Abstract
Previously we reported that a 5-hour exposure of 6-day-old (P6) rhesus macaques to isoflurane triggers robust neuron and oligodendrocyte apoptosis. In an attempt to further describe the window of vulnerability to anesthetic neurotoxicity, we exposed P20 and P40 rhesusmacaques to 5 h of isoflurane anesthesia or no exposure (control animals). Brains were collected 3 h later and examined immunohistochemically to analyze neuronal and glial apoptosis. Brains exposed to isoflurane displayed neuron and oligodendrocyte apoptosis distributed throughout cortex and white matter, respectively. When combining the two age groups (P20 + P40), the animals exposed to isoflurane had 3.6 times as many apoptotic cells as the control animals. In the isoflurane group, approximately 66% of the apoptotic cells were oligodendrocytes and 34% were neurons. In comparison, in our previous studies on P6 rhesusmacaques, approximately 52% of the dying cells were glia and 48% were neurons. In conclusion, the present data suggest that the window of vulnerability for neurons is beginning to close in the P20 and P40 rhesus macaques, but continuing for oligodendrocytes.
Keywords: Developmental anesthesia neurotoxicity, Nonhuman primates, Apoptosis, Pediatric anesthesia, Isoflurane
1. Introduction
Worldwide, millions of young children undergo surgery and anesthesia each year. It is becoming increasingly common for pediatric patients of all ages to receive general anesthesia not only for surgical procedures, but also to facilitate long diagnostic procedures and minimally invasive interventions. There is conflicting clinical evidence regarding a potential association between exposure to anesthesia in early childhood and deleterious neurodevelopmental outcomes. While children initially exposed to anesthesia in the first few years of childhood have been shown to have an increased risk of neurodevelopmental deficits in language and cognition, (Ing et al., 2012; Backeljauwet al., 2015) a recent cohort study found that an initial anesthesia exposure after age 3 had no effects on language or cognitive function, but did have deleterious effects on motor function (Ing et al., 2014). Results from two recently published clinical studies suggest that single, short anesthetic episodes may be of less concern (Sun et al., 2016a; Davidson et al., 2016). Today, parents and caregivers often decide to postpone elective pediatric surgery until the child is older with the hope of avoiding any potential neurotoxic anesthetic effects; however, uncertainty remains as to when this window of vulnerability ends.
Substantial animal research suggests that exposure to anesthetics is neurotoxic to the developing brain causing acute apoptosis of neurons/ oligodendrocytes and long-term behavior and learning impairment (Jevtovic-Todorovic et al., 2003; Creeley et al., 2014; Brambrink et al., 2012a; Brambrink et al., 2010). The rodent window of vulnerability for neuroapoptosis coincides with the brain growth spurt, a period that encompasses the first two weeks of life in rodents but extends from midgestation to several years after birth in humans. One investigation found that rats exposed to a combination of midazolam, nitrous oxide, and isoflurane on postnatal day 7 (P7) exhibit increased neuroapoptosis, synaptic dysfunction, and long-term cognitive deficits (Jevtovic-Todorovic et al., 2003). In another study, propofol exposure of P5 and P10 rats significantly decreased pyramidal neuronal spine density, whereas similar exposures induced an increase in spine density when administered to P15, 20, or 30 rats (Briner et al., 2011). However, differences in brain maturation rates between rats and humans make translation of data to the clinical setting challenging. Compared to the rodent, the nonhuman primate (NHP) brain more closely parallels human anatomy and neurodevelopment. In addition, the NHP model allows one to more closely approximate clinical conditions such as intubation, mechanical ventilation, and constant monitoring of vital signs and thus the ability to maintain physiologic homeostasis. In NHPs, a single study has investigated whether the vulnerability to anesthetic neurotoxicity decreases as animals age (Slikker et al., 2007). As part of a larger study, these authors compared the effects of a 24-hour exposure to ketamine on NHPs at P5 or P35 and found that the frontal cortices of the P5 animals showed apoptosis while those of P35 animals did not (Slikker et al., 2007). Of note, brain development in P5 and P35 monkeys approximates that of 5 and 9 months of age, respectively, in human infants (Dobbing & Sands, 1979;Workman et al., 2013).
We have previously shown that 5 hour isoflurane exposure of the P6 neonatal rhesus macaque (Macaca mulatta) triggered apoptotic neurodegeneration, and led to apoptosis of oligodendrocytes (Brambrink et al., 2012a; Brambrink et al., 2010). These histopathology findings are similar to those found following P6 NHP exposure to ketamine or propofol (Brambrink et al., 2012b; Creeley et al., 2013). Not only do these anesthesia exposures cause histopathologic changes, but they have also been found to lead to long-lasting cognitive and behavioral deficits in NHPs (Raper et al., 2015; Paule et al., 2011; Coleman et al., 2016). However, the effects of anesthetics on the brains of older NHPs remain unclear. In order to help further describe the window of vulnerability to the neurotoxic effects of anesthetics, the present study was performed to investigate whether the developing brain of nonhuman primates remains vulnerable on postnatal days 20 (P20) or 40 (P40). Of note, the brain development of the P20 rhesus macaque is equivalent to that of a 7-month-old human infant, whereas the P40 approximates that of a 9.5-month-old infant (Workman et al., 2013). Using the same experimental protocols as in our prior studies in P6 animals, we exposed P20 and P40 NHPs to 5 h of isoflurane anesthesia (Brambrink et al., 2012a; Brambrink et al., 2010).
2. Materials and methods
2.1. Animals and experimental procedures
All animal procedures and study protocols were conducted in full accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees of the Oregon National Primate Research Center and Washington University in St. Louis School of Medicine. The subjects for these experiments were 7 female and 5 male infant rhesus macaques that were on average 20 or 40 days old. We included at least one male and one female in each group, and thus did not analyze sex differences. The infant macaques (n = 3/group, Table 1) were exposed to either isoflurane for 5 h or no anesthesia.
Table 1.
Infant nonhuman primate sex distribution by group.
| Male | Female | |
|---|---|---|
| P20 Control | n = 1 | n = 2 |
| P20 Isoflurane | n = 1 | n = 2 |
| P40 Control | n = 2 | n = 1 |
| P40 Isoflurane | n = 1 | n = 2 |
Infant macaques were taken to an operating room immediately after their dams received intramuscular ketamine sedation (5 to 10 mg/kg; Ketathesia, Butler Schein Animal Health, Dublin, OH). Upon arrival in the operating room, the infant body weight and rectal temperature were recorded. Prior to induction of anesthesia, animals were gently restrained to allow placement of 22-gauge catheter (Introcan Safety, B Braun Medical, Melsungen, Germany) in the saphenous or cephalic vein. Venous blood (0.3 mL) withdrawn from the saphenous vein catheter was used for baseline point-of-care analysis (CG4+ and EC8+ cartridges, iSTAT, Abbott Point of Care, Princeton, NJ). Following blood collection, the catheter was secured in place with tape and maintained throughout the duration of the exposure period in both anesthesia exposed and control animals. Isoflurane general anesthesia was administered as previously described (Creeley et al., 2014; Brambrink et al., 2012a; Brambrink et al., 2010). Briefly, anesthesia was induced by administering isoflurane via facemask, after which animals were tracheally intubated and mechanically ventilated. Isoflurane (end tidal 1.3–2.5 vol%; Piramal Healthcare, Andhra, India) general anesthesia was regulated to maintain a surgical plane of anesthesia as determined by no movement and not >10% increase in blood pressure or heart rate in response to a mosquito-clamp pinch of the hand or foot (assessed every 30 min). During general anesthesia, the following vital signs were recorded every 15 min: peripheral oxygen saturation, noninvasive blood pressure, heart rate, continuous electrocardiogram, body temperature, end-tidal carbon dioxide, and respiratory rate. Blood gases and metabolic profiles were determined a minimum of every 2 h throughout general anesthesia.
Upon completion of 5 h of isoflurane anesthesia, the isoflurane was discontinued, and animals were extubated when protective reflexes and spontaneous movements returned. The animals were then observed for 3 h in an intensive care unit system (Snyder ICU cage; Snyder MFG, Centennial, CO), which allowed visual monitoring, as well as recording of vital signs, blood gases, and metabolic profiles. Subsequently, the animals received intravenous ketamine (20mg/kg) and pentobarbital (25 mg/kg). After confirmation of loss of reflexes and deep anesthesia level, the chest was opened, the heart was cannulated, and animals were transcardially perfusion-fixed (4% paraformaldehyde) in preparation for histopathological brain analysis.
Animals that were randomized to the control, no anesthesia group underwent similar handling. The infants were removed from their dams, received an IV catheter, and had baseline vital signs and blood values measured to mimic the stress that animals in the experimental group experienced prior to the induction of anesthesia. Physiologic parameters were collected at two additional time points to mirror isoflurane exposed animals. Eight hours following baseline data collection, final measurements were collected and the awake animals were transferred to pathology for transcardial perfusion as described above.
2.2. Histopathology studies
Following in vivo perfusion fixation with 4% paraformaldehyde in phosphate buffer, brains were serially cut into 70 µM sections on a vibratome. Sections were cut coronally in the cerebrum (approximately 800 sections) and sagittally in the cerebellum (approximately 200 sections). Approximately 30 sections per brain (cerebrum + cerebellum) were selected at 2.24 mm intervals and stained using activated caspase 3 (AC3) immunohistochemistry (marker for apoptosis) as previously described (Creeley et al., 2014; Brambrink et al., 2012a; Brambrink et al., 2010).
2.3. Quantification of apoptosis
An experienced neurohistologist blinded to treatment condition counted AC3 positive neurons and oligodendrocytes. Serial brain sections were analyzed via light microscopy with a 10× objective lens and a computer-assisted Microbrightfield Stereo Investigator system (Microbrightfield, Inc., Williston, VT) to record the location and number of dying cells and the dimensions of the field. Location of the cell (white vs. gray matter) and morphological features were used to differentiate AC3 stained neurons fromglia. The AC3-positive cell counts were divided by volume in mm3 to obtain final densities.
2.4. Statistical analysis
Physiological variables are presented as median and range; all other data are presented as means ± standard errors of the mean (SEM). A two-way analysis of variance (ANOVA) using animal age (P20 or P40) and treatment group (isoflurane or control) as factors was performed for both neuronal and oligodendrocyte counts followed by a Bonferroni correction using GraphPad Prism statistical software (GraphPad Software, Inc., La Jolla, CA).
3. Results
3.1. Animal response to isoflurane exposure
All animals survived the experimental protocol and active warming of the infants, together with continuous fluid and glucose administration, ensured homeostasis throughout the experimental period. Physiologic variables are listed in Table 2. No adverse events were noted.
Table 2.
Physiologic parameters of infant rhesus macaques at 20 (P20) or 40 (P40) days of age or before, during, and after control versus isoflurane anesthesia.
| Baseline (0 h) | 1 h | 2.5 h | 5 h | |
|---|---|---|---|---|
| Heart rate (min−1) | ||||
| P20 Control | 95 (93–95) | 95 (95–95) | 96 (95–99) | 96 (95–99) |
| P20 Isoflurane |
97 (96–98) | 97 (97–99) | 98 (97–99) | 99 (98–100) |
| P40 Control | 99 (97–100) | 99 (98–99) | 96 (95–99) | 98 (97–100) |
| P40 Isoflurane |
97 (97–99) | 98 (97–100) | 99 (99–100) | 100 (98–100) |
| MAP (mm Hg) | ||||
| P20 Control | 125 (102–147) | nc | 69 (68–105) | 99 (60–102) |
| P20 Isoflurane |
106 (103–108) | 42 (42–42) | 35 (29–41) | 48 (28–60) |
| P40 Control | 75 (75–75) | 62 (45–78) | 84 (65–98) | 71 (70–96) |
| P40 Isoflurane |
71 (45–89) | 38 (26–46) | 33 (26–36) | 36 (35–52) |
| SpO2 (%) | ||||
| P20 Control | 95 (93–95) | 95 (95–95) | 96 (95–99) | 96 (95–99) |
| P20 Isoflurane |
97 (96–98) | 97 (97–99) | 98 (97–99) | 99 (98–100) |
| P40 Control | 99 (97–100) | 99 (98–99) | 96 (95–99) | 98 (97–100) |
| P40 Isoflurane |
97 (97–99) | 98 (97–100) | 99 (99–100) | 100 (98–100) |
| Temperature (rectal, °C) | ||||
| P20 Control | 37.3 (36.7–37.9) |
nc | 38.3 (38.3–38.5) |
38.6 (38.4–38.7) |
| P20 Isoflurane |
37.3 (36.9–38.0) |
35.5 (35.0–37.0) |
37.6 (37.6–38.0) |
37.1 (36.0–38.4) |
| P40 Control | 37.1 (36.9–38.4) |
38.5 (38.1–38.8) |
38.7 (38.2–38.7) |
38.6 (37.4–38.7) |
| P40 Isoflurane |
37.2 (36.4–37.8) |
36.6 (36.3–38.4) |
37.4 (37.4–37.6) |
38.4 (38.2–38.8) |
| Glucose (mg/dL) | ||||
| P20 Control | 103 (97–183) | nc | 84 (63–97) | 80 (56–94) |
| P20 Isoflurane |
88 (77–94) | 98 (86–100) | 84 (64–184) | 77 (60–106) |
| P40 Control | 84 (74–89) | nc | 75 (73–76) | 67 (40–69) |
| P40 Isoflurane |
73 (69–100) | 91 (84–178) | 68 (66–122) | 68 (65–70) |
Data are presented as median (range: min-max);
nc = not collected; SpO2 = peripheral capillary oxygen saturation; MAP = mean arterial pressure (noninvasive).
3.2. P20 animal histopathology
Qualitative comparison of P20 control and isoflurane treated animals revealed a strikingly different pattern of apoptosis between treatment groups (Fig. 1). In control animals, occasional AC3 positive neurons were randomly distributed in all major divisions of the cortex. A few subcortical apoptotic neurons were observed in the thalamus, while a small number of AC3 positive oligodendrocytes were primarily confined to the corona radiata with sporadic distribution (Fig. 1, Panel A). This pattern is characteristic of physiologic apoptosis, the background rate of programmed cell death that serves to prune unneeded cells and synapses during brain development.
