Introduction
Self-regulation is central to developmental psychopathology, but progress has been impeded by varying terminology and meanings across fields and literatures. The present review attempts to move that discussion forward by noting key sources of prior confusion such as measurement-concept confounding, and then arguing the following major points. First, the field needs a domain-general construct of self-regulation that encompasses self-regulation of action, emotion, and cognition and involves both top-down and bottom-up regulatory processes. This does not assume a shared core process across emotion, action, and cognition, but is intended to provide clarity on the extent of various claims about kinds of self-regulation. Second, top-down aspects of self-regulation need to be integrated. These include (a) basic processes that develop early and address immediate conflict signals, such as cognitive control and effortful control, and (b) complex cognition and strategies for addressing future conflict, represented ty the regulatory application of complex aspects of executive functioning. Executive function and cognitive control are not identical to self-regulation because they can be used for other activities, but account for top-down aspects of self-regulation at the cognitive level. Third, impulsivity, risk-taking, and disinhibition are distinct although overlapping; a taxonomy of the kinds of breakdowns of self-regulation associated with psychopathology requires their differentiation. Fourth, different aspects of the self-regulation universe can be organized hierarchically in relation to granularity, development, and time. Lower-level components assemble into higher-level components. This hierarchical perspective is consistent across literatures. It is hoped that the framework outlined here will facilitate integration and cross-talk among investigators working from different perspectives, and facilitate individual differences research on how self-regulation relates to developmental psychopathology.
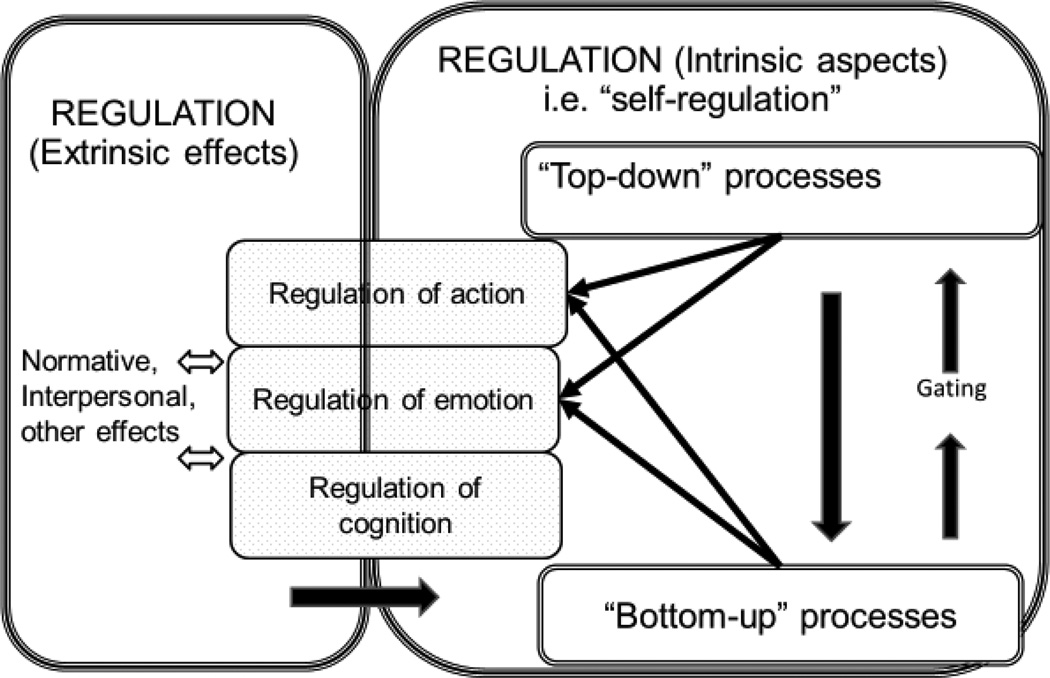
Regulation is the ongoing, dynamic, and adaptive modulation of internal state (emotion, cognition) or behavior, mediated by central and peripheral physiology.1 It draws upon numerous aspects of the mind, including such capacities as executive functioning. It includes regulation of and by others (called extrinsic) particularly in early life (Cox, Mills-Koonce, Propper, & Gariepy, 2010) but also throughout development and into adulthood (Gross, 2015). Regulation of and by oneself (intrinsic) emerges increasingly with development and is called self-regulation (SR) (Eisenberg & Zhou, 2016). Figure 1 shows that SR is a portion of ‘regulation’ and includes top-down (deliberate) and bottom-up (automatic) aspects discussed later. This review is about SR.
Figure 1.
SR entails regulation by one-self (called intrinsic) as opposed to regulating or being regulated via others. SR has top-down and bottom-up components that mutually influence one another. Bottom-up processes are both targets of and sources of regulation (not shown). Bottom-up processes can interfere with top-down SR, but they also help regulate one another and regulate top-down control via a threshold referred to as ‘gating’ which implies continual information updating. Extrinsic regulatory effects can work via both bottom-up and top-down intrinsic processes; the arrow at the bottom illustrates their cross talk via reactive (bottom-up) processes, which are most heavily studied in child development (e.g., behavioral inhibition as described in the text or parental soothing of a child). The present review is concerned only with intrinsic processes or SR, not regulation generally. Not to scale.
SR holds almost unparalleled importance to mental health. Poor SR in some form is related to ADHD and internalizing and externalizing psychopathology (Calkins, Graziano, & Keane, 2007; Eisenberg et al., 2009; Espy, Sheffield, Wiebe, Clark, & Moehr, 2011; Martel & Nigg, 2006; Olson, Sameroff, Kerr, Lopez, & Wellman, 2005; Petitclerc et al., 2015; Rothbart & Bates, 2006; Wakschlag et al., 2012), addiction (Zucker, Heitzeg, & Nigg, 2011), depression (Wang, Cassin, Eisenberg, & Spinrad, 2015), bipolar disorder risk (Tseng et al., 2015), schizophrenia, autism spectrum disorder, obsessive-compulsive and habit disorders (Fineberg et al., 2014), eating disorders, some personality disorders (Nigg, Silk, Stavro, & Miller, 2005) and others. Processes of SR are integral to the emergence of psychopathology (Rothbart, 2011), and, exemplifying a developmental cascade of risk, problems in SR predict future obesity, unintentional injury, homicide, and suicide in youth (Barkley & Cox, 2007; Prager, 2009; van den Ban et al., 2014). Children’s SR informs social and intellectual developmental milestones (Blair & Razza, 2007; Kochanska, 1997; Padilla-Walker & Christensen, 2011; Spinrad et al., 2006), and is a leading predictor of academic outcome, and occupational success, and health (Clausen, 1995; Tangney, Baumeister, & Boone, 2004).
While it is clearly important, different aspects of SR may relate to different outcomes in distinct ways (Morris, Keane, Calkins, Shanahan, & O’Brien, 2014; Wakschlag et al., 2014). Unfortunately, integration of findings is impeded by differences in terminology, levels of analysis, and measurement among related constructs across fields. SR (or equivalent) is studied heavily in personality, social, and cognitive psychology, developmental science, clinical psychology, psychiatry, economics, sociology, neuroscience, and medicine.
Investigators in these fields have approached SR in multiple ways without a consensus framework for decades (Karoly, 1993). Some of this variation represents useful sub-division of the construct domain for analysis from different perspectives. Further, because SR reflects an adaptive, dynamic, complex process and underlying system or systems, verbal formulas or linear equations will at best capture only part of it. Thus, some variation is understandable.
Nonetheless, the range of constructs can be confusing. It includes (with key reviews for background): executive functioning (Diamond, 2013), emotion-, mood-, and affect-regulation (Gross, 2015), temperament (Rothbart & Derryberry, 1981; Rothbart 2011); effortful control (Eisenberg et al., 2013; Rothbart, 2011), ego under-control (J. H. Block & Block, 1980), reactive control (Derryberry & Rothbart, 1997), behavioral inhibition (Kagan & Snidman, 2004), impulse control and impulsivity (Madden & Bickel, 2010), risk-taking (Luciana, 2013); cognitive control (Botvinick & Cohen, 2014), working memory (Baddeley, 2012; D'Esposito & Postle, 2015; Kane & Engle, 2002), inhibition (Logan & Cowan, 1984; Nigg, 2000; Simpson et al., 2012), delay of gratification (Sethi, Mischel, Aber, Shoda, & Rodriguez, 2000), will/willpower (Ainslie, 2005), venturesomeness, constraint or conscientiousness (Sharma, Markon, & Clark, 2014); planfulness (Clausen, 1995), and more. Many of these are related but there is no agreement on how so. Table 1 provides a glossary of key terms.
Table 1.
Definitions and Glossary of major terms as relevant to in the current essay
| Action Selection: Cognitive or mental process of determining which of two possible actions to take (turn left or turn right). |
| Attentional inhibition: See inhibition, attentional. |
| Behavioral inhibition: The bottom-up interruption of a behavior sequence in response to novel, ambiguous, or threatening stimulus; mediated by internal state of anxiety. A component of bottom-up and reactive aspects of SR. Also referred to as reactive inhibition. See Nigg (2000) for more explanation and Kagan and Snidman (2004) for in depth elaboration. This term is sometimes incorrectly used interchangeably with ‘response inhibition,’ but behavioral inhibition is a bottom-up process and response inhibition is a top-down process (Nigg, 2000). |
| Behavioral under-control: A high level trait connoting spontaneous response to internal and external stimuli. See Block and Block (1980) and Zucker et al. (2011) for further conceptualization. Could be related to the meta-trait of ‘Stability’ in the Big Five framework. |
| Cognitive control: It has been defined as ‘the ability to flexibly adjust behavior in the context of dynamically changing goals and task demands’ (C. S. Carter & Krus, 2012) (pg.89); as ‘a set of superordinate functions that encode and maintain representation of the current task….[and engage] working memory…attention…action selection and inhibition’ (Botvinick & Braver, 2015) (p. 85); while related to executive functioning, greater emphasis on working memory, e.g., it is ‘active maintenance of goals and means to achieve them’ (E. K. Miller & Cohen, 2001). Herein, closely related to ‘lower level’ EF and enables ‘higher level’ EF. |
| Control, Behavioral: See behavioral under-control |
| Control, Cognitive: See Cognitive control. |
| Control, Executive. See Executive control. |
| Control, Interference: See interference control. |
| Control, proactive: See Proactive control. |
| Control, reactive: See reactive control. |
| Conflict: A general term that refers to the following kinds of conflict: (a) prediction failure (conflict between expected and actual event or outcome of an action), (b) perceptual (interference between goal relevant and goal-irrelevant but similar-seeming information), (c) response (two responses are triggered by stimulus, but only one is goal-relevant), (d) goal conflict (available action advances one goal at the expense of another goal), (e) activation of irrelevant cognition, emotion, or action to a goal. At the neural level, neural networks are always working in parallel and frequently if not constantly in competition as part of their mutual SR. |
| Conflict monitoring: Detection of any conflict, i.e., between expected and actual outcome, between perceptual targets and distractors, or between two conflicting response triggers. Often associated with activation in anterior cingulate cortex. |
| Effortful control: a dispositional trait-level representation that represents the tendency to be able to employ top-down control to self-regulate. It is seen as emerging from one aspect of EF or cognitive control, executive attention, but also, with development, including other capabilities (Rothbart, 2011). I argue here that effortful control maps at the cognitive level onto cognitive control, that is, basic controlled operations that underpin complex cognition. When cognitive control is used in the service of SR, that essentially is effortful control. |
| Ego control. See behavioral under-control. |
| Ego resiliency: The capacity to modulate self-control (or ego-control) in either direction to adapt to a situation. Flexible adaptation and use of different problem-solving strategies is a hallmark of ego resiliency (Block & Block, 1980). Broadly overlaps the domain space taken by SR but quite distinct in terms of theoretical basis and specification and adds the critical component of flexibility in application of control. |
| Emotion regulation: Adjustment of emotional state or expression to meet goals or to maintain homeostatic or allostatic state; includes both top-down (strategic) efforts (e.g., redirect attention) and bottom-up (e.g., arousal state) processes (Gross, 2014); it includes both intrinsic and extrinsic regulation and therefore is partially outside the domain of SR described here. |
| Executive Attention: Overcoming attention to a competing stimulus, to focus attention on a goal-relevant stimulus. A top-down form of attention, similar to endogenous attention and focused attention in other literatures. |
| Executive control: Synonym for top-down cognition, closely related to cognitive control and to executive function. |
| Executive function: Partially independent, top-down cognitive functions involved in top- down control of behavior, emotion, and cognition; support goal-directed behavior and cognition; and can be employed for top-down SR (Barkley, 2012). Other definitions emphasize rule- governed cognition; all definitions agree that EF reflects cognitive operations responding to an internal (top-down) goal. Dispute continues on whether there is a unitary ‘core’ to all EF. Includes ‘lower level’ operations like working memory and inhibition that overlap with cognitive control, as well as elaborated combinations of these operations that create complex cognition (Diamond, 2013) and include planning and coping strategies. |
| Impulsivity: Non-reflective stimulus-driven action when a later-rewarding goal-relevant response was also available. May be adaptive or maladaptive depending on context and degree of inflexibility as context changes. Mediated by both bottom-up processes (e.g., spontaneous reward valuation/discounting) and top-down process (e.g., biasing from prior goals; response inhibition). |
| Inhibition, Attentional: Ignoring a stimulus that is competing for attention to enable focus on goal-relevant information—in that sense, closely related to interference control and to executive attention. However, this top-down function is controversial; computational models suggest that inhibition may not be necessary to focus attention as simple inactivation of competing signals may be enough. Note that although not used in this way typically, attention can be inhibited by bottom- up signals as well (e.g., anxiety driving attention away from an immediate stimulus and toward another one). |
| Inhibition, Reactive: From Nigg (2000), reactive inhibition was use to designate the bottom-up interruption of a behavior, thus, here it is the same as bottom-up behavioral inhibition, and also closely related to reactive control (below). |
| Inhibition, Response: Top-down ability to intentionally or effortfully suppress a triggered behavior to sustain behavior toward a goal; a component of effortful control and of executive function. See (Logan & Cowan, 1984; Nigg, 2000; Simpson et al., 2012). |
| Interference control. Ignoring (inhibiting, suppressing, or de-activating) internal or external competing information to protect working memory or to focus attention on goal-relevant information; it is related to selective attention and attentional inhibition (Diamond, 2013). |
| Proactive control: In the cognitive literature, refers to expectancy-based activation of cognitive control (maintaining goal activation to bias responding) prior to an anticipated conflict or challenge (Braver, 2012). It is in contrast to reactive control, which is the activation of cognitive control after a change or conflict is detected. |
| Reactivity. From Rothbart’s model, means sensitivity to negative affects (fear, frustration, distress, sadness) and positive affects (smiling, laughter, approach). Related to bottom-up appetitive processes as described in the text. |
| Reactive control. Has two meanings in two literatures. In the cognitive literature, is differentiated from proactive control, where it refers to the activation of cognitive control after an error or a sudden change in conditions (i.e., response/goal conflict or other conflict) is detected, while proactive control means activation of control in expectation of challenge or conflict (Braver, 2012). In the developmental literature, it refers to bottom-up control, closely related to behavioral inhibition (see behavioral inhibition) (Derryberry & Rothbart, 1997). |
| Reactive aspects of SR: refers to bottom-up or relatively automatic responses that suppress behavior or alter regulatory state, such as anxious interruption of behavior in response to novelty, change in arousal level in response to potential reward, or spontaneous estimation of reward value; and spontaneous attentional capture by salient stimuli. |
| Reactive inhibition: See inhibition, reactive |
| Response inhibition: See inhibition, response |
| Risk-taking: Adaptive or maladaptive selection of rewarding behavioral option in the face of high probability of loss, attributed to steep discounting of loss probability (Reward sensitivity+punishment insensitivity). Also defined as the option with the most variable outcome; this is only true if a high possible loss is paired with a high possible gain. May be bottom-up (e.g., ‘impulsive’) or top-down (i.e., ‘deliberative’ or strategic); adaptation value depends on behavioral payoff. |
| Self-control: Has been used both in a very specific sense in the developmental literature to connote the capacity to resist temptation or inhibit a dominant response or activate a subdominant response (Diamond, 2013; Rothbart, 2011). In that sense it means over-ride of a stronger, stimulus-driven representation with a weaker, memory-driven representation. It has also been used in the social and other literatures in a broad sense to refer to voluntary cognition and behavior or effectively, top-down aspects of SR (Duckworth & Kern, 2011; Fujita, 2011); here it encompasses also control strategies such as re-appraisal, pre-commitment, and others. |
| Self-regulation: The intrinsic processes aimed at adjusting mental and physiological state adaptively to context. Encompasses cognitive control, emotion regulation, and top-down and bottom-up processes that alter emotion, behavior, or cognition to attempt to enhance adaptation (or to achieve an explicit or implicit goal or goal state). Also involves physiological systems (Calkins & Fox, 2002) as well as homeostatic and allostatic mechanisms in response to stress, challenge, or new information; encompasses strategic/deliberative as well as reactive/automatized processes and their reciprocal influences. However, the present review excludes allostatic and homeostatic processes and some theorists deny these are part of SR. |
| Sensation seeking: for present purposes, risk-taking, due to their high correlation and similar factor loadings. However, sensation seeking can also be framed as a higher-order personality factor. |
| Signal detection. Perceptual distinction of target signal from noise (is that my friend I hear speaking in the crowd?), differentiating two different stimuli (is it a bear or moose?), or signal detection (did I just hear the phone?). Amenable to mathematical modeling and therefore useful in process and mechanism models of components of SR. |
| Switching. Attention switching means looking at the same problem from two different perspectives; task switching means changing tasks in response to a meta-goal. Both are examples of top-down cognition or of executive functioning. |
| Venturesomeness: for present purposes, risk-taking at the personality trait level. |
| Will / will-power. The term will-power is rarely used but when it is, the usage varies between (a) interchangeable with effortful-control as defined here and (b) interchangeable with the high level self-control capacities of persistence and long-term strategies, what Duckworth and colleagues call ‘grit.’ Thus, while it generally refers to a deliberate resistance to an immediately-activated stimulus-driven response (Ainslie, 2005; Diamond, 2013; Kross & Mischel, 2010; Zhou et al., 2012), in some usages ‘will’ also refers to grit or conscientiousness. |
| Working memory: Has multiple definitions. Fundamentally, the ability to hold multiple things in mind at once, while mentally manipulating one or more of them (Baddeley, 2012). While different models are related, the model adopted in the text is closely related to that offered by Engle (Kane & Engle, 2002) as the combination of short-term memory and controlled or ‘executive’ attention or interference control; that is, working memory in that model requires inhibition of competing information to protect working memory. I do not assume that working memory requires attentional inhibition but do assume it requires executive attention. |
Calls for clarification have multiplied and been helpful (Blair & Ursache, 2011; Diamond, 2013; Eisenberg, Smith, & Spinrad, 2011; Rothbart, 2011; Sharma et al., 2014; Welsh & Peterson, 2014; Zhou, Chen, & Main, 2012). Yet the effort remains incomplete. Morrison and Grammer (2016) colorfully warn of ‘conceptual clutter’ and ‘measurement mayhem.’ This review offers a roadmap for a unified approach in developmental psychopathology and clinical science. A set of constructs is proposed within which further subdomains can be added for particular purposes, to foster integration, delineation of mechanisms, and prediction.
General issues
Definition of terms and scope of review
Rather than a systematic review or meta-analysis, I briefly orient readers to historical linkages and then emphasize recent literature. I draw primarily on cognitive science and neuroscience, developmental science, and social-personality psychology in addition to the clinical psychopathology literature, and prioritize constructs related to psychopathology. Table 1 defines the most common terms as intended herein. Before moving to important concept distinctions, I note selected background issues that have caused confusion.
First, SR is not static. It develops through critical periods from early life to adulthood, in non-linear and stage-sequenced fashion, via a hierarchical, cascade process. Lower-level capacities assemble into more complex capabilities, congruently with development of physical and neural systems and the gradual internalization of control during childhood (Cox et al., 2010; Masten & Cicchetti, 2010; L. B. Smith & Thelen, 2003; Thelen, Schoner, Scheier, & Smith, 2001). Adolescence, for example, is typified by asynchronous, non-linear development across different kinds or aspects of SR (Casey, 2015), moderated by emotion context (Cohen et al., 2016). This principle of hierarchical differentiation helps with integrating constructs later.
Second, needless confusion arises from confounding measurement and construct—they are far from isomorphic. The same measures can tap related constructs; and laboratory-based observations, computerized reaction time and accuracy tasks, and rating scales have weak inter-correlations (McAuley, Chen, Goos, Schachar, & Crosbie, 2010), although coherent patterns can be identified (Duckworth & Kern, 2011). My focus is on concepts, not measures.
Third, models of SR typically start with a dual-process logic making it important to clarify what is regulated, versus what is regulating, in a given context. Dual process conceptions emanated from the classic distinction between automatic and deliberate attention (Posner & Snyder, 1975; Schneider & Shiffrin, 1977), then generalized to most domains of psychology (Evans, 2008; Evans & Stanovich, 2013). While ultimately a dual-process account is insufficient, it is valuable. The dual-processes are described in different ways, such as automatic/deliberate; bottom-up/top-down; exogenous/endogenous; implicit/explicit; gist/verbatim; unconscious/conscious; and to remain agnostic, Type I/Type II. Models of SR propose at least one top-down ‘control’ aspect and one or more bottom-up processes, the latter as targets of regulation (Gross, 2014; Rothbart, 1981; Shulman et al., 2016) but also as regulating. I explain further below.
Fourth, while a mechanistic model is not my goal, the process literature needs to be recognized as crucial, as it will aid in future concept clarification. I borrow from it selectively. It includes psychological models (Baumeister & Heatherton, 1996; Carver & Scheier, 2001; Gross, 2015; Mischel & Shoda, 1995); a range of mathematical decision models including signal detection (D. M. Green & Swets, 1966; Sergeant, Oosterlaan, & van der Meere, 1999; Wickens, 2002), timing (Gibbon, Church, Fairhurst, & Kacelnik, 1988; Wearden & Lejeune, 2008), discounting (Mitchell, Wilson, & Karalunas, 2015), foraging (Stevens & Stephens, 2010), accumulative models (Huang-Pollock, Karalunas, Tam, & Moore, 2012; P. L. Smith & Ratcliff, 2004); their computational extensions (Botvinick & Cohen, 2014), and neural accounts (Petersen & Posner, 2012). Feedback principles have been fundamental to understanding brain function for nearly a century (von Uexküll, 1926); and to process models of SR for half a century up to the present (Gross, 2015; G. A. Miller, 1956; Verbruggen, McLaren, & Chambers, 2014). Computational models also operationalize the recursive optimization logic consistent with cybernetics—including in particular input from bottom-up processes (Botvinick & Cohen, 2014)2. Most theorists therefore agree that bottom-up processes must participate in SR (thus, Figure 1).
I therefore next clarify the crucial bottom-up and top-down concepts. Then, I address three clarifications: (a) domain general SR; (b) similarity of executive functioning, effortful control, and cognitive control; and (c) distinctions among impulsivity, inhibition, and risk-taking. I conclude with a proposed descriptive organization of the SR domain.
Target/source of SR in relation to top-down/bottom-up process
While this distinction is extremely useful, it has to be noted that bottom-up (automatic, reactive, or Type I) and top-down (deliberate, or Type II) are on a continuum, not absolute categories (Evans & Stanovich, 2013; MacLeod, 1991; Nigg, 2000; Posner & Snyder, 1975; Schneider & Shiffrin, 1977) in part due to successive reciprocal neural feedback loops (Fuster, 1997). For instance, top-down systems can activate, suppress, or bias bottom-up responses (Avital-Cohen & Tsal, 2016; Corbetta, Patel, & Shulman, 2008; Ochsner et al., 2009). In turn, bottom-up systems can activate goal-related behavior via priming or other effects, limiting the effects of top-down process (Verbruggen, McAndrew, Weidemann, Stevens, & McLaren, 2016).
Type I (‘Bottom-up’ or automatic) processes in relation to SR
Type I processes are automatic, stimulus driven, rapid, and do not require mental capacity. They are called ‘bottom-up’ as an evocative shorthand because they are elicited by sensory (external) stimuli and because human brain imaging and primate single cell recording studies link them with ‘feed-forward’ neural signaling (e.g., subcortical to cortical, or posterior to anterior cortical signaling) (Depue & Lenzenweger, 2006; E. K. Miller & Buschman, 2012). Bottom-up processes are not unitary (Evans & Stanovich, 2013), as shown in Table 2. These are classically the targets of SR; for example, a child must regulate her excitement. However, they also can be regulatory in at least four ways. Because this is not always recognized, I elaborate briefly.
Table 2.
Key Bottom-up mental processes that can be targets of regulation or can be regulatory
| Process | Example |
|---|---|
| Innate and unconditioned response | child reaches directly for an object rather than around an obstacle; attention is captured by a looming object |
| Reflexes | startle to a loud noise |
| Single stimulus learning (habituation, sensitization) |
learning to ignore an uninformative bell or command; e.g., a child ignores repeatedly un-enforced warnings of punishment |
| Habit formation/associative learning (classical conditioning) |
A child learns to wake up when his bladder is full |
| Operant learning | A dog learns to sit to earn a treat; A child learns to say please to earn parental praise |
| Appetitive approach (positive affect; excitement, hope; but also possibly anger) |
Drawing upon both conditioned and learned signals; e.g., a child comes to the kitchen upon smelling cookies; a smoker stops the car upon seeing a party store. |
| Fear avoidance* (negative affect; anxiety, fear) |
Converse to approach; a child avoids a stranger; driver spontaneously takes foot off gas on seeing police |
Approach and avoidance, of course, also operate at other levels of analysis, such as reflex (pulling hand away from the stove), and higher order goal setting. Here, the terms pertain specifically to the appetitive systems. The term fear can be controversial; I bypass that here but further debate on the best term for reactive motivations is likely to occur. Crucially, fear and anxiety can be distinguished, but that also is beyond my scope here.
The biological validity of approach and avoidance affect is supported by scalp electrical recordings: Approach (‘positive’ but including aspects of anger) emotions are associated with left-lateralized frontal EEG activation, and avoidance emotions (fear, anxiety, possibly sadness and other aspects of anger) with right-lateralized frontal EEG activation (Compton & Heller, 1998; Davidson, 1992); as well as by experimental mathematical representations as described later in the text.
The neural bases of these bottom-up operations are extensively described in the literature but not described here due to space limitations. Note that debate remains about how many systems exist; for example, while habit formation is a well-described phenomenon important to psychopathology, controversy remains about whether associative or implicit learning is a separate system (Shanks, 2010).
First, they modulate and optimize one another. For example, incentive approach and avoidance are modulated by associative and instrumental learning history (a baby’s excitement when a mother pulls out a toy they played with before). Second, bottom-up processes can prime, activate, or modulate top-down processing (Bargh & Ferguson, 2000). Nigg (2000, 2001), following earlier authors (Derryberry & Rothbart, 1997; Eisenberg et al., 1997; Rosenberg & Kagan, 1989; Rothbart, 1981), highlighted that bottom-up, reactive processes related to (a) approach and (b) fear/avoidance are a particularly important part of SR of action and cognition. For example, Table 2’s fear avoidance, also called behavioral inhibition (Table 1) is activated in response to novelty, uncertainty, or other cues for possible harm. It inhibits action and redirects attention (cognition) to more quickly adapt to uncertainty (Depue & Lenzenweger, 2006; Gray, 1982; Kagan & Snidman, 2004). A child spontaneously lowers his voice and redirects attention when a strange child enters the room. Third, top-down operations can be automatized for particular contexts (e.g., due to learning; Bargh & Ferguson, 2000); now automatic, they remain regulatory (Dijksterhuis & Strick, 2016; Marien et al., 2012; Papies & Aarts, 2011; Verbruggen et al., 2014). In fact, we automatize as much as possible (Bargh & Ferguson, 2000), due to costs of control; control depends on motivation, capacity or both (Botvinick & Braver, 2015). Fourth, they support top-down regulation by providing goal-relevant information (threat or reward cue information; prior learned associations) to working memory, when cued. Figure 1 therefore includes bottom-up processes, which access top-down, goal-directed processing via gating mechanisms as part of SR. We return to them when considering impulsivity and risk-taking later.
Type II (‘Top-down’ or deliberate) processes in relation to SR
Most SR literature focuses on Type II (top-down or deliberate) processes. Top-down processes are subjectively deliberate, slow, sequential, require working memory, and are capacity-limited (the separate question of resource-limitation is complex (Hagger & Chatzisarantis, 2016); for discussion see the online supplementary information, Appendix S1). They engage to address novel problems, to resolve conflict or co-activation (Table 1), or to prepare for an anticipated goal or challenge. They are called ‘top-down’ because they respond to internal mental representations (such as a goal or a rule) rather than external (sensory) stimuli, and because human imaging and primate single cell recording data link them to ‘feed backward’ neural signaling (i.e., cortical to subcortical or anterior to posterior cortical) (Depue & Lenzenweger, 2006; E. K. Miller & Buschman, 2012).
Various top-down operations involved in SR engage overlapping and distinct neural regions, underscoring the importance of some differentiation of component functions despite ongoing debate about a possible shared ‘core’ of top-down processing. A distributed network, parallel, recursive processing model of the brain defies strict localization. However, brain nodes important in top-down processes include the anterior cingulate cortex (ACC) (Botvinick & Braver, 2015; Shenhav, Botvinick, & Cohen, 2013); ventromedial prefrontal cortex (VMPFC) (Cohen & Lieberman, 2010); ventrolateral prefrontal cortex (Egner, 2011); and the dorsolateral prefrontal cortex (DLPFC) (Diamond, 2013). These nodes in turn are embedded in circuits (such as the ACC-DLPFC circuit); the circuits in turn are embedded within distributed functional and anatomical cortical-subcortical networks. Two important networks include the cingulo-opercular and fronto-parietal (Petersen & Posner, 2012). The cingulo-opercular network, broadly, includes the inferior regions in lateral PFC (including ventrolateral PFC), regions of the ACC, the insula, and other nodes. The fronto-parietal network, broadly, involves superior regions in lateral PFC, including dorsolateral PFC, as well as regions of posterior parietal and inferior temporal cortex and subcortical connections. Cross-talk with additional brain networks is important, including the default mode network (which includes the ventromedial PFC) and a noradrenergic, locus-coeruleus ‘alertness’ network involved in arousal regulation (Aston-Jones & Cohen, 2005). Subcortical regions, particularly the thalamus and hippocampus, also participate indirectly in top-down regulation via information salience and updating.
Specific conceptual confounds and recommended clarifications
With the preceding in mind, I move on to three key conceptual challenges in the field.
SR and self-control as domain general
The first issue is the need for a domain-general construct while separating broad and narrower meanings of SR and of self-control. I take these together because they are used at times interchangeably (Kelley, Wagner, & Heatherton, 2015; McClelland & Cameron, 2012) to mean top-down control. Matters also have been confused by sometimes conflating emotion with regulation (so handling an emotionally challenging task=SR) and cognition with control (so that handling a cognitively challenging task=self-control). I begin by arguing for a domain general meaning of SR across emotion, action, and cognition (Figure 1).
Emotion regulation is ‘any process that influences the onset, offset, magnitude, duration, intensity, or quality of one or more aspects of emotion response’ (Gross, 2014). Koole et al (2011) distinguish SR of action (which they call ‘SR’) from emotion regulation. I take SR of action to mean optimization of overt motor, ocular, or vocal response (excluding physiological changes) for purposes of adaptation (‘a goal’). Whereas it is clear that attention (and other cognitive functions) are regulating (Posner, Rothbart, Sheese, & Voelker, 2014), some definitions of SR extend it to include cognition as also regulated (Karoly, 1993). I agree and use the term SR of cognition3 to connote the modification of attention, memory, or working memory to try to enhance adaptation or achieve a goal in the absence of overt behavior or salient emotion regulation. Counting my breaths to clear my mind is regulating contents of working memory, with secondary regulation of affect; attending to my friend’s voice while keeping my eyes on my child regulating attention; in neither case is there action or primary emotion regulation. These examples are top-down, but when attention is captured by a threat cue, it is regulated by a bottom-up process to enhance survival.
Thus, although some definitions of ‘SR’ are domain specific (either distinguishing it from, or else equating it with, regulation of emotion) or narrow (limiting to overcoming temptation), and while it remains important to distinguish SR from the meta-skills or component processes that enable it, it is most helpful to treat ‘SR’ as broader and domain-general (Bell & Calkins, 2012; Blair, Raver, & Finegood, 2016; Karoly, 1993). We can then use specific terms to designate SR of emotion, action, or cognition. Note that this is not to postulate a single core process (an empirical question), but that we need an umbrella concept to prevent confusion of domain-specific versus domain-general claims.
