Abstract
Elevated vertebral bone marrow fat (BMF) among individuals with osteoporosis has been established in histomorphometric studies. Several studies have found a negative correlation between BMF and bone mineral density (BMD) at the spine in men and women across different age groups. Animal studies have also observed bone loss with increased BMF in mice with induced diabetes. Our study objective was to test the hypothesis that the association between BMF and BMD varies by diabetic status. We performed a cross-sectional study of 156 men aged 74–96 years from the Osteoporotic Fractures in Men study (MrOS) at the Pittsburgh clinical site. All men had spine BMF scans using proton magnetic resonance spectroscopy and spine and hip BMD scans by dual-energy x-ray absorptiometry. BMF was expressed as lipid to “lipid + water” ratio (%). Men were considered diabetic if they self-reported a physician diagnosis of diabetes, diabetes medication or had a fasting glucose ≥ 126mg/dl. Men with diabetes (n=38) had a significantly higher spine BMF (58.9 vs. 54.6 %, p=0.0035), spine BMD (1.20 vs. 1.10 g/cm2, P=0.007)) and total hip BMD (0.99 vs. 0.94 g/cm2, p=0.04) than those without, while no differences were observed for body weight, body mass index or waist circumference. Pearson correlation tests showed no significant correlation of spine BMF with age or BMD in non-diabetics. Significant inverse correlations were observed between BMF and BMD (−0.30 for femoral neck and −0.39 for total hip) among diabetic men. In conclusion, men with diabetes had a higher BMF compared to non-diabetic men. The correlation between BMF and BMD differed by diabetes status. Further investigation of the association of diabetes with BMF and BMD may provide a better understanding of the high fracture rates among individuals with diabetes despite their higher BMD.
Keywords: bone-fat interactions, general population studies, DXA
Introduction
Osteoblasts and adipocytes share common precursors, mesenchymal stem cells (MSC), and the differentiation of MSC occurs in the bone marrow. With advancing age, hematopoietic marrow is progressively replaced by fatty marrow with a higher conversion rate into fatty marrow in the bone cavity of the appendicular skeleton [1]. In the past decade, studies have begun to evaluate the bone-fat association targeting bone marrow fat (BMF) of the lumbar spine and femur. Histomorphometric studies performed on iliac crest biopsies demonstrated not only a positive correlation between BMF and age, but also an elevated BMF level in persons with osteoporosis or low trabecular bone volume [2, 3]. Advancements in imaging technology have enabled researchers to non-invasively quantify BMF using proton magnetic resonance spectroscopy (1H-MRS) [4–10]. Results from 1H-MRS studies have shown a positive association between BMF and age in both sexes [6, 7, 9] as well as higher BMF content among individuals with osteoporosis than those without [4–6, 10]. However, the linear association between BMF with bone mineral density (BMD) is not well understood. Several studies have reported a significant and negative correlation of BMF with areal BMD [4–6, 10, 11], while another study showed no correlation once age was accounted for [7].
Bone marrow fat has also been linked to higher prevalence of vertebral fractures [3, 9, 12, 13]. Bone loss and increased BMF have been observed in mice with induced diabetes [14–16], but much less is known about this association in humans. In a small study of sixteen adults with type 1 diabetes and 12 controls, there was no differences in BMF by diabetes status [17]. However, there was a negative correlation between femur BMF and both femoral neck and total hip BMD in all subjects [17]. Patsch et al showed that diabetics with a prevalent fracture had lower unsaturated fat and higher saturated fat than both non-diabetic controls and diabetics without a fracture [18]. Subjects with type 2 diabetes are at increased risk of fracture despite their high BMD[19, 20]. Many mechanisms have been proposed, but they explain only a fraction of this paradoxical relationship [19, 20]. Further research on the interrelationships between fat, diabetes and skeletal health may improve our understanding of the underlying mechanisms whereby diabetics are at an increased risk of fracture. Our current cross-sectional study aimed to examine the relationship of BMF with hip and spine BMD and to determine whether diabetic status influences this association among men enrolled in the Osteoporotic Fractures in Men study (MrOS).
Materials and Methods
Study population
Participants in this study were recruited from the Pittsburgh site of the Osteoporotic Fractures in Men (MrOS) study. The MrOS study is a prospective cohort study designed to identify risk factors associated with osteoporosis and osteoporotic fractures in older men. From March 2000 to April 2002, 5,994 older men were recruited from 6 sites across the US (Birmingham, AL, Minneapolis, MN, Palo Alto, CA, Pittsburgh, PA, Portland, OR, and San Diego, CA). Recruitment was accomplished primarily through targeted mailings based on motor vehicle registration, voter registration, and the Veterans Administration database. To be eligible for the MrOS study, men needed to be age 65 years and older, be able to walk without assistance from another person, and have had no bilateral hip replacement. Details of the study have been published [21, 22].
