Abstract
Background & Aims
Concurrent to development of more effective drugs for treatment of hepatitis C virus (HCV), infection, there has been an increase in the incidence of non-alcoholic fatty liver disease (NAFLD). Data indicate that liver transplantation prolongs survival times of patient with acute hepatitis associated with alcoholic liver disease (ALD). We compared data on disease prevalence in the population with data from liver transplantation waitlists to evaluate changes in the burden of liver disease in the United States.
Methods
We collected data on the prevalence of HCV from the National Health and Nutrition Examination Survey (NHANES), from the 2010 and 2013–2014 cycles. We also collected data from the HealthCore Integrated Research Database on patients with cirrhosis and chronic liver failure (CLF) from 2006 through 2014, and data on patients who received transplant from the United Network for Organ Sharing (UNOS), from 2003 through 2015. We determined percentages of new waitlist members and transplant recipients with HCV infection, stratified by indication for transplant, modeling each calendar year as a continuous variable using the Spearman rank correlation, non-parametric test of trends, and linear regression models.
Results
In an analysis of data from the NHANES (2013–2014), we found that the proportion of patients with a positive HCV antibody who had a positive HCV RNA was 0.5 (95% CI, 0.42–0.55); this value was significantly lower than in 2010 (0.64; 95% CI, 0.59–0.73) (P=.03). Data from the HealthCore databased revealed significant changes (P<.05 for all), over time, in percentages of patients with compensated cirrhosis (decreases in percentages of patients with cirrhosis from HCV or ALD, but increase in percentages of patients with cirrhosis from non-alcoholic steatohepatitis [NASH]), CLF (decreases in percentages of patients with CLF from HCV or ALD, with an almost 3-fold increase in percentage of patients with CLF from NASH), and hepatocellular carcinoma (HCC) (decreases in percentages of patients with HCC from HCV or ALD and a small increase in HCC among persons with NASH). Data from the UNOS revealed that among patients new to the liver transplant waitlist, or undergoing liver transplantation, for CLF, there was a significant decrease in the percentage with HCV infection and increases in percentages of patients with NAFLD or ALD. Among patients new to the liver transplant waitlist, or undergoing liver transplantation, for HCC, proportions of those with HCV infection, NAFLD, or ALD did not change between 2003 and 2015
Conclusions
In an analysis of 3 different databases (NHANES, HealthCore, and UNOS), we found the proportion of patients on the liver transplant waitlist or undergoing liver transplantation for chronic HCV infection to be decreasing, and fewer patients to have cirrhosis or CLF. However, the percentages of patients on the waitlist or receiving liver transplants for NASH or ALD are increasing, despite different relative burdens of disease among the entire population of patients with cirrhosis.
Keywords: population analysis, US, DAA therapy, obesity
Introduction
It is estimated that 2-3 million people in the United States are infected with chronic hepatitis C virus (HCV). 1 HCV has been the most common indication for liver transplantation (LT) in North America and Western Europe for the last twenty years.2-4 Chronic HCV infection can necessitate liver transplantation through two main mechanisms: chronic liver failure (CLF) manifesting as synthetic dysfunction and complications of portal hypertension (i.e., ascites) and hepatocellular carcinoma (HCC).
Since 2011, the landscape of HCV treatment has evolved rapidly, with the sequential approval by the US Food and Drug Administration (FDA) of six direct-acting antiviral agents (DAA), with progressive advances in efficacy and, as importantly, tolerability. The NS3/4A HCV protease inhibitors telaprevir5 and boceprevir6, were the first DAAs to be approved in 2011. Drug-drug interactions, toxicities of these medications, and the need to co-administer interferon, greatly limited the utility and impact of these agents in patients with advanced liver disease. The therapeutic landscape was altered again in December 2013, when the FDA approved two new agents: sofosbuvir7 and simeprevir8, with efficacy against HCV genotype 1. The safety and efficacy of sofosbuvir and simeprevir, together and independently, among patients with cirrhosis has been documented in several multicenter studies, with cure rates exceeding 90%.9-11 Recent studies have demonstrated similar cure rates in patients with more severe liver disease, including patients with Child-Turcotte-Pugh (CTP) classification B and C stages of disease, yielding improvements or stabilization in liver disease, as measured by the Model for End-Stage Liver Disease (MELD) and CTP scores in the great majority of treated patients.12,13
The reported improvement in MELD and CTP scores among treated patients with advanced liver disease suggest that the advent of highly effective and well tolerated DAAs clearly could, in theory, reduce the frequency of HCV and HCV-related complications in the general population, and as a result, as an indication for LT. Such an effect has not been reported to date. Furthermore, it is possible that that the impact of treatment with DAAs may differentially affect waitlist additions and LT for HCV for CLF, as cure may lead to immediate stabilization or improvement in liver function, as compared with HCC, which would have a more delayed benefit in the broader HCV population, and those with more advanced disease.
