pregnancy from obstetric clinics at the University of Colorado Anschutz Medical Campus. Women were eligible if they were ≥16 years of age, <24 weeks pregnant with a single fetus, had no chronic medical conditions (Type 2 diabetes, cancer, etc), and no history of obstetric complications (prior stillbirth or birth <25 weeks). Participants completed research visits in early pregnancy (median 17 weeks), mid-pregnancy (median 27 weeks) and at delivery (median 1 day post-birth). The original protocol included a postnatal phone interview (median 5.2 months), which was converted to an in-person visit in January 2011. The study was approved by the Colorado Multiple Institutional Review Board and all women provided written informed consent.
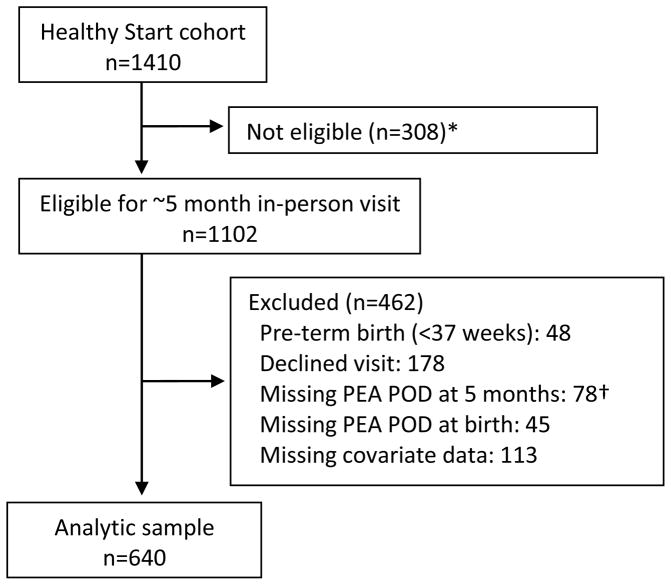
We enrolled 1410 women in Healthy Start, of which 1102 were eligible for the in-person infant visit when it was added to the protocol (Figure; available at www.jpeds.com). Participants were eligible for the current analysis if infants were full-term (gestational age ≥37 weeks), had body composition measurements at birth and ~5 months, and complete data on all covariates.
Figure.
Study flow diagram.
*5 month postnatal visit introduced later in study protocol.
†76 declined measure, 2 exceeded PEA POD weight limit of 10 kg.
Exposure assessments
Women self-reported demographic information at enrollment including date of birth, race/ethnicity, education, annual household income, and gravidity. At the pregnancy and delivery visits, women self-reported smoking, alcohol consumption, and vitamin use. Following delivery, we obtained data on pre-pregnancy body mass index (BMI), gestational weight gain, diagnosis of gestational diabetes, date of delivery, mode of delivery, offspring sex, birth weight, birth length, and gestational age at birth from clinical records. At the ~5 month visit, women reported breastfeeding status, use of formula (including infant age at introduction and relative proportion of formula to breastmilk), and introduction of solid foods (i.e., age at which the infant began consuming foods other than breastmilk, formula, or water on a daily basis). We quantified breastfeeding with breastmilk-months, a metric which reflects both duration and exclusivity of breastfeeding. For example, 6 months of exclusive breastfeeding equates to 6 breastmilk-months while 4 months of exclusive breastfeeding followed by 2 months of 50% breastmilk and 50% formula equates to 5 breastmilk-months. To account for differences in age at the infant visit, we divided breastmilk-months by offspring age to obtain the percent time each infant was exclusively breastfed. This data can be interpreted on a continuous scale, such that 0% indicates exclusively formula-fed, 50% indicates half breastfed, half formula-fed, and 100% indicates exclusively breastfed from birth to the ~5 month visit.