Between December 2009 and December 2010, recruitment for the BMF ancillary study took place at the Pittsburgh clinic with a goal of 150 participants. This sample size of 150 men provided sufficient power to detect a minimum correlation of 0.23 with 80 % power and two sided alpha of 0.05. Men were eligible for the BMF study if they had no implants that are contraindicated for the magnetic resonance (MR) examination. Implants that might create a health risk or other problem during an MR exam include: 1) cardiac pacemaker or implantable defibrillator, 2) catheter that has metal components that may pose a risk of a burn injury, 3) a ferromagnetic metal clip placed to prevent bleeding from an intracranial aneurysm, 4) an implanted medication pump (such as that used to deliver insulin or a pain-relieving drug), and 5) a cochlear implant. At the beginning of the BMF study recruitment, the Pittsburgh MrOS clinic had 745 active (74%), 222 deceased (22%), and 38 withdrawn (4%) participants. Of 745 active participants, 53 men were not able to participate in clinic visits because they lived out–of-the-area, agreed to post card follow-up only, or were in the care of someone else. The recruitment goals were reached after a total of 364 men were contacted for participation in this study. Among them, 130 were ineligible for MR (36%), 73 refused to participate (20%), and 159 (44%) completed the BMF study. We excluded two scans that were corrupted during file transfer and one scan with extremely low BMF likely due to treatment of the individual’s illness. Therefore, our analytical sample size for this study was 156. All men had dual energy x-ray absorptiometry (DXA) scans for BMD, and a clinical interview/examination for anthropometric measurements, medical history, and lifestyle information. Fasting blood samples were collected for all participants. The institutional review board at the University of Pittsburgh approved the study protocol. All participants gave written informed consent.
Proton magnetic resonance spectroscopy (1H-MRS)
Acquiring BMF at the peripheral skeletal sites was not suitable for our study population due to the lack of fatty and hematopoietic marrow conversion in long bones at this age. Therefore, BMF content was quantified at three vertebral levels of the lumbar spine. 1H-MRS was performed on two Trio Siemens (3.0 Tesla) MRI scanners equipped with multi-channel reception capabilities. Subjects were positioned head first in the magnet bore in the supine position with their lumbar region over the spine matrix coil. After a triplane localizer sequence, a voxel measuring 15×15×20 mm was placed within the body of L1, L2 and L3. Single voxel 1H-MRS data was then acquired using point-resolved spatially localized spectroscopy (PRESS) pulse sequence with the following parameters: TE 37 msec., TR 3000 msec., 16 averages, 1024 data points and receiver bandwidth of 2000 Hz. Fitting of the spectra was done with jMRUI software. Water and fat peaks were identified based on published prior knowledge with water at ~ 4.6 ppm and methylene at~ 1.3 ppm [1]. Fitting parameters included line width, amplitude and shift of each peak. Fat content was defined as percent total fat over the sum of total fat and water: Fat content = (Ifat)/(Ifat+ Iwater) * 100% [23].
Fat content was measured from each vertebra from L1 to L3. As the BMF of each vertebra was strongly associated with each other (L1 vs L2, r=0.86, p<0.0001; L2 vs. L3, r=0.85, p<0.0001; L1 vs. L3, r=0.82, p<0.0001), the average fat content across all 3 levels was computed. When one value was missing due to vertebrae inhomogeneity or poor fitting (8 missing for L1, 2 for L2, 3 for L3 out of the 156 subjects), the mean was computed on the other 2 levels. A test-retest trial was performed prior to recruitment to ensure scan quality within and between the two MR machines. Volunteers were scanned twice at each machine and were repositioned between the first and second set of scans by the same technician. The mean coefficient of variation (CV) for 15 pairs of vertebral BMF was 3.8% with a Pearson correlation coefficient of 0.98. The intra-scanner CV (between the 2 MR machines) was 6.3% with a Pearson correlation of 0.99.
Bone mineral density
Areal BMD of the lumbar spine, total hip and femoral neck were measured using dual-energy X-ray absorptiometry (DXA) (QDR 4500W, Hologic Inc., New Bedford, MA). All measurements of hip BMD were made on the right hip, unless the participant reported a right hip replacement or metal objects in the right leg, in which case the left hip was measured. Extensive quality assurance protocols were used throughout the study including central training and certification of technicians and regular phantom scans within and across centers.
Volumetric BMD
Volumetric BMD was measured using peripheral quantitative computed tomography (pQCT). A pQCT scan of the radius and tibia was performed using the Stratec XCT-2000 (Stratec Medizintechnik, Pforzheim, Germany). Trained technicians followed a standardized protocol for subject positioning and scanning. A scout view was obtained prior to the pQCT scan to define an anatomic reference line for the relative location of the subsequent scans at the radius and tibia. Tibia length was determined from the medial malleolus to the medial condyle of the tibia, and forearm length was determined from the olecranon to the ulna styloid process. Scans were taken at four different sites: 4% and 33% of the total length of radius and tibia. The scans at the 4% radius and tibia sites represent predominantly trabecular bone, whereas the scans at the 33% site represent predominantly cortical bone. A single axial slice of 2.5-mm thickness with a voxel size of 0.5 mm and a speed of 20 mm/s was taken at all locations. Image processing was performed by a single investigator using the Stratec software package (version 5.5E). Daily phantom scans were analyzed to ensure long-term scanner stability.
Laboratory assays
Serum specimens were collected after an overnight fast and stored at −70°C until assay. Leptin, adiponectin, insulin and glucose were measured in the Heinz Nutrition Laboratory at the Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh. Leptin, adiponectin and insulin were measured using RIA developed by Linco Research, Inc. (St. Charles, MO). For leptin, a complete set of blank, standards (0.5 ng/ml to 100 ng/ml) and quality controls were run with each assay, and the intrassay and interassay CV were 6.6 ± 0.8% and 5.5 ± 0.9%, respectively. The CV for adiponectin and insulin were 8.0% and 2.6%, respectively. Serum glucose was quantitatively determined by an enzymatic method in a procedure similar to that described by Bondar and Mead [24] and utilized the coupled enzyme reactions catalyzed by hexokinase and glucose-6-phosphate dehydrogenase. The coefficient of variation was 1.8%.