Concurrent to rapid advancements in the treatment of HCV, the incidence of liver failure and HCC related to non-alcoholic fatty liver disease (NAFLD) have been reported to be increasing dramatically,14 with associated increases in the prevalence of NAFLD-related liver disease as an indication for liver transplantation.15 In addition to the impact of improved therapy for HCV and the steady increase in the frequency of NAFLD and as a cause of liver failure and HCC, alcohol-related liver disease (ALD) has been reported as the most common cause of chronic liver disease, rather than HCV or NAFLD,16 with recent evidence that liver transplantation for acute alcoholic hepatitis (which almost always occur in the setting of advanced fibrosis) confers substantial survival benefits with a low frequency of relapse of clinically apparent alcohol use following liver transplantation in carefully selected patients. The impact of these three emerging trends on the overall need for and relative frequency of indications for liver transplantation is likely to be substantial, but must be weighed in the face of recently published data suggesting that patients with liver disease related to NAFLD or ALD are significantly less likely to be waitlisted compared to patients with HCV, while patients with cirrhosis complicated by HCC are 2-5 times more likely than patients with CLF.17
We sought to assess the impact of new advents in HCV treatment and emerging trends in liver disease in the broader population with cirrhosis and the more limited waitlist population, we sought to synthesize data from three distinct datasets. We evaluated data from the National Health and Nutrition Evaluation Survey (NHANES), from a nationally-representative database that includes a broad cohort of patients with cirrhosis, and national transplant data from 2003-2015. In combining data from these three datasets, our overarching goal was to evaluate temporal trends in the burden of liver disease from HCV, NASH, and ALD through the continuum of liver disease: chronic liver disease->compensated cirrhosis->chronic liver failure (decompensated cirrhosis) and hepatocellular carcinoma->waitlisting for transplantation->transplant.
Study participants and methods
Population-based data for on the changing epidemiology of chronic HCV
We first sought to assess changes in the prevalence of chronic HCV in the era of DAA therapy using NHANES data in the 2013-2014 cycle. The details on NHANES methods and procedures have been previously published.1 The NHANES collects nationally representative data on the non-institutionalized US civilian population.18 With respect to HCV, all individuals 6 years and older are tested for the presence of antibodies to HCV (anti-HCV). Individuals testing HCV positive undergo further testing with HCV RNA and if positive, HCV genotype. There were no changes to the lab method, lab equipment, or lab site for Hepatitis C RNA and Hepatitis C genotype in the NHANES 2013-2014 cycle compared to the previous NHANES survey cycles.
Population-based data of compensated cirrhosis, chronic liver failure, and HCC
The next step in the pathway from chronic liver disease to end-stage liver disease is the development of cirrhosis, followed by either decompensated cirrhosis (chronic liver failure) or HCC. In order to evaluate temporal trends in the etiologies of liver disease for cirrhosis and advanced liver disease, we assessed the burden of cirrhosis from HCV, ALD, and NASH using a nationally representative database of commercially insured patients: HealthCore Integrated Research Database (HIRD) from 2006-2014. HealthCore is a wholly owned subsidiary of Anthem, Inc. serving members in all 50 states. It is a nationally-representative dataset of commercially insured patients, with longitudinal medical and pharmacy claims on 25 million patients, and has been used for prior studies evaluating population-level outcomes in patients with chronic liver failure and HCC.19-22 This study sample has been previously described.21,22 HIRD patients with cirrhosis were categorized as: compensated cirrhosis, CLF, or HCC. These categories were based on severity of cirrhosis at entry into the study cohort, and were defined using algorithms based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes that have been validated to have positive predictive values of >85%.23-27 Using HIRD data enabled us to estimate the emerging trends in cirrhosis and advanced liver disease from HCV, ALD, and NASH.
Compensated cirrhosis was defined based on having ≥1 inpatient or ≥2 outpatient ICD-9-CM codes for cirrhosis (571.2, 571.5) in the absence of a complication of portal hypertension, HCC, or laboratory abnormalities consistent with severe liver synthetic dysfunction (MELD score ≥15 and/or total serum bilirubin ≥3mg/dL).23-27 CLF was defined as having ≥1 inpatient or ≥2 outpatient ICD-9-CM codes for a complication of portal hypertension (ascites, bleeding esophageal varices, ascites, and/or spontaneous bacterial peritonitis) occurring after the diagnosis of cirrhosis. HCC required a diagnosis of cirrhosis and ≥1 inpatient or ≥2 outpatient ICD-9-CM code for HCC (ICD-9-CM code: 155.0). In the subset of cirrhotic patients without HCC or a complication of portal hypertension, we used laboratory criteria to define hepatocellular dysfunction/chronic liver failure (calculated MELD score ≥15 and/or a total serum bilirubin ≥3mg/dL.) Although this cohort may not fully reflect the entire US population, it was a uniform cohort over time that allowed us to evaluate trends in cirrhosis, CLF, and HCC in a broader population with chronic liver disease. Etiology of liver disease was based on ICD-9-CM coding for patients with HCV or ALD (patients with HCV and ALD were categorized as HCV). NASH was assigned as the etiology of liver disease in patients without a specific etiology of liver disease based on ICD-9-CM codes, in the setting of ≥1 of the following: ICD-9-CM code for obesity/overweight, diabetes mellitus, impaired glucose tolerance, hyperlipidemia, hypertension, or a hemoglobin A1c ≥6.5% if such laboratory data were available. Patients were required to have at least 90 days of complete follow-up in the HIRD in order to be included in the study. Of note, the HIRD does include patients with certain Medicare Advantage plans, but this represents <5% of patients, and in our HIRD population, only 44.6% were ≥65 years of age.