Outcome assessments
Offspring body composition was measured at the in-person delivery and infant visits via air displacement plethysmography (PEA POD, COSMED, Rome, Italy), which has excellent validity and reliability in infants.14, 15 The PEA POD device measures total body volume by detecting differences in air pressure between a test chamber in which the infant is placed and a control chamber with known air pressure.16 Body mass and volume measurements are used to calculate body density, and fat and fat-free mass density coefficients17 are used to obtain estimates of fat-free mass (g), fat mass (g), and adiposity (percent fat mass). Trained research personnel measured each offspring outcome twice, with a third measurement obtained when percent fat mass differed by >2.0%. The average of the two closest readings was used for analysis. We quantified early infant growth by calculating birth and ~5 month sex-specific weight-for age z-scores (WAZ) using the 2006 World Health Organization growth charts. Rapid growth was defined as a change >0.67 in WAZ from birth to the ~5 month visit, which corresponds to upward crossing of a percentile band on standardized growth charts and indicates a clinically significant increase in weight.18
Data analyses
Analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC, USA). We fit separate multivariable linear regression models for the outcomes of fat-free mass, fat mass, and adiposity. The primary predictors of interest were maternal pre-pregnant BMI, gestational weight gain, and percent time of exclusive breastfeeding. We used a backwards stepwise model-fitting approach, wherein non-significant covariates were removed, starting with the highest p-value. As covariates, we considered the continuous variables of maternal age, gravidity, weeks of daily prenatal vitamin use, gestational age at birth, and offspring age at the postnatal visit; categorical variables of race/ethnicity, education, and household income; and binary variables for introduction of solids by the ~5 month visit, diagnosis of gestational diabetes, any maternal smoking during pregnancy, any maternal alcohol consumption during pregnancy, mode of delivery, and offspring sex. We also considered second- and third-order interactions, hypothesized a priori, between maternal pre-pregnant BMI, gestational weight gain, and percent time of exclusive breastfeeding. Examination of jackknifed studentized residuals confirmed the assumptions of normality and homoscedasticity. Variance inflation factors were inspected to confirm that multicollinearity between predictors was not prohibitive (all <2.0). We removed covariates which were statistically non-significant (p>0.05) for all body composition measures, including the interaction terms, and present three models for interpretation. Model 1 includes the primary predictors (pre-pregnant BMI, gestational weight gain, breastfeeding exclusivity) and significant covariates (race/ethnicity and sex). Model 2 includes Model 1 predictors plus the respective body composition measure at birth (e.g. fat-free mass at birth for the model predicting fat-free mass at ~5 months). Model 3 includes Model 2 predictors and additionally adjusts for the binary status of rapid growth in WAZ from birth to ~5 months.
Results
Of the 1102 participants eligible for the postnatal exam, complete data were available for 640 participants (Figure). As shown in Table I, these 640 participant-dyads were similar to the larger eligible cohort with respect to maternal-offspring characteristics, including race/ethnicity, education, pre-pregnancy BMI, gestational weight gain, infant feeding, and infant size and body composition at birth and ~5 months.
Table 1.
Characteristics of mothers and offspring participating in the Healthy Start study.
| Postnatal cohort n=1102 |
Analytic cohort n=640 |
|
|---|---|---|
| Maternal characteristics | ||
| Age at delivery (years) | 28.8 (6.1) | 29.1 (6.0) |
| Race | ||
| Hispanic | 261 (24%) | 155 (24%) |
| Non-Hispanic white | 613 (56%) | 364 (57%) |
| Non-Hispanic black | 153 (14%) | 85 (13%) |
| Other | 75 (7%) | 36 (6%) |
| Education | ||
| <12 years | 130 (12%) | 77 (12%) |
| High school degree | 191 (17%) | 94 (15%) |
| College classes or college degree | 781 (71%) | 469 (73%) |
| Household income | ||
| <$40,000 | 304 (28%) | 179 (28%) |
| $40,000–$70,000 | 219 (20%) | 127 (20%) |
| >$70,000 | 387 (35%) | 226 (35%) |
| Missing/don’t know | 192 (17%) | 108 (17%) |
| Gravidity (n) | 1.4 (1.5) | 1.4 (1.6) |
| Smoking during pregnancy (n) | 81 (7%) | 41 (6%) |
| Alcohol during pregnancy (n) | 220 (20%) | 130 (20%) |
| Gestational diabetes (n) | 44 (4%) | 24 (4%) |
| Pre-pregnant BMI (kg/m2) | 25.7 (6.1) | 25.7 (6.3) |
| Underweight | 35 (3%) | 20 (3%) |
| Lean | 564 (51%) | 324 (51%) |
| Overweight | 288 (26%) | 174 (27%) |
| Obese | 215 (20%) | 122 (19%) |
| Gestational weight gain (kg) | 13.6 (6.5) | 13.9 (6.2) |
| Inadequate (n) | 270 (25%) | 138 (22%) |
| Adequate (n) | 319 (29%) | 196 (31%) |
| Excessive (n) | 513 (47%) | 306 (48%) |
| Prenatal multivitamins (weeks of daily use) | 35.0 (13.4) | 35.6 (13.5) |
| Offspring characteristics | ||
| Female (n) | 536 (49%) | 324 (51%) |
| Gestational age at birth (weeks) | 39.4 (1.6) | 39.6 (1.1) |
| Cesarean delivery (n) | 226 (21%) | 129 (20%) |
| Body size, composition at birth | ||
| Weight (g) | 3237 (498) | 3281 (422) |
| Weight-for-age z-score | −0.17 (0.92) | −0.05 (0.89) |
| Fat-free mass (g) | 2841 (346) | 2846 (325) |
| Fat mass (g) | 297 (152) | 296 (147) |
| Adiposity (%) | 9.2 (3.9) | 9.1 (3.9) |
| Age at postnatal visit (months) | 5.5 (1.5) | 5.1 (1.2) |
| Breastfeeding category (n) | ||
| Exclusive breastfeeding | 393 (42%) | 287 (45%) |
| Formula only | 60 (6%) | 39 (6%) |
| Mixed feeding | 492 (52%) | 312 (49%) |
| Percent time exclusively breastfed | 0.73 (0.37) | 0.72 (0.36) |
| Started solids (n) | 387 (43%) | 296 (46%) |
| Body size, composition at ~5 months | ||
| Weight (g) | 6929 (980) | 6818 (864) |
| Weight-for-age z-score | −0.46 (0.94) | −0.49 (0.91) |
| Fat-free mass (g) | 5147 (650) | 5149 (618) |
| Fat mass (g) | 1649 (493) | 1669 (491) |
| Adiposity (%) | 24.0 (5.4) | 24.2 (5.3) |
| Early infant growth | ||
| Change in weight-for-age z-score ≥0.67 (n) | 153 (17%) | 74 (12%) |
Data are mean (SD) or n (%).
The associations of maternal, perinatal, and infant feeding characteristics with offspring body composition at ~5 months is reported in Table II in sequentially adjusted models. The interactions between pre-pregnant BMI, gestational weight gain, and breastfeeding were all statistically non-significant; thus we present and interpret only the main effects.
Table 2.
Predictors of infant body composition at ~5 months of age.
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| β (SE) | p | β (SE) | p | β (SE) | p | |
| Fat-free mass (g) | ||||||
| Race (reference = NHW) | ||||||
| Hispanic | 79 (45) | 0.08 | 53 (38) | 0.16 | 18 (36) | 0.62 |
| Black | 93 (56) | 0.10 | 201 (49) | <0.001 | 165 (46) | <0.001 |
| Other | 19 (77) | 0.80 | 44 (66) | 0.51 | 2 (63) | 0.97 |
| Pre-pregnant BMI (kg/m2) | 6 (3) | 0.05 | −1 (3) | 0.72 | 0 (2) | 0.99 |
| Gestational weight gain (kg) | 6 (3) | 0.03 | 0 (3) | 0.94 | 1 (2) | 0.56 |
| Sex (ref = male) | −482 (35) | <0.001 | −345 (31) | <0.001 | −355 (30) | <0.001 |
| GA at birth (ref = 37 weeks) | 112 (16) | <0.001 | 8 (16) | 0.61 | 16 (15) | 0.29 |
| Percent time exclusively breastfed (%) | −311 (53) | <0.001 | −417 (46) | <0.001 | −375 (44) | <0.001 |
| Fat-free mass at birth (%) | 1 (0) | <0.001 | 1 (0) | <0.001 | ||
| Rapid growth in WAZ (ref = no) | 411 (47) | <0.001 | ||||
| R2 | 0.50 | 0.64 | 0.68 | |||
| Fat mass (g) | ||||||
| Race (reference = NHW) | ||||||
| Hispanic | 267 (47) | <0.001 | 272 (46) | <0.001 | 221 (42) | <0.001 |
| Black | −141 (59) | 0.02 | −110 (59) | 0.06 | −169 (53) | 0.002 |
| Other | 88 (81) | 0.28 | 101 (80) | 0.21 | 36 (72) | 0.62 |
| Pre-pregnant BMI (kg/m2) | 3 (3) | 0.30 | 0 (3) | 0.98 | 1 (3) | 0.67 |
| Gestational weight gain (kg) | 5 (3) | 0.08 | 4 (3) | 0.26 | 6 (3) | 0.03 |
| Sex (ref = male) | 50 (37) | 0.18 | 25 (37) | 0.50 | −15 (33) | 0.65 |
| GA at birth (ref = 37 weeks) | 34 (17) | 0.05 | 21 (17) | 0.23 | 40 (16) | 0.01 |
| Percent time exclusively breastfed (%) | 224 (56) | <0.001 | 204 (55) | 0.0003 | 276 (50) | <0.001 |
| Fat mass at birth (%) | 1 (0) | <0.001 | 1 (0) | <0.001 | ||
| Rapid growth in WAZ (ref = no) | 651 (54) | <0.001 | ||||
| R2 | 0.13 | 0.16 | 0.31 | |||
| Adiposity (%) | ||||||
| Race (reference = NHW) | ||||||
| Hispanic | 2.72 (0.50) | <0.001 | 2.80 (0.49) | <0.001 | 2.36 (0.46) | <0.001 |
| Black | −1.93 (0.63) | 0.002 | −1.70 (0.62) | 0.01 | −2.23 (0.59) | 0.0002 |
| Other | 1.08 (0.86) | 0.21 | 1.22 (0.85) | 0.15 | 0.67 (0.80) | 0.40 |
| Pre-pregnant BMI (kg/m2) | 0.00 (0.03) | 0.94 | −0.03 (0.03) | 0.43 | −0.01 (0.03) | 0.63 |
| Gestational weight gain (kg) | 0.04 (0.03) | 0.25 | 0.02 (0.03) | 0.49 | 0.05 (0.03) | 0.13 |
| Sex (ref = male) | 2.30 (0.39) | <0.001 | 1.92 (0.39) | <0.001 | 1.56 (0.37) | <0.001 |
| GA at birth (ref = 37 weeks) | −0.01 (0.18) | 0.93 | −0.08 (0.18) | 0.64 | 0.10 (0.17) | 0.55 |
| Percent time exclusively breastfed (%) | 3.51 (0.59) | <0.001 | 3.36 (0.59) | <0.001 | 4.00 (0.55) | <0.001 |
| Adiposity at birth (%) | 0.22 (0.05) | <0.001 | 0.29 (0.05) | <0.001 | ||
| Rapid growth in WAZ (ref = no) | 5.57 (0.59) | <0.001 | ||||
| R2 | 0.17 | 0.19 | 0.28 | |||
NHW, non-Hispanic White; BMI, body mass index; GA, gestational age; WAZ, weight-for-age z-score
Estimates obtained from multivariable linear regression models conducted separately for each outcome. Other predictors considered and removed due to statistical non-significance include: maternal age, education, household income, gravidity, gestational diabetes, prenatal smoking, prenatal alcohol consumption, weeks of daily prenatal vitamin use, mode of delivery, introduction to solids, and second- and third-order interactions between pre-pregnant BMI, gestational weight gain, and breastfeeding.
Pre-pregnant BMI and gestational weight gain
Increasing pre-pregnant BMI and gestational weight gain were each associated with increasing offspring fat-free mass at ~5 months (Model 1, +6g, p<0.05 for both), but these associations were attenuated to non-significance after adjustment for fat-free mass at birth (Model 2) and remained non-significant with additional adjustment for rapid infant growth (Model 3). In contrast, increasing gestational weight gain was associated with increasing absolute fat mass at ~5 months (Model 1, +5g, p=0.08), and this association was independent of both fat mass at birth (Model 2, +4g, p=0.26) and rapid infant growth (Model 3, +6g, p<0.05). However, neither pre-pregnant BMI nor gestational weight gain were associated with fat mass percent (adiposity) at 5 months.
Exclusive breastfeeding
A greater percent time of exclusive breastfeeding was associated with reduced fat-free mass (−311g, p<0.001) and increased fat mass (244g, p<0.001), resulting in significantly greater adiposity (3.51%, p<0.001). These associations were consistent across all three models, thus independent of race/ethnicity, sex, maternal factors, body composition at birth and rapid infant growth status.