Other measurements
All participants completed a self-administered questionnaire, which included self-reported medical history, health behaviors, and medication inventory. Height was measured on a wall-mounted Harpenden stadiometer, and weight was measured on a standard balance beam scale using standard protocols. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2). Waist circumference was measured at the level of the umbilicus. In the current study, diabetes was defined by self-reported physician’s diagnosis of the disease, use of diabetes medication(s) or a fasting plasma glucose level ≥126 mg/dl.
Statistical analysis
Characteristics of study participants by diabetic status were compared using two-sample t-tests for normally distributed continuous variables, Wilcoxon rank sum test for non-normally distributed continuous variables, and chi-squared test for categorical variables. Since we observed a higher level of BMF among men with diabetes than those without, analysis of covariance (ANCOVA) was used to examine whether the difference in BMF was independent of confounders. Age, race, and BMI were included in our base model. Leptin, adiponectin, or insulin concentrations were added to the base model individually to examine whether adjustment for these attenuates the difference in BMF by diabetic status. The associations of BMF with age and BMD were first evaluated using the Pearson correlation tests. A separate Pearson correlation test was done with stratification for diabetic status. We further used linear regression models to examine the association between BMF and BMD adjusting for age, race, BMI. The interaction between BMF and diabetes was also examined. All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
The overall distribution of BMF is shown in Figure 1. The mean (±SD) BMF was 55.7 ± 11.0 (%) (range: 23.5% to 76.4%) among 156 men aged between 74 and 96 years old (mean age: 80.5 ± 5.4 years), Table 1. There were only three men of African descent. The prevalence of diabetes was 24% (n=38). Although body weight, BMI and waist circumference appeared to be higher for men with diabetes, there was no significant difference compared to men without diabetes. There was no differences in smoking, alcohol intake, total calcium intake (diet plus supplements), history of rheumatoid arthritis or use of osteoporosis medication or oral corticosteroids between diabetic and non-diabetic men. Men with diabetes had a significantly higher BMF than those without (58.9% vs. 54.6%), p=0.035. This difference was slightly greater than 1/3 of one standard deviation. Compared to the non-diabetic men, diabetic men also had a significantly greater total hip, p=0.04 and lumbar spine BMD, p=0.007 but there were no differences in trabecular and cortical vBMD between diabetic and non-diabetic men. In addition, levels of insulin were significantly higher among individuals with diabetes with no difference in leptin or adiponectin.
Fig 1.
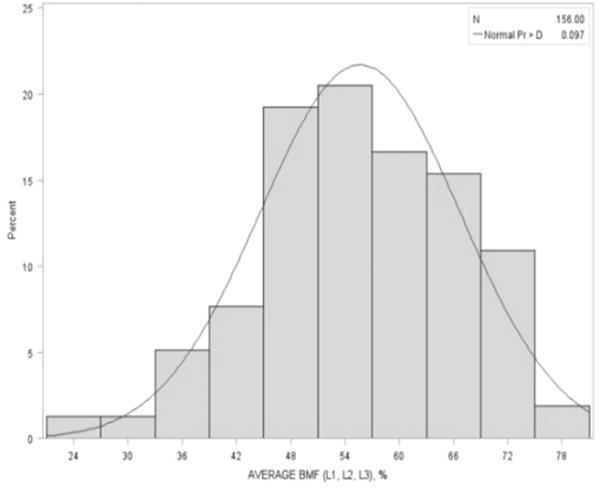
Distribution of vertebral bone marrow fat.
Table 1.
Subject characteristics: Overall and by diabetes status.
| Characteristics |
Overall (N=156) |
Diabetes (N=38) |
Non-Diabetes (N=118) |
P-valuea |
|---|---|---|---|---|
| Age | 80.5 ± 4.8 | 80.2 ± 5.4 | 80.6 ± 4.6 | 0.65 |
| African American (%) | 3 (1.9) | 1 (2.6) | 2 (1.7) | 0.57 |
| Weight (kg) | 83.8 ± 12.5 | 86.6 ± 10.4 | 82.9 ± 13.0 | 0.11 |
| BMI (kg/m2) | 28.1 ± 3.7 | 28.8 ± 3.1 | 27.9 ± 3.9 | 0.17 |
| Waist circumference (cm) | 102.8 ± 10.4 | 104.5 ± 10.4 | 102.2 ± 10.3 | 0.23 |
| Smoking | ||||
| Past | 95 (60.9) | 25 (65.8) | 70 (59.3) | 0.76 |
| Current | 4 (2.6) | 1 (2.6) | 3 (1.7) | — |
| Alcohol (≥1 drink/week) | 80 (51.6) | 19 (50.0) | 61 (52.1) | 0.82 |
| Total calcium intake (mg/d) | 1097.9 (525.9) | 1171.7 (681.1) | 1073.8 (466.1) | 0.41 |
| Rheumatoid arthritis | 6 (3.9) | 0 (0) | 6 (5.1) | 0.34 |
| Osteoporosis medication use | 7 (4.5) | 2 (5.3) | 5 (4.2) | 0.69 |
| Oral corticosteroid use | 3 (1.9) | 0 (0) | 3 (2.5) | 0.43 |
| Bone marrow fat (%) | 55.7 ± 11.0 | 58.9 ± 10.8 | 54.6 ± 10.9 | 0.035 |
| Areal BMD (g/cm2) | ||||
| Femoral neck | 0.78 ± 0.13 | 0.81 ± 0.14 | 0.77 ± 0.12 | 0.08 |
| Total hip | 0.95 ± 0.15 | 1.00 ± 0.16 | 0.94 ± 0.14 | 0.04 |
| Total spine | 1.12 ± 0.20 | 1.20 ± 0.23 | 1.10 ± 0.18 | 0.007 |
| Volumetric BMD (mg/cm3) | ||||
| Radius 4% trabecular | 195.6 ± 43.9 | 200.1 ± 47.3 | 194.3 ± 43.0 | 0.50 |
| Radius 33% cortical | 229.0 ± 37.1 | 236.6 ± 39.9 | 226.5 ± 35.9 | 0.15 |
| Tibia 4% trabecular | 1153.1 ± 38.3 | 1161.7 ± 33.7 | 1150.5 ± 39.3 | 0.14 |
| Tibia 33% cortical | 1130.7 ± 33.3 | 1137 ± 29.1 | 1128.5 ± 34.4 | 0.16 |
| Leptin (ng/ml) b | 10.7 (7.3 – 15.8) | 11.2 (8.4 – 17.4) | 10.4 (7.0 – 14.9) | 0.19 |
| Adiponectin (ug/ml) b | 10.8 (7–15.3) | 10.6 (7.0 – 16.4) | 10.9 (7.0 – 15.2) | 0.95 |
| Insulin (uU/ml) b | 14.0 (11.2 – 18.9) | 16.4 (12.5 – 23.0) | 13.3 (11.0 – 17.7) | 0.014 |
| Diabetes medication (%) | 21 (13.5) | 21 (55.3) | 0 (0.0) | <0.0001 |
| Metformin (%) | — | 10 (26.3) | — | — |
| Thiazolidinedione (%) | — | 2 (5.3) | — | — |
| Sulfonylureas (%) | — | 9 (23.27) | — | — |
Values are unadjusted mean ± SD or frequency (%), unless indicated otherwise;
P-values are for comparison of characteristics between diabetic and non-diabetic groups
Values are median (interquartile range).
To further investigate whether the difference in BMF between men with and without diabetes was independent of confounders, we compared the mean BMF between the two groups after adjusting for age, race and BMI (Table 2).
Table 2.
Adjusted means for bone marrow fat by type 2 diabetes status.
| Models | Diabetes (N = 38) |
Non-Diabetes (N = 118) |
P-value |
|---|---|---|---|
| Age | 58.96 ± 1.77 | 54.60 ± 1.01 | 0.034 |
| Age + Race | 59.19 ± 3.53 | 54.83 ± 3.26 | 0.035 |
| Base Model: Age + Race + BMI | 59.18 ± 3.54 | 54.80 ± 3.28 | 0.036 |
| Base Model + Leptin | 57.56 ± 3.55 | 53.20 ± 3.30 | 0.034 |
| Base Model + Adiponectin | 59.05 ± 3.56 | 54.72 ± 3.30 | 0.038 |
| Base Model + Insulin | 59.17 ± 3.56 | 54.80 ± 3.30 | 0.042 |
We found that BMF remained significantly higher for men with diabetes, p=0.034. Additional inclusion of potential mediators like leptin, adiponectin or insulin did not change the observed difference. We excluded the 2 men who reported thiazolidinediones (TZD) use and the BMF remained higher in those with diabetes (58.54%) compared to non-diabetics (54.61%), although the difference was borderline significant, p=0.06.
Correlations of BMF with age and skeletal parameters are presented in Table 3. There were no significant correlations between BMF and age. The correlations between BMF and BMD were not significant in the total sample. When stratified by diabetes status, BMF was inversely correlated with total hip BMD (r= −0.39, p=0.017) and femoral neck BMD (r= −0.30, p=0.064) but not spine BMD among men with diabetes but not among non-diabetics. Results from linear regression (Figure 2) showed that the negative correlation between BMF and total hip BMD was stronger in men with diabetes than those without (test for interaction between BMF and diabetes, p=0.07). There was no association between BMD and trabecular and cortical volumetric BMD overall or stratified by diabetes status.
Table 3.
Pearson correlations between bone marrow fat and age and bone mineral density in older men
| Models | Overall (N = 156) |
Diabetes (N = 38) |
Non-Diabetes (N = 118) |
|---|---|---|---|
| Age | 0.04 | 0.06 | 0.03 |
| Areal BMD(g/cm2) | |||
| Femoral neck | −0.05 | −0.30a | 0.003 |
| Total hip | −0.08 | −0.39b | −0.01 |
| Total spine | −0.08 | −0.08 | −0.13 |
| Volumetric BMD (mg/cm3) | |||
| Radius 4% trabecular | 0.08 | 0.07 | 0.07 |
| Radius 33% cortical | −0.08 | 008 | −0.06 |
| Tibia 4% trabecular | −0.07 | −0.19 | −0.14 |
| Tibia 33% cortical | −0.09 | 0.07 | −0.16 |
p < 0.10;
p < 0.05.
Fig 2.