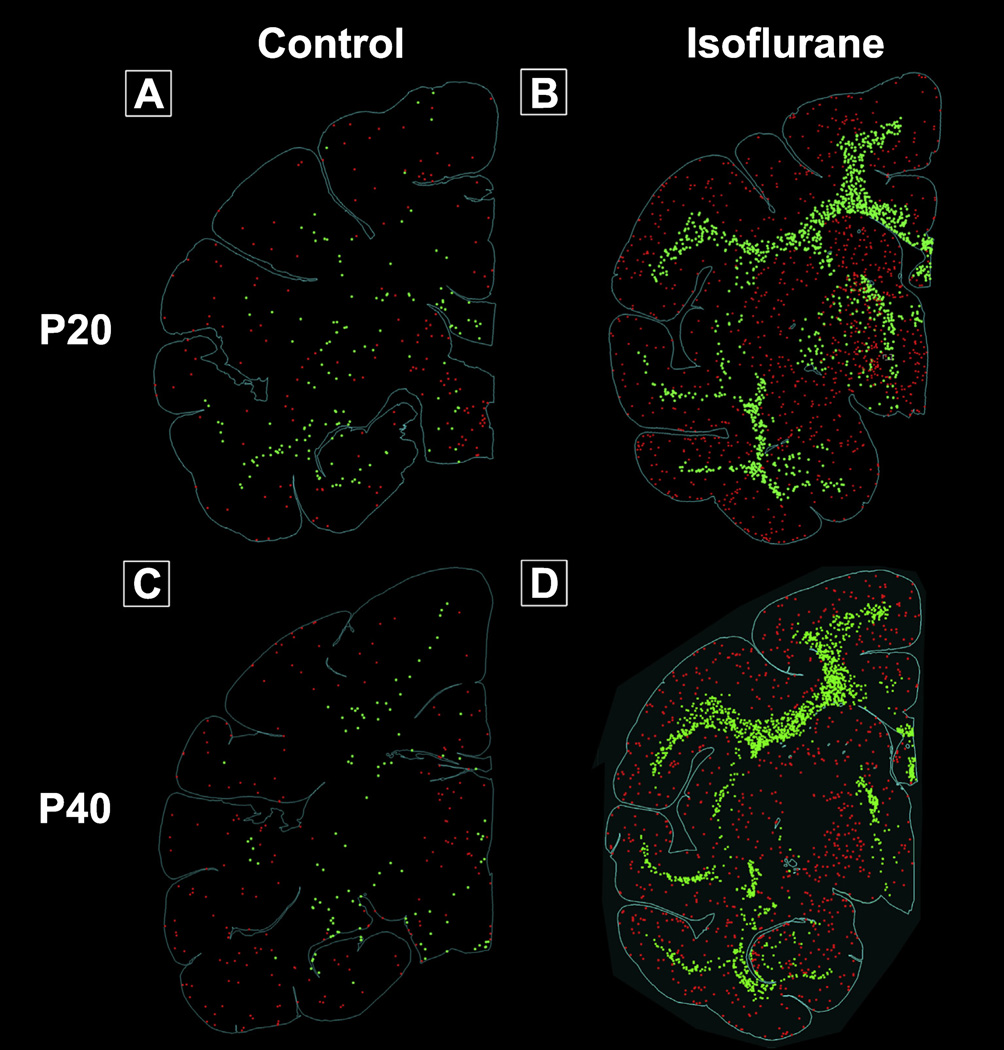
Fig. 1.
Computer plots of representative brain slices portraying apoptotic cells. See text for further details. Panel A: Control, P20; Panel B: Isoflurane, P20; Panel C: Control P40; Panel D: Isoflurane P40. Red: apoptotic neurons; green: apoptotic oligodendrocytes. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In contrast, 5 hour isoflurane exposure of P20 animals caused an acute apoptotic reaction throughout the extent of the neuraxis (Fig. 1, Panel B). Neuroapoptosis was diffuse with apoptotic profiles scattered within frontal, motor, somatosensory, temporal, and V1 and V2 visual cortex. However, we observed organized and dense subcortical apoptosis in the caudate, putamen, and thalamus of P20 NHPs.
While AC3 positive oligodendrocytes in control animals were sporadic and randomly distributed, we observed widespread oligodendrocyte apoptosis concentrated in large white matter tracts in P20 isoflurane treated animals. Specifically, oligodendrocyte apoptosis was markedly pronounced in the internal capsule, corpus callosum, and corona radiata. Computer plots of oligodendrocyte apoptosis in the P20 isoflurane treated brain (Fig. 1, Panel B) demonstrate a high degree of organized oligodendrocyte apoptosis that, in essence, outlines the extent of the internal capsule, corpus callosum, and corona radiata.
3.3. P40 animal histopathology
Physiological apoptosis in P40 control animals followed a similar pattern to that observed in the P20 control cohort. Briefly, neurons and oligodendrocytes undergoing apoptosis were sparsely distributed throughout cortex and white matter, respectively (Fig. 1, Panel C).
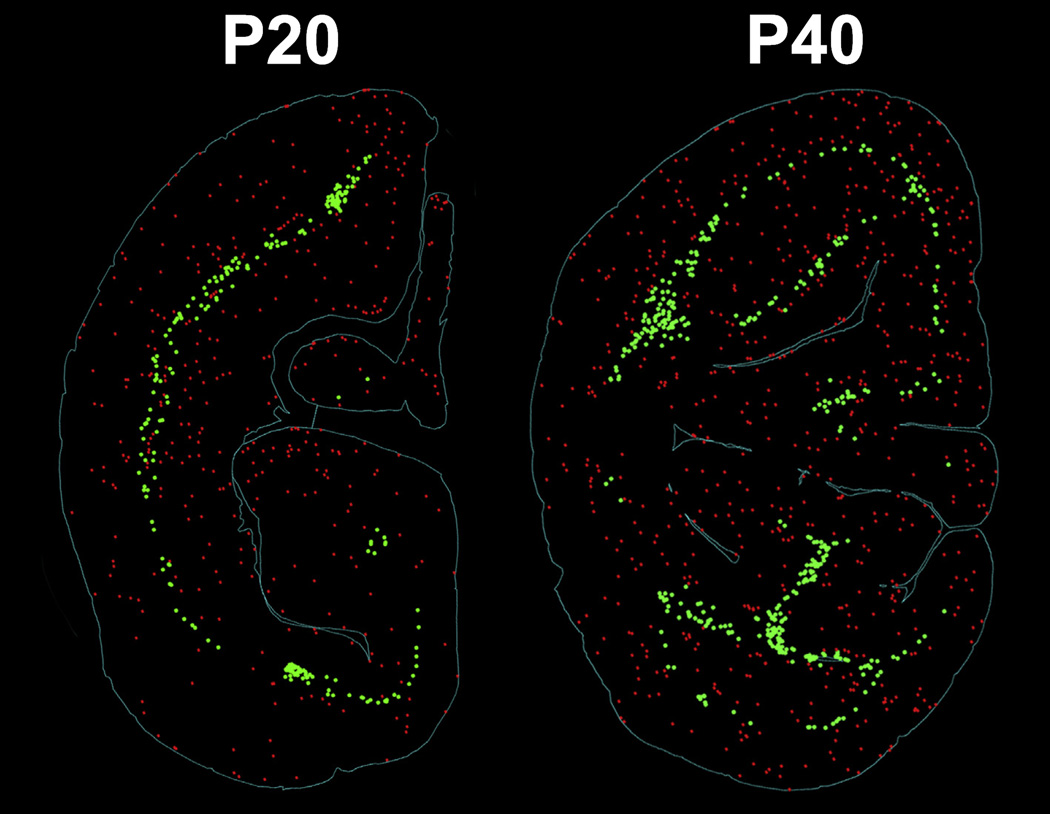
At P40, no major cortical regions were spared after 5 h of isoflurane exposure (Fig. 1, Panel D). We observed densely concentrated AC3 positive neurons in the cingulate, motor, somatosensory, and insular cortex. Furthermore, neuroapoptosis extended to temporal and occipital cortices, including V1 and V2. We previously reported that exposure to a similar duration of propofol or isoflurane anesthesia at P6 caused a robust, laminar pattern of neuroapoptosis encompassing Layers II and IV of the somatosensory and auditory cortex, as well as Layers II and V of the visual cortex (Brambrink et al., 2012a; Creeley et al., 2013). On the other hand, in both the P20 and P40 animals, we observed that the apoptotic cortical patterning in these regions was isotropic and nonlaminar. (Fig. 2) The white matter injury appear similar in the P20 and P40NHPs, and mimics what we had previously reported after isoflurane exposure in P6 NHPs (Brambrink et al., 2012a). Apoptotic neurons were randomly distributed throughout all neocortical layers, likely indicating changes in vulnerability with increased developmental age. Similar to P20 animals, P40 animals exposed to isoflurane also exhibited neuroapoptosis in subcortical nuclei including the caudate, putamen, and thalamus (Fig. 1, Panel D).
Fig. 2.
Computer plots of representative brain slices portraying representative patterns of neuroapoptosis in the cortex of infant NHPs. The images represent the isotropic and non-laminar apoptotic cortical patterning following isoflurane exposure at P20 (left) and P40 (right). This is in contrast to the laminar pattern of gray matter apoptosis that we previously reported in isoflurane-exposed P6NHPs (Brambrink et al., 2012a; Creeley et al., 2013). Red: apoptotic neurons; green: apoptotic oligodendrocytes. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The distribution of oligodendrocyte apoptosis in the corona radiata of the P40 isoflurane-treated cohort was mostly indistinguishable from that observed at P20 (Fig. 1, Panel D). Apoptotic oligodendrocytes were densely concentrated throughout the entirety of the corona radiata in all sections examined. However, oligodendrocyte apoptosis was not as pronounced in the internal capsule and corpus callosum of isoflurane-treated P40 animals versus P20 animals.
3.4. Quantitative histopathology
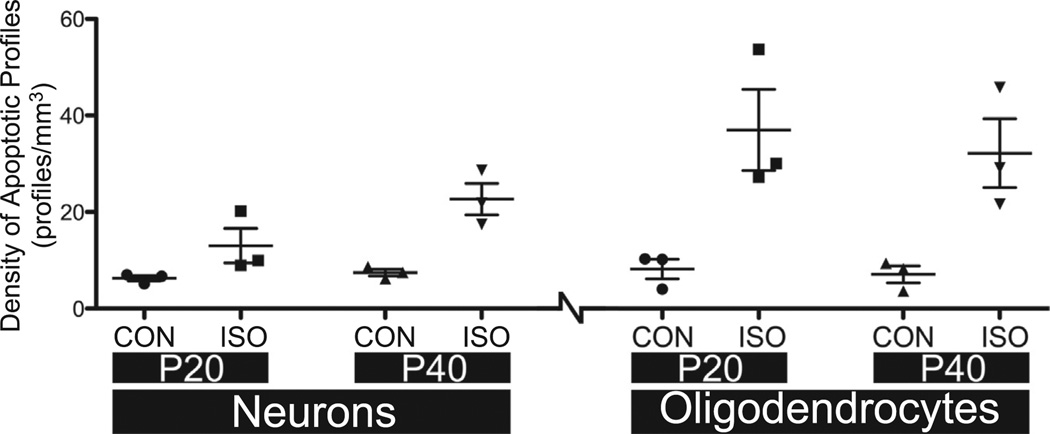
Densities of AC3-positive cells were analyzed using a two-way ANOVA using age and treatment as factors. (Fig. 3) For neurons, results revealed a main effect for treatment (F (Ing et al., 2012; Brambrink et al., 2012a) = 19.78, p < 0.01), but no interaction (F (Ing et al., 2012; Brambrink et al., 2012a) = 2.961, p > 0.05) or main effect for age (F (Ing et al., 2012; Brambrink et al., 2012a) = 4.784, p > 0.05). This indicates that isoflurane exposure significantly increased neuronal apoptosis, but age (P20 vs. P40) had no overall effect on the expression of toxicity. The lack of interaction indicates that isoflurane exposure produced similar levels of neuronal apoptosis at both ages. Similarly, for oligodendrocytes, results revealed a main effect for treatment (F (Ing et al., 2012; Brambrink et al., 2012a) = 22.62, p < 0.01), but no interaction (F (Ing et al., 2012; Brambrink et al., 2012a)=0.1073, p > 0.05) or main effect for age (F (Ing et al., 2012; Brambrink et al., 2012a) = 0.2724, p > 0.05). This suggests that isoflurane significantly increased oligodendrocyte apoptosis, but the ages tested did not vary with respect to sensitivity to this effect. The lack of interaction suggests that isoflurane exposure produced similar levels of oligodendrocyte toxicity at P20 and P40 in NHPs.
Fig. 3.
Density of apoptotic cell profiles in the infant nonhuman primate after anesthesia exposure at age P20 or P40. Densities (profiles/mm3) of AC3-positive cells are presented as scatter plots. The groups are divided by cell type (neurons or oligodendrocytes), age (P20 or P40) and treatment (control vs. isoflurane). Data were analyzed using a two-way ANOVA using age and treatment as factors. Only the isoflurane treatment was significantly different (p < 0.01) at both ages and in both cell types; none of the other comparisons were statistically different. CON: controls (no anesthesia); ISO: isoflurane anesthesia.
Due to the lack of a main effect for age, data were collapsed across this factor for both neurons and oligodendrocytes. For neurons, the densities of apoptotic cells for control and isoflurane exposed animals (P20 + P40; mean ± SEM) were 6.89 ± 0.5 neurons/mm3 and 17.86±3.1 neurons/mm3 respectively (a 2.6-fold increase in isoflurane vs. control). For oligodendrocytes, the densities of apoptotic cells for control and isoflurane exposed animals were 7.67 ± 1.2 neurons/mm3 and 34.59 ± 5.0 neurons/mm3 respectively (a 4.5-fold increase in isoflurane vs. control). In the control group, there was almost an equal number of apoptotic neurons compared to apoptotic oligodendrocytes. Of the total apoptotic cells in the control animals, 53% were oligodendrocytes and 47% were neurons. On the other hand, the number of apoptotic oligodendrocytes was almost twice the number of apoptotic neurons following isoflurane treatment. Of the total apoptotic cells in the isoflurane-exposed animals, 66% were oligodendrocytes and 34% were neurons.
4. Discussion
Our findings demonstrate that exposing P20 or P40 infant rhesus macaques to a moderate surgical plane of isoflurane anesthesia maintained for 5 h leads to increases in apoptotic neuronal and glial cell death. Isoflurane-induced apoptosis of neurons and glia has been previously described in the fetal (gestational age 120 days) and P6 neonatal NHP brain, (Creeley et al., 2014; Brambrink et al., 2012a; Brambrink et al., 2010) but this is the first report describing the susceptibility of older infant monkeys (P20 and P40) to the neurotoxic effects of clinically relevant isoflurane anesthesia. The overall number of apoptotic profiles (neurons+ oligodendrocytes) in the isoflurane-exposed brains in this study was 3.6 times greater than in the control, unexposed brains. To put this in the context of our prior investigations, we found that, when compared to unexposed controls: P6 NHPs exposed to isoflurane had a 13× more apoptotic profiles; (Brambrink et al., 2010) isoflurane exposed G120 NHPs had 4.1× more apoptotic profiles; (Creeley et al., 2014) P6 NHPs exposed to ketamine had 3.8× more apoptotic profiles; (Brambrink et al., 2012b) G120 NHPs exposed to ketamine had 4.9× more apoptotic profiles; (Brambrink et al., 2012b) P6 NHPs exposed to propofol had 3.8× more apoptotic profiles; (Creeley et al., 2013) and G120 NHPs exposed to propofol had 2.4× more apoptotic profiles (Creeley et al., 2013). Of the total number of apoptotic cells in the isoflurane-exposed brains in the current investigation, 34% were neurons and 66% were oligodendrocytes. This is in contrast to our prior study of the younger, P6NHP brain in which the percentage of apoptosis was 48% neurons and 52% oligodendrocytes (Brambrink et al., 2012a). In the present study, there was more oligodendrocyte apoptosis compared to neuronal apoptosis. Of note, because nonhuman primates are a limited and valuable resource, the sample size restriction in this model was a function of ethical considerations. Our analysis was limited to a single survival time of 3 h following discontinuation of isoflurane exposure, designed to capture early apoptosis. However, it is possible that initiation of apoptosis may continue for intervals longer than 3 h after anesthetic exposure, which we are unable to determine from our current investigation.
The present data suggest that the window of vulnerability for neurons may be slowly declining in the P20–P40 NHP, but continues for oligodendrocytes. This is consistent with a rodent investigation in which ethanol (a GABA agonist and NMDA antagonist like isoflurane) exposure led to neuroapoptosis that peaked at P7 but decreased rapidly after P14, whereas oligodendrocyte apoptosis began around P7 and peaked around P14 (Olney et al., 2005). Based on these findings and our new data, it is expected that the window of vulnerability for oligodendrocytes would extend well beyond that of neurons. The lack of a statistically significant interaction also suggests toxicity for both cell types occurs similarly at P20 and P40 and older animals need to be tested in future research.
The pattern of neuronal degeneration induced by isoflurane in the P20 and P40 NHP brain is different from the pattern reported previously in the P6 NHP brain in which a laminar arrangement of neuroapoptosis was present in the cortical layers (Brambrink et al., 2010). In contrast, though there is significant cortical toxicity in the P20 and P40 animals, it is more evenly scattered. Also, there is more subcortical neuroapoptosis in regions such as the basal ganglia and the thalamus. Finally, the oligodendrocyte apoptosis is densely but evenly distributed in the white matter tracts (i.e. corona radiata). Evidence that the regional pattern of cell apoptosis and type of cell apoptosis (neurons vs. oligodendrocytes) varies as a function of age at time of anesthetic exposure (newborn [P6] vs. older infants [P20–40]) suggests that long-term cognitive and behavioral deficits might also vary as a function of age at time of anesthetic exposure. This should be a focus for future NHP and clinical studies.
There are increasing numbers of retrospective clinical studies that suggest exposure to anesthetic agents in early childhood is associated with subsequent behavioral and learning disorders. However, other clinical cohort studies have found no association between childhood anesthetic exposure and academic performance (Hansen et al., 2011). Recently, using a sibling-matched cohort design, the Pediatric Anesthesia Neurodevelopment Assessment (PANDA) study found no differences in IQ in healthy children with a single anesthesia exposure (median duration 80 min) before 3 years of age when compared to a sibling without an anesthesia exposure (Sun et al., 2016b). Another ongoing population-based birth cohort study, the Mayo Anesthesia Safety in Kids (MASK) study, will compare neurodevelopmental abnormalities in children exposed to anesthesia prior to age 3 years and a reference population of propensity-matched children without anesthesia exposure (Gleich et al., 2015). In the first randomized controlled equivalence trial, infants were randomly assigned to receive regional anesthesia or general anesthesia for inguinal herniorrhaphy (General Anesthesia compared to Spinal anesthesia (GAS) trial) (Davidson et al., 2016). An interim analysis (secondary outcomes) of this trial found no evidence that general anesthesia in infancy increases the risk of adverse neurodevelopmental outcome at 2 years of age when compared to regional anesthesia. The primary outcome of this ongoing trial will compare measures of intelligence at age 5 years.
In summary, our new results suggest that at age P20–P40 NHPs are still vulnerable to isoflurane-induced apoptosis, but at this developmental age almost twice as many oligodendrocytes as neurons are eliminated. As oligodendrocytes become the dominant cell type undergoing apoptosis, neurodevelopmental impairment (if present) may also change. Since each oligodendrocyte myelinates several axons, this may produce widespread demyelination of white matter tracts and impaired neuronal communication, which may underlie neurodevelopment deficits linked to infant anesthesia exposure. This is especially concerning considering that myelination continues beyond late adolescence in humans (Miller et al., 2012). It is possible that not only are infants at risk for anesthetic-induced neurotoxicity, but toddlers and adolescents are also at risk resulting in a different type of brain injury at that time of development. Whether human infants and children are susceptible to deleterious effects of isoflurane or other inhalational or intravenous anesthetics cannot be definitively answered in the nonhuman primate model, and the window of vulnerability in humans needs to be delineated in human studies. There are ongoing prospective clinical studies, such as the MASK study and the GAS trial, that promise to further shed light on this phenomenon. In the meantime, compelling data from this and other NHP studies support the hypothesis that inhalational anesthetics, such as isoflurane, cause differential deleterious effects on the developing brain dependent on age and the stage of brain development. Future studies focusing on NHPs at later stages of brain development, other anesthetic agents, and repeated exposures are urgently needed in this model.
Acknowledgments
This work was supported by National Institute of Child Health and Human Development grants HD052664 (KKN), HD052664S (KKN), the Intellectual and Developmental Disabilities Research Center at Washington University in St. Louis (NIH/NICHD U54-HD087011) (KKN), an NIH-funded BIRCWH K12 award made possible through the Eunice Kennedy Shriver National Institute of Child Health & Human Development and the Office of Research on Women's Health (K12 HD 043488) (KJS), the Frontiers in Anesthesia Research Award (FARA 2012) by the International Anesthesia Research Society (IARS) (AMB), and by NIH grant 8P51OD011092-that supports the operation of the Oregon National Primate Research Center.
Footnotes
Transparency Document
The Transparency document associated with this article can be found, in the online version.