Demographic characteristics
Race/ethnicity
In Model 1, compared with offspring of non-Hispanic white mothers, offspring of Hispanic mothers had significantly greater fat mass (+267g, p<0.001) and adiposity (2.72%, p<0.001), while offspring of non-Hispanic black mothers had significantly lower fat mass (+141g, p=0.02) and adiposity (−1.93%, p<0.001), independent of maternal pre-pregnant BMI, gestational weight gain, and breastfeeding exclusivity. These effects persisted after adjustment for body composition at birth (Model 2) and rapid infant growth (Model 3); however, with these additional adjustments, offspring of non-Hispanic black mothers also displayed significantly higher fat-free mass (+165g, p<0.001) compared with offspring of non-Hispanic white women.
Infant sex
In Model 1, compared with male offspring, female offspring had lower fat-free mass (−482g, p<0.001), with no significant differences in absolute fat mass levels, thus resulting in increased adiposity at ~5 months (+2.30%, p<0.001), independent of race/ethnicity, maternal BMI and gestational weight gain. Further adjustment for body composition at birth (Model 2) and rapid growth status (Model 3) did not influence these results.
Body composition measures at birth were significant predictors of the respective body composition measures at ~5 months, independent of prenatal and postnatal factors. For example, a 1% increase in adiposity at birth was associated with a 0.3% increase in adiposity at ~5 months, independent of demographic maternal, infant feeding characteristics and rapid growth.
Rapid infant growth
Finally, offspring who exhibited rapid growth in WAZ had significantly greater fat-free mass, fat mass, and adiposity at ~5 months. Of note, these effects were independent of body composition measures at birth.
Other predictors that were considered and removed due to statistical non-significance include: maternal age, education, household income, gravidity, gestational diabetes, prenatal smoking, prenatal alcohol consumption, weeks of daily prenatal vitamin use, mode of delivery, introduction to solids, and second- and third-order interactions between pre-pregnant BMI, gestational weight gain, and breastfeeding.
Discussion
We found that increasing maternal gestational weight gain was significantly associated with an increase in offspring fat mass at ~5 months, independent of race/ethnicity, pre-pregnant BMI, fat mass at birth, breastfeeding exclusivity and rapid infant growth. However, due to similar effects on fat free mass, these did not translate in a significant increase in relative fat mass (adiposity). Increased breastfeeding was associated with reduced fat-free mass, but increased fat mass, and increased relative fat mass (adiposity). Offspring who grew rapidly from birth to ~5 months gained substantially more fat mass than fat-free mass, resulting in 5% more adiposity at ~5 months, independent of all other pre and postnatal factors considered. Importantly, our data provided evidence that increased infant adiposity at birth persists through 5 months of age, independent of demographic, maternal, and early infant feeding and growth characteristics.
We previously reported that maternal pre-pregnant BMI and gestational weight gain were each independent predictors of offspring fat mass, fat-free mass, and adiposity at birth.3 Here we show that maternal pre-pregnant BMI is no longer significantly associated with fat mass and adiposity ~5 months, and the association with fat free mass is attenuated to non-significance by adjustment for fat-free mass at birth. Furthermore, although gestational weight gain was a significant predictor of fat mass at ~5 months, independent of both fat mass at birth, breastfeeding, and early infant growth, the increase in fat mass did not translate into a corresponding increase in adiposity (fat mass percent). Thus, pre-pregnant BMI and gestational weight gain were no longer significantly associated with adiposity at ~5 months. However, they did predict greater adiposity at birth,3 which in turn, as we show here, is a significant predictor of adiposity at ~5 months, regardless of early growth patterns. As offspring continue to grow, adopt more postnatal behaviors modeled by mothers/families, and transition through other critical developmental periods such as puberty, it is possible that associations between maternal and offspring obesity will again become apparent. Given the complexity of early life influences on infant body composition, further work is needed to understand how infant body composition is related to future health and disease risk.