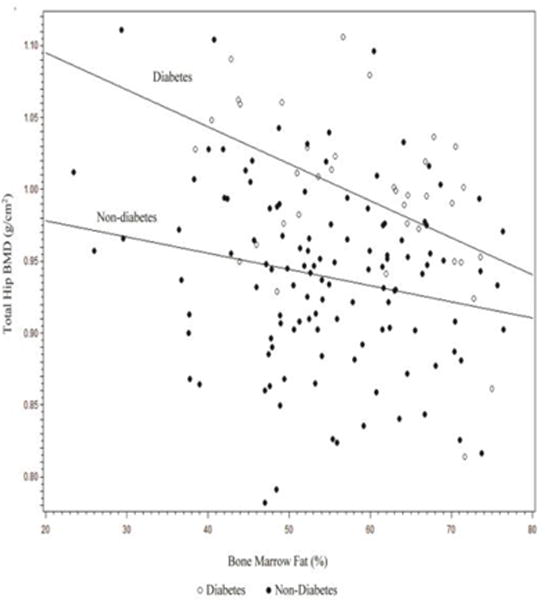
Scatterplot of the association between bone marrow fat and total hip bone mineral density stratified by diabetes status.
Discussion
Our study examined the relationship between vertebral BMF and BMD at the lumbar spine and hip measured by DXA, and whether diabetes influenced these associations. We found a higher BMF content among men with diabetes than those without. Unlike previous studies, the overall correlation between BMF and BMD in our study was weak and not statistically significant. However, when participants were stratified by type 2 diabetes status, negative and moderate correlations were found with femoral neck and total hip BMD and BMF among men with diabetes.
The use of the 1H-MRS technique enabled us to measure BMF content and evaluate its relationship with skeletal parameters. The overall BMF content in our study was similar to that reported in older Chinese men [25] and Icelandic men [12], but lower than that for postmenopausal Chinese women [25].
Studies investigating this relationship often included postmenopausal women only or both genders with a wide age range that spanned several decades. Studies by Griffith and colleagues found a higher vertebral BMF content among osteoporotic Chinese men and women aged 60 and older, when compared with those with low or normal BMD [4–6]. The BMF content in the osteoporotic group was 8%, or approximately one SD, higher than the normal BMD group [4, 5].
The majority of studies examining the BMF and BMD relationship in men were conducted in a Chinese population in Hong Kong, where a correlation of −0.21 (p=0.05) with spine BMD was found among men aged 61–90 years old [25]. Among the non-Asian population, this relationship was primarily found in women. One study reported a correlation of −0.39 between vertebral BMF and trabecular BMD among premenopausal Caucasian women [26], while another reported a correlation of −0.45 among postmenopausal Caucasian women [27]. When BMF was measured using CT at the pelvis, its correlation with pelvic, whole-body and spine BMD was between −0.41 and −0.56 for men and women aged 40–88 years old. However, the correlations were attenuated (−0.35 to −0.40) after adjusting for age, weight and body fat [11]. Although the lack of an overall association between BMF and BMD in our study is unclear, it is possible that this relationship diminishes with older age and differs in men and women. Griffin and colleagues showed that when correlations between vertebral BMF and lumbar spine BMD were stratified by age in 10 year increments among Chinese men and women aged 61 to 90 years old, the correlations were weakened and no longer statistically significant for men age ≥71 years [25].
We found no correlation between BMF and lumbar spine aBMD which may have reflected the larger proportion of trabecular bone in the spine. However, we found no correlation between radial and tibial trabecular and cortical vBMD overall or stratified by diabetic status. It is not clear why there was an association with hip aBMD but not vBMD. Future studies will need to replicate these findings.
Although results from our study may not support the inverse correlation between BMF and BMD overall as reported in previous studies, the significant and inverse correlations between BMF and hip BMD were only found among men with diabetes. A similar study design using the same MR technique reported an association between areal BMD and BMF in women but not men [12]. The lack of association with spine BMD may reflect the presence of artifact in lumbar area that elevates spine BMD in older adults such as osteoarthritis, scoliosis or aortic calcifications.
To our knowledge, our study is one of a few to compare BMF and BMD correlations by diabetes status. Baum and colleagues reported no significant difference in BMF between postmenopausal women with (n=13) and without (n=13) diabetes; however, they also found a stronger correlation between vertebral BMF and lumbar spine volumetric BMD (measured by QCT) among diabetes than controls (−0.70 vs. −0.58) [27]. Changes in BMF after gastric bypass were compared in 11 morbidly obese women by diabetic status [28]. Despite large declines in body weight in both groups, there was little change in BMF in non-diabetic women but decreased by 7.5% over 6 months in diabetic women. The authors hypothesized that the decline in BMF in diabetics may reflect better diabetes control due to their weight loss. The underlying mechanism that drives the stronger bone-fat relationship in diabetic individuals is unclear and merits further investigation.
While the positive association between type 2 diabetes and BMD has been widely reported [19, 20], the relationship between BMF and diabetes remains largely unexplored. The differentiation process of MSC into osteoblasts and adipocytes is determined by several transcriptional regulators including peroxisome proliferator activated receptor gamma 2 (PPARɤ2) [29]. A class of diabetes medications, TZD, activates PPARɤ2 and has been shown to increase adipocytes at the expense of osteoblasts in mouse models [30]. Two previously published studies reported divergent results on the association between TZDs and BMF. One study reported that the use of pioglitazone increased BMF after 6 months in both men and women [31], while another found a decrease in BMF after 14 weeks of rosiglitzone treatment among women [30]. In our study, men with diabetes had a higher BMF content than those without; however, this observation did not appear to be due to TZD use, since a sensitivity analysis, which removed two participants taking TZDs, yielded similar results. However, our lack of historical information on TZD use limits our ability to definitely exclude the possible role of TZDs in higher BMF among diabetics in our study.
The activation of PPARɤ2 is also known to increase insulin sensitivity and adipokines [29]. We also evaluated whether adipokines related to insulin sensitivity had a role of mediating the association between BMF and diabetes status. Both leptin and adiponectin are secreted by adipocytes and have been known to affect bone metabolism. Leptin is highly correlated with body fat mass [32, 33], and increases bone formation through the proliferation of osteoblasts and inhibition of osteoclasts [34]; however, its association with BMD has not been consistently reported. Positive [35–38], negative [39–41] and null [42, 43] associations have been found. Adiponectin is known to regulate insulin sensitivity, and low level of this adipocyte-producing hormone has been associated with the progression of diabetes. In addition, high adiponectin levels have been linked to lower BMD [38, 44, 45] and greater BMD loss [46, 47], and its role in stimulating the receptor activator of nuclear factor- κB ligand (RANKL) pathway and inhibiting the production of the decoy receptor for RANKL and osteoprotegerin may contribute greatly to this observation [48]. With the potential interplay of leptin, adiponectin and insulin with bone and type 2 diabetes, we further tested the effect of these factors on the observed difference in BMF content among diabetic and non-diabetic men. Results showed that the differences in BMF by diabetic status were independent of leptin, adiponectin or insulin. More studies are needed to identify other pathways and factors that might bridge the connection between BMF and diabetes.
Strengths of our study include a relatively large sample size of older men compared to previous studies and use of non-invasive techniques to measure BMF. However, several limitations must be acknowledged. The inclusion of primarily Caucasian men limits the generalization of study results to other populations, especially among those of African ancestry who have a greater prevalence and risk for diabetes. Second, our study participants were over the age of 70. Additional studies with men of African ancestry and/or younger age should be carried out to confirm the association between BMF and BMD and its interaction with diabetes. We had no information on the duration and severity of diabetes or other risk factors for osteoporosis in older men including 25-hydroxyvitamin D or prevalence of hypogonadism. Lastly, although our findings suggest an important role of diabetes on the relationship between BMF and BMD, the small number of diabetic cases limited our ability and statistical power to investigate this complex interplay.
In conclusion, we found no association between BMF and aBMD overall in the cohort. However, we found that this relationship may differ by diabetic status. Compared to men without diabetes, a higher BMF and a stronger inverse correlation between BMF and BMD at the hip were found for men with diabetes. Our findings may suggest a novel pathway of BMF in bone metabolism among subjects with diabetes. Individuals with type 2 diabetes are known to be more susceptible to fracture despite their high BMD [19]. The link between BMF and diabetes may provide insight into the paradoxical relationship among BMD, fracture and diabetes. With the increase in life expectancy and the epidemic of diabetes, understanding the underlying mechanisms that links diabetes with BMF and how it may affect fracture risk is essential. Future studies should also seek to determine whether BMF can serve as a biomarker for fracture prediction.
Highlights.
Men with diabetes had significantly higher bone marrow fat than non-diabetics.
There was no association between bone marrow fat and bone mineral density (BMD) in non-diabetic men.
Bone marrow fat was negatively correlated with BMD in diabetic men.
Further understanding of bone marrow fat, BMD and diabetes may improve our understanding of the etiology of fractures in diabetics.
Acknowledgments
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. Additional funding was obtained from NIAMS for Dr. Cauley’s grant entitled, “Epidemiologic Study of Bone Marrow Fat and Osteoporosis”, RC1 AR058162.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors state that they have no conflicts of interest.
References
- 1.Machann J, Stefan N, Schick F. (1)H MR spectroscopy of skeletal muscle, liver and bone marrow. Eur J Radiol. 2008;67(2):275–84. doi: 10.1016/j.ejrad.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J Clin Pathol. 2002;55(9):693–8. doi: 10.1136/jcp.55.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2(3):165–71. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- 4.Griffith JF, Yeung DK, Antonio GE, Lee FK, Hong AW, Wong SY, Lau EM, Leung PC. Vertebral bone mineral density, marrow perfusion, and fat content in healthy men and men with osteoporosis: dynamic contrast-enhanced MR imaging and MR spectroscopy. Radiology. 2005;236(3):945–51. doi: 10.1148/radiol.2363041425. [DOI] [PubMed] [Google Scholar]
- 5.Griffith JF, Yeung DK, Antonio GE, Wong SY, Kwok TC, Woo J, Leung PC. Vertebral marrow fat content and diffusion and perfusion indexes in women with varying bone density: MR evaluation. Radiology. 2006;241(3):831–8. doi: 10.1148/radiol.2413051858. [DOI] [PubMed] [Google Scholar]