References
- Backeljauw B, Holland SK, Altaye M, Loepke AW. Cognition and brain structure following early childhood surgery with anesthesia. Pediatrics. 2015;136:e1–e12. doi: 10.1542/peds.2014-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, Olney JW. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambrink AM, Back SA, Riddle A, Gong X, Moravec MD, Dissen GA, Creeley CE, Dikranian KT, Olney JW. Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain. Ann. Neurol. 2012a;72:525–535. doi: 10.1002/ana.23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Martin LD, Dissen GA, Creeley CE, Olney JW. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology. 2012b;116:372–384. doi: 10.1097/ALN.0b013e318242b2cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briner A, Nikonenko I, De Roo M, Dayer A, Muller D, Vutskits L. Developmental stage-dependent persistent impact of propofol anesthesia on dendritic spines in the rat medial prefrontal cortex. Anesthesiology. 2011;115:282–293. doi: 10.1097/ALN.0b013e318221fbbd. [DOI] [PubMed] [Google Scholar]
- Coleman K, Robertson ND, Dissen GA, Neuringer MD, Martin LD, Cuzon Carlson VC, Kroenke C, Fair D, Brambrink AM. Isoflurane anesthesia has long-term consequences on motor and behavioral development in infant rhesus macaques. Anesthesiology. 2016;1 doi: 10.1097/ALN.0000000000001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeley C, Dikranian K, Dissen G, Martin L, Olney J, Brambrink A. Propofol-induced apoptosis of neurones and oligodendrocytes in fetal and neonatal rhesus macaque brain. Br. J. Anaesth. 2013;110(Suppl. 1):i29–i38. doi: 10.1093/bja/aet173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeley CE, Dikranian KT, Dissen GA, Back SA, Olney JW, Brambrink AM. Isoflurane-induced apoptosis of neurons and oligodendrocytes in the fetal rhesus macaque brain. Anesthesiology. 2014;120:626–638. doi: 10.1097/ALN.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Disma N, de Graaff JC, Withington DE, Dorris L, Bell G, Stargatt R, Bellinger DC, Schuster T, Arnup SJ, Hardy P, Hunt RW, Takagi MJ, Giribaldi G, Hartmann PL, Salvo I, Morton NS, von Ungern Sternberg BS, Locatelli BG, Wilton N, Lynn A, Thomas JJ, Polaner D, Bagshaw O, Szmuk P, Absalom AR, Frawley G, Berde C, Ormond GD, Marmor J, McCann ME. GAS consortium, Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2016;387:239–250. doi: 10.1016/S0140-6736(15)00608-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum. Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Gleich SJ, Flick R, Hu D, Zaccariello MJ, Colligan RC, Katusic SK, Schroeder DR, Hanson A, Buenvenida S, Wilder RT, Sprung J, Voigt RG, Paule MG, Chelonis JJ, Warner DO. Neurodevelopment of children exposed to anesthesia: design of the Mayo Anesthesia Safety in Kids (MASK) study. Contemp. Clin. Trials. 2015;41:45–54. doi: 10.1016/j.cct.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TG, Pedersen JK, Henneberg SW, Pedersen DA, Murray JC, Morton NS, Christensen K. Academic performance in adolescence after inguinal hernia repair in infancy: a nationwide cohort study. Anesthesiology. 2011;114:1076–1085. doi: 10.1097/ALN.0b013e31820e77a0. [DOI] [PubMed] [Google Scholar]
- Ing C, DiMaggio C, Whitehouse A, Hegarty MK, Brady J, von Ungern-Sternberg BS, Davidson A, Wood AJ, Li G, Sun LS. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 2012;130:e476–e485. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- Ing CH, DiMaggio CJ, Whitehouse AJ, Hegarty MK, Sun M, von Ungern-Sternberg BS, Davidson AJ, Wall MM, Li G, Sun LS. Neurodevelopmental outcomes after initial childhood anesthetic exposure between ages 3 and 10 years. J. Neurosurg. Anesthesiol. 2014;26:377–386. doi: 10.1097/ANA.0000000000000121. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J. Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DJ, Duka T, Stimpson CD, Schapiro SJ, Baze WB, McArthur MJ, Fobbs AJ, Sousa AM, Sestan N, Wildman DE, Lipovich L, Kuzawa CW, Hof PR, Sherwood CC. Prolonged myelination in human neocortical evolution. Proc. Natl. Acad. Sci. U. S. A. 2012;109:16480–16485. doi: 10.1073/pnas.1117943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Young C, Qin YQ, Dikranian K, Farber NB. Ethanol-induced Developmental Glioapoptosis in Mice and Monkeys. 916.7. Society for Neuroscience Annual Meeting; Washington, DC. 2005. [Google Scholar]
- Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, Hanig JP, Patterson TA, Slikker W, Jr, Wang C. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol. Teratol. 2011;33:220–230. doi: 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Alvarado MC, Murphy KL, Baxter MG. Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology. 2015;123:1084–1092. doi: 10.1097/ALN.0000000000000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slikker W, Jr, Zou X, Hotchkiss CE, Divine RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC, Patterson TA, Hanig JP, Paule MG, Wang C. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol. Sci. 2007;98:145–158. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- Sun LS, Li G, Miller TL, Salorio C, Byrne MW, Bellinger DC, Ing C, Park R, Radcliffe J, Hays SR, DiMaggio CJ, Cooper TJ, Rauh V, Maxwell LG, Youn A, McGowan FX. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312–2320. doi: 10.1001/jama.2016.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. Modeling transformations of neurodevelopmental sequences across mammalian species. J. Neurosci. 2013;33:7368–7383. doi: 10.1523/JNEUROSCI.5746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]