Infants with a greater percent time of exclusive breastfeeding were smaller overall at ~5 months: fat-free mass was lower by 311g and fat mass was higher by 224g resulting in a net body size decrease of 98g among infants exclusively breastfed for 100% of the time versus 0% during the first ~5 months. Although these exclusively breastfed babies are smaller overall, they have an increased relative fat mass (adiposity). These findings are seemingly in contrast to the collective body of evidence suggesting a small but consistent protective effect of breastfeeding against obesity19 and adiposity20 across the life course. However, our findings are consistent with a 2012 meta-analysis of 15 smaller studies that reported reduced fat-free mass, greater fat mass, and greater adiposity among breastfed offspring at 3–6 months compared with formula-fed offspring.21 Further, by 12 months of age, formula-fed infants have significantly more fat-free mass and a tendency towards increased fat mass (p=0.16) and adiposity (p=0.07).21 Differences in size and adiposity between feeding groups could be explained by the higher protein content of formula compared with breastmilk, or by differences in appetite control and satiety that become more pronounced as in later infancy as offspring are weaned onto solid foods. We have shown here that breastfeeding has complex and diverging effects on infant size and composition at ~5 months, and further studies are needed to determine whether differences in body composition early in life associated with breastfeeding exclusivity influence future obesity and chronic disease risk.
We also noted significant differences in infant body composition according to race/ethnicity and sex. As compared with non-Hispanic whites, offspring of Hispanic mothers had increased fat mass and adiposity, while offspring of non-Hispanic black mothers had lower fat mass and adiposity, independent of all other pre-and postnatal factors considered. The racial/ethnic differences we observed could reflect genetics or other unmeasured cultural differences,22 including beliefs surrounding ideal body size for children or nuances in early infant feeding that were not assessed in the present study. The sex differences we observed are consistent with prior studies.23 Further research is needed to understand both the causes and long-term significance of racial/ethnic and sex differences in early infant body composition.
Our study has limitations and strengths. Our analysis included only 58% of the eligible cohort, mostly due to pre-term birth exclusions, missing data on covariates, or missed postnatal visit. The lower sample size may have reduced power to detect significant effects. However, the analytic sample was similar to the eligible cohort in terms of maternal characteristics, perinatal predictors, and body composition at birth, mitigating potential concerns regarding loss to follow-up bias. A strength of the study is the prospective pre-birth cohort design, which enabled us to collect detailed data on exposures throughout pregnancy and early infancy. Measuring infant body composition by air displacement plethysmography is also a strength, as this method provides better estimates of overall adiposity than skinfold thicknesses, BMI, or abdominal ultrasound.
In summary, we report differences in infant body composition at ~5 months by demographic, prenatal and early postnatal characteristics. We show that infant body composition, particularly increased adiposity, persists from birth to ~5 months, independent of other early life factors, and that offspring who grow rapidly accumulate more fat mass than fat-free mass, resulting in substantially greater adiposity. These findings warrant further research into the long-term implications of differences in infant body composition at a very early age.
Acknowledgments
Supported by the National Institutes of Health (R01DK076648, UL1TR001082, T32DK07658, R01GM121081, K99ES025817).
The authors thank the participants, study coordinator Ms. Mercedes Martinez, and the Healthy Start staff for their contributions to this work.