- 6.Yeung DK, Griffith JF, Antonio GE, Lee FK, Woo J, Leung PC. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study. J Magn Reson Imaging. 2005;22(2):279–85. doi: 10.1002/jmri.20367. [DOI] [PubMed] [Google Scholar]
- 7.Shih TT, Chang CJ, Hsu CY, Wei SY, Su KC, Chung HW. Correlation of bone marrow lipid water content with bone mineral density on the lumbar spine. Spine (Phila Pa 1976) 2004;29(24):2844–50. doi: 10.1097/01.brs.0000147803.01224.5b. [DOI] [PubMed] [Google Scholar]
- 8.Schellinger D, Lin CS, Lim J, Hatipoglu HG, Pezzullo JC, Singer AJ. Bone marrow fat and bone mineral density on proton MR spectroscopy and dual-energy X-ray absorptiometry: their ratio as a new indicator of bone weakening. AJR Am J Roentgenol. 2004;183(6):1761–5. doi: 10.2214/ajr.183.6.01831761. [DOI] [PubMed] [Google Scholar]
- 9.Schellinger D, Lin CS, Hatipoglu HG, Fertikh D. Potential value of vertebral proton MR spectroscopy in determining bone weakness. AJNR Am J Neuroradiol. 2001;22(8):1620–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Tang GY, Lv ZW, Tang RB, Liu Y, Peng YF, Li W, Cheng YS. Evaluation of MR spectroscopy and diffusion-weighted MRI in detecting bone marrow changes in postmenopausal women with osteoporosis. Clin Radiol. 2010;65(5):377–81. doi: 10.1016/j.crad.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Shen W, Scherzer R, Gantz M, Chen J, Punyanitya M, Lewis CE, Grunfeld C. Relationship between MRI-measured bone marrow adipose tissue and hip and spine bone mineral density in African-American and Caucasian participants: the CARDIA study. J Clin Endocrinol Metab. 2012;97(4):1337–46. doi: 10.1210/jc.2011-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz AV, Sigurdsson S, Hue TF, Lang TF, Harris TB, Rosen CJ, Vittinghoff E, Siggeirsdottir K, Sigurdsson G, Oskarsdottir D, Shet K, Palermo L, Gudnason V, Li X. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. J Clin Endocrinol Metab. 2013;98(6):2294–300. doi: 10.1210/jc.2012-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wehrli FW, Hopkins JA, Hwang SN, Song HK, Snyder PJ, Haddad JG. Cross-sectional study of osteopenia with quantitative MR imaging and bone densitometry. Radiology. 2000;217(2):527–38. doi: 10.1148/radiology.217.2.r00nv20527. [DOI] [PubMed] [Google Scholar]
- 14.Botolin S, McCabe LR. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology. 2007;148(1):198–205. doi: 10.1210/en.2006-1006. [DOI] [PubMed] [Google Scholar]
- 15.Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50(2):546–52. doi: 10.1016/j.bone.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Botolin S, Faugere MC, Malluche H, Orth M, Meyer R, McCabe LR. Increased bone adiposity and peroxisomal proliferator-activated receptor-gamma2 expression in type I diabetic mice. Endocrinology. 2005;146(8):3622–31. doi: 10.1210/en.2004-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slade JM, Coe LM, Meyer RA, McCabe LR. Human bone marrow adiposity is linked with serum lipid levels not T1-diabetes. J Diabetes Complications. 2012;26(1):1–9. doi: 10.1016/j.jdiacomp.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Patsch JM, Li X, Baum T, Yap SP, Karampinos DC, Schwartz AV, Link TM. Bone marrow fat composition as a novel imaging biomarker in postmenopausal women with prevalent fragility fractures. J Bone Miner Res. 2013;28(8):1721–8. doi: 10.1002/jbmr.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strotmeyer ES, Cauley JA. Diabetes mellitus, bone mineral density, and fracture risk. Curr Opin Endocrinol Diabetes Obes. 2007;14(6):429–35. doi: 10.1097/MED.0b013e3282f1cba3. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz AV, Sellmeyer DE. Diabetes, fracture, and bone fragility. Curr Osteoporos Rep. 2007;5(3):105–11. doi: 10.1007/s11914-007-0025-x. [DOI] [PubMed] [Google Scholar]
- 21.Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26(5):557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Kugel H, Jung C, Schulte O, Heindel W. Age- and sex-specific differences in the 1H-spectrum of vertebral bone marrow. J Magn Reson Imaging. 2001;13(2):263–8. doi: 10.1002/1522-2586(200102)13:2<263::aid-jmri1038>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 24.Bondar RJ, Mead DC. Evaluation of glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides in the hexokinase method for determining glucose in serum. Clin Chem. 1974;20(5):586–90. [PubMed] [Google Scholar]
- 25.Griffith JF, Yeung DK, Ma HT, Leung JC, Kwok TC, Leung PC. Bone marrow fat content in the elderly: a reversal of sex difference seen in younger subjects. J Magn Reson Imaging. 2012;36(1):225–30. doi: 10.1002/jmri.23619. [DOI] [PubMed] [Google Scholar]
- 26.Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, Rosen CJ, Klibanski A, Miller KK. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring) 2011;19(1):49–53. doi: 10.1038/oby.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baum T, Yap SP, Karampinos DC, Nardo L, Kuo D, Burghardt AJ, Masharani UB, Schwartz AV, Li X, Link TM. Does vertebral bone marrow fat content correlate with abdominal adipose tissue, lumbar spine bone mineral density, and blood biomarkers in women with type 2 diabetes mellitus? J Magn Reson Imaging. 2012;35(1):117–24. doi: 10.1002/jmri.22757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schafer AL, Li X, Schwartz AV, Tufts LS, Wheeler AL, Grunfeld C, Stewart L, Rogers SJ, Carter JT, Posselt AM, Black DM, Shoback DM. Changes in vertebral bone marrow fat and bone mass after gastric bypass surgery: A pilot study. Bone. 2015;74:140–5. doi: 10.1016/j.bone.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawai M, Devlin MJ, Rosen CJ. Fat targets for skeletal health. Nat Rev Rheumatol. 2009;5(7):365–72. doi: 10.1038/nrrheum.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazarenko OP, Rzonca SO, Hogue WR, Swain FL, Suva LJ, Lecka-Czernik B. Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology. 2007;148(6):2669–80. doi: 10.1210/en.2006-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grey A, Beckley V, Doyle A, Fenwick S, Horne A, Gamble G, Bolland M. Pioglitazone increases bone marrow fat in type 2 diabetes: results from a randomized controlled trial. Eur J Endocrinol. 2012;166(6):1087–91. doi: 10.1530/EJE-11-1075. [DOI] [PubMed] [Google Scholar]
- 32.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104(4):531–43. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 33.Flier JS. Clinical review 94: What’s in a name? In search of leptin’s physiologic role. J Clin Endocrinol Metab. 1998;83(5):1407–13. doi: 10.1210/jcem.83.5.4779. [DOI] [PubMed] [Google Scholar]
- 34.Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140(4):1630–8. doi: 10.1210/endo.140.4.6637. [DOI] [PubMed] [Google Scholar]
- 35.Weiss LA, Barrett-Connor E, von Muhlen D, Clark P. Leptin predicts BMD and bone resorption in older women but not older men: the Rancho Bernardo study. J Bone Miner Res. 2006;21(5):758–64. doi: 10.1359/jbmr.060206. [DOI] [PubMed] [Google Scholar]
- 36.Pasco JA, Henry MJ, Kotowicz MA, Collier GR, Ball MJ, Ugoni AM, Nicholson GC. Serum leptin levels are associated with bone mass in nonobese women. J Clin Endocrinol Metab. 2001;86(5):1884–7. doi: 10.1210/jcem.86.5.7417. [DOI] [PubMed] [Google Scholar]
- 37.Thomas T, Burguera B, Melton LJ, 3rd, Atkinson EJ, O’Fallon WM, Riggs BL, Khosla S. Role of serum leptin, insulin, and estrogen levels as potential mediators of the relationship between fat mass and bone mineral density in men versus women. Bone. 2001;29(2):114–20. doi: 10.1016/s8756-3282(01)00487-2. [DOI] [PubMed] [Google Scholar]
- 38.Barbour KE, Zmuda JM, Boudreau R, Strotmeyer ES, Horwitz MJ, Evans RW, Kanaya AM, Harris TB, Cauley JA, Health ABCS. The effects of adiponectin and leptin on changes in bone mineral density. Osteoporos Int. 2012;23(6):1699–710. doi: 10.1007/s00198-011-1768-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jurimae J, Jurimae T, Leppik A, Kums T. The influence of ghrelin, adiponectin, and leptin on bone mineral density in healthy postmenopausal women. J Bone Miner Metab. 2008;26(6):618–23. doi: 10.1007/s00774-008-0861-5. [DOI] [PubMed] [Google Scholar]
- 40.Lorentzon M, Landin K, Mellstrom D, Ohlsson C. Leptin is a negative independent predictor of areal BMD and cortical bone size in young adult Swedish men. J Bone Miner Res. 2006;21(12):1871–8. doi: 10.1359/jbmr.060814. [DOI] [PubMed] [Google Scholar]
- 41.Ruhl CE, Everhart JE. Relationship of serum leptin concentration with bone mineral density in the United States population. J Bone Miner Res. 2002;17(10):1896–903. doi: 10.1359/jbmr.2002.17.10.1896. [DOI] [PubMed] [Google Scholar]
- 42.Morberg CM, Tetens I, Black E, Toubro S, Soerensen TI, Pedersen O, Astrup A. Leptin and bone mineral density: a cross-sectional study in obese and nonobese men. J Clin Endocrinol Metab. 2003;88(12):5795–800. doi: 10.1210/jc.2003-030496. [DOI] [PubMed] [Google Scholar]
- 43.Martini G, Valenti R, Giovani S, Franci B, Campagna S, Nuti R. Influence of insulin-like growth factor-1 and leptin on bone mass in healthy postmenopausal women. Bone. 2001;28(1):113–7. doi: 10.1016/s8756-3282(00)00408-7. [DOI] [PubMed] [Google Scholar]
- 44.Basurto L, Galvan R, Cordova N, Saucedo R, Vargas C, Campos S, Halley E, Avelar F, Zarate A. Adiponectin is associated with low bone mineral density in elderly men. Eur J Endocrinol. 2009;160(2):289–93. doi: 10.1530/EJE-08-0569. [DOI] [PubMed] [Google Scholar]
- 45.Jurimae J, Jurimae T. Adiponectin is a predictor of bone mineral density in middle-aged premenopausal women. Osteoporos Int. 2007;18(9):1253–9. doi: 10.1007/s00198-007-0365-5. [DOI] [PubMed] [Google Scholar]
- 46.Jurimae J, Kums T, Jurimae T. Adipocytokine and ghrelin levels in relation to bone mineral density in physically active older women: longitudinal associations. Eur J Endocrinol. 2009;160(3):381–5. doi: 10.1530/EJE-08-0673. [DOI] [PubMed] [Google Scholar]
- 47.Araneta MR, von Muhlen D, Barrett-Connor E. Sex differences in the association between adiponectin and BMD, bone loss, and fractures: the Rancho Bernardo study. J Bone Miner Res. 2009;24(12):2016–22. doi: 10.1359/JBMR.090519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo XH, Guo LJ, Xie H, Yuan LQ, Wu XP, Zhou HD, Liao EY. Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J Bone Miner Res. 2006;21(10):1648–56. doi: 10.1359/jbmr.060707. [DOI] [PubMed] [Google Scholar]