Self-control, although it should not be limited to cognition, is a narrower concept. All theorists refer to self-control in relation to top-down process exclusively (Baumeister & Heatherton, 1996; Carver & Scheier, 1982, 2001; Magen & Gross, 2010; Mischel & Shoda, 1995). Common ‘narrow’ definitions include: (a.1) top-down overcoming of a stimulus-driven response to execute a goal-relevant response (Baumeister & Heatherton, 1996; Diamond, 2013) and more specifically, (a.2) selecting a later, larger reward over an earlier, tempting, but ultimately smaller reward (Rachlin & Green, 1972). However, (b) a broader definition is proposed by others, including (b.1) any deliberate action that promotes long-term adaptation (Fujita, 2011); (b.2) any voluntary alteration of responses (Baumeister, Vohs, & Tice, 2007); or (b.3) voluntary self-governance (Duckworth & Kern, 2011). Such broad definitions implicitly simplify self-control to the top-down aspects of SR, although to avoid confusion it is essential that researchers spell out, rather than assume or only imply, their intended definition.
Executive functioning, effortful control, and cognitive control are closely related
How do the top-down aspects of SR relate to executive functioning, effortful control, and cognitive control, as well as other related terms like executive attention and working memory (Table 1)? I will argue that EF, effortful control, and cognitive control are closely related but arise from different intellectual traditions in clinical, developmental, and cognitive sciences.
Executive Functioning (EF) or Executive Functions (EFs)
EF as a description of top-down cognition emerged from studies of frontal lobe functioning and clinical neuropsychology of adults (Luria, 1966; Pribram, 1973; Shallice, 1982; Shallice & Burgess, 1991; Stuss & Benson, 1986), and was initially operationalized with clinical laboratory measures (Lezak, Howieson, & Lorine, 2004; Strauss, Sherman, & Spreen, 2006). It has accrued a welter of overlapping definitions, as recently reviewed by Barkley (2012). Contemporary definitions describe (a) a set of at least partially independent top-down functions that support goal-directed action (Banich, 2009; Blair et al., 2016; Diamond, 2013; Friedman & Miyake, 2016; Miyake et al., 2000); or in a computational context, rule-governed behavior (guided by internal goals or rules rather than by external stimuli) (Verbruggen et al., 2014); and (b) complex cognition including manipulating two things in mind at once, reasoning, temporal projection, and complex mental and action sequences (Barkley, 1997; Diamond, 2013). EF is invoked when automatized routines will not work or are not possible (e.g., novel situations). Applying the EF to young children has been facilitated by child-specific reviews (Diamond, 2013; Garon, Bryson, & Smith, 2008; Jurado & Rosselli, 2007), developmental conceptual models (Diamond, 2013; Wiebe et al., 2011; Zelazo, Carter, Reznick, & Frye, 1997), and measures (Garon et al., 2008; Willoughby, 2015).
Even pigeons can learn complex behavior chains and carry them out in the face of familiar cues. Humans, however, can assemble a complex sequence of actions based solely on a mental representation, even for novel problems for which automatized behavioral chains cannot be used (unless they are re-assembled or re-mapped to a new context). EF thus allows people both to resolve immediate conflict (e.g., ignoring a distractor), and to manage, in the present, future conflicts/goals (e.g., preparing for tomorrow’s meeting or next year’s marathon).
Because EF is too broad to enable computational implementation or consensus measurement, it must either be operationalized more narrowly or fractionated to be useful for research. Fuster (1997) proposed that the prefrontal cortex supports three functions necessary for higher order EF: working memory (which he associated with involvement of dorsolateral prefrontal cortex), set maintenance and shifting (‘motor attention’), and inhibition/interference control (suppressing interfering information; which he associated with orbitomedial prefrontal cortex and its connections). In a pioneering empirical report supporting that model, Miyake et al. (2000) reported that working memory, response inhibition, and task/set shifting formed three related but distinct latent factors. They did not claim to have used an exhaustive set of EF measures. Subsequently, commonly cited components include response inhibition (stopping a prepotent response), interference control (resisting internal or external attentional distraction), switching (seeing a problem from two different angles as well as changing task set in relation to higher order rule or goal), working memory, planning, fluency, and many others (Diamond, 2013; Friedman & Miyake, 2016; Garon et al., 2008; Sharma et al., 2014).
Distinctions among EFs are clearly sensible. In addition to employing partially distinct neural regions, EF component functions develop hierarchically (Rueda, Posner, & Rothbart, 2005). In very young children, EFs hardly extend beyond temporarily overcoming a stimulus-driven response (Garon et al., 2008). Diamond (2013) outlined how key components can be organized developmentally. The earliest to develop she called ‘low level EF’: working memory and response inhibition. These enable a subsequently developing intermediate level, cognitive flexibility (which overlaps with set shifting and task shifting). They combine still later in development to enable ‘high level EFs,’ including reasoning, problem solving, and planning.
EF and Emotion
Historically, EF has generally referred to cognitive operations without explicit reference to emotional regulation. However, interest has grown in the role of EF in the SR of emotion, blurring an apparent distinction between cognitive and emotion-related top-down operations. For example, Gross (2014) notes that emotion regulation includes the use of strategies like reframing or pro-active planning that clearly rely on EF. Barkley (2012) proposed that EF be defined broadly as any ‘self-directed action’ aimed at reaching a goal or goal state, equating it with top-down SR. Casey (2015) notes that computationally, emotion is another type of information on which top-down cognition operates. Zelazo and Cunningham (2007) consider EF as directly involved in emotion self-regulation.
None of those writers, however, intended an equivalence in regulating emotional and non-emotional information. It is generally recognized that, neurobiologically, while absolute separation of emotion-related from other top-down cognition is problematic, partially distinct neural nodes in the ACC are engaged by top-down regulation in the face of emotionally salient but not cognitively complex conflict (the rostral ACC), versus cognitively complex but not emotionally salient conflict (the caudal ACC) (Beckmann, Johansen-Berg, & Rushworth, 2009). Relatedly the prefrontal cortex can be functionally segregated into dorsal and medial regions (Blumenfeld, Nomura, Gratton, & D'Esposito, 2013; Fuster, 1997) with differential association to emotionally-laden tasks. Further, developmentally, the trajectory of behavioral self-regulation differs for emotional and non-emotional stimuli (Botdorf, Rosenbaum, Patrianakos, Steinberg, & Chein, 2016; Casey, 2015; Cohen et al., 2016).
Even so, growing recognition of the overlap and distinction of EF in various self-regulation contexts including emotion has led to new terminology. The most widely-used formulation in a developmental context is ‘hot’ versus ‘cool’ EF (Zelazo & Carlson, 2012; Zelazo & Muller, 2002). Zelazo and colleagues suggest that these are not two systems but ends of a continuum; in most situations, both are partially involved. Hot EF is top-down processing (including regulation) of emotional or incentive signals. It is unclear whether it has any different meaning than top-down SR of emotion—but it serves to emphasize that EFs are involved in regulating emotion. Cool EF is top-down processing (including regulation) of salient information signals that presumably have minimal incentive and/or emotional intensity. This distinction does face familiar difficulties, such as different definitions used by different authors, confounding of task with construct, and failure of efforts at psychometric validation (Welsh & Peterson, 2014). Nonetheless, the constructs have been generative and led to new theoretical formulations. For example, Petrovic and Castellanos (2016), while agreeing that the EFs may be domain-general, marshal brain-imaging data to argue that much psychopathology occurs along a neurobiologically mapped hot/cool gradient. For instance, ADHD and conduct disorder may involve breakdown along different parts of that neurobiological gradient.
Summary
EFs are inter-related abilities that emerge hierarchically in development, although their precise fractionation varies somewhat across statistical (Miyake et al., 2000), computational (Vandierendonck, 2016), and developmental approaches (Diamond, 2013; Marcovitch & Zelazo, 2009; Wiebe et al., 2011), perhaps in part because components vary with development. EF means the top-down processing of information, including emotion information, and therefore is involved in a domain general way in all top-down aspects of SR. Consistent with this view, Zelazo and Cunningham (2007) proposed that when the task is SR, then EF is the same as SR. However, that claim depends on agreeing that SR includes top-down strategies, such as a priori plans to avoid a conflict in the first place via pre-commitment (Ainslie, 1975; Fujita, 2011), antecedent regulation (Gross, 1998), implementation intentions, appraisal, construal, and others that are related to emotion SR (Duckworth, Gendler, & Gross, 2016; Fujita, 2011; Gross, 1998)—because all of these depend on aspects of EF. In other words, when EFs are employed in the service of SR, then EF and SR are practically the same.
Yet, EF should not simply be equated with top-down SR. SR is an adaptive change in internal state, emotion, thought, or action, whereas EF is a set of cognitive capacities that when implemented can enable SR to occur. Crucially, from this perspective EF is available for purposes other than SR. For example, solving a mental math problem requires EF, but is not self-regulating.
Cognitive control
Cognitive control is an umbrella term that at a molar level stands in the EF space within cognitive psychology, although with far less emphasis on complex cognition. It emerged initially in cognitive science as early as the 1960’s with the identification of controlled attention as noted earlier (Posner & Snyder, 1975), although only later becoming a defined focus, as reviewed in detail by Botvinick and Cohen (2014). It has been defined recently as ‘the ability to flexibly adjust behavior in the context of dynamically changing goals and task demands’ (C. S. Carter & Krus, 2012) (pg.89); as ‘a set of superordinate functions that encode and maintain representation of the current task….marshalling to that task subordinate functions including working memory…attention…action selection and inhibition’ (Botvinick & Braver, 2015) (p. 85); as ‘active maintenance of goals and means to achieve them’ (E. K. Miller & Cohen, 2001); and as real-time optimization of responses (emotion, attention, motor) to a goal (Botvinick & Cohen, 2014). Thus, despite a close relation to EF, cognitive control is narrower than EF with a particular emphasis on resource allocation, information maintenance (working memory), and executive attention, rather than complex cognition (Botvinick & Cohen, 2014; Niendam et al., 2012). Cognitive control can be taken to mean the basic top-down operations from which complex EF emerges.
Conflict is essential to activate cognitive control. It (Table 1) can mean response conflict (two responses have been primed), perceptual conflict (i.e., interference from task-irrelevant information), cognitive conflict (i.e., interference from task-irrelevant associations or thoughts), or goal conflict (task-switching, or a single action will support one goal but defeat another). While highlighted in research on cognitive control, conflict also triggers EF.
Cognitive control is also fractionated in different ways. Proposed elements include executive attention, working memory, response inhibition, and interference control—all overlapping with lower level elements of EF. However, working memory receives considerable emphasis. Proposals using an information processing approach specify other components, such as signal detection, action selection, and action execution.
Summary
For purposes of highlighting integrations, cognitive control is domain general and is the same as ‘lower-level’ EF as described by Diamond (2013) (possibly including switching). EF depends on cognitive control, but EF extends beyond cognitive control to include complex cognition as well as emotion. Like EF, cognitive control can be employed both for SR and for other purposes; when employed for SR it is the cognitive part of top-down SR.
Effortful control (EC)
The extension of SR to children is heavily influenced by research on temperament pioneered by Mary Rothbart and colleagues (Rothbart, 1981; Rothbart & Derryberry, 1981). SR was seen in a temperament model as comprising the interplay of reactivity (bottom-up) and self-regulation (top-down). Factor analysis of parent ratings of child temperament revealed a higher order factor comprising focusing and shifting of attention, inhibitory control, perceptual sensitivity, and low threshold for pleasure called effortful control (EC) (Rothbart, Ahadi, Hershey, & Fisher, 2001). Subsequently, Rothbart, Ellis, Rueda, and Posner (2003) offered a more general definition: ‘a broad factor involving attentional focusing, attentional shifting, and inhibition and activation control of behavior’ …and added that EC is ‘the ability to inhibit a dominant response (inhibitory control)…to detect errors…and to engage in planning’ (p. 57). They identified correlations between ratings of EC and laboratory measures of attention (orienting and later executive attention) that established a link between EC and brain development (Rothbart et. al. 2003, 2014). Neural implementation was seen as via an ACC-DLPFC circuit and the associated cingulo-opercular network (Posner & Rothbart, 2000, 2007; 2009; Posner et al., 2014). Although EC regulates emotional response, its constituent items include regulation of action and cognition as well. Thus, it is domain general. EC was also operationalized with laboratory tasks (Eisenberg et al., 2011; Kochanska, Coy, & Murray, 2001; Reed, Pien, & Rothbart, 1984; Rothbart, 2011; Rothbart, Sheese, & Posner, 2014), some of which overlap with EF tasks such as response inhibition.
By its link with basic temperament traits, EC excludes complex cognitions and strategies. Historically, working memory has also been excluded, but Zhou and colleagues (Eisenberg & Zhou, 2016; Zhou et al., 2012) concluded in their literature reviews that working memory is probably part of or closely related to EC. Whether this is due to a shared attention component is unclear. The nascent confirmatory and exploratory factor analytic literature, while complex, generally puts EC and EF trait/task measures, including measures of working memory, on the same factor when measurement type is taken into account (Duckworth & Kern, 2011; Eisenberg & Zhou, 2016; Sulik et al., 2009).
Planning was included as a component of EC in some papers by Rothbart and colleagues, as cited earlier. However, planning entails complex cognition. It is likely that planning is only related to EC due to shared involvement of executive attention and that planning itself goes outside the EC construct (Mary Rothbart, personal communication, 9/12/2016). In short, as identified empirically, EC involves the act of top-down control for purposes of self-regulation, and excludes high level EF. EC therefore appears to map closely onto cognitive control at the trait level.
Summary
EC, identified as top down aspects of self-regulation, represents at a trait level many of the cognitive control aspects of EF, and in particular executive attention. While empirical examination likely will lead to inclusion of working memory, EC is narrower than EF. While questions remain about precise empirical mapping, careful consideration is warranted for a proposal that EC is a trait level that maps onto the use of cognitive control in SR. It will likely correlate with EF measures of complex cognition, but this would be attributable to their shared dependence on executive attention.
EF, EC, and cognitive control: Summary
EF, EC, and cognitive control are conceptually domain-general in relation to self-regulation of action, emotion, and cognition (although partially distinct neural systems are engaged in those different regulatory contexts). Cognitive control is essentially the same as lower level, or basic EF functions. Higher-level EFs involve complex cognition and are extended elaborations of cognitive control. Many strategies for self-regulation, such as pre-commitment, are part of, uses of, or elaborations of EF. EC, while still undergoing empirical investigation of its relations with cognitive control and EF, can be understood provisionally as the trait level representation of the use of cognitive control for SR.
To reiterate the justification for this, it requires addressing working memory and planning. While in the past omission of working memory from EC made a linkage of EC with cognitive control problematic, and while more empirical work is needed, we can provisionally conclude based on recent empirical work that working memory is part of EC—although it’s unclear if this is due to shared involvement of executive attention. At the same time, inclusion of planning in some renditions of EC can be reversed, as because EC does not include complex cognition (high level EF). While EC may be correlated with planning and other complex EF, this is explained by their mutual dependence on executive attention (or, likely, cognitive control generally). This simplification, that EC is trait level of cognitive control, while not without qualifications, enables basic linkage of literatures.
Impulsivity, response disinhibition, and risk-taking should be distinguished
Distinguishing constructs
When SR works well, we successfully navigate response conflict (Table 1) to maximize our adaptiveness (or its mediators—goals, resource gain, and so on). SR can break down in numerous ways. One of the most often mentioned is breakdown in response inhibition (‘disinhibition’). Impulsivity and risk-taking are multi-componential behaviors also typically interpreted as breakdowns in SR. both are often confused with disinhibition. Helpfully, theories of impulsivity and risk-taking have described SR failures in a decision-making framework. While that is only one of several approaches (Fineberg et al., 2014), the relevant mathematical and computational analyses help describe the SR domain in relation to psychopathology (Nigg & Nagel, 2016) (Appendix S1 provides details on the psychophysical basis for this approach as well as its limitations). Avoiding the common muddling of impulsivity, disinhibition, and risk-taking is fundamental to mapping the different ways that SR can break down in psychopathology. (It is also important to relate compulsivity to these constructs but space allows me only a brief mention below; see Fineberg et al., 2014, for more discussion).
Response inhibition is often carelessly used interchangeably with impulsivity. It refers to preventing or interrupting a response--regardless of discounting, stimulus valence, or decision context (Aron, Robbins, & Poldrack, 2004; Logan & Cowan, 1984; Nigg, 2000; Simpson et al., 2012) (Table 1); it is closely related to inhibition-switch, in which one action is replaced by another. It is an early developing component of top-down aspects of SR (Diamond, 1990; 2013). It is one component of impulsivity but also of compulsivity. In the case of cue-activated automatic behavior, disinhibition and impulsivity are the same. But other cases of impulsivity include discounting of delayed rewards and are not reducible to disinhibition.
Impulsivity in turn is multi-componential and variously defined, but has two meanings for purposes of this discussion (see Appendix S1 for background on the specifiers given in what follows). One meaning is non-reflective selection of the stimulus-evoked response (cue-activated behavior), e.g., the frontal-lobe patient who automatically strikes a match when shown a cigarette or the dysregulated individual who blurts out an unflattering comment upon being surprised by a friend’s weight gain. As noted above, this is the same as disinhibition. The other meaning is non-reflective selection or preference for the immediately rewarding response, (motivated decision style), e.g., the drinker who despite a resolve to be at work on time, decides to swing by the tavern on the way home (immediate reward prioritized).
Thus, impulsivity in the second instance is more than disinhibition: it also reflects the (implicit as well as explicit) weighting of immediate versus delayed reward. It is modulated both by bottom-up reward valuation (part of the incentive approach system) and top-down biasing related to goals. Notably, immediate reward, not merely earlier reward, is what invokes a distinct approach response system (Depue & Lenzenweger, 2006; McClure et al., 2004; Mitchell et al., 2015). It depends on computations involving time.
Risk-taking, in the decision-making context, entails weighting on probability (rather than temporal) features of a choice; it is the action that discounts the probability of negative consequences relative to probability of positive consequences. (Appendix S1 discusses other definitions). Either possible reward is excessively valued (computationally, over-predicted) or possible harm is excessively devalued (under-predicted) or both (Casey, 2015; Ernst, 2014). Whereas risk-taking is clearly related to strong reward motivation (approach), its distinguishing feature in fact may be concurrently suppressed estimation of punishment-probability. Casey (2015) reviewed animal and human studies indicating that adolescents, while more sensitive to cues for reward and punishment than children or adults, have suppressed sensitivity to punishment context (i.e., ambiguity risk). This may be a bottom-up effect, as animal work she reviewed suggests reduced hippocampal signaling to the amygdala in that context.
Risk-taking can be impulsive (clamoring up a risky cliff face for a view without stopping to think) or reflective (planning an expedition on Mt. Everest). It is risk-taking to the extent that chance of loss is devalued relative to chance of reward. The Everest expedition multiplies one’s chances of an early death in return for a thrilling experience. It may be ill-advised, inflexibly chosen, or maladaptive. However, after a year of planning, it is anything but impulsive.
Temporal and probability computations behave differently (L. Green & Myerson, 2010). One example is the differential magnitude effect: when discounting by time, people discount small rewards more steeply than large rewards. But when discounting by probability, the reverse occurs—people discount large rewards more than small rewards (Mitchell & Wilson, 2010). Further, responses in the two experiments are uncorrelated. Impulsivity entails computations about timing of outcome (immediate is favored) regardless of valence (though research usually focuses on reward). Risk-taking entails computations about probability, with relative probability of punishment or loss discounted, regardless of immediacy.
Personality structure also distinguishes impulsivity and risk-taking. Eysenck (1993) suggested that two basic personality dimensions were venturesomeness and constraint (he called it psychoticism, a misnomer in today’s nomenclature). In more contemporary work, venturesomeness is related to extraversion and to incentive approach (Table 2), with relative devaluation of uncertainty or possible loss (Sharma et al., 2014), regardless of immediacy. The core theme is psychological movement toward reward (approach) while disregarding potential ‘downside’—i.e., risk-taking. Constraint entails focusing on long-term payoff and avoiding immediate temptation, among many other lower-order facets. It is the inverse of impulsivity.
Developmental patterns also differ for impulsivity and risk-taking. Impulsivity declines in linear fashion from childhood to adulthood (Shulman et al., 2016). Risk-taking, in contrast, has a curvilinear pattern, tending (with cultural variation) to peak in adolescence, and decline again in adulthood (Casey, 2015; Mata, Josef, Samanez-Larkin, & Hertwig, 2011). Evolutionary theorists conceive this adolescent risk-taking shift as adaptive for human development (Sercombe, 2014). In support, risk-taking’s non-linear developmental pattern is also quite plastic to environmental context (Mata, Josef, & Hertwig, 2016).
Table 3 summarizes the contrast between impulsivity and risk-taking across multiple levels of analysis. While readily distinguished in concept and empirically, they can overlap in practice, which is impulsive risk-taking. It is recommended that clinical researchers diligently distinguish (a) impulsivity, (b) risk-taking, and (c) their overlap, impulsive risk-taking. Further, it is important to articulate whether or not we intend to operationalize a general but not rigid response style/tendency, versus an inflexible response pattern.
Table 3.
The distinction between impulsive and risk-taking choice across cognitive neuroscience and personality literatures
| Impulsivity | Risk-taking | |
|---|---|---|
| Classic personality | Impulsivity | Venturesomeness |
| Current personality | Constraint-disinhibition | Extraversion/sensation-seeking |
| Preferred reward choice | Soonest/immediate | Biggest |
| Computational Parameter | Time | Reward size |
| Devalued parameter | Reward size | Reward/punishment uncertainty |
| Reflection | No | Neutral (yes or no) |
| Mathematical function | Temporal discounting | Probability discounting |
| Magnitude effect rewarda | forward (small>large) | backward (large>small) |
| Variation across outcomesb | yes | no |
| Developmental coursec | linear | non-linear |
| Heuristic pharmacologyd | Serotonergic tone | Dopaminergic tone |
| Meta-traitse | Stability | Plasticity |
For more discussion see reviews by L. Green and Myerson (2010) and Evenden (1999). The classic personality model is that of Eysenck (Eysenck, 1993); the contemporary rendition is taken from the meta-analysis by (Sharma et al., 2014).
= magnitude effect means larger rewards are discounted less steeply than smaller rewards over time (‘forward’ effect); but more steeply over odds against their occurrence (‘backward’ effect). If they are governed by the same system then this reversal is puzzling because longer delay is generally correlated with odds against occurrence.
=monetary rewards and consumables (e.g., food) have different discounting rates for temporal discounting but the same discounting rates for probability (or odds against)(Estle, Green, Myerson, & Holt, 2007; Mitchell et al., 2015).
=response control develops with linear growth to adulthood, whereas risk-taking peaks in adolescence and then declines in a nonlinear path (Shulman et al., 2016).
=A psychobiological model proposes that Constraint (but not sensation seeking or risk-taking) is related to serotonergic tone (Carver, Johnson, & Joormann, 2009; Depue & Spoont, 1986; Evenden, 1999) while venturesomeness reflects a dopaminergically-mediated approach system (Depue and Collins (1999). Neurotransmitter systems involve complex feedback loops that cannot be reduced to a single chemical, but these emphases are heuristic.
=For review see (DeYoung, 2011).
Psychopathology and SR breakdowns
Figure 2 schematizes the overlaps and distinctions among these three major instances of faulty SR. It illustrates that while response disinhibition, impulsivity, and risk-taking can certainly overlap, they can also be differentiated in different behaviors or psychopathologies. These distinctions and their overlaps are quite helpful in characterizing the relation of SR to psychopathology. SR aims to serve adaptation (‘meet a goal’ of state or attainment). Impulsivity, crucially, may be adaptive or not (see Appendix S1 for further discussion). For example, an inflexible impulsive style (immediate decision or reaction, based on immediate stimuli and payoff) may be adaptive for an elite special forces team leader in a combat environment; but then become maladaptive when the soldier returns to civilian life and is needlessly reactive in benign situations (Carey, 2016).4 The example illustrates that it is misleading to label impulsivity or risk taking globally as psychopathological or dysregulatory without specifying the context.
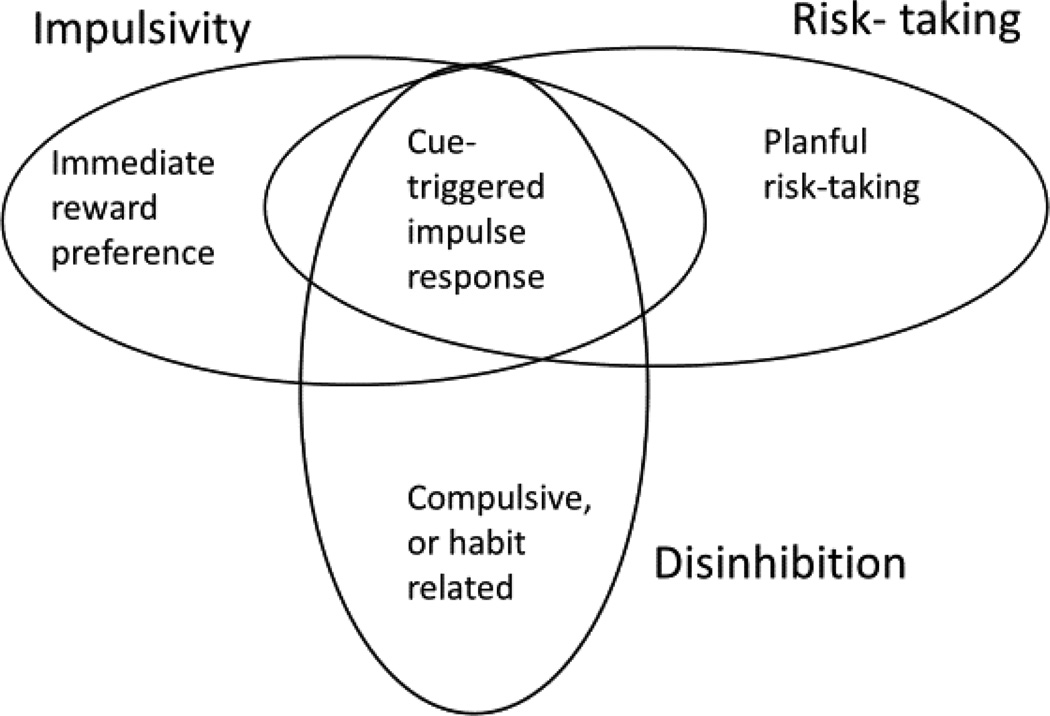
Figure 2.
Schematic of the partially differentiable constructs of impulsivity, disinhibition, and risk-taking. Some behaviors represent inhibition as well as impulsivity, but other impulsive behaviors include processes beyond response disinhibition, and some disinhibitory problems are not impulsive in the usual sense of the word (e.g., ruminative obsession). Risk taking can be impulsive, or not.
Figure 2 notes overlap as well. For example, children with ADHD show both steeper discounting of future rewards (Patros et al., 2016), and riskier style on gambling tasks (Dekkers, Popma, Agelink van Rentergem, Bexkens, & Huizenga, 2016) compared to typically developing children. Thus, impulsive risk-taking is risk-taking without reflection, in response to immediate opportunity. It too is often, but not inevitably, maladaptive. Psychopathological risk-taking is maladaptive because it is inappropriately impulsive or inflexible.
The distinction between impulsivity and response disinhibition is similarly relevant. Faulty response inhibition is apparent in multiple conditions that are not necessarily impulsive in relation to discounting, e.g., anxiety (failure to inhibit response to punishment cue), and habit disorders (e.g., obsessive-compulsive disorder, trichotillomania)(Pinto, Steinglass, Greene, Weber, & Simpson, 2014). Compulsive and habit disorders overlap with impulsivity in that they involve breakdowns in response inhibition, but they do not necessarily involve breakdown in delay discounting or in decision preferences for earlier versus later payoffs. Addictions can begin as impulsivity characterized by altered delay discounting, but end as disorders of disinhibition (inability to inhibit response to drink cue). Neurobiology is partially shared in conditions with poor response inhibition (Fineberg et al., 2014). Thus, response inhibition is part of impulsivity but not identical to it; disorders of response inhibition are not necessarily characterized by impulsivity.
Table 4 further summarizes this complex but crucial set of distinctions in relation to psychopathology. Note that impulsivity and risk-taking are only related to failed SR if a modifier is attached, such as ‘inflexible.’ Deciding to go with the flow at a party may be impulsive without reflecting an SR failure, in that it may lead to adaptive optimization of state to context. It is crucial to clarify, in research and in the clinic, the difference between a behavior (selecting the immediate reward, or selecting the option with the biggest potential downside), and an inflexible application of that behavior. The latter is probably maladaptive on average; but can be adaptive in particular niches or contexts (see Appendix S1 for more discussion of the multi-layered issue of specifying adaptiveness; see Block & Block, 1980, for a related perspective).
Table 4.
Toward a taxonomy of SR breakdown: Subtypes of impulsivity and risk-taking and relation to response disinhibition
| Low Regulation |
Maladaptive | Valence Trigger |
Discounting computation |
Response Disinhibition |
|
|---|---|---|---|---|---|
| Impulsivity | Maybe | Not necessarily | R | time | Maybe |
| Inflexible impulsivity | Yes | Yes (average) | R | time | Yes |
| Risk-taking | Maybe | Not necessarily | R/P | chance | No |
| Impulsive risk-taking | Yes | Yes (average) | R/P | both | Yes |
| GAD | Yes | Yes (average) | P | NA | Maybe |
| OCD (habit disorders) | Yes | Yes (average) | Spec | NA | Yes |
R=Reward, P=Punishment; time=temporal discounting of future relative to immediate/soon; chance=discounting of probability of punishment relative to probability of reward. OCD=obsessive compulsive disorder; GAD=generalized anxiety disorder.
‘average = over an infinite number of contexts this response preference would lead to loss of advantage a majority of the time. It may lead to occasional advantage by luck, coincidence, or niche-specific learning.
Spec—the stimulus for OCD and other habit disorders is a specific sensation or stimulus that serves as a reward cue; it is specific—that is, not generalized to a response style as in a putative ‘impulsive style.’ The role of discounting is variable in habit conditions; for example, OCD is associated with normal temporal discounting of reward; on the other hand, drug addiction is associated with steep discounting of future reward.
Integration
Hierarchical integration
A principle of development, and thus of the organization of cognition and personality (response dispositions) is that it is hierarchical. Development proceeds in a cascade in which sub-routines, abilities, or traits combine or share features that can be described in terms of higher order abilities, routines, or traits (Cicchetti & Tucker, 1994; Cox et al., 2010; Masten & Cicchetti, 2010; L. B. Smith & Thelen, 2003; Thelen et al., 2001). Computational models feature a similar hierarchical structure for the cross-sectional assembly of self-regulation.
A hierarchical structure is also seen in which nearly all psychopathology shares a general or ‘P’ factor, while specific components of psychopathology fit into two or more, correlated, lower-level domains in adults (Caspi et al., 2014; Lahey et al., 2012) and in children (Noordhof, Krueger, Ormel, Oldehinkel, & Hartman, 2015) in a bifactor structure.5 These sub-domains may also have a bifactor structure (Martel, Roberts, Gremillion, von Eye, & Nigg, 2011) consistent with a cascade in which breakdown in one domain leads to further breakdowns (Martel et al., 2009).
A hierarchical structure also holds for personality and temperament. For example, the ‘Big Five’ personality traits are correlated into two ‘super-factors’ labeled in earlier research as avoid and approach but more recently as ‘stability’ and ‘plasticity’ (Sharma et al., 2014; Tsukayama et al., 2013). The ‘stability’ factor comprises low Neuroticism (N), high Conscientiousness (C), and high Agreeableness (A). It is related to Constraint in the 3-factor model of personality (Tellegen, 1985).6 Plasticity comprises high Extraversion (E) and Openness to Experience (O) in the big Five framework. Each of the Big Five comprise several psychometrically coherent lower-order trait facets. These higher order personality traits appear to assemble hierarchically in development by building on lower order constituents of SR. For example, the trait of Conscientiousness may emerge from earlier development of effortful control (Eisenberg, Duckworth, Spinrad, & Valiente, 2014; Nigg, 2006) or even earlier from elaboration of sensitivity to negative avoidance signals supporting emergence of EC (Nigg, 2006). It is notable that the hierarchical model of personality appears to be related to the hierarchical model of psychopathology (Tackett et al., 2013).
Updated Tripartite Model
It is illuminative to convert this picture schematically from a dual-process model to the classic tripartite model that characterized this literature for over a generation, illustrated in Figure 3. This helps us link the bottom-up and top-down conceptions of SR with models of personality and with cognitive neuroscience, and is helpful in bridging literatures.
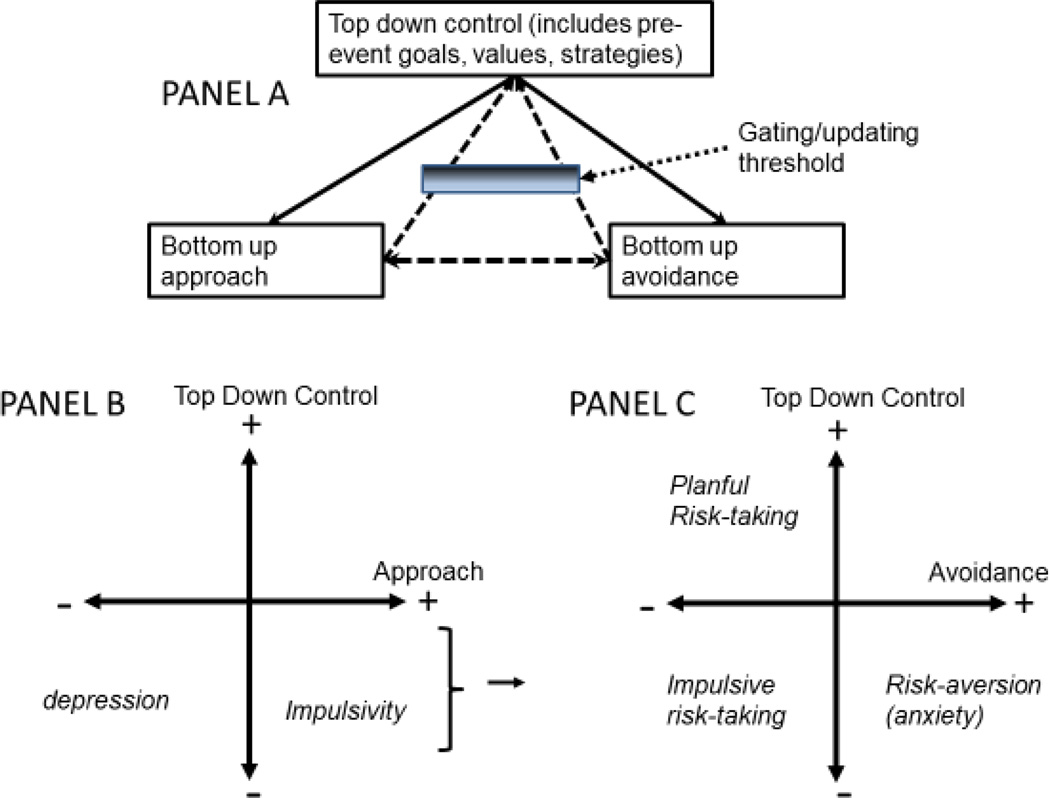
Figure 3.
Tripartite model: Figure illustrates the integration of a top-down and bottom-up processes within a model from the psychobiology of personality that parallels what is seen in the cognitive neuroscience and developmental literature. Panel A: The fundamental tripartite conception. The bidirectional arrows indicate that the two bottom-up systems are mutually regulating, and that both are regulated by top-down control. At the same time, via gating and cybernetic checking mechanisms, the bottom-up processes also regulate the level of top-down control. Panel B: The interplay of top-down SR and bottom-up approach response creates opportunities for both depression (low top-down capacity and low bottom-up motivation), and for impulsivity (low top-down control with high bottom-up motivation). Panel C: The interplay of top-down SR processes and bottom-up avoidance response (fear/anxiety). Their interplay creates opportunity for anxiety (when avoidance is high and top-down regulatory capacity is low) and impulsive risk taking (when avoidance is low, top-down control is low, and approach is also elevated—thus the arrow from panel A’s lower right quadrant). Panel A is based on material in Carver, Johnson & Joorman, (2009), Hofmann, Friese, & Strack, (2009), Gray (1982), Verbruggen et al (2014) and other sources they cite. Panel B and Panel C are based on material in Nigg (2006) and other sources cited therein.
Panel 3.A illustrates the model as described, with variations, by numerous authors (Carver et al., 2009; Depue & Lenzenweger, 2006; Ernst, 2014; Gray, 1982; Nigg, 2006). Two basic bottom-up appetitive processes are emphasized; they are each modulated by top-down control. Either can also interrupt or update top-down processes, thus contributing to (or else disrupting) SR. A gating mechanism allows bottom-up signals to update goal representations.
Panel 3.B shows that impulsivity lies at the conjunction of low top-down control and high bottom-up approach (e.g., excitement). Note that approach is related to immediacy valuation in the discounting models discussed earlier. In the other lower quadrant of Panel 3.B., low top-down control and low bottom-up approach motivation converge to heighten depression risk (Clark, Watson, & Mineka, 1994). The upper right quadrant of panel 3.B (high control, high approach) might represent a tendency for deliberate or planful preference for immediate rewards based on learning history. For example, some youth in dangerous inner city environments appear to adaptively discount the future (Ramos, Victor, Seidl-de-Moura, & Daly, 2013) and as noted, soldiers may learn to make snap decisions as a successful adaptation to frequent combat (Carey, 2016).
Panel 3.C illustrates that risk-taking is low-control and low avoidance (e.g., low fear). Impulsive risk-taking is likely with low avoidance and low control, provided sufficient approach motivation (thus the small arrow from 3.B. to 3.C). The upper left quadrant of Panel 3.C. illustrates that low avoidance motivation with strong top-down control might be associated with planful risk-taking, as in expert mountain-climbing. While most research has prioritized a focus on the high reward response seen in risk-taking (Shulman et al 2016), as noted earlier emerging literature suggests that low avoidance motivation (discounting of punishment probability) is associated with risk-taking (Casey, 2015; Van Leijenhorst et al., 2010).
SR: levels of analysis and temporal variation
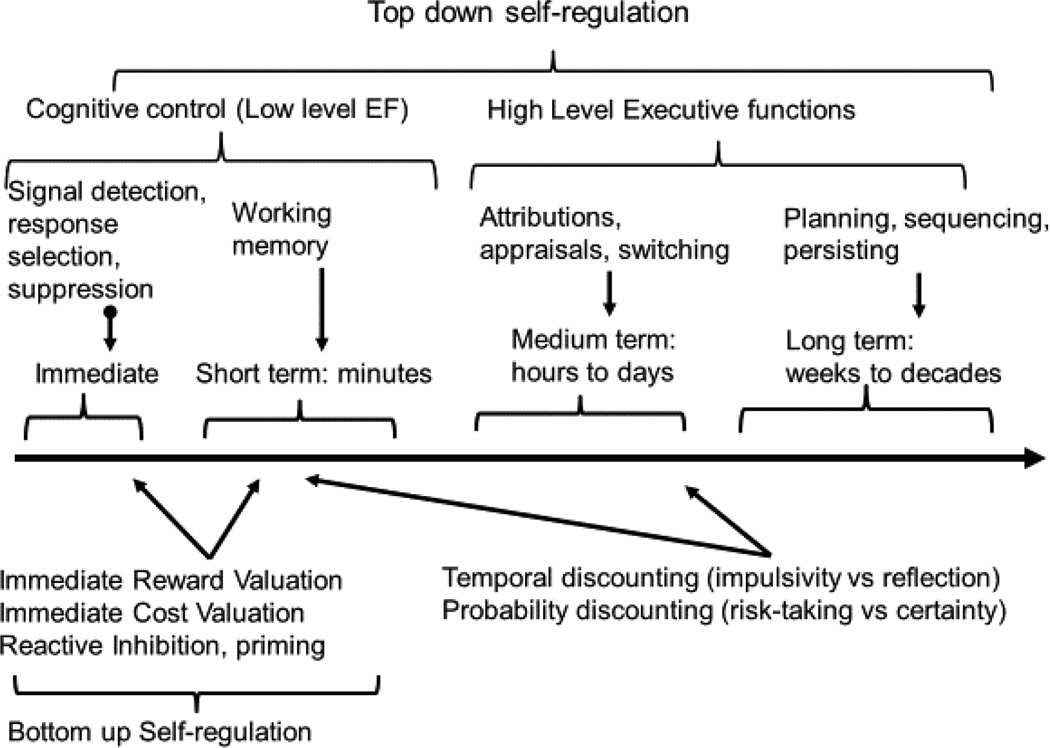
On a similar principle, the different terms occupying the conceptual space related to top-down aspects of SR are distinguished, in part, by hierarchical variation in specificity, coverage, or granularity, in temporal focus, and in developmental emergence. Their hierarchical arrangement was outlined by Diamond (2013) as alluded to earlier, and suggests that low level operations like response inhibition and working memory support emergence of more complex operations like higher order EF. Another way of looking at hierarchical integration is to recognize that different aspects of SR differ in their timescale (Duckworth & Gross, 2014; Newell, 1990; Verbruggen et al., 2014). Figure 4 illustrates one view of how the domains fit together temporally. Immediate and very short-term conflict requires engagement of response inhibition and working memory (bottom-up regulatory processes are usually immediate, or stimulus-driven7). Medium and longer time frames involve higher-level EF (strategies and planning) in the face of future conflict or to prepare for future challenge. The long-term future (beyond hours) is largely the domain of higher order EF. Higher order traits of temperament and personality refer to the tendency to utilize the short-term and long-term strategies and describe an individual’s typical response bias (e.g., appetitive approach--extraversion; cost-avoidance--neuroticism; exertion of top-down control—effortful control). Thus, a temporal perspective provides a complementary way of organizing ‘lower’ and ‘higher’ order constructs.
Figure 4.
A hierarchical view of different functions involved in SR this time from the view of time scale of the goal or conflict (immediate or future). Contrast with Figure 2 that showed a hierarchy view related to complexity or developmental level for the same functions. Immediate stimuli activate immediate bottom-up valuation mechanisms; their strength is balanced by top-down operations including such as response inhibition. Short-term goals are also supported by ‘low level’ executive functions subsumed under cognitive control, such as working memory, but as time spans increase, additional operations are brought to bear to support SR. The examples here are simply illustrative. Bottom-up SR is principally active in immediate and short-term response optimization. Discounting of time and probability effects likely reflects a different combination of bottom-up and top-down processes depending on the species and the particular time frames. The cognitive operations (e.g., lower and higher order EF) can also be employed for purposes other than SR; here only their regulatory application is depicted.
Joint integration and recommendations
Table 5 combines the preceding perspectives in a tabular outline format. The developmental illustrations here are only suggestive; more granular discussions of developmental timing of capacities are provided by others (Colombo, 2001; Diamond, 2013; Garon et al., 2008; Huang-Pollock, Carr, & Nigg, 2002; Rothbart, 2011). Here my intent is simply to recall that (a) bottom-up processes emerge and mature earlier than top-down processes and (b) different aspects of SR mature at different rates within both bottom-up and top-down domains. Earlier-developing aspects are part of the assembly of later-developing aspects. Further, the temporal reach of SR increases with development. With these general schematics as support, I move to recommendations for a framework of constructs for use in developmental psychopathology research.
Table 5.
Combined nesting of aspects of SR in level of abstraction/molarity and temporality
| Developmental Emergence |
Maturation | Temporal Focus |
|
|---|---|---|---|
| Bottom-up (examples) | |||
| a. Attentional capture | 3 months* | childhood | immediate |
| b. Behavioral inhibition | 6 months | childhood | immediate |
| c. Salience | unclear | unclear | immediate |
| d. Immediate valuation | unclear | unclear | immediate |
| Top-down (examples) | |||
| Low level EF (cognitive control) | |||
| i. Res. Inhibition | 6–9 months | adolescence | immediate |
| ii. Exec Attention | toddler | adolescence | immediate |
| iii. WM | preschool | adolescence | immediate |
| iv. Delay Reg** | unclear | adulthood | both |
| v. Risk Reg** | unclear | adulthood | both |
| e. High Level | |||
| i. Strategies | toddler | adulthood | both |
| ii. Sequencing | preschool | adulthood | future |
| iii. Planning | preschool | adulthood | future |
Developmental onsets and maturation are schematic and arguable. See Diamond (2013) for more detailed discussion. The point here is to illustrate that bottom-up processes emerge and mature earlier than top-down processes and that different components mature at different rates.
regulation of temporal and probability variation involves both bottom-up valuation mechanisms that are typically quite automatic (and thus behave similarly across species) and control mechanisms that bias those set points in relation to goals in humans.
EF=executive functions
Necessary concepts
I suggest that from the basic concepts listed below, researchers and theoreticians should specify which concept their construct intends to represent or how it is distinct from these basic ideas. Note that this set is not proposed as a formal model—the boundaries between, for example, low-level and high-level EF will be fuzzy, and I already noted that even top-down and bottom-up are not absolute distinctions. This outline is meant as a descriptive framework for linking literatures.
General SR
This should be a domain-general construct that encompasses all SR (including bottom up aspects). I propose ‘self-regulation’ here. Within it, particular sub-domains can include SR of emotion, of action, and of cognition. This is not meant to propose there is a shared SR process across all forms of SR (that remains a key empirical question); rather my intent is to stimulate clarification of the extent of various claims about SR-related processes, determinants, or effects.
Top Down (Deliberate) SR
This concept should designate the family of top-down SR processes (voluntary or limited-capacity regulation of the self by the self), including both simple processes like response inhibition, and complex processes like preparatory planning to regulate future behavior. It thus includes EC, as well as the SR application of EF. The term ‘top-down’ has the advantage of being translatable to a conceptual nervous system and schematic computational models. The term ‘deliberate’ is more easily understood but evades formal definition or computational/neural mapping.
Basic top-down processes (cognitive control and EC but not complex EF)
This concept is the lower level, basic, and early developing components of deliberate SR. It is generally congruent with EC and with cognitive control (which can be used for non SR purposes also), but not with the complex cognition aspect of EF or associated future-oriented strategies. I tentatively suggest that the defining characteristic here be handling immediate challenge or conflict that is represented by an immediate stimulus (a piece of candy in front of me), but not future challenge or conflict that is represented only internally (tomorrow’s meeting). Additional fractionation can be done either in an information processing way to accommodate computational modeling (e.g., signal detection, action selection) or in a behavioral way to accommodate molar behavioral and developmental studies (e.g., response inhibition, response activation, executive attention, working memory) or the components of EC.
Complex cognition and strategies
This concept comprises the complex or higher-level EF (Diamond, 2013) such as reasoning and planning, but also coping strategies that are adopted strategically or ‘intentionally’ to self-regulate emotion or action, and which can address future conflict (mental preparation) as described by Gross (2015). These may be defined as oriented toward future or internally represented conflict or challenge, rather than immediately cued conflict. (Note, therefore, that regulatory ‘strategies’ can refer to both immediate and future conflict, but complex cognition typically addresses future oriented conflict. For example, a child diverting her attention by looking away from an immediately tempting but forbidden toy is showing a type of strategy, but not using complex cognition). This higher-order domain of complex cognition can be further subdivided as needed (e.g., planning, reasoning). Higher order EF are combinations of lower order processes that assemble developmentally as well as behaviorally and computationally. For example, planning is an extended application of working memory and action selection; mental flexibility depends on inhibition and working memory; SR coping strategies often entail planning.
Bottom-up or reactive processes
A separate designation is needed for bottom-up processes. These were noted earlier (Table 2). Recall that the involvement of bottom-up (or reactive) processes in SR is complex. It requires consideration of when bottom-up interruption of behavior or cognition is regulating versus regulated, as well as recognition of the ambiguity in differentiating top-down and bottom-up in the SR context. However, particularly important for psychopathology models are the incentive response systems of approach and fear (or, in animal models, threat avoidance). Other systems participate in self-regulation by providing updating information to top-down processes (e.g., associative learning informing working memory in relation to a goal step) and in other ways, as noted earlier. Most important is specification of whether investigators mean to address only top-down SR, or all SR.
Psychopathological breakdowns of SR
A separate domain that overlaps with the preceding is specific to psychopathology, and concerns psychopathological breakdowns in SR that emanate from joint involvement of top-down and bottom-up processes. A complete taxonomy of these breakdowns might include problems largely bypassed here, such as compulsive behavior, obsessions, rumination, post-traumatic symptoms, and self-harm—adding constructs only if needed. The first step, highlighted here to start this discussion, is distinguishing between inflexible impulsivity (immediate stimulus response), impulsive or inflexible/compulsive (but not deliberative or calculated) risk-taking, and breakdowns in response inhibition. Inflexible impulsivity and impulsive risk-taking are not, however, a complete converse of SR, because impulsivity and risk-taking are defined in relation to decision contexts.
However, SR, including top-down control, is broader than impulsivity or risk taking, as it refers to state or response optimization even when there is no decision context (e.g., shooting a free throw; hitting the musical note to set the tone for a group to sing; balancing on a bicycle).
Complete set and usage
This set of constructs is proposed as a sufficient descriptive framework for SR (though other components are needed for process models of how SR works, such as gating and updating). For description, additional constructs are sub-components of one of these levels of analysis or refer to their implementation in a particular regulatory or decision context or at a different level of analysis (e.g., neural, cognitive, trait). The considerations in this essay may assist researchers in defining their constructs, and readers in integrating literatures.
These constructs can be used in two ways. One is to help map related constructs across theoretical models and to help in defining what different constructs are represented by a variable. Another is to help in defining targets for study of individual differences, and their correlations, including the structure of the relations among effortful control and impulsivity, or between top down and bottom up forms of control.