Abbreviations
- WAZ
weight-for-age z-score
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey KM, Reynolds RM, Prescott SL, Nyirenda M, Jaddoe VW, Eriksson JG, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2016 doi: 10.1016/S2213-8587(16)30107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starling AP, Brinton JT, Glueck DH, Shapiro AL, Harrod CS, Lynch AM, et al. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study. Am J Clin Nutr. 2015;101:302–9. doi: 10.3945/ajcn.114.094946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hull HR, Thornton JC, Ji Y, Paley C, Rosenn B, Mathews P, et al. Higher infant body fat with excessive gestational weight gain in overweight women. Am J Obstet Gynecol. 2011;205:211, e1–7. doi: 10.1016/j.ajog.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stuebe AM, Landon MB, Lai Y, Spong CY, Carpenter MW, Ramin SM, et al. Maternal BMI, glucose tolerance, and adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;207:62, e1–7. doi: 10.1016/j.ajog.2012.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andres A, Shankar K, Badger TM. Body fat mass of exclusively breastfed infants born to overweight mothers. J Acad Nutr Diet. 2012;112:991–5. doi: 10.1016/j.jand.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 7.Estampador AC, Pomeroy J, Renstrom F, Nelson SM, Mogren I, Persson M, et al. Infant body composition and adipokine concentrations in relation to maternal gestational weight gain. Diabetes Care. 2014;37:1432–8. doi: 10.2337/dc13-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andres A, Hull HR, Shankar K, Casey PH, Cleves MA, Badger TM. Longitudinal body composition of children born to mothers with normal weight, overweight, and obesity. Obesity (Silver Spring) 2015;23:1252–8. doi: 10.1002/oby.21078. [DOI] [PubMed] [Google Scholar]
- 9.Ay L, Van Houten VA, Steegers EA, Hofman A, Witteman JC, Jaddoe VW, et al. Fetal and postnatal growth and body composition at 6 months of age. J Clin Endocrinol Metab. 2009;94:2023–30. doi: 10.1210/jc.2008-2045. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson B, Lof M, Forsum E. Body composition in full-term healthy infants measured with air displacement plethysmography at 1 and 12 weeks of age. Acta Paediatr. 2010;99:563–8. doi: 10.1111/j.1651-2227.2009.01665.x. [DOI] [PubMed] [Google Scholar]
- 11.Ode KL, Gray HL, Ramel SE, Georgieff MK, Demerath EW. Decelerated early growth in infants of overweight and obese mothers. J Pediatr. 2012;161:1028–34. doi: 10.1016/j.jpeds.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heerman WJ, Bian A, Shintani A, Barkin SL. Interaction between maternal prepregnancy body mass index and gestational weight gain shapes infant growth. Acad Pediatr. 2014;14:463–70. doi: 10.1016/j.acap.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crume TL, Ogden L, Maligie M, Sheffield S, Bischoff KJ, McDuffie R, et al. Long-term impact of neonatal breastfeeding on childhood adiposity and fat distribution among children exposed to diabetes in utero. Diabetes Care. 2011;34:641–5. doi: 10.2337/dc10-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellis KJ, Yao M, Shypailo RJ, Urlando A, Wong WW, Heird WC. Body-composition assessment in infancy: air-displacement plethysmography compared with a reference 4-compartment model. Am J Clin Nutr. 2007;85:90–5. doi: 10.1093/ajcn/85.1.90. [DOI] [PubMed] [Google Scholar]
- 15.Ma G, Yao M, Liu Y, Lin A, Zou H, Urlando A, et al. Validation of a new pediatric air-displacement plethysmograph for assessing body composition in infants. Am J Clin Nutr. 2004;79:653–60. doi: 10.1093/ajcn/79.4.653. [DOI] [PubMed] [Google Scholar]
- 16.Demerath EW, Fields DA. Body composition assessment in the infant. Am J Hum Biol. 2014;26:291–304. doi: 10.1002/ajhb.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fomon SJ, Haschke F, Ziegler EE, Nelson SE. Body composition of reference children from birth to age 10 years. Am J Clin Nutr. 1982;35:1169–75. doi: 10.1093/ajcn/35.5.1169. [DOI] [PubMed] [Google Scholar]
- 18.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320:967–71. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harder T, Bergmann R, Kallischnigg G, Plagemann A. Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol. 2005;162:397–403. doi: 10.1093/aje/kwi222. [DOI] [PubMed] [Google Scholar]
- 20.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115:1367–77. doi: 10.1542/peds.2004-1176. [DOI] [PubMed] [Google Scholar]
- 21.Gale C, Logan KM, Santhakumaran S, Parkinson JR, Hyde MJ, Modi N. Effect of breastfeeding compared with formula feeding on infant body composition: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95:656–69. doi: 10.3945/ajcn.111.027284. [DOI] [PubMed] [Google Scholar]
- 22.Caprio S, Daniels SR, Drewnowski A, Kaufman FR, Palinkas LA, Rosenbloom AL, et al. Influence of race, ethnicity, and culture on childhood obesity: implications for prevention and treatment: a consensus statement of Shaping America’s Health and the Obesity Society. Diabetes Care. 2008;31:2211–21. doi: 10.2337/dc08-9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fields DA, Krishnan S, Wisniewski AB. Sex differences in body composition early in life. Gend Med. 2009;6:369–75. doi: 10.1016/j.genm.2009.07.003. [DOI] [PubMed] [Google Scholar]