Waitlist and transplant data
The final pathway of advanced liver disease is waitlisting for transplantation, and transplantation in the sickest patients with chronic liver disease. To assess this, we performed analyses using Organ Procurement and Transplantation Network (OPTN)/United Network for Organ Sharing (UNOS) data. For analyses of new waitlistings, we included all adult patients (≥18 years of age) added to the waiting list between February 27, 2002 and December 31, 2015. Patients listed for re-transplantation and/or listed after being removed from the waitlist were included. For transplant analyses, we included all adult (≥18 years of age) transplant recipients between February 27, 2002 and December 31, 2015, including re-transplant patients. All new waitlistings and transplant recipients with acute liver failure and/or status 1 designation were excluded in order to identify a cohort with chronic liver disease.
Etiology of liver disease was based on OPTN/UNOS diagnostic coding, HCV serostatus data, and free-texting of diagnoses for patients without a specified diagnosis based on OPTN/UNOS coding. Re-listings or re-transplant recipients were assigned an etiology of liver disease based on coding for the initial transplant. Patients were then categorized into the three main etiologies of liver disease (HCV, ALD, NASH/cryptogenic), with the remaining patients categorized as “other.” Analyses were stratified into transplant recipients with chronic liver failure (CLF; including decompensated cirrhosis and other manifestations of chronic liver disease excluding status 1) and hepatocellular carcinoma (HCC; based on diagnostic codes and HCC exception points) to evaluate differences in indications for transplants for patients with CLF versus HCC.
Statistical analysis of NHANES data
We used appropriate published sampling weights as recommended by the NCHS for the NHANES survey. To determine if any change in prevalence may be attributable to treatment effect, we compared the proportion of HCV RNA-to-HCV antibodies in 2010 and 2014, knowing that drugs only affect HCV RNA but not HCV antibodies. These proportions were compared using the chi-square test.
Statistical analyses of OPTN/UNOS data
We first evaluated the absolute number of new waitlistings and transplant recipients by disease category, stratified by CLF versus HCC. For each calendar year, we then calculated the percentage of new waitlisting and transplant recipients with versus without HCV, stratified by indication for transplant. We first tested whether the percentage of new waitlistings and transplant recipients with HCC within each category (CLF vs HCC) were significantly different; such statistical tests assessed whether the percentage with versus without HCC differed among the 14 calendar years. To then test for statistical trends over time (rather than differences in percentage but temporal trends), we evaluated the percentage of new waitlistings and transplant recipients with HCV stratified by indication for transplant, modeling calendar year as a continuous variable, in three ways: 1) Spearman rank correlation with the dependent variable as the percentage of within a specified category with HCV and the independent variable being calendar year; 2) non-parametric test of trends; and 3) linear regression models with the outcome of percentage within a specified category with HCV and the exposure calendar year as a continuous variable. All analyses were performed using Stata 14.0 (College Station, TX)
Results
Burden of chronic HCV in the population
There were 8,291 subjects included in the 2010-2014 NHANES analysis. The median age was 35 years (IQR 16-56), with a majority of subjects being female (51.5%) and non-Hispanic white (63.2%). HCV RNA was detectable in the serum of 67 study participants, which yielded a weighted prevalence of 0.65% (95% CI: 0.5%-0.8%), corresponding to approximately 1.7 million individuals in the US general population. The unweighted proportion of HCV RNA-to-HCV antibody in the 2013-2014 cycle was 0.5 (95% CI: 0.42-0.55) which was significantly lower (p=0.03) than the proportion in 2010 of 0.64 (95% CI: 0.59-0.73) in 2010. The prevalence of HCV RNA was significantly higher in men (P=0.001), non-Hispanic blacks (P = 0.001), people who serve/served in the US military (P = 0.02), individuals below the poverty line (P = 0.01) and those without health insurance (P = 0.001). The prevalence among individuals born between 1945 and 1965 was 1.7% (95% CI: 1.2%-2.4%).
Distribution of HCV, ALD, and NASH in patients with compensated cirrhosis, chronic liver failure, and HCC
Using the HIRD data to evaluate the causes of cirrhosis, CLF, and HCC, we identified 41,082 patients in the HIRD between 2006 and 2014 who had cirrhosis, CLF, or HCV. Of this nationally representative population of commercially insured patients, 24,258 (59.0%) had compensated cirrhosis, 14,971 (36.4%) had CLF (decompensated cirrhosis), and 1,853 (4.5%) had HCC. Of 41,082 patients, 39,800 (96.9%) had at least six months of total follow-up time, and 36,939 (89.9%) had at least 90 days of follow-up in the HIRD before being diagnosis with cirrhosis.
Of the 24,258 patients in the HIRD with compensated cirrhosis, 5,806 (23.9%) had HCV, 5,708 (23.5%) had ALD, and 3,521 (14.5%) had possible NASH (based on having no specific liver diagnosis in the setting of a evidence for a medical or metabolic risk factor for NASH). An additional 6,731 (27.8%) of patients with compensated cirrhosis had no identifying etiology of cirrhosis (cryptogenic cirrhosis), and no medical co-morbidities that allowed us to say the patient had possible NASH with a high degree of confidence. Among the 14,971 patients with chronic liver failure, 4,007 (26.8%) had HCV, 6,469 (43.2%) had ALD, and 2,474 (16.5%) had presumed NASH. Among the 1,853 patients with cirrhosis and HCC, 1,138 (61.4%) had HCV, 329 (17.8%) had ALD, and 187 (10.1%) had presumed NASH. There were temporal changes in the distribution of these diagnoses within each category: 1) compensated cirrhosis—decrease in the percentage with HCV and ALD in the face of increasing NASH (Figure 1a); 2) CLF—decreased HCV and ALD with a near tripling in the percentage of these patients having NASH to the point that the percentage with HCV and presume NASH cross in calendar year 2014 (Figure 1b); 3) HCC—decreases in the percentage with HCV and ALD and a small increase with NASH (Figure 1c).
Figure 1 (three panels).
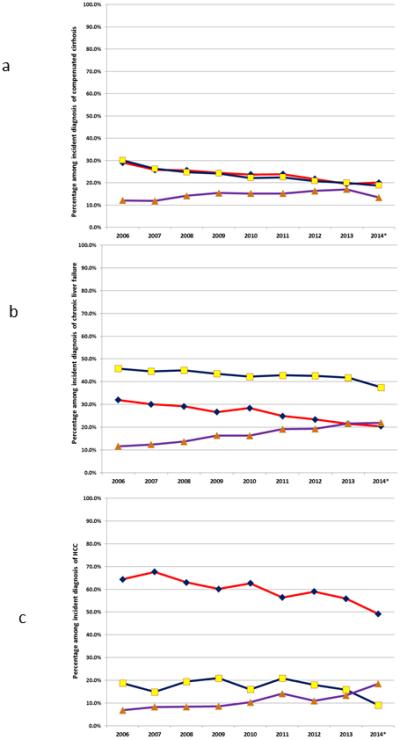
a. Figure 1a: Diagnosis distribution among patients with an incident diagnosis of compensated cirrhosis in HealthCore, 2006-2014
b. Figure 1b: Diagnosis distribution among patients with an incident diagnosis of chronic liver failure in HealthCore, 2006-2014
c. Figure 1v: Diagnosis distribution among patients with an incident diagnosis of hepatocellular carcinoma in HealthCore, 2006-2014
d. Figure legend
e. Figure footnote: *data through June 30, 2014
Waitlist and transplant data
We evaluated the final pathway in the continuum from chronic liver disease to CLF and HCC severe enough to require a transplant using OPTN data from February 27, 2002 through December 31, 2015. During this period, there was an absolute increase in the number of new waitlistings for LT and the number of liver transplants. Among new waitlistings with CLF, the absolute number with HCV was stable between 2004 and 2012, and then precipitously dropped over the next three calendar years (Figure 2a). At the same time, there was a progressive, continued increase in the absolute number of new waitlistings with alcoholic liver disease and NASH/cryptogenic cirrhosis. By contrast, the absolute number of new waitlistings for HCC increased dramatically for patients with HCV between 2002 and 2014, and only increased modestly for other causes of liver disease (Figure 2b).
Figure 2 (four panels).
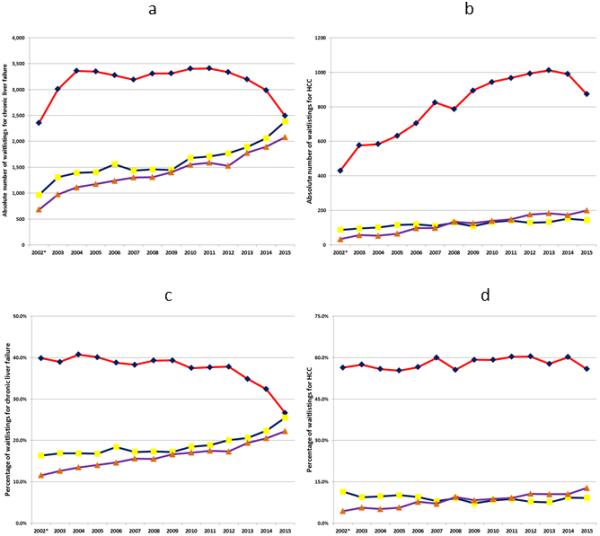
a. Figure 2a: Absolute number of new waitlistings for chronic liver failure in the US by etiology of liver disease, 2002-2015
b. Figure 2b: Absolute number of new waitlistings for hepatocellular carcinoma in the US by etiology of liver disease, 2002-2015
c. Figure 2c: Percentage of new waitlistings for chronic liver failure in the US by etiology of liver disease, 2002-2015
d. Figure 2d: Percentage of new waitlistings for hepatocellular carcinoma in the US by etiology of liver disease, 2002-2015
e. Figure legend
f. Figure footnote: *data began on February 27, 2002
There was a significant difference in the percentage of new waitlistings for CLF who had HCV across the 14 calendar years (p<0.001; Table 1, Figure 2c). This manifested in a significant decrease in the percentage of new waitlistings with HCV among patients with CLF in all three statistical tests of trends (Table 1). By contrast, among patients with HCC, despite there being a statistically significant difference in the percentage with HCV across the study period (p=0.008), there was no statistical trend in the percentage of new HCC waitlistings with HCV relative to increasing calendar year (Table 1; Figure 2d).
Table 1.
Trend analyses for new waitlistings and liver transplants in the US from 2003-2015
| Initial waitlistings | Transplant recipients | |||
|---|---|---|---|---|
| % with HCV among CLF waitlistings | % with HCV among HCC waitlistings | % with HCV among CLF transplant recipients | % with HCV among HCC transplant recipients | |
| Chi-square test | p<0.001 | p=0.008 | p<0.001 | p=0.20 |
| Spearman rank correlation | ρ=−0.86; p<0.001 | ρ=0.42; p=0.13 | ρ=−0.80; p<0.001 | ρ=0.73; p=0.003 |
| Non-parametric test of trends | p=0.002 | p=0.13 | p=0.004 | p=0.009 |
| Linear regression | β=−0.0071; p=0.001 | β =0.0021; p=10 | β=−0.0067; p<0.001 | β =0.0027; p=0.008 |
There was a rise in the absolute number of transplants for CLF in patients with HCV from 2002-2006, with a progressive decrease in the absolute number from 2007-2015 (Figure 3a). This decrease between 2007 and 2015 corresponded to an increase in the absolute number of liver transplants for CLF in patients with alcoholic liver disease and NASH/cryptogenic cirrhosis. Among transplant recipients with HCC, there was a steady linear increase in the absolute number of transplants for HCC among patients with HCC, with a small increase in the absolute number among the other three diagnostic categories (Figure 3b).
Figure 3 (four panels).
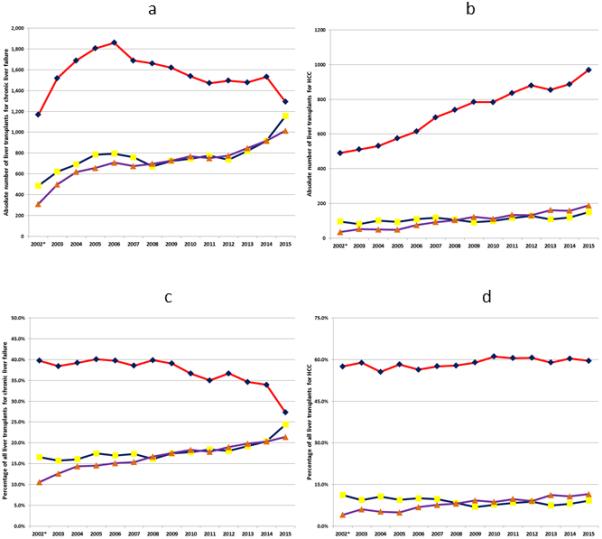
a. Figure 3a: Absolute number of liver transplants for chronic liver failure in the US by etiology of liver disease, 2002-2015
b. Figure 3b: Absolute number of liver transplants for hepatocellular carcinoma in the US by etiology of liver disease, 2002-2015
c. Figure 3c: Percentage of liver transplants for chronic liver failure in the US by etiology of liver disease, 2002-2015
d. Figure 3d: Percentage of liver transplants for hepatocellular carcinoma in the US by etiology of liver disease, 2002-2015
e. Figure legend
f. Figure footnote: *data began on February 27, 2002
There was a significant difference in the percentage of HCC transplant recipients with HCV across the study period (p<0.001; Table 1, Figure 3c). Over the study period, all three tests of trends demonstrated a significant decrease in the percentage of CLF transplant recipients with HCV (Table 1). By contrast, among HCC transplant recipients, there was not a statistically significant difference in the percentage with HCV across the study period (p=0.20). Despite this, there was a statistically significant trend over time, with an increase in the percentage of HCC transplant recipients with HCV relative to increasing calendar year (Table 1; Figure 3d).
Utilization of livers from HCV-positive donors
Over the last five years, there has been an increasing number of deceased donors with HCV (increased from 319 in 2011 to 530 in 2015 according to OPTN/UNOS data). Despite the decreased proportion of transplants for CLF among patients with HCV, the continued increase in HCV-related HCC has not decreased utilization of livers from these HCV-positive donors (196 [61.4%) HCV-positive donor livers used in 2011 compared to 399 [75.3%] in 2015). In 2011, 60.9% of HCV-positive livers were used in HCV-positive patients with CLF, compared to 56.9% in 2015.
New prescriptions for HCV therapy in Medicare beneficiaries
Data on the total number of prescriptions for DAAs in the broader population are not available, and are proprietary data by each drug manufacturer. However, there are publicly available data on the number of prescriptions for all medications for Medicare beneficiaries with Part D prescription coverage (approximately 70% of Medicare beneficiaries). These data demonstrate that between 2011 and 2013, the number of HCV prescriptions was stable; yet between 2013 and 2015 the number of prescriptions for DAAs increased by a factor of 5, which correlated with the time period that there were decreasing numbers of patients waitlisted and transplanted for chronic liver failure in the setting of HCV (Supplementary Figure 1).
Discussion
By using three distinct databases, we have demonstrated dramatically changing trends in etiologies of chronic liver disease and chronic liver failure, which are associated with profound changes in the frequency of HCV, NASH and alcoholic liver disease as indications for liver transplantation. The NHANES data clearly demonstrates that the burden of active HCV infection has decreased since the introduction of highly efficacious DAA therapy. Concurrent to advances in HCV treatment that allow for high cure rates, even in the setting of cirrhosis, is reflected by a decreased incidence of cirrhosis related to HCV (compensated or decompensated) in the broader population with chronic liver disease. Simultaneous to the decrease in the burden of cirrhosis from HCV, there has been an increase in the burden related to NASH to a degree almost as great as the decline for HCV, occurring in parallel with the well described increase in prevalence of obesity. Finally, when waitlisting and transplantation data were evaluated, there was a decreased burden of CLF due to HCV, which mirrors the data on the decreased prevalence of chronic HCV in the NHANES data and cirrhosis and CLF from HCV in the HIRD. At the same time, HCC waitlistings related to HCV increased, which likely reflects that even when patients with HCV and cirrhosis are treated, the benefit on HCC is more delayed. Parallel to the changes in HCV in the cirrhotic population, we saw increased waitlistings and transplants for NASH, which reflects the increasing burden of NASH in the broader population with compensated cirrhosis and CLF. For reasons that are less clear, the frequency of liver transplantation for ALD has increased even more rapidly than that for NASH. This is even more striking because among cirrhotic patients with Medicaid or commercial health insurance, those with ALD are significantly less likely to be waitlisted than patients with HCV.21 This fact, as discussed below, likely reflects changes in behavior and attitudes with respect to waitlisting and transplant for patients with ALD. All of these emerging trends have changed the face of chronic liver disease, cirrhosis, CLF, and liver transplantation in the US.
Chronic liver disease due to HCV, NASH, or ALD occurs over many years, with continued liver damage and inflammation leading to fibrosis and progressive liver failure. For patients with ALD, there is the potential for rapid improvement in liver function with alcohol cessation, even in the setting of advanced fibrosis or cirrhosis. For HCV and NASH, it is clear that fibrosis can regress in the early stages of disease, but improvements in liver function are more modest in the presence of advanced disease. For these reasons, interventions to prevent disease before cirrhosis has developed are critical. Given the high costs of DAA therapy, therapy with DAAs was initially targeted towards patients with advanced fibrosis/cirrhosis to prevent disease progression and decompensated liver disease.28 Over time, patients at both ends of the spectrum (early-stage disease and chronic liver failure) have been considered for therapy. These data would suggest that with the advent of improved HCV treatments, the burden of HCV has decreased (NHANES data), leading to fewer patients with cirrhosis and/or decompensated cirrhosis (HIRD), and ultimately, fewer patients waitlisted and/or transplanted for chronic liver failure due to HCV (UNOS data). For these reasons, continuing efforts to diagnose HCV in those who are unaware of their diagnoses, and treating their HCV upon diagnosis are needed. An element of reversibility of severity of liver disease following successful DAA therapy in patients with decompensated liver disease due to HCV infection has been demonstrated recently in large, prospective studies.29,30 More than 75% of patients with decompensated cirrhosis who achieve an SVR experience a decline in MELD and CTP within the first three months following completion of DAA therapy. It is thus plausible that some part of the dramatic decline in wait listing for and transplantation for liver failure secondary to HCV is on the basis of treating patients with more advanced liver disease. 29,30
Changes in waitlistings and transplants for HCC have been much more modest than those observed for liver failure secondary to chronic HCV infection. This is not unexpected, because hepatic fibrosis regression occurs over a process of many years and as the need for liver transplantation due to HCC is independent of intrinsic liver disease, such as indicated by the calculated MELD score. This underscores the fact that even if HCV is cured, continued HCC surveillance is needed in patients with HCV cirrhosis. Furthermore, even though advances in HCV treatment, even in the setting of cirrhosis, will be expected to continue to decrease the need for transplant for chronic liver failure due to HCV, we should not expect HCV to disappear as a major indication for transplant until efforts continue to focus on treating patients with HCV before advanced fibrosis has developed. It is worth noting that although the proportion of patients with HCC who had HCV in the HIRD cohort decreased over time, the proportion waitlisted for HCC in the setting of HCC increased. The data do not allow us to determine the exact reason for this, but it may simply be due to the fact that the relative proportion of HCCs due to HCV are so much higher, in the setting of where these patients are preferentially listed, that more patients with HCV are waitlisted. Second, it also may reflect the small sample size of HCC patients in HIRD on a year-to-year basis. Third, it may be that there is a lag between HCC diagnosis and subsequently being placed on the waitlist such that the changes in HCC listings relative to HCV may lag behind that in the general cirrhotic population.
There have been several previous reports describing the epidemic of NASH in the US. The increased burden of NASH in the overall population is reflected by the increasing percentage of patients with cirrhosis (compensated or decompensated cirrhosis in the HIRD) due to NASH. Not surprisingly, this has translated to an increased proportion of new waitlistings for liver transplant, and actual transplants in the setting of NASH (although this may reflect greater awareness and labeling of NASH, it does reflect the rising prevalence of obesity and NASH in the general population). These population and waitlisting trends are all consistent with one another. The same cannot be said for ALD. In the broader population (HIRD), the percentage of patients with compensated cirrhosis, decompensated cirrhosis, or HCC who have ALD has decreased over time. As a result, one would have expected fewer waitlistings and transplants due to ALD. However, this was not the case. The percentage of new waitlistings and transplants for ALD has mirrored that of NASH, despite a decreasing overall prevalence in the general population. This speaks to changes in selection criteria for waitlisting and transplanting patients with ALD, rather than a growing burden of ALD. We do not feel these changes in waitlist reflect broader temporal trends in improvement in alcohol abstinence rates. Although there was a highly publicized randomized controlled trial of baclofen therapy as a pharmacological means to cause sustained abstinence,31 this has not led to widespread use. Furthermore, there are data from the transplant field that alcohol abstinence and recidivism rates are unchanged, with as many as 25% of waitlisted patients with alcohol-induced liver disease continuing to drinking while waitlisted.32,33 Lastly, there have been other failed clinical trials for medications such as selective serotonin reuptake inhibitors (i.e., citalopram) to help with alcohol abstinence,34 which all together would suggest changes in waitlisting for ALD reflect selection criteria for waitlisting rather than changes in abstinence rates for ALD.
This study has limitations. First, the NHANES data only captured HCV prevalence in a random subset of the US population, excluding populations that may be enriched with HCV without access to care (i.e., homeless, incarcerated). This may lead to an underestimate of the burden of HCV, but this sampling in NHANES has been constant over time so would not explain the temporal changes we noted. Furthermore, the NHANES data does not include patients with NASH and/or ALD. Second, the data on the broader population with cirrhosis was based on patients with commercial health insurance, and thus may not generalize to patients with government-sponsored health insurance.
In conclusion, this analysis of multiple different datasets highlights novel trends in the burden of chronic liver disease. Chronic HCV seems to be decreasing with the advent of new therapies, which is manifesting as a lower burden of cirrhosis and chronic liver failure among waitlist additions and new transplant recipients. At the same time, NASH and ALD are becoming relatively more common among waitlisted patients and transplant recipients, despite different relative burdens of disease in the broader cirrhotic population.
Supplementary Material
Acknowledgments
Grant Support
1. Dr. Goldberg was funded by the National Institutes of Health (K08-DK098272).
2. This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C.
Abbreviations
- HCV
Hepatitis C virus
- CLF
Chronic liver failure
- LT
Liver transplantation
- HCC
Hepatocellular carcinoma
- FDA
Food and Drug Administration
- DAA
Direct-acting antiviral agents
- CTP
Child-Turcotte-Pugh
- MELD
Model for End-Stage Liver Disease
- NAFLD
Non-alcoholic fatty liver disease
- ALD
Alcohol-related liver disease
- NHANES
National Health and Nutrition Evaluation Survey
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- OPTN
Organ Procurement and Transplantation Network
- UNOS
United Network for Organ Sharing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None of the authors have any relevant financial, professional, and/or personal conflicts of interest with respect to this manuscript.
Author Contributions
David Goldberg: Study concept and design, acquisition of data, analysis and interpretation of the data, drafting of the manuscript, drafting and critical revision of the manuscript, statistical analysis
Ivo C. Ditah: Study concept and design, acquisition of data analysis and interpretation of the data, critical revision of the manuscript, statistical analysis
Kia Saeian: Analysis and interpretation of the data, statistical analysis
Mona Lalehzari: Analysis and interpretation of the data, statistical analysis
Andrew Aronsohn: Study concept and design, acquisition of data, critical revision of the manuscript
Emmanuel C. Gorospe: Study concept and design, acquisition of data, critical revision of the manuscript
Michael Charlton: Study concept and design, acquisition of data, analysis and interpretation of the data, drafting of the manuscript, drafting and critical revision of the manuscript
References
- 1.Ditah I, Ditah F, Devaki P, et al. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol. 2014;60(4):691–698. doi: 10.1016/j.jhep.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13(17):2436–2441. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 4.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36(5 Suppl 1):S35–46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 5. [December 9, 2014];FDA Approves Telaprevir for HCV. http://www.medscape.com/viewarticle/743192.
- 6.FDA News Release: FDA approves Victrelis for Hepatitis C.
- 7. [December 9, 2014];FDA News Release: FDA approves Sovaldi for chronic hepatitis C. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm377888.htm.
- 8. [December 9, 2014];FDA News Release: FDA approves new treatment for hepatitis C virus. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm376449.htm.
- 9.Fried MW, Buti M, Dore GJ, et al. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naive genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58(6):1918–1929. doi: 10.1002/hep.26641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. The New England journal of medicine. 2013;368(20):1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 11.Manns M, Marcellin P, Poordad F, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384(9941):414–426. doi: 10.1016/S0140-6736(14)60538-9. [DOI] [PubMed] [Google Scholar]
- 12.Charlton M, Gane E, Manns MP, et al. Sofosbuvir and Ribavirin for Treatment of Compensated Recurrent Hepatitis C Virus Infection After Liver Transplantation. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Curry MP, Forns X, Chung RT, et al. Sofosbuvir and Ribavirin Prevent Recurrence of HCV Infection After Liver Transplantation: An Open-Label Study. Gastroenterology. 2014 doi: 10.1053/j.gastro.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 14.Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001-2013. Gastroenterology. 2015;149(6):1471–1482. e1475. doi: 10.1053/j.gastro.2015.07.056. quiz e1417-1478. [DOI] [PubMed] [Google Scholar]
- 15.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 16.Setiawan VW, Stram DO, Porcel J, Lu SC, Le Marchand L, Noureddin M. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: The multiethnic cohort. Hepatology (Baltimore, Md.) 2016 doi: 10.1002/hep.28677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg D, French B, Newcomb C, et al. Patients With Hepatocellular Carcinoma Have Highest Rates of Wait-listing for Liver Transplantation Among Patients With End-Stage Liver Disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2016;14(11):1638–1646. e1632. doi: 10.1016/j.cgh.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezzati TM, Massey JT, Waksberg J, Chu A, Maurer KR. Sample design: Third National Health and Nutrition Examination Survey. Vital Health Stat 2. 1992;(113):1–35. [PubMed] [Google Scholar]
- 19.Schelleman H, Bilker WB, Strom BL, et al. Cardiovascular events and death in children exposed and unexposed to ADHD agents. Pediatrics. 2011;127(6):1102–1110. doi: 10.1542/peds.2010-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Platt R, Wilson M, Chan KA, Benner JS, Marchibroda J, McClellan M. The new Sentinel Network--improving the evidence of medical-product safety. The New England journal of medicine. 2009;361(7):645–647. doi: 10.1056/NEJMp0905338. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg D, French B, Newcomb C, et al. Patients With Hepatocellular Carcinoma Have Highest Rates of Wait-listing for Liver Transplantation Among Patients With End-Stage Liver Disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2016 doi: 10.1016/j.cgh.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldberg DS, French B, Sahota G, Wallace AE, Lewis JD, Halpern SD. Use of Population-Based Data to Demonstrate How Waitlist-Based Metrics Overestimate Geographic Disparities in Access to Liver Transplant Care. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016 doi: 10.1111/ajt.13820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldberg DS, Lewis JD, Halpern SD, Weiner MG, Lo Re V., 3rd. Validation of a coding algorithm to identify patients with hepatocellular carcinoma in an administrative database. Pharmacoepidemiology and drug safety. 2013;22(1):103–107. doi: 10.1002/pds.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldberg D, Lewis J, Halpern S, Weiner M, Lo Re V., 3rd. Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiology and drug safety. 2012;21(7):765–769. doi: 10.1002/pds.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nehra MS, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol. 2013;47(5):e50–54. doi: 10.1097/MCG.0b013e3182688d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lo Re V, 3rd, Lim JK, Goetz MB, et al. Validity of diagnostic codes and liver-related laboratory abnormalities to identify hepatic decompensation events in the Veterans Aging Cohort Study. Pharmacoepidemiol Drug Saf. 2011;20(7):689–699. doi: 10.1002/pds.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27(3):274–282. doi: 10.1111/j.1365-2036.2007.03572.x. [DOI] [PubMed] [Google Scholar]
- 28.Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology (Baltimore, Md.) 2015;62(3):932–954. doi: 10.1002/hep.27950. [DOI] [PubMed] [Google Scholar]
- 29.Charlton M, Everson GT, Flamm SL, et al. Ledipasvir and Sofosbuvir Plus Ribavirin for Treatment of HCV Infection in Patients With Advanced Liver Disease. Gastroenterology. 2015;149(3):649–659. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Curry MP, O'Leary JG, Bzowej N, et al. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis. The New England journal of medicine. 2015;373(27):2618–2628. doi: 10.1056/NEJMoa1512614. [DOI] [PubMed] [Google Scholar]
- 31.Addolorato G, Leggio L, Ferrulli A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370(9603):1915–1922. doi: 10.1016/S0140-6736(07)61814-5. [DOI] [PubMed] [Google Scholar]
- 32.Weinrieb RM, Van Horn DH, Lynch KG, Lucey MR. A randomized, controlled study of treatment for alcohol dependence in patients awaiting liver transplantation. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2011;17(5):539–547. doi: 10.1002/lt.22259. [DOI] [PubMed] [Google Scholar]
- 33.Weinrieb RM, Lucey MR. Treatment of addictive behaviors in liver transplant patients. 13. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2007;(11 Suppl 2):S79–82. doi: 10.1002/lt.21340. [DOI] [PubMed] [Google Scholar]
- 34.Charney DA, Heath LM, Zikos E, Palacios-Boix J, Gill KJ. Poorer Drinking Outcomes with Citalopram Treatment for Alcohol Dependence: A Randomized, Double-Blind, Placebo-Controlled Trial. Alcoholism, clinical and experimental research. 2015;39(9):1756–1765. doi: 10.1111/acer.12802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