Conclusion
Clarifying description and process in the domain of SR is paramount to understanding much of psychopathology. However, progress has been impeded by (a) using separate constructs for regulation of emotion and action without a commensurate domain-general construct that can connect literatures, (b) failing to clearly differentiate top-down and bottom-up contributions to SR, (c) excessive separation of EF, EC, and cognitive control; (d) confounding of impulsivity, disinhibition, and risk-taking; and (e) confounding of constructs across levels of analysis. It is hoped that this essay provides a framework for shared integration across studies and advances our effort to unify knowledge relevant to developmental psychopathology. Future work will continue to refine the neural and physiological concomitants of these processes as well as their mathematical and computational representation. That work can proceed more effectively with a shared terminological roadmap and nomenclature.
Supplementary Material
Key points.
Self-regulation is of central importance to the field but defined differently across literatures
A broad meaning of self-regulation includes top down and bottom up processes
A close relationship exists between executive functioning, cognitive control, and effortful control
Effortful control is hypothesized to be a trait level mapping of cognitive control
Impulsivity, risk taking, and disinhibition are distinct concepts, but too often conflated
Components of self-regulation vary in their developmental assembly and functional time course
Acknowledgments
Work on this paper was supported by grant R37MH59105 by the National Institute of Mental Health. This review was invited by the Editors of this journal, who offered a small honorarium to cover expenses. It has been externally peer reviewed.
The author is indebted for incisive comments on prior drafts of this manuscript to: BJ Casey, Adele Diamond, Nancy Eisenberg, James Gross, Cynthia Huang-Pollock, Sarah Karalunas, Michele Martel, Suzanne Mitchell, Mary Rothbart, Mike Posner, and Laurie Wakschlag. This does not mean any commentators necessarily agree with any particular claims in this paper.
Footnotes
The author declares that he has no competing or potential conflicts of interest.
Supporting Information
Additional Supporting Information may be found on the online version of this article.
Appendix S1: Additional reading and background considerations.
Physiological parameters, such as the cardiac respiratory sinus arrhythmia, as well as hormonal indicators such as cortisol levels, are powerful measures of regulatory process and central to much of that literature (Bell & Calkins, 2012; Calkins & Fox, 2002). They are ignored in this review to constrain its scope; here I focus on the psychological constructs while noting their neural and computational aspects only briefly.
An early connectionist model included: (a) a top-down “working memory” mechanism that represents and maintains a goal, (b) a conflict or outcome monitoring mechanism that can influence the level of resource or energy to the control mechanism, and (c) a gating mechanism that allows bottom-up feedback to correct or update the goal representation to enable recursive optimization (Cohen, Dunbar, & McClelland, 1990). Later models recognized that cybernetic updating also is accommodated by bottom-up learning mechanisms which are readily implemented in connectionist architecture (Botvinick & Cohen, 2014) but the basic elements of conflict or outcome monitoring, goal representation, and gating typically remain. Failures of top-down regulation may be due to any of these components. For example, experimental evidence suggests that children with ADHD have poor conflict/outcome monitoring (McLoughlin et al., 2009). Symbolic models are a class of computational models that emphasize the nested rule sets and information processing steps in moment-by-moment regulation (Wang, Liu, & Fan, 2012); they are underutilized in relation to psychopathology or development.
The idea of cognitive SR is noted in passing by Blair and Razza (2007) in the context of executive functioning but its use as I take it here is not widespread.
This learned style means something different than a style arrived at by neural injury (e.g. the case of Phineas Gage). In the former case, the “maladjustment” is created by the change in context and entails difficulty shifting automatized routines to a new learning context. In the case of Gage, it entails a context independent style that was not learned and fails to adapt to any context. A disorder like ADHD is presumed to reflect a developmental, not learned dysfunction that is maladaptive in most contexts. The classic distinction between inherited versus acquired, or between biological versus learned, breaks down on closer examination. At the same time, pursuant to the question of adaptation, psychopathology theory must wrestle with the elusive distinction of attempts at adaptation that fail in a new context (efficient fat storage in an abundant environment leading to obesity; intense vigilance in a violent home leading to relationship breakdown in a safe haven), versus a system overwhelmed and failing (death after excess fever response to infection; psychosis after failed coping psychological or moral trauma).
A bifactor model is mathematically and conceptually distinct from a second order factor. (Both are called hierarchical, creating needless confusion). This distinction, crucial for some aspects of psychopathology theory, does not materially alter the current argument related to organizing constructs of self-control as relevant for psychopathology.
It is interesting to consider this model in relation to J. H. Block and Block (1980) 2-factor model of personality. However, J. Block (1995) objected, noting that these two traits are conceptualized rather differently. In particular, the “plasticity” trait has rather different connotations in this model than does “ego resiliency” in the Blocks’ model.
Bottom-up systems can become chronically activated (anxiety disorders, post-traumatic stress disorder), but they then are not regulating but a target of regulation.
References
- Ainslie G. Specious reward: A behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82(4):463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Ainslie G. Breakdown of will. New York, NY, US: Cambridge University Press; 2001. [Google Scholar]
- Ainslie G. Precis of breakdown of will. Behav Brain Sci. 2005;28(5):635–650. doi: 10.1017/S0140525X05000117. discussion 650–673. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Avital-Cohen R, Tsal Y. Top-down processes override bottom-up interference in the flanker task. Psychological Science. 2016 doi: 10.1177/0956797616631737. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: Theories, models, and controversies. Annu Rev Psychol. 2012;63(1):1–29. doi: 10.1146/annurev-psych-120710-100422. doi: [DOI] [PubMed] [Google Scholar]
- Banich MT. Executive function: The search for an integrated account. Current Directions in Psychological Science. 2009;18(2):89–94. [Google Scholar]
- Bargh JA, Ferguson MJ. Beyond behaviorism: On the automaticity of higher mental processes. Psychol Bull. 2000;126(6):925–945. doi: 10.1037/0033-2909.126.6.925. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of adhd. Psychol Bull. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Executive functions: What they are, how they work, and why they evolved. Guilford Press; 2012. [Google Scholar]
- Barkley RA, Cox D. A review of driving risks and impairments associated with attention-deficit/hyperactivity disorder and the effects of stimulant medication on driving performance. Journal of Safety Research. 2007;38:113–128. doi: 10.1016/j.jsr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Baum WM. On two types of deviation from the matching law: Bias and undermatching. J Exp Anal Behav. 1974;22(1):231–242. doi: 10.1901/jeab.1974.22-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF, Heatherton TF. Self-regulation failure: An overview. Psychological Inquiry. 1996;7(1):1–15. [Google Scholar]
- Baumeister RF, Vohs KD, Tice DM. The strength model of self-control. Current Directions in Psychological Science. 2007;16(6):351–355. [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. The Journal of neuroscience. 2009;29(4):1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MA, Calkins SD. Attentional control and emotion regulation in early development. In: Posner MI, editor. Cognitive neuroscience of attention. 2nd. New York: Guilford Press; 2012. pp. 322–330. [Google Scholar]
- Berg JM, Latzman RD, Bliwise NG, Lilienfeld SO. Parsing the heterogeneity of impulsivity: A meta-analytic review of the behavioral implications of the upps for psychopathology. Psychol Assess. 2015;27(4):1129–1146. doi: 10.1037/pas0000111. [DOI] [PubMed] [Google Scholar]
- Blair C, Raver CC, Finegood ED. Self-regulation and developmental psychopathology: Experiential canalization of brain and behavior. In: Cicchetti, editor. Developmental psychopathology. 3rd. III. New York: John Wiley & Sons, Inc; 2016. pp. 484–522. [Google Scholar]
- Blair C, Razza RP. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Dev. 2007;78(2):647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Blair C, Ursache A. A bidirectional model of executive functions and self regulation. In: vohs kd, bauermeister rf., editors. handbook of self regulation. 2nd. NY: Guilford Press; 2011. [Google Scholar]
- Blanchard TC, Hayden BY. Monkeys are more patient in a foraging task than in a standard intertemporal choice task. PLoS One. 2015;10(2):e0117057. doi: 10.1371/journal.pone.0117057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block J. A contrarian view of the five-factor approach to personality description. Psychol Bull. 1995;117(2):187–215. doi: 10.1037/0033-2909.117.2.187. [DOI] [PubMed] [Google Scholar]
- Block JH, Block J. The role of ego-control and ego-resiliency in the origination of behavior. In: Collings wA., editor. The Minnesota Symposia on Child Psychology. Vol. 13. Hillsdale, NJ: Erlbaum; 1980. [Google Scholar]
- Blumenfeld RS, Nomura EM, Gratton C, D’Esposito M. Lateral prefrontal cortex is organized into parallel dorsal and ventral streams along the rostro-caudal axis. Cereb Cortex. 2013;23(10):2457–2466. doi: 10.1093/cercor/bhs223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botdorf M, Rosenbaum GM, Patrianakos J, Steinberg L, Chein JM. Adolescent risk-taking is predicted by individual differences in cognitive control over emotional, but not non-emotional, response conflict. Cognition and Emotion. 2016:1–8. doi: 10.1080/02699931.2016.1168285. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Braver T. Motivation and cognitive control: From behavior to neural mechanism. Annu Rev Psychol. 2015;66:83–113. doi: 10.1146/annurev-psych-010814-015044. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Cohen JD. The computational and neural basis of cognitive control: Charted territory and new frontiers. Cognitive Science. 2014;38(6):1249–1285. doi: 10.1111/cogs.12126. [DOI] [PubMed] [Google Scholar]
- Braver TS. The variable nature of cognitive control: A dual mechanisms framework. Trends Cogn Sci. 2012;16(2):106–113. doi: 10.1016/j.tics.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Fox NA. Self-regulatory processes in early personality development: A multilevel approach to the study of childhood social withdrawal and aggression. Dev Psychopathol. 2002;14(3):477–498. doi: 10.1017/s095457940200305x. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Graziano PA, Keane SP. Cardiac vagal regulation differentiates among children at risk for behavior problems. Biol Psychol. 2007;74(2):144–153. doi: 10.1016/j.biopsycho.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey B. Those with multiple tours of duty overseas struggle at home. The New York Times. 2016 May 29; Retrieved from http://www.nytimes.com/2016/05/30/health/veterans-iraq-afghanistan-psychology-therapy.html?_r=0. [Google Scholar]
- Carter CS, Krus MK. Dynamic cognitive control and frontal-cingulate interactions. In: Posner MI, editor. Cognitive neuroscience of attention. 2nd. New York: Guilford Press; 2012. pp. 88–98. [Google Scholar]
- Carter EC, Kofler LM, Forster DE, McCullough ME. A series of meta-analytic tests of the depletion effect: Self-control does not seem to rely on a limited resource. Journal of Experimental Psychology: General. 2015;144:796–815. doi: 10.1037/xge0000083. [DOI] [PubMed] [Google Scholar]
- Carter EC, Pedersen EJ, McCullough ME. Reassessing intertemporal choice: Human decision-making is more optimal in a foraging task than in a self-control task. Frontiers in Psychology. 2015;6 doi: 10.3389/fpsyg.2015.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J. Two-mode models of self-regulation as a tool for conceptualizing effects of the serotonin system in normal behavior and diverse disorders. Curr Dir Psychol Sci. 2009;18(4):195–199. doi: 10.1111/j.1467-8721.2009.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Scheier MF. Attention and self-regulation: A control-theory approach to human behavior. New York: Springer-Verlag; 1981. [Google Scholar]
- Carver CS, Scheier MF. Control theory: A useful conceptual framework for personality-social, clinical, and health psychology. Psychol Bull. 1982;92(1):111–135. [PubMed] [Google Scholar]
- Carver CS, Scheier MF. On the self-regulation of behavior. Cambridge University Press; 2001. [Google Scholar]
- Casey BJ. Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu Rev Psychol. 2015;66:295–319. doi: 10.1146/annurev-psych-010814-015156. [DOI] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington HL, Israel S, Moffitt TE. The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clin Psychol Sci. 2014;2(2):119–137. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Tucker D. Development and self-regulatory structures of the mind. Dev Psychopathol. 1994;6(04):533–549. doi: [Google Scholar]
- Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. J Abnorm Psychol. 1994;103(1):103–116. [PubMed] [Google Scholar]
- Clausen J. American lives. Looking back at the children of the great depression. Berkeley CA: University of California Press; 1995. [Google Scholar]
- Cohen J, Dunbar K, McClelland JL. On the control of automatic processes: A parallel distributed processing account of the stroop effect. Psychol Rev. 1990;97(3):332–361. doi: 10.1037/0033-295x.97.3.332. [DOI] [PubMed] [Google Scholar]
- Cohen J, Lieberman MD. The common neural basis of exerting self-control in multiple domains. In: Hassin RR, Ochsner KN, Trope Y, editors. Self control in society, mind, and brain. New York, NY, US: Oxford University Press; 2010. pp. 141–160. [Google Scholar]
- Cohen SO, Breiner K, Steinberg L, Casey BJ. When is an adolescent an adult? Assessing cognitive control in emotional and non-emotional contexts. Psychological Science. 2016;27:549–562. doi: 10.1177/0956797615627625. [DOI] [PubMed] [Google Scholar]
- Colombo J. The development of visual attention in infancy. Annu Rev Psychol. 2001;52:337–367. doi: 10.1146/annurev.psych.52.1.337. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Heller W. Brain asymmetry. Edited by richard j. Davidson and kenneth hugdahl. Cambridge, ma: Mit press, 1995. Psychophysiology. 1998;35(4):479–481. [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58(3):306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MJ, Mills-Koonce R, Propper C, Gariepy JL. Systems theory and cascades in developmental psychopathology. Development and Psychopathology. 2010;22(3):497–506. doi: 10.1017/S0954579410000234. [DOI] [PubMed] [Google Scholar]
- Crone EA, van Duijvenvoorde AC, Peper JS. Annual research review: Neural contributions to risk-taking in adolescence - developmental changes and individual differences. J Child Psychol Psychiatry. 2016;57(3):353–368. doi: 10.1111/jcpp.12502. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR. The cognitive neuroscience of working memory. Annu Rev Psychol. 2015;66:115–142. doi: 10.1146/annurev-psych-010814-015031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain Cogn. 1992;20(1):125–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- de Ridder DT, Lensvelt-Mulders G, Finkenauer C, Stok FM, Baumeister RF. Taking stock of self-control: A meta-analysis of how trait self-control relates to a wide range of behaviors. Pers Soc Psychol Rev. 2012;16(1):76–99. doi: 10.1177/1088868311418749. [DOI] [PubMed] [Google Scholar]
- Dekkers TJ, Popma A, Agelink van Rentergem JA, Bexkens A, Huizenga HM. Risky decision making in attention-deficit/hyperactivity disorder: A meta-regression analysis. Clin Psychol Rev. 2016;45:1–16. doi: 10.1016/j.cpr.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behav Brain Sci. 1999;22(3):491–517. doi: 10.1017/s0140525x99002046. discussion 518–469. [DOI] [PubMed] [Google Scholar]
- Depue RA, Lenzenweger MF. A multidimensional neurobehavioral model of personality disturbance. In: Tackett RFKJL, editor. Personality and psychopathology. New York, NY, US: Guilford Press; 2006. pp. 210–261. [Google Scholar]
- Depue RA, Spoont MR. Conceptualizing a serotonin trait. A behavioral dimension of constraint. Ann N Y Acad Sci. 1986;487:47–62. doi: 10.1111/j.1749-6632.1986.tb27885.x. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Rothbart MK. Reactive and effortful processes in the organization of temperament. Dev Psychopathol. 1997;9(4):633–652. doi: 10.1017/s0954579497001375. [DOI] [PubMed] [Google Scholar]
- DeYoung CG. Impulsivity as a personality trait. In: Vohs KD, Baumeister RF, editors. Handbook of self-regulation: Research, theory, and applications. 2. New York: Guilford Press; 2011. [Google Scholar]
- Diamond A. Developmental time course in human infants and infant monkeys and the neural bases of inhibitory control in reaching. Annals of the ny academy of sciences, 608: 637–676. Annals of the NY Academy of Sciences. 1990;608:637–676. doi: 10.1111/j.1749-6632.1990.tb48913.x. [DOI] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annu Rev Psychol. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman SJ. Functional and dysfunctional impulsivity: Personality and cognitive correlates. J Pers Soc Psychol. 1990;58(1):95–102. doi: 10.1037//0022-3514.58.1.95. [DOI] [PubMed] [Google Scholar]
- Dijksterhuis A, Strick M. A case for thinking without consciousness. Perspectives on Psychological Science. 2016;11(1):117–132. doi: 10.1177/1745691615615317. [DOI] [PubMed] [Google Scholar]
- Duckworth AL, Gendler TS, Gross JJ. Situational strategies for self-control. Perspectives on Psychological Science. 2016;11(1):35–55. doi: 10.1177/1745691615623247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth AL, Gross JJ. Self-control and grit: Related but separable determinants of success. Current Directions in Psychological Science. 2014;23(5):319–325. doi: 10.1177/0963721414541462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth AL, Kern ML. A meta-analysis of the convergent validity of self-control measures. J Res Pers. 2011;45(3):259–268. doi: 10.1016/j.jrp.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T. Right ventrolateral prefrontal cortex mediates individual differences in conflict-driven cognitive control. J Cogn Neurosci. 2011;23(12):3903–3913. doi: 10.1162/jocn_a_00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Duckworth AL, Spinrad TL, Valiente C. Conscientiousness: Origins in childhood? Dev Psychol. 2014;50(5):1331–1349. doi: 10.1037/a0030977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Edwards A, Spinrad TL, Sallquist J, Eggum ND, Reiser M. Are effortful and reactive control unique constructs in young children? Dev Psychol. 2013;49(11):2082–2094. doi: 10.1037/a0031745. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Guthrie IK, Fabes RA, Reiser M, Murphy BC, Holgren R, Losoya S. The relations of regulation and emotionality to resiliency and competent social functioning in elementary school children. Child Dev. 1997;68(2):295–311. [PubMed] [Google Scholar]
- Eisenberg N, Smith CL, Spinrad TL. Effort control: Relations with emotion regulation, adjustment, and socialization in childhood. In: vohs kd, bauermeister rf., editors. handbook of self regulation. 2nd. NY: Guilford Press; 2011. pp. 263–283. [Google Scholar]
- Eisenberg N, Valiente C, Spinrad TL, Liew J, Zhou Q, Losoya SH, Cumberland A. Longitudinal relations of children’s effortful control, impulsivity, and negative emotionality to their externalizing, internalizing, and co-occurring behavior problems. Dev Psychol. 2009;45(4):988–1008. doi: 10.1037/a0016213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Zhou Q. Conceptions of executive function and regulation: When and to what degree do they overlap? In: Griffin JA, McCardle P, Freund LS, editors. Executive function in preschool-age children: Integrating measurement, neurodevelopment, and translational research. Washington, DC, US: American Psychological Association; 2016. pp. 115–136. [Google Scholar]
- Ernst M. The triadic model perspective for the study of adolescent motivated behavior. Brain Cogn. 2014;89:104–111. doi: 10.1016/j.bandc.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espy KA, Sheffield TD, Wiebe SA, Clark CA, Moehr MJ. Executive control and dimensions of problem behaviors in preschool children. J Child Psychol Psychiatry. 2011;52(1):33–46. doi: 10.1111/j.1469-7610.2010.02265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estle SJ, Green L, Myerson J, Holt DD. Discounting of monetary and directly consumable rewards. Psychological Science. 2007;18(1):58–63. doi: 10.1111/j.1467-9280.2007.01849.x. [DOI] [PubMed] [Google Scholar]
- Evans JS. Dual-processing accounts of reasoning, judgment, and social cognition. Annu Rev Psychol. 2008;59:255–278. doi: 10.1146/annurev.psych.59.103006.093629. [DOI] [PubMed] [Google Scholar]
- Evans JS, Stanovich KE. Dual-process theories of higher cognition: Advancing the debate. Perspect Psychol Sci. 2013;8(3):223–241. doi: 10.1177/1745691612460685. [DOI] [PubMed] [Google Scholar]
- Evenden JL. The pharmacology of impulsive behaviour in rats vii: The effects of serotonergic agonists and antagonists on responding under a discrimination task using unreliable visual stimuli. Psychopharmacology (Berl) 1999;146(4):422–431. doi: 10.1007/pl00005487. [DOI] [PubMed] [Google Scholar]
- Eysenck SBG. The i7: Development of a measure of impulsivity and its relationship to the superfactors of personality. In: McCown WG, Johnson JL, Shure MB, editors. The impulsive client: Theory, research, and treatment. Washington, DC, US: American Psychological Association; 1993. pp. 141–149. [Google Scholar]
- Fineberg NA, Chamberlain SR, Goudriaan AE, Stein DJ, Vanderschuren LJ, Gillan CM, Potenza MN. New developments in human neurocognition: Clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS Spectr. 2014;19(1):69–89. doi: 10.1017/S1092852913000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman NP, Miyake A. Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex. 2016 doi: 10.1016/j.cortex.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K. On conceptualizing self-control as more than the effortful inhibition of impulses. Personality and Social Psychology Review. 2011;15(4):352–366. doi: 10.1177/1088868311411165. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex: Anatomy, physiology, and neuropsychology of the frontal lobe. 3rd. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- Garon N, Bryson SE, Smith IM. Executive function in preschoolers: A review and integrative framework. Psychological bulletin. 2008;134(1):31–60. doi: 10.1037/0033-2909.134.1.31. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Church RM, Fairhurst S, Kacelnik A. Scalar expectancy theory and choice between delayed rewards. Psychol Rev. 1988;95(1):102–114. doi: 10.1037/0033-295x.95.1.102. [DOI] [PubMed] [Google Scholar]
- Gray JA. The neuropsychology of anxiety: An enquiry into the functions of the septohippocampal system. New york: Clarendon Press? Oxford University Press; 1982. [Google Scholar]
- Gray JA. But the schizophrenia connection. Behavioral and Brain Sciences. 1999;22(03):523–524. doi: [Google Scholar]
- Green DM, Swets JA. Signald detection theory and psychophysics. New York: Wiley; 1966. [Google Scholar]
- Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychological bulletin. 2004;130(5):769–792. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J. Experimental and correlational analyses of delay and probability discounting. In: Bickel GJMWK, editor. Impulsivity: The behavioral and neurological science of discounting. Washington, DC, US: American Psychological Association; 2010. pp. 67–92. [Google Scholar]
- Green L, Myerson J. How many impulsivities? A discounting perspective. J Exp Anal Behav. 2013;99(1):3–13. doi: 10.1002/jeab.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. J Pers Soc Psychol. 1998;74(1):224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: Conceptual and empirical foundations. Handbook of emotion regulation. 2014;2:3–20. [Google Scholar]
- Gross JJ. Emotion regulation: Current status and future prospects. Psychological Inquiry. 2015;26(1):1–26. [Google Scholar]
- Hagger MS, Chatzisarantis NLD. A multi-lab preregistered replication of the ego-depletion effect. Perspectives on Psychological Science. 2016;11:546–573. doi: 10.1177/1745691616652873. [DOI] [PubMed] [Google Scholar]
- Hagger MS, Wood C, Stiff C, Chatzisarantis NLD. Ego depletion and the strength model of self-control: A meta-analysis. Psychological Bulletin. 2016;136:495–525. doi: 10.1037/a0019486. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock C, Carr TH, Nigg JT. Development of selective attention: Perceptual load influences early versus late attentional selection in children and adults. Dev Psychol. 2002;38(3):363–375. [PubMed] [Google Scholar]
- Huang-Pollock C, Karalunas SL, Tam H, Moore AN. Evaluating vigilance deficits in adhd: A meta-analysis of cpt performance. J Abnorm Psychol. 2012;121(2):360–371. doi: 10.1037/a0027205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang-Gu SL, Gau SS. Interval timing deficits assessed by time reproduction dual tasks as cognitive endophenotypes for attention-deficit/hyperactivity disorder. PLoS One. 2015;10(5):e0127157. doi: 10.1371/journal.pone.0127157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado MB, Rosselli M. The elusive nature of executive functions: A review of our current understanding. Neuropsychol Rev. 2007;17(3):213–233. doi: 10.1007/s11065-007-9040-z. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N. The long shadow of temperament. Cambridge, Massachusetts: Harvard University Press; 2004. [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychon Bull Rev. 2002;9(4):637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Karoly P. Mechanisms of self-regulation: A systems view. Annu Rev Psychol. 1993;44(1):23–52. doi: [Google Scholar]
- Kelley WM, Wagner DD, Heatherton TF. In search of a human self-regulation system. Annu Rev Neurosci. 2015;38:389–411. doi: 10.1146/annurev-neuro-071013-014243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen PR. An additive-utility model of delay discounting. Psychol Rev. 2009;116(3):602–619. doi: 10.1037/a0016414. [DOI] [PubMed] [Google Scholar]
- Killeen PR. The logistics of choice. J Exp Anal Behav. 2015;104(1):74–92. doi: 10.1002/jeab.156. [DOI] [PubMed] [Google Scholar]
- Killeen PR, Russell VA, Tannock R. Neuroenergetics. Current Directions in Psychological Science. 2016;25(2):124–129. [Google Scholar]
- Kochanska G. Multiple pathways to conscience for children with different temperaments: From toddlerhood to age 5. Dev Psychol. 1997;33(2):228–240. doi: 10.1037//0012-1649.33.2.228. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Coy KC, Murray KT. The development of self-regulation in the first four years of life. Child Dev. 2001;72(4):1091–1111. doi: 10.1111/1467-8624.00336. [DOI] [PubMed] [Google Scholar]
- Koole SL, Van Dillen LF, Sheppes G. The self-regulation of emotion. In: Vohs KD, Baumeister RF, editors. Handbook of self-regulation : Research, theory, and applications. 2. New York: Guilford Press; 2011. pp. 22–40. [Google Scholar]
- Kross E, Mischel W. From stimulus control to selfcontrol: Towards an integrative understanding of the processes underlying willpower. In: Hassin RR, Ochsner KN, Trope Y, editors. Self control in society, mind, and brain. Oxford, New York: Oxford University Press; 2010. pp. 428–446. [Google Scholar]
- Lahey BB, Applegate B, Hakes JK, Zald DH, Hariri AR, Rathouz PJ. Is there a general factor of prevalent psychopathology during adulthood? J Abnorm Psychol. 2012;121(4):971–977. doi: 10.1037/a0028355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Lorine DW. Neuropsychological assessment. 4th. New York: Oxford University Press; 2004. [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: A theory of an act of control. Psychological Review. 1984;91(3):295. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Luciana M. Adolescent brain development in normality and psychopathology. Dev Psychopathol. 2013;25(402):1325–1345. doi: 10.1017/S0954579413000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria AR. Higher cortical functions in man. New York: Basic Books; 1966. [Google Scholar]
- MacLeod CM. Half a century of research on the stroop effect: An integrative review. Psychol Bull. 1991;109(2):163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Bickel WK. Impulsivity: The behavioral and neurological science of discounting. Washington DC: American Psychological Association; 2010. [Google Scholar]
- Magen E, Gross JJ. Getting our act together: Toward a general model of self-control. Self-control in society, mind and brain. 2010:335–353. [Google Scholar]
- Marcovitch S, Zelazo PD. A hierarchical competing systems model of the emergence and early development of executive function. Developmental Science. 2009;12(1):1–18. doi: 10.1111/j.1467-7687.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marien H, Custers R, Hassin RR, Aarts H. Unconscious goal activation and the hijacking of the executive function. J Pers Soc Psychol. 2012;103(3):399–415. doi: 10.1037/a0028955. [DOI] [PubMed] [Google Scholar]
- Martel MM, Nigg JT. Child adhd and personality/temperament traits of reactive and effortful control, resiliency, and emotionality. J Child Psychol Psychiatry. 2006;47(11):1175–1183. doi: 10.1111/j.1469-7610.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- Martel MM, Pierce L, Nigg JT, Jester JM, Adams K, Puttler LI, Zucker RA. Temperament pathways to childhood disruptive behavior and adolescent substance abuse: Testing a cascade model. J Abnorm Child Psychol. 2009;37(3):363–373. doi: 10.1007/s10802-008-9269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MM, Roberts B, Gremillion M, von Eye A, Nigg JT. External validation of bifactor model of adhd: Explaining heterogeneity in psychiatric comorbidity, cognitive control, and personality trait profiles within dsm-iv adhd. J Abnorm Child Psychol. 2011;39(8):1111–1123. doi: 10.1007/s10802-011-9538-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten AS, Cicchetti D. Developmental cascades. Dev Psychopathol. 2010;22(Special Issue 03):491–495. doi: 10.1017/S0954579410000222. doi: [DOI] [PubMed] [Google Scholar]
- Mata R, Josef AK, Hertwig R. Propensity for risk taking across the life span and around the globe. Psychological Science. 2016 doi: 10.1177/0956797615617811. [DOI] [PubMed] [Google Scholar]
- Mata R, Josef AK, Samanez-Larkin GR, Hertwig R. Age differences in risky choice: A meta-analysis. Ann N Y Acad Sci. 2011;1235:18–29. doi: 10.1111/j.1749-6632.2011.06200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley T, Chen S, Goos L, Schachar R, Crosbie J. Is the behavior rating inventory of executive function more strongly associated with measures of impairment or executive function? J Int Neuropsychol Soc. 2010;16(3):495–505. doi: 10.1017/S1355617710000093. [DOI] [PubMed] [Google Scholar]
- McClelland MM, Cameron CE. Self-regulation in early childhood: Improving conceptual clarity and developing ecologically valid measures. Child Development Perspectives. 2012;6(2):136–142. [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695):503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- McLoughlin G, Albrecht B, Banaschewski T, Rothenberger A, Brandeis D, Asherson P, Kuntsi J. Performance monitoring is altered in adult adhd: A familial event-related potential investigation. Neuropsychologia. 2009;47(14):3134–3142. doi: 10.1016/j.neuropsychologia.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Buschman TJ. Top-down control of attention by rhythmic neural computations. In: Posner MI, editor. Cognitive neuroscicence of attention. 2nd. New York: Guilford; 2012. pp. 229–241. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller GA. The magical number seven, plus or minus two: Some limits on our capacity for processing information. Psychological Review. 1956;63(2):81–97. [PubMed] [Google Scholar]
- Mischel W, Shoda Y. A cognitive-affective system theory of personality: Reconceptualizing situations, dispositions, dynamics, and invariance in personality structure. Psychol Rev. 1995;102(2):246–268. doi: 10.1037/0033-295x.102.2.246. [DOI] [PubMed] [Google Scholar]
- Mitchell SH, Wilson VB. The subjective value of delayed and probabilistic outcomes: Outcome size matters for gains but not for losses. Behav Processes. 2010;83(1):36. doi: 10.1016/j.beproc.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SH, Wilson VB, Karalunas SL. Comparing hyperbolic, delay-amount sensitivity and present-bias models of delay discounting. Behav Processes. 2015;114:52–62. doi: 10.1016/j.beproc.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex ‘frontal lobe’ tasks: A latent variable analysis. Cogn Psychol. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Morris N, Keane S, Calkins S, Shanahan L, O’Brien M. Differential components of reactivity and attentional control predicting externalizing behavior. Journal of Applied Developmental Psychology. 2014;35(3):121–127. doi: 10.1016/j.appdev.2014.02.002. doi: http://dx.doi.org/10.1016/j.appdev.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison FJ, Grammer JK. Conceptual clutter and measurement mayhem: Proposals for cross-disciplinary integration in conceptualizing and measuring executive function. In: Griffin JA, McCardle P, Freund LS, editors. Executive function in preschool-age children: Integrating measurement, neurodevelopment, and translational research. Washington, DC, US: American Psychological Association; 2016. pp. 327–348. [Google Scholar]
- Namboodiri VM, Mihalas S, Hussain Shuler MG. Analytical calculation of errors in time and value perception due to a subjective time accumulator: A mechanistic model and the generation of weber’s law. Neural Comput. 2016;28(1):89–117. doi: 10.1162/NECO_a_00792. [DOI] [PubMed] [Google Scholar]
- Namboodiri VM, Mihalas S, Marton TM, Hussain Shuler MG. A general theory of intertemporal decision-making and the perception of time. Front Behav Neurosci. 2014;8:61. doi: 10.3389/fnbeh.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell A. Unified theories of cognition. MA: Harvard University Press; 1990. [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12(2):241–268. doi: 10.3758/s13415-011-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT. On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychology Bulletin. 2000;126(2):220–246. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Is adhd a disinhibitory disorder? Psychol Bull. 2001;127(5):571–598. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Temperament and developmental psychopathology. Journal of Child Psychology and Psychiatry. 2006;47(3–4):395–422. doi: 10.1111/j.1469-7610.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Nagel BJ. Commentary: Risk taking, impulsivity, and externalizing problems in adolescent development - commentary on crone et al. 2016. J Child Psychol Psychiatry. 2016;57(3):369–370. doi: 10.1111/jcpp.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Silk KR, Stavro G, Miller T. Disinhibition and borderline personality disorder. Dev Psychopathol. 2005;17(4):1129–1149. doi: 10.1017/s0954579405050534. [DOI] [PubMed] [Google Scholar]
- Noordhof A, Krueger RF, Ormel J, Oldehinkel AJ, Hartman CA. Integrating autism-related symptoms into the dimensional internalizing and externalizing model of psychopathology. The TRAILS study. J Abnorm Child Psychol. 2015;43(3):577–587. doi: 10.1007/s10802-014-9923-4. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RR, Hughes B, McRae K, Cooper JC, Weber J, Gross JJ. Bottom-up and top-down processes in emotion generation: Common and distinct neural mechanisms. Psychol Sci. 2009;20(11):1322–1331. doi: 10.1111/j.1467-9280.2009.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson SL, Sameroff AJ, Kerr DC, Lopez NL, Wellman HM. Developmental foundations of externalizing problems in young children: The role of effortful control. Development and Psychopathology. 2005;17(1):25–45. doi: 10.1017/s0954579405050029. [DOI] [PubMed] [Google Scholar]
- Padilla-Walker LM, Christensen KJ. Empathy and self-regulation as mediators between parenting and adolescents’ prosocial behavior toward strangers, friends, and family. Journal of Research on Adolescence. 2011;21(3):545–551. doi: 10.1111/jora.12362. [DOI] [PubMed] [Google Scholar]
- Papies EK, Aarts H. Nonconscious self-regulation or the automatic pilot of human behavior. In: Vohs KD, Baumeister RF, editors. Handbook of self-regulation: Research, theory, and applications. 2. New York: Guilford Press; 2011. pp. 125–142. [Google Scholar]
- Patros CH, Alderson RM, Kasper LJ, Tarle SJ, Lea SE, Hudec KL. Choice-impulsivity in children and adolescents with attention-deficit/hyperactivity disorder (adhd): A meta-analytic review. Clin Psychol Rev. 2016;43:162–174. doi: 10.1016/j.cpr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitclerc A, Briggs-Gowan MJ, Estabrook R, Burns JL, Anderson EL, McCarthy KJ, Wakschlag LS. Contextual variation in young children’s observed disruptive behavior on the db-dos: Implications for early identification. J Child Psychol Psychiatry. 2015;56(9):1008–1016. doi: 10.1111/jcpp.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Castellanos FX. Top-down dysregulation - from adhd to emotional instability. Front Behav Neurosci. 2016;10 doi: 10.3389/fnbeh.2016.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A, Steinglass JE, Greene AL, Weber EU, Simpson HB. Capacity to delay reward differentiates obsessive-compulsive disorder and obsessive-compulsive personality disorder. Biol Psychiatry. 2014;75(8):653–659. doi: 10.1016/j.biopsych.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Developing mechanisms of self-regulation. Journal of Development and Psychopathology. 2000;12(3):427–441. doi: 10.1017/s0954579400003096. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annu Rev Psychol. 2007;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Toward a physical basis of attention and self regulation. Phys Life Rev. 2009;6(2):103–120. doi: 10.1016/j.plrev.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK, Sheese BE, Voelker P. Developing attention: Behavioral and brain mechanisms. Adv Neurosci (Hindawi) 2014;2014:405094. doi: 10.1155/2014/405094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Snyder CRR. Attention and cognitive control. In: Solso RL, editor. Information processing and cognition: The loyola symposium. Lawrence: Erlbaum; 1975. [Google Scholar]
- Prager LM. Depression and suicide in children and adolescents. Pediatrics in Review. 2009;30:199–205. doi: 10.1542/pir.30-6-199. [DOI] [PubMed] [Google Scholar]
- Pribram KH. The primate frontal cortex-executive of the brain. In: Pribram AH, Luria AR, editors. Psychophysiology of the frontal lobes. New York: Academic Press; 1973. pp. 293–314. [Google Scholar]
- Rachlin H, Green L. Commitment, choice and self-control. J Exp Anal Behav. 1972;17(1):15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos D, Victor T, Seidl-de-Moura ML, Daly M. Future discounting by slum-dwelling youth versus university students in rio de janeiro. Journal of Research on Adolescence. 2013;23(1):95–102. [Google Scholar]
- Redish AD, Kurth-Nelson Z. Neural models of delay discounting. In: Bickel GJMWK, editor. Impulsivity: The behavioral and neurological science of discounting. Washington, DC, US: American Psychological Association; 2010. pp. 123–158. [Google Scholar]
- Reed MA, Pien DP, Rothbart MK. Inhibitory self-control I preschool children. Merrill-Palmer Quarterly. 1984;30:131–147. [Google Scholar]
- Reis RS, Dalle Molle R, Machado TD, Mucellini AB, Rodrigues DM, Bortoluzzi A, Silveira PP. Impulsivity-based thrifty eating phenotype and the protective role of n-3 pufas intake in adolescents. Transl Psychiatry. 2016;6:e755. doi: 10.1038/tp.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg AA, Kagan J. Physical and physiological correlates of behavioral inhibition. Dev Psychobiol. 1989;22(8):753–770. doi: 10.1002/dev.420220802. [DOI] [PubMed] [Google Scholar]
- Rothbart MK. Measurement of temperament in infancy. Child Dev. 1981;52(2):569–578. [Google Scholar]
- Rothbart MK. Becoming who we are: Temperament and personality in development. New York: Guilford Press; 2011. [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey K, Fisher P. Investigations of temperament at three to seven years: The children’s behavior questionnaire. Child Development. 2001;72:1394–1408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Damon W, Lerner R, Eisenberg N, editors. Handbook of child psychology (6th ed.): (Vol 3: Social, emotional, and personality development. New York: Wiley; 2006. pp. 99–176. (Volume Ed.) [Google Scholar]
- Rothbart MK, Derryberry D. Development of individual differences in temperament. In: Lamb ME, Brown AL, editors. Advances in Developmental Psychology. Vol. 1. Hillsdale, NJ: Erlbaum; 1981. pp. 37–86. [Google Scholar]
- Rothbart MK, Ellis LK, Rueda MR, Posner MI. Developing mechanisms of temperamental effortful control. J Pers. 2003;71(6):1113–1143. doi: 10.1111/1467-6494.7106009. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Sheese BE, Posner MI. Handbook of emotion regulation. 2nd. New York, NY, US: Guilford Press; 2014. Temperament and emotion regulation; pp. 305–320. [Google Scholar]
- Rueda MR, Posner MI, Rothbart MK. The development of executive attention: Contributions to the emergence of self-regulation. Dev Neuropsychol. 2005;28(2):573–594. doi: 10.1207/s15326942dn2802_2. [DOI] [PubMed] [Google Scholar]
- Schneider W, Shiffrin RM. Controlled and automatic human information processing: I. Detection, search, and attention. Psychological Review. 1977;84(1):1–66. [Google Scholar]
- Scholer A, Higgins ET. Conflict and control at different levels of self-regulation. In: Ran Hassin KO, Trope Yaacov, editors. Self control in society, mind, and brain. Oxford Scholarship Online: Oxford University Press; 2010. pp. 312–355. [Google Scholar]
- Sercombe H. Risk, adaptation and the functional teenage brain. Brain Cogn. 2014;89:61–69. doi: 10.1016/j.bandc.2014.01.001. doi: http://dx.doi.org/10.1016/j.bandc.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Sergeant JA, Oosterlaan J, van der Meere JJ. Information processing and energetic factors in attention deficit/hyperactivity disorder. In: Quay HC, Hogan AE, editors. Handbook of disruptive behavior disorders. New York: Kluwer Academic/Plenum; 1999. pp. 75–104. [Google Scholar]
- Sethi A, Mischel W, Aber JL, Shoda Y, Rodriguez ML. The role of strategic attention deployment in development of self-regulation: Predicting preschoolers’ delay of gratification from mother-toddler interactions. Dev Psychol. 2000;36(6):767–777. [PubMed] [Google Scholar]
- Shallice T. Specific impairments of planning. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 1982;298(1089):199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess PW. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114(Pt 2):727–741. doi: 10.1093/brain/114.2.727. [DOI] [PubMed] [Google Scholar]
- Shanks DR. Learning: From association to cognition. Annu Rev Psychol. 2010;61:273–301. doi: 10.1146/annurev.psych.093008.100519. [DOI] [PubMed] [Google Scholar]
- Sharma L, Markon KE, Clark LA. Toward a theory of distinct types of ‘impulsive’ behaviors: A meta-analysis of self-report and behavioral measures. Psychol Bull. 2014;140(2):374–408. doi: 10.1037/a0034418. [DOI] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron. 2013;79(2):217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman EP, Smith AR, Silva K, Icenogle G, Duell N, Chein J, Steinberg L. The dual systems model: Review, reappraisal, and reaffirmation. Dev Cogn Neurosci. 2016;17:103–117. doi: 10.1016/j.dcn.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson A, Riggs KJ, Beck SR, Gorniak SL, Wu Y, Abbott D, Diamond A. Refining the understanding of inhibitory processes: How response prepotency is created and overcome. Developmental Science. 2012;15(1):62–73. doi: 10.1111/j.1467-7687.2011.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LB, Thelen E. Development as a dynamic system. Trends Cogn Sci. 2003;7(8):343–348. doi: 10.1016/s1364-6613(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Smith PL, Ratcliff R. Psychology and neurobiology of simple decisions. Trends Neurosci. 2004;27(3):161–168. doi: 10.1016/j.tins.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Spinrad TL, Eisenberg N, Cumberland A, Fabes RA, Valiente C, Shepard SA, Guthrie IK. Relation of emotion-related regulation to children’s social competence: A longitudinal study. Emotion. 2006;6(3):498–510. doi: 10.1037/1528-3542.6.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JR, Stephens DW. The adaptive nature of impulsivity. In: Madden GJ, Bickel WK, editors. Impulsivity: The Behavioral and Neurological Science of Discounting. Washington DC: American Psychological Association; 2010. pp. 361–388. [Google Scholar]
- Strauss E, Sherman E, Spreen O. A compendium of neuropsychological tests. 3rd. New York: Oxford University Press; 2006. [Google Scholar]
- Stuss DT, Benson DF. The frontal lobes. New York: Raven Press; 1986. [Google Scholar]
- Sulik MJ, Huerta S, Zerr AA, Eisenberg N, Spinrad TL, Valiente C, Taylor HB. The factor structure of effortful control and measurement invariance across ethnicity and sex in a high-risk sample. J Psychopathol Behav Assess. 2009;32(1):8–22. doi: 10.1007/s10862-009-9164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tackett JL, Lahey BB, van Hulle C, Waldman I, Krueger RF, Rathouz PJ. Common genetic influences on negative emotionality and a general psychopathology factor in childhood and adolescence. J Abnorm Psychol. 2013;122(4):1142–1153. doi: 10.1037/a0034151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangney JP, Baumeister RF, Boone AL. High self-control predicts good adjustment, less pathology, better grades, and interpersonal success. J Pers. 2004;72(2):271–324. doi: 10.1111/j.0022-3506.2004.00263.x. [DOI] [PubMed] [Google Scholar]
- Tellegen A. Structures of mood and personality and their relevance to assessing anxiety, with an emphasis on self-report. In: Maser AHTJD, editor. Anxiety and the anxiety disorders. Hillsdale, NJ, England: Lawrence Erlbaum Associates, Inc; 1985. pp. 681–706. [Google Scholar]
- Thelen E, Schoner G, Scheier C, Smith LB. The dynamics of embodiment: A field theory of infant perseverative reaching. Behav Brain Sci. 2001;24(1):1–34. doi: 10.1017/s0140525x01003910. discussion 34–86. [DOI] [PubMed] [Google Scholar]
- Thorndike EL. A proof of the law of effect. Science. 1933;77(1989):173–175. doi: 10.1126/science.77.1989.173-a. [DOI] [PubMed] [Google Scholar]
- Tseng WL, Guyer AE, Briggs-Gowan MJ, Axelson D, Birmaher B, Egger HL, Brotman MA. Behavior and emotion modulation deficits in preschoolers at risk for bipolar disorder. Depress Anxiety. 2015;32(5):325–334. doi: 10.1002/da.22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukayama E, Duckworth AL, Kim B. Domain-specific impulsivity in school-age children. Dev Sci. 2013;16(6):879–893. doi: 10.1111/desc.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadillo MA, Gold N, Osman M. The bitter truth about sugar and willpower: The limited evidential value of the glucose model of ego-depletion. Psychological Science. doi: 10.1177/0956797616654911. (in press) [DOI] [PubMed] [Google Scholar]
- van den Ban E, Souverein P, Meijer W, van Engeland H, Swaab H, Egberts T, Heerdink E. Association between adhd drug use and injuries among children and adolescents. European Child and Adolescent Psychiatry. 2014;23:95–102. doi: 10.1007/s00787-013-0432-8. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Gunther Moor B, Op de Macks ZA, Rombouts SA, Westenberg PM, Crone EA. Adolescent risky decision-making: Neurocognitive development of reward and control regions. Neuroimage. 2010;51(1):345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Vandierendonck A. A working memory system with distributed executive control. Perspectives on Psychological Science. 2016;11(1):74–100. doi: 10.1177/1745691615596790. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, McAndrew A, Weidemann G, Stevens T, McLaren IPL. Limits of executive control: Sequential effects in predictable environments. Psychological Science. 2016 doi: 10.1177/0956797616631990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, McLaren IPL, Chambers CD. Banishing the control homunculi in studies of action control and behavior change. Perspectives on Psychological Science. 2014;9(5):497–524. doi: 10.1177/1745691614526414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Uexküll J. Theoretical biology. Harcourt, Brace & Company, Inc; 1926. [Google Scholar]
- Wakschlag LS, Briggs-Gowan MJ, Choi SW, Nichols SR, Kestler J, Burns JL, Henry D. Advancing a multidimensional, developmental spectrum approach to preschool disruptive behavior. J Am Acad Child Adolesc Psychiatry. 2014;53(1):82.e83–96.e83. doi: 10.1016/j.jaac.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Choi SW, Carter AS, Hullsiek H, Burns J, McCarthy K, Briggs-Gowan MJ. Defining the developmental parameters of temper loss in early childhood: Implications for developmental psychopathology. J Child Psychol Psychiatry. 2012;53(11):1099–1108. doi: 10.1111/j.1469-7610.2012.02595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FL, Chassin L, Eisenberg N, Spinrad TL. Effortful control predicts adolescent antisocial-aggressive behaviors and depressive symptoms: Co-occurrence and moderation by impulsivity. Child Development. 2015;86:1812–1829. doi: 10.1111/cdev.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liu X, Fan J. Symbolic and connectionist models of attention. Cognitive Neuroscience of Attention. 2012:47–56. [Google Scholar]
- Wearden JH, Lejeune H. Scalar properties in human timing: Conformity and violations. Q J Exp Psychol (Hove) 2008;61(4):569–587. doi: 10.1080/17470210701282576. [DOI] [PubMed] [Google Scholar]
- Welsh M, Peterson E. Issues in the conceptualization and assessment of hot executive functions in childhood. J Int Neuropsychol Soc. 2014;20(2):152–156. doi: 10.1017/S1355617713001379. [DOI] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30(4):669–689. doi: http://dx.doi.org/10.1016/S0191-8869(00)00064-7. [Google Scholar]
- Wickens T. Elementary signal detection theory. New York: Oxford University Press; 2002. [Google Scholar]
- Wiebe SA, Sheffield T, Nelson JM, Clark CA, Chevalier N, Espy KA. The structure of executive function in 3-year-olds. J Exp Child Psychol. 2011;108(3):436–452. doi: 10.1016/j.jecp.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby MW, Blair C. Longitudinal measurement of executive function in preschoolers. In: Freund PML, Griffin J, editors. Executive function in preschool age children: Integrating measurement, neurodevelopment, and translational research. Washington D.C.: APA; 2015. [Google Scholar]
- Zelazo PD, Carlson SM. Hot and cool executive function in childhood and adolescence: Development and plasticity. Child Development Perspectives. 2012;6(4):354–360. [Google Scholar]
- Zelazo PD, Carter A, Reznick JS, Frye D. Early development of executive function: A problem-solving framework. Review of General Psychology. 1997;1(2):198–226. [Google Scholar]
- Zelazo PD, Cunningham W. Executive function: Mechanisms underlying emotion regulation. In: Gross JJ, editor. Handbook of emotion regulation. New York, NY: Guilford; 2007. pp. 135–158. [Google Scholar]
- Zelazo PD, Muller U. Executive function in typical and atypical development. In: Goswami U, editor. Handbook of childhood cognitive development. Oxford, UK: Blackwell; 2002. pp. 445–469. [Google Scholar]
- Zhou Q, Chen SH, Main A. Commonalities and differences in the research on children’s effortful control and executive function: A call for an integrated model of self-regulation. Child Development Perspectives. 2012;6(2):112–121. [Google Scholar]
- Zucker RA, Heitzeg MM, Nigg JT. Parsing the undercontrol/disinhibition pathway to substance use disorders: A multilevel developmental problem. Child Dev Perspect. 2011;5(4):248–255. doi: 10.1111/j.1750-8606.2011.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.