Abstract
Preterm birth adversely affects postnatal brain development. In order to investigate the critical gestational age at birth (GAB) that alters the developmental trajectory of gray and white matter structures in the brain, we investigated diffusion tensor and quantitative T2 mapping data in 43 term-born and 43 preterm-born infants. A novel multivariate linear model—the change point model, was applied to detect change points in fractional anisotropy, mean diffusivity, and T2 relaxation time. Change points captured the “critical” GAB value associated with a change in the linear relation between GAB and MRI measures. The analysis was performed in 126 regions across the whole brain using an atlas-based image quantification approach to investigate the spatial pattern of the critical GAB. Our results demonstrate that the critical GABs are region- and modality-specific, generally following a central-to-peripheral and bottom-to-top order of structural development. This study may offer unique insights into the postnatal neurological development associated with differential degrees of preterm birth.
Keywords: change point analysis, preterm birth, gestational age at birth, diffusion MRI, T2 mapping, neonatal brain atlas
1. Introduction
Medical complications due to preterm birth are the leading cause of death and disability among children under five years of age, and preterm births occur 5–18% globally, leading to almost 1 million deaths per year according to the World Health Organization, 2015 (http://www.who.int/mediacentre/factsheets/fs363/en/). Preterm is defined as babies born alive before 37 weeks of pregnancy are completed. Sub-categories include: extremely preterm (<28 weeks of gestational age), very preterm (28 to <32 weeks) and moderate-to-late preterm (32 to <37 weeks). The adverse impact of preterm birth on neurological development has been demonstrated in a number of cognitive, behavioral, and neuroimaging studies (Atkinson and Braddick, 2007; Cheon et al., 2011; Fryer et al., 2008; Ortibus et al., 2012; Van Braeckel et al., 2008; Volpe, 2009). Nevertheless, the effect of gestational age at birth (GAB) on regional brain development has not been fully characterized. For example, it is not clear how moderate-to-late preterm birth affects the postnatal development of different brain structures (e.g., commissural fibers versus the association fibers). Considering the variations in the developmental trajectories of the gray and white matter and their differential maturity levels at birth (Mukherjee et al., 2001; Saksena et al., 2008; Trivedi et al., 2009; Yap et al., 2013), we can expect that the GAB may differentially affect the development of brain structures. Therefore, knowledge of the regional vulnerability to GAB may offer additional insights into the risk assessment for preterm labor or induced delivery.
Magnetic resonance imaging (MRI) is an ideal tool to investigate postnatal neurological development, as well as structural and functional alterations induced by preterm birth (Akazawa et al., 2016; Anjari et al., 2007; Ball et al., 2013; Dubois et al., 2008a; Huppi et al., 1998; Melbourne et al., 2014; Padilla et al., 2015; Partridge et al., 2004; Rose et al., 2008; Thompson et al., 2011; Yoo et al., 2005) and other perinatal events (Ball et al., 2010; Gao et al., 2012; Lepomaki et al., 2013). Structural MRI, including T1- and T2-weighted imaging and quantitative T1 and T2 mapping techniques, have been employed in neonatal studies since the 1980s (Barkovich et al., 1988; Holland et al., 1986; Johnson et al., 1983; Lee et al., 1986), and are used widely for clinical monitoring of brain development (Baratti et al., 1999; Ding et al., 2004; Ment et al., 2009; Woodward et al., 2006). Brain maturation is marked by T1 and T2 shortening during childhood, which is thought to reflect the progression of myelination in white matter tracts. However, at the long echo-times (>20ms) that are commonly used, it is unlikely that the myelin water signal with an extremely short T2 (<10ms) is directly captured on conventional T2-weighted images. In quantitative T2 studies, the reduction in T2 relaxation time has been attributed to the secondary maturation processes of myelin, such as tightening of the myelin spiral around the axon (Barkovich, 1995), and the increased concentration of macromolecules and membranes, which may modify the chemical composition (Baratti et al., 1999).
More recently, diffusion MRI, especially diffusion tensor imaging (DTI) (Basser et al., 1994), has become a popular tool for the characterization of neonatal brain development, as it provides superior contrast between gray and white matter tissues in the premature brain. DTI-measures and DTI-based tractography have been extensively used to detect anatomical abnormalities in the neonatal brain (Miller et al., 2007; Mourmans et al., 2006; Neil et al., 2002; Nijman et al., 2013; Oishi et al., 2013; Okumura et al., 2008; Padilla et al., 2014; Panigrahy and Bluml, 2007; Paquette et al., 2013; Parmar et al., 2004; Pogribna et al., 2013; Porter et al., 2010), including aberrant neurological development in preterm infants (Akazawa et al., 2016; Huppi et al., 1998; Partridge et al., 2004; Yoo et al., 2005). Mean diffusivity (MD) decreases and fractional anisotropy (FA) increases rapidly during the first two years of life, and these measurements continue to change through childhood and adulthood (Brown et al., 2012; Mukherjee et al., 2001; Saksena et al., 2008; Yap et al., 2013; Zhang et al., 2005). Relative to term born infants, preterm infants showed lower FA values and higher MD in white matter regions, such as the corpus callosum (Alexandrou et al., 2014; Anjari et al., 2007; Huppi et al., 1998; Rose et al., 2008; Shim et al., 2012; Thompson et al., 2011). The age-dependent decreases in MD during normal development is likely due to the concomitant decrease in overall water content, as well as changes in cellularity and axonal packing that further hinder water motion (Martin et al., 2014). FA is thought to reflect microstructural organization: in the white matter, the increase in FA during brain maturation is related to both ‘premyelination’ changes (increase in axon diameter and axonal membrane changes) (Wimberger et al., 1995), and increased axonal coherence from the myelination process (Huppi and Amato, 2001; Nelson et al., 1998); conversely, in the cortical gray matter, the FA decrease may be related to the growing complexity of the dendritic architecture (Neil et al., 2002). DTI-based indices and relaxation time values reveal complementary information about the structural properties in the brain; together, these metrics may offer a more comprehensive profile of neuronal development and preterm-birth related abnormalities.
To extend our understanding of the MRI signatures of perinatal development, we employed a novel statistical model, namely, the change point analysis (Miller et al., 2015; Younes et al., 2014), aiming to detect changes in the correlation between GAB and MRI measures in preterm and term-born infants. To capture regional variability, rather than choosing selected regions of interest (ROI) or tracts of interest, we performed a data-driven analysis using automated whole-brain segmentation based on neonatal DTI brain atlases (Oishi et al., 2011) and advanced image registration techniques (Christensen et al., 1996; Miller et al., 1993). We hypothesized that (1) the neurodevelopment trajectory is altered at a given threshold that is determined by the GAB of the infant, and (2) that each brain structure has its characteristic threshold, or “change point”. Our findings demonstrate the whole-brain pattern of critical GABs that may alter the neurodevelopmental trajectories. The structure-dependent change points may reflect the regional variability of brain development associated with different degrees of preterm birth and possible additional influences from typical co-morbid medical conditions associated with preterm birth.
2. Methods
2.1 Participant selection and characteristics
Brain MRI data were obtained from infants recruited and scanned at the University of Hawaii and the Queen’s Medical Center MR Research Center in Honolulu, HI, as described in our prior work (Akazawa et al., 2016; Chang et al., 2016). The infants’ parents or legal guardians provided written and verbal informed consent for the study, which was approved by the Cooperative Institutional Review Board (IRB) of the Queen’s Medical Center and the University of Hawaii, as well as the IRB at the Johns Hopkins University. Participants were screened by telephone initially and again by a physician on the day of the scans for the following criteria. They were excluded if the mothers were <18 years of age, or were unable to fully understand English, which would have precluded informed consent. Inclusion criteria for the infants were: 1) new born male or female child of any ethnicity; 2) born prematurely at <37 weeks gestational weeks (for the preterm infants) or born at 37–42 weeks gestation (for term born infants); 3) had parental/legal guardian consents. Exclusion criteria for the term-born infants included: 1) prolonged intensive care (>7 days); 2) intracranial hemorrhage; 3) neonatal hypoxic-ischemic encephalopathy; 4) known toxoplasmosis; other (syphilis, varicella-zoster, parvovirus B19); and rubella, Cytomegalovirus (CMV), and Herpes infections (TORCH); and 5) congenital heart disease or other anomaly; or 6) any chromosomal anomaly. Preterm-born infants were excluded if they 1) required supplementary oxygen or mechanical ventilation during the time of scanning; 2) had a circulatory, respiratory or airway abnormality; or 3) were diagnosed with fever, epilepsy, or active infection. The infants were also excluded if they had any contraindications for MR studies (e.g., metallic or electronic implants).
43 preterm-born infants and 72 term-born infants fulfilled all study criteria and had acceptable DTI scans were included in this study initially. Since more term-born infants were available than preterm-born infants, and this unbalanced GAB distribution may affect the change point analysis, we matched the number of term-born infants to that of the preterm-born infants by randomly sub-sampling the term-born infant population. Therefore, 43 preterm and 43 term-born infants were used in the final analysis. The demographic and clinical characteristics of these infants are listed in Table 1, including their gestational ages, weight, height, head circumference at birth and at scan, sex, race, delivery method, complications during neonatal period, neurological examination results, as well as related parental information.
Table 1.
Clinical Characteristics of the Infants and Parent or Primary Caregiver. Data are presented as number (%) or median [min—max].
| Full-Term (n=43) | Pre-Term (n=43) | p-value | |
|---|---|---|---|
| Characteristics of infants at birth | |||
| Sex: Female (%) / Male (%) | 22 (51.16%) / 21 (48.84%) | 19 (44.2%) / 24 (55.8%) | 0.517 |
| Race: | 0.038 | ||
| Asian (%) | 1 (2.32%) | 6 (13.95%) | |
| More than one race (%) | 31 (72.09%) | 22 (51.16%) | |
| Native Hawaiian / other Pacific Islander (%) | 6 (13.95%) | 3 (6.97%) | |
| White (%) | 2 (4.65%) | 9 (20.93%) | |
| Black or African American (%) | 2 (4.65%) | 3 (6.97%) | |
| American Indian or Alaska Native (%) | 1 (2.32%) | 0 (0%) | |
| Ethnicity Hispanic (%) / Non Hispanic (%) | 15 (34.88%) / 28 (65.12%) | 7 (16.28%) / 36 (83.72%) | 0.048 |
| Delivery method: | 0.006 | ||
| C-Section (%) | 9 (20.93%) | 23 (53.48%) | |
| Vaginal delivery (%) | 26 (60.46%) | 17 (39.53) | |
| Unknown (%) | 8 (18.60%) | 3 (6.97%) | |
| Gestational age (weeks) | 39.6 (37–41.6) | 32.6 (23.7–36.9) | <0.001 |
| Weight (kg) | 3.32 (2.336–4.825) | 1.76 (0.561–3.079) | <0.001 |
| Length (cm) | 50.8 (45–56) | 43.0 (31.0–50.2) | <0.001 |
| Body mass index | 12.8 (10.7–15.6) | 9.5 (5.8–12.2) | <0.001 |
| Head circumference (cm) | 34.3 (31.5–36.5) | 29.5 (13.0–35.0) | <0.001 |
| *APGAR score (1 minute) | 8 (1–9) | 8 (1–9) | 0.255 |
| *APGAR score (5 minutes) | 9 (8–9) | 9 (6–9) | 0.15 |
| Diagnoses reported per Infants’ Birth Record | |||
| Hemorrhage | 0 | 4 | |
| Patent ductus arteriosus | 0 | 2 | |
| Intrauterine growth restriction | 0 | 3 | |
| Retinopathy of prematurity | 0 | 8 | |
| Neonatal sepsis | 0 | 7 | |
| Bronchopulmonary dysplasia | 0 | 1 | |
| Jaundice (phototherapy) | 2 | 26 | |
| Twin pregnancy | 0 | 17 | |
| Characteristics of infants at baseline imaging | |||
| Post-menstrual age (weeks) | 42.1 (38.3–57.7) | 42.4 (37.3–52.1) | 0.731 |
| Weight (kg) | 3.99 (2.53–6.59) | 3.975 (2.13–6.25) | 0.421 |
| Length (cm) | 53.34 (45.72–62.23) | 52.07 (43.18–58.42) | 0.039 |
| Head circumference (cm) | 36.25 (33.0–41.0) | 36.5 (31.5–40.5) | 0.883 |
| Clinical Characteristics of the Parent or Primary Caregiver | |||
| Mother’s age at birth (years) | 25 (19–40) | 32 (20–42) | 0.001 |
| Mother’s pregnancy weight gain (kg) | 18.14 (0–36.29) | 13.61 (−9.07–46.27) | 0.144 |
| Mother’s head circumference (cm) | 57.2 (53.3–66.0) | 56.0 (52.7–61.0) | 0.079 |
| Mother’s Body Mass Index by self-report | 31 (20.0–45.5) | 29.1 (18.6–56.5) | 0.288 |
| Socioeconomic status (index of social position) | 61 (18–69) | 33 (11–69) | <0.001 |
Activity, Pulse, Grimace, Appearance, & Respiration
2.2 MRI data
The infants were scanned without sedation. A vacuum immobilization mat (Noras MRI Products, Hoechberg, Germany) was used to minimize infant motion, and earmuffs were used to attenuate the scanner noise. Images were acquired using a 3.0 Tesla Siemens TIM Trio scanner (Siemens Medical Solutions, Erlangen, Germany) equipped with a 12-channel phased-array RF coil for parallel imaging.
For DTI, a single-shot echo-planar imaging (EPI) acquisition was used with sensitivity-encoding (SENSE) at two-fold acceleration. The imaging parameters were as follows: imaging matrix = 80 × 80 with a field-of-view (FOV) of 160 × 160 mm, which resulted in a 2 × 2 mm in-plane resolution; axial slices of 2.5 mm thickness and 40–50 slices to cover the entire brain; echo time (TE) = 106 ms and repetition time (TR) = 7- 9 s, depending on specific absorption rate limitations; and two signal averages. Diffusion weighting was applied along 12 independent directions with b = 1000 s/mm2, in addition to a minimally diffusion-weighted (b0) image.
A dual-echo fast spin echo sequence was used to obtain the T2 maps, with the following imaging parameters: imaging matrix = 128 × 128 and FOV = 250 × 250 mm, resulting in a 1.95 × 1.95 mm in-plane resolution, axial slices of 2.5 mm thickness and 40–50 slices to cover the entire brain, and TR/TE1/TE2 = 4550/24/130 ms.
T1- weighted images were acquired with three-dimensional (3D) magnetization-prepared rapid gradient-echo (MPRAGE) sequence, with TE/TI/TR of 4.15/1400/3200 ms, a flip angle of 7°, an imaging matrix of 176 × 256 × 160, and 1 mm isotropic resolution. T2-weighted images were acquired with 3D Sampling Perfection with Application optimized Contrast using different flip angle Evolutions (SPACE) sequence, with TE/TR of 386/3200ms, an imaging matrix of 120 × 204 × 256, and 1 mm isotropic resolution. All scans were quality assured upon acquisition by the experienced MR technical staff and repeated as needed. A neuroradiologist (D.L., see Acknowledgments) read all anatomical images (T1- and T2-weighted) to exclude subjects with anatomical abnormalities, and all scans used in this study were read as normal, except for scans from four preterm infants who had minimal or small amounts of residual hemosiderin from their intracerebral hemorrhages.
2.3 Data preprocessing
2.3.1 DTI data segmentation
The raw diffusion-weighted images were first co-registered to the minimally diffusion-weighted images separately for each dataset, using linear Automated Image Registration (AIR) (Woods et al., 1998). From the co-registered diffusion-weighted images, six elements of the diffusion tensor were calculated for each pixel, with log-linear fitting using DtiStudio (www.mriStudio.org) (Jiang et al., 2006). We used an automated outlier rejection function (Li et al., 2013) in the DtiStudio to reject slices with a relative fitting error of more than 3%. An experienced neurologist (K.O.) performed the second quality check, based on visual inspection of the color-coded orientation maps calculated from the tensor field, to ensure the data had no noticeable motion artifacts.
The FA and MD images from individual neonates were then transformed to the JHU-neonate single brain DTI atlas (Oishi et al., 2011), first through linear AIR transformation followed by Large Deformation Diffeomorphic Metric Mapping (LDDMM) (Christensen et al., 1996; Miller et al., 1993), using the dual-channel FA and MD contrasts. The multi-contrast JHU-neonate atlas then automatically segmented the transformed DTI data into 126 regions of interests (ROIs). The FA and MD values were extracted from the ROIs with a MD threshold of 2×10−3 mm2/s, to exclude the cerebrospinal fluid (CSF) component. The DTI images were further inspected for registration failure, and none of the data showed visible registration failure. The axial and radial diffusivities (AD and RD) were also calculated from the tensor field to help with the interpretation of the change points in FA and MD.
2.3.2 T2 map segmentation
The T2 maps of individual infants were calculated by calculating T2 relaxation times from the two echoes of the dual-echo sequence (Duncan et al., 1996). The proton-weighted images (first echo) were first skull-stripped, using the Brain Extraction Tool (BET) (Smith, 2002), followed by manual editing performed on ROIEditor (www.mristudio.org), and the resultant brain-mask was used to skull-strip the second-echo T2-weighted images of the T2 mapping sequence. The skull-stripped second-echo T2-weighted images were transformed to the minimally-diffusion-weighted images from the DTI data, which carried a similar T2-weighted contrast, through AIR transformation followed by LDDMM. The second-echo T2-weighted images and T2 maps that were co-registered to the corresponding DTI images in their native space were then transformed to the JHU-neonate atlas, using the transformation matrix obtained in 2.3.1. The T2 relaxation times could then be extracted from the 126 ROIs segmented from the atlas. The concatenated registration procedure was performed instead of directly registering the T2 mapping data to the JHU T2 atlas, because 1) the T2 mapping data and the DTI data were acquired from the same individuals, and the contrasts between the second-echo T2-weighted image and the b0 image of the DTI data were close enough to drive the cross-modality co-registration (Huang et al., 2008); and 2) DTI-to-DTI co-registration was more accurate than the T2-to-T2 co-registration due the high white matter contrast in the FA maps (Oishi et al., 2012).
2.4 Change point analysis
2.4.1 Change point model
We developed a change point analysis model to examine changes in structural MRI measurements (FA, MD, or T2 relaxation time) in relation to GAB, in order to estimate the critical GAB, when the slopes of linear correlations between MRI measures and GAB significantly change. For this purpose, we established a multivariate model that regresses the response variable y (MRI measures) on the GAB (g), PMA at scan (p), and sex (s ):
| Equation 1 |
where k is the subject index, Δ is the change point (in unit of GAB), [a0, a1, a2, a3, a4], are the model parameters, and ∊ is the noise that follows Gaussian distribution with zero mean. H is an indicator (Heaviside) function with H(x) = 1 if x>0 and H(x) = 0 otherwise. Therefore, in Equation 1, the change point is explicitly modeled as an additional linear change (a2) of y against GAB that takes effect only when GAB is greater than the change point Δ, in addition to the baseline change (a1).
The model parameters are estimated using maximum likelihood estimation (MLE) (Miller et al., 2015; Younes et al., 2014), at each Δ with log-likelihood L(Δ). The optimal change point is obtained at Δ* when L(Δ) reaches the maximum, where Δ ranges from a GAB of 30–40 weeks with a step size of 0.1. The null hypothesis is a2 = 0, and a significant change point is found when a2 ≠ at Δ*. Notice that we have more term-born infants (n=72) than preterm-born infants (n=43), and the GAB of the term-born infants fell within a narrow range (39.62 ± 1.07 weeks).
2.4.2 Significance tests
The significance of the change points was evaluated using a permutation test (Nichols and Hayasaka, 2003), as detailed previously (Miller et al., 2015; Younes et al., 2014). Briefly, a test statistic is computed as the log-likelihood difference between the null hypothesis (LH0) and the general hypothesis (LH1), namely, S = LH1 – LH0. To compute the p-value, the test statistic is computed for a large number (M = 10,000) of permutations, by randomizing the model residuals to obtain M numbers of Sπ. If v is the number of permutations, π , for which the value of Sπ > S, the p-value is given by . The significance of the change points was detected at a 5% false discovery rate (FDR) after correcting for multiple comparisons (Benjamini and Hochberg, 1995).
2.4.3 Bootstrap
We assessed the variance of the change point estimation using a bootstrap operation (Miller et al., 2015; Younes et al., 2014). Briefly, in each bootstrap operation, the data (k = 1, …, K) were resampled with resubstitution, and the change point () was estimated based on the resampled data. By repeating this procedure for a large number of times (N = 10,000), the standard deviation of the change point of Δ* can be obtained based on the N numbers of to evaluate the precision of the detected change points.
2.4.4 Effects of chronological age
External stimuli and environmental changes that occur after birth are known to have significant effects to the neurological development (Citri and Malenka, 2008; Morishita and Hensch, 2008; Tau and Peterson, 2010). The chronological ages of preterm-born infants were older than that of term-born infants; and thereby, the preterm-born infants had longer periods of exposure to the external environment. We did not directly include the chronological age (from birth to PMA at scan) into the change point model, since it depends on PMA at scan and GAB, which are already included in the model. Therefore, correlations between chronological age and the MRI measures (FA, MD, and T2 after correcting for PMA at scan and gender) were investigated separately from the change point analysis, to help interpretation of the change points identified in this study.
3. Results
3.1 Change point analysis of the FA data
Figure 1 shows several white and gray matter structures with significant change points based on FA measurements. The FA values were plotted against GAB, after correction for PMA at scan and gender, based on the multivariate regression model; and therefore, the bi-phasic linear changes in FA observed in the plots were primarily dependent on GAB. The red dots denote data from preterm and blue dots from term-born neonates. The black solid curves represent the fitted FA data based on GAB (second and third terms in Equation 1), and the black dashed vertical lines indicate the positions of the change points. 49 of the 126 brain structures showed significant change points (familywise p<0.05), and in the majority of these structures (39 out of 49), FA increased with GAB up to the change point, with relatively small changes thereafter (Figure 1A, only those with most significant change points (familywise p<0.05) were plotted). The increase in FA with GAB seen before the change point was due to steeper RD reduction compared to AD (Figure 1A in reference (Wu et al., 2017)). However, an opposite pattern was observed in the remaining 10 of the 49 structures, including the bilateral posterior limb of the internal capsule, bilateral posterior corona radiata, bilateral globus pallidus, bilateral midbrain, right middle occipital gyrus, and left cuneus. In these structures, FA values were relatively stable before the change point but tended to decrease with GAB after the change points (Figure 1B), which was associated with the slight increase of RD with GAB after the change points (Figure 1B in reference (Wu et al., 2017)).
Figure 1.
Change point analyses in the white and gray matter structures that showed GAB-dependent FA changes with significant change points (familywise p<0.05). (A–B) First row for each structure: the x-axes represent GAB in unit of weeks, and the y-axes represent FA after correcting for PMA at scan and gender, based on the multivariate regression change point model. The red and blue dots denote data from preterm and term-born neonates, respectively. The black lines show FA values fitted to GAB only (first and second terms in Equation 1), and dashed lines indicate the change points. Second row for each structure: the x-axes represent chronological age (age after birth) in unit of weeks, and the y-axes represent FA after correcting for PMA at scan and gender. The black lines indicated the linear regression between FA and chronological age with both the term and preterm-born infants, and the orange lines showed the linear fitting with only the preterm data. In a majority of structures (n=39), FA increased with GAB before the change point, and then remained relatively stable after the change point (A). However, in some structures (n=10), the FA was relatively stable before the change point and then decreased with GAB after the change point (B). For the first pattern (A), only the structures with most significant change points (familywise p<0.01) were plotted. For the structures that were bilaterally significant, only one side (left side) was presented; for those that were significant only in one side, the laterality is indicated in the plots.(C): Change point values of FA in regions with significant change points (family-wise p<0.05), overlaid on the JHU neonate FA atlas. The color bar indicates the change points in units of GAB (weeks). Abbreviations: SCP – superior cerebellar peduncle; ITG –inferior temporal gyrus; CP –cerebral peduncle; MB – midbrain; SS –sagittal striatum; IFO – inferior fronto-occipital fasciculus; GP –globus pallidus; Put –putamen; Thal –thalamus; Cu – cuneus; ALIC –anterior limb of internal capsule; PLIC – posterior limb of internal capsule; RLIC – retrolenticular part of internal capsule; TAP –tapetum; PCR – posterior corona radiata; PrCu – precuneus; SLF –superior longitudinal fasciculus; CGC – cingulum cingular part.
The change points of FA from individual ROIs were mapped onto the JHU-neonate atlas, and thresholded with a significance level of 0.05 (permutation test followed by FDR correction) (Figure 1C). A whole brain map of FA-based change point maps without thresholding can be found in Supplementary Figure 1A. A bottom-up and central-to-peripheral order of structural maturation can be appreciated from these maps: 1) the brainstem structures, such as the midbrain, pons, and medial lemniscus (yellow arrows in Figure 1C), showed the earliest change points around 30–34 weeks of GAB; 2) the projection tracts, such as the internal capsule (orange arrows in Figure 1C), showed an early change point around 34 weeks of GAB; 3) the association tracts, such as the superior longitudinal fascicles, uncinate fasciculus, cingulum, and inferior fronto-occipital fasciculus (blue arrows in Figure 1C), had relatively late change points around 36–40 weeks of GAB; 4) several deep brain gray matter structures also showed an early change point around 34 weeks of GAB, e.g., the striatum and globus pallidus. The change points of all structures along with their p-values and standard deviations are listed in Supplementary Table 1.
3.2 Change point analysis of MD data
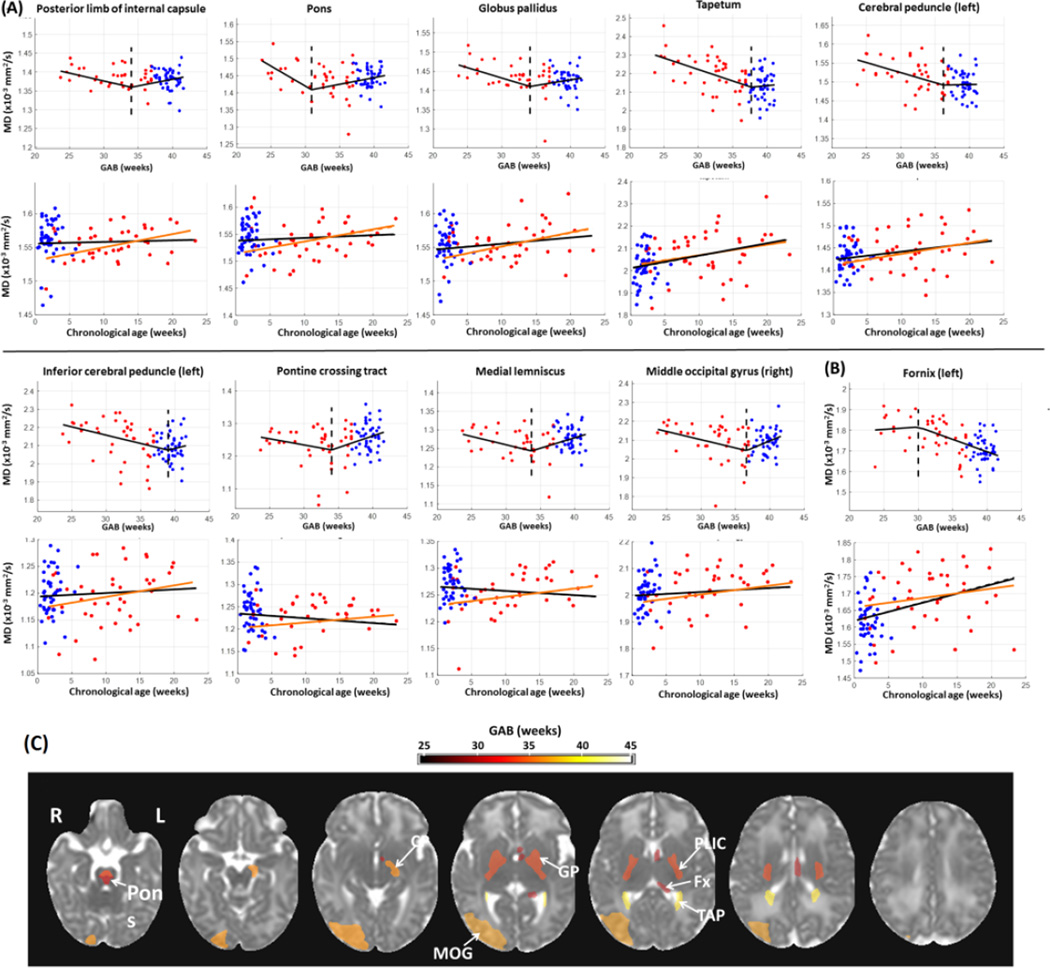
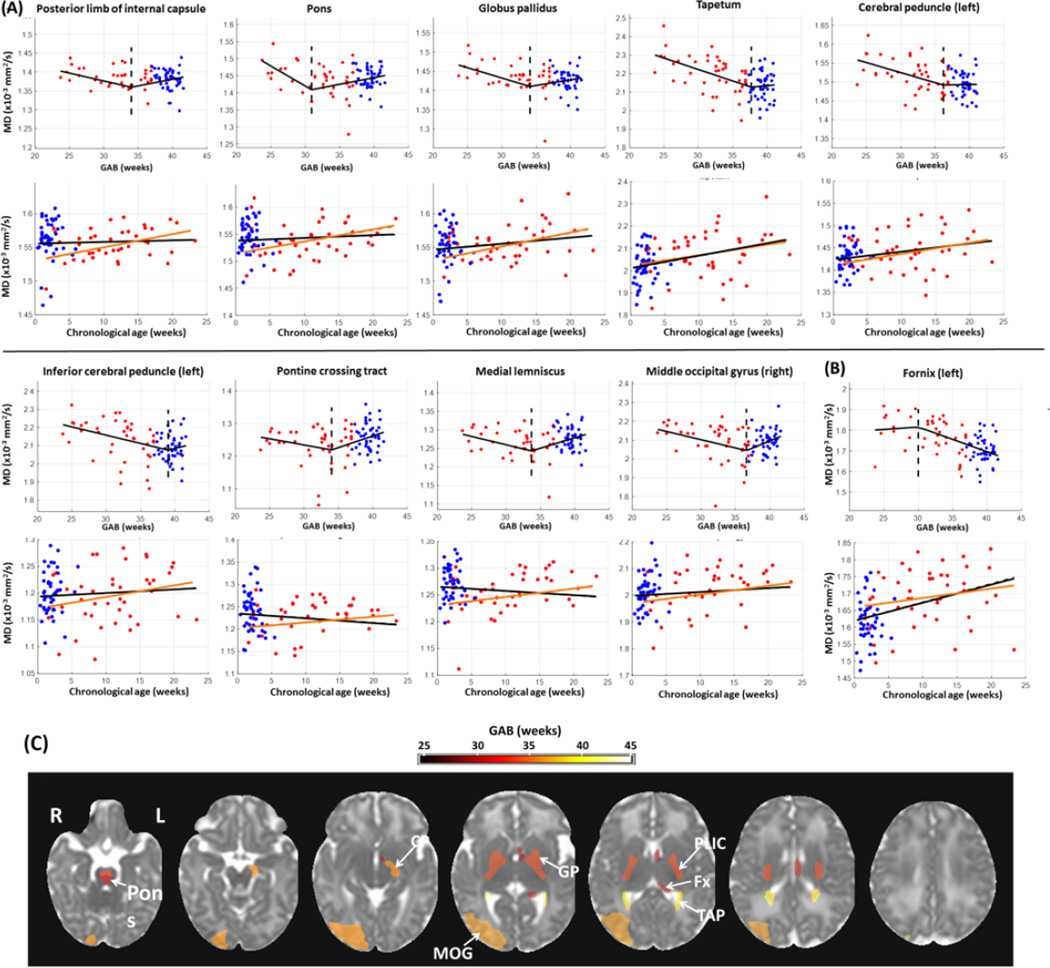
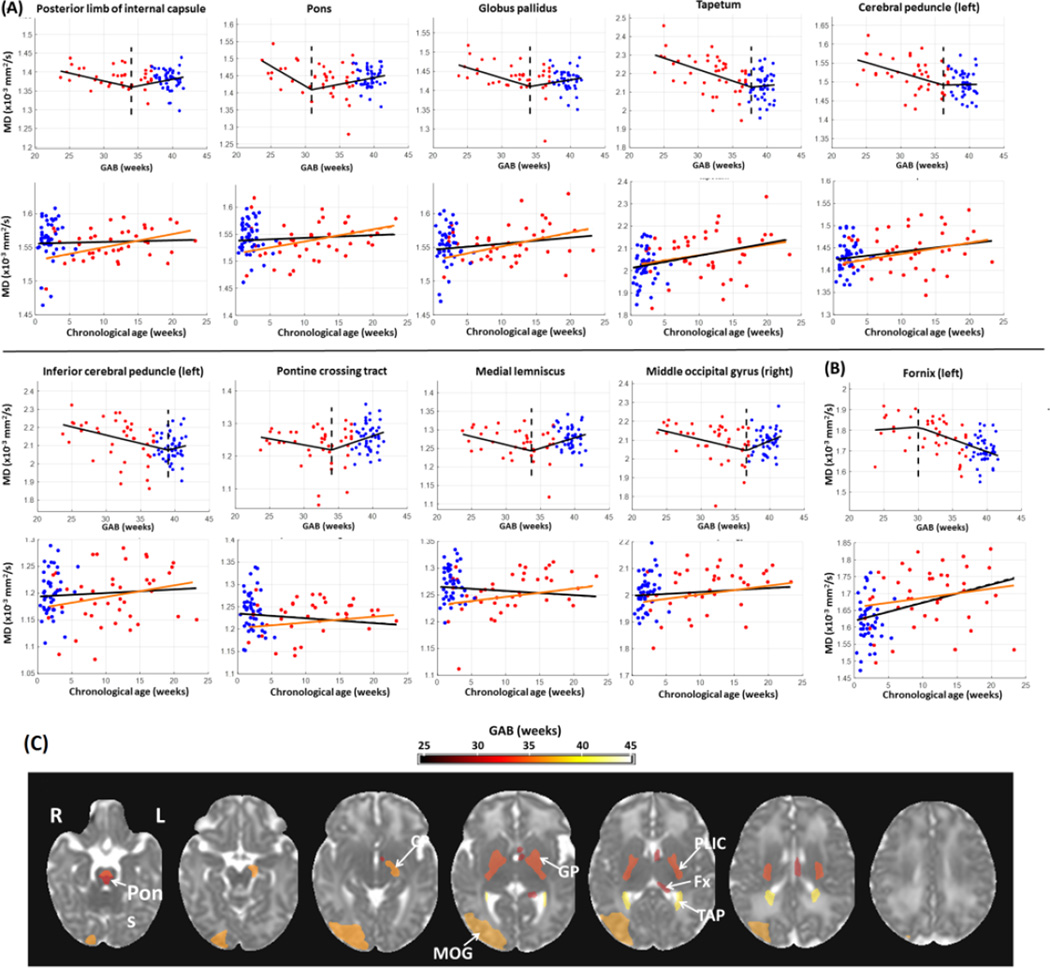
Compared to FA, fewer structures showed significant change points based on MD measurements (16 structures from MD analysis versus 49 from FA). In most of these structures, MD values (after correcting for PMA at scan and gender) decreased with GAB before the change points and then remained relatively stable afterwards (Figure 2A). This is similar to the first pattern of GAB-dependent change observed in FA (Figure 1A), although MD showed GAB-dependent decreases while FA showed GAB-dependent increases prior to the change point. An exception was found in the left fornix, where the MD values were stable before the change points but underwent a rapid decrease with GAB after the change points (Figure 2B). Figure 2C shows the MD-based change points mapped onto an MD image from the JHU-neonate atlas, with a FDR-corrected p-value threshold of 0.05. The pons had the earliest change point around 31 weeks of GAB. A few deep brain structures showed relatively early change points at 34–36 weeks of GAB, including the posterior limb of the internal capsule and globus pallidus. Whole brain maps of MD-based change points in all structures are shown in Supplementary Figure 1B, and their statistical results are listed in Supplementary Table 1.
Figure 2.
Change point analysis of MD measurements (corrected for PMA at scan and gender) in the structures with significant change points (familywise p<0.05). (A–B) First row for each structure: change of MD against GAB, after correcting for PMA at scan and gender. The red and blue dots denote data from preterm and term-born neonates, respectively. The black lines show FA values fitted to GAB only, and dashed lines indicate the change points. Second row for each structure: change of MD against chronological age, after correcting for PMA at scan and gender. The black lines indicated the linear regression between FA and chronological age with both the term and preterm-born infants, and the orange lines showed the linear fitting with only the preterm data. For the structures that were bilaterally significant, only one side (left side) was presented; for those that were significant only in one side, the laterality is indicated in the plots. In a majority of regions (n=15), MD decreased with GAB before the change point, and remained relatively stable after the change point (A). Conversely, MD values in left fornix (B) were relatively stable before the change point and then decreased with GAB after the change point. (C): Change point values of MD in regions with significant change points (family-wise p<0.05), overlaid on the JHU neonate MD atlas. The color bar indicates change points in units of GAB (weeks). Abbreviations: CP –cerebral peduncle; MOG –middle occipital lobe; GP –globus pallidus; PLIC –posterior limb of internal capsule; Fx –fornix; TAP –tapetum.
3.3 Change point analysis of the T2 relaxation times
The T2 relaxation times, after correcting for PMA at scan and gender, showed an initial increase, followed by a decrease after the change point. 11 brain regions showed significant change points from the T2 analysis (Figure 3A). In contrast to the FA and MD results, these structures were mostly located in the cortex (Figure 3B), including the bilateral postcentral gyrus, precuneus, medial fronto-orbital gyrus and gyrus rectus. These cortical regions demonstrated similar change points around 32 weeks of GAB. Whole brain maps of T2-based change points in all structures are shown in Supplementary Figure 1C, and their statistical results are listed in Supplementary Table 1.
Figure 3.
Change point analysis of T2 relaxation times (corrected for PMA at scan and gender) in the structures with significant change points (familywise p<0.05). (A) First row for each structure: change of T2 relaxation time against GAB, after correcting for PMA at scan and gender. The red and blue dots denote data from preterm and term-born neonates, respectively. The black lines show T2 relaxation time fitted to GAB only, and dashed lines indicate the change points. Second row for each structure: change of T2 relaxation time against chronological age, after correcting for PMA at scan and gender. The black lines indicated the linear regression between T2 relaxation time and chronological age with both the term and preterm-born infants, and the orange lines showed the linear fitting with only the preterm data. For the structures that were bilaterally significant, only one side (left side) was presented; for those that were significant only in one side, the laterality is indicated in the plots. In all regions (n = 11), T2 relaxation times increased slightly before the change point and then shortened with GAB after the change point. (B): Change point values of T2 relaxation time in regions that showed significant change points (FDR p<0.05), overlaid on the JHU neonate T2-weighted atlas. The color bar indicates the change points in units of GAB (weeks). Abbreviations: MFOG – medial fronto-orbaital gyrus; RG – gyrus rectus; SCP –superior cerebellar peduncle; Fx –fornix; PrCu – precuneus; PoCG –postcentral gyrus.
Note that only the left fornix showed significant change points across all three modalities (FA, MD, and T2 relaxation-time). In addition to left fornix, 13 structures were significant for both FA and MD measurements, including the bilateral posterior limb of the internal capsule, bilateral tapetum, bilateral globus pallidus, bilateral pontine crossing tract, bilateral pons, bilateral medial lemniscus, left cerebral peduncle; and 4 structures were significant for both the FA and T2 relaxation time measurements, including the bilateral superior cerebellar peduncle and bilateral precuneus.
3.4 Effect of postnatal external stimuli on FA, MD, and T2 relaxation time
The FA, MD, and T2 relaxation times were plotted against chronological age to investigate the impact of external stimuli on postnatal brain development (Figures 1–3, plots in the second rows for each structure), in addition to the effect of GAB that was analyzed in the change point model. The red and blue dots denote data from preterm and term-born neonates, respectively, after correcting for PMA at scan and gender. The black lines indicated the linear fitting against chronological age with all data, and the orange lines showed the linear fitting with only the preterm data. The FA measurements demonstrated two types of chronological age dependent changes. For the structures in Figure 1A, which had increased FA before the change point, the FA values showed a tendency to decrease with chronological age; whereas for the structures in Figure 1B, which had decreased FA after the change point, FA tended to increase with chronological age. Although the linear regression between FA and chronological age was not significant, after FDR correction, the different tendency seen in the two types of structures indicated that the chronological age had higher impact on the second type of structures. The MD values also exhibited two types of relations with chronological age. For the structures in Figure 2A, which had decreased MD before the change point, the MD values showed little change or slight increase but no significant correlation with chronological age; whereas in the left fornix (Figure 2B), the only structure that had decreased MD after the change point, the MD values significantly increased with chronological age (familywise p<0.001). The T2 relaxation time showed a positive correlation with chronological age in all structures that had significant change points (Figure 3A, black lines in the second row plots for each structure), and the correlation was significant in the left fornix, bilateral superior cerebral peduncle, and bilateral gyrus rectus (familywise p<0.05). However, there was a disparity between term and preterm populations, and the preterm infants alone showed a slight tendency decreased T2 relaxation time with chronological age (orange lines, no statistical significance), which indicated that changes in T2 relaxation time were mainly attributed to prematurity from preterm birth instead of the effect of chronological age.
4. Discussion
Brain immaturity associated with preterm birth may pose risks for neurological disabilities and impairments throughout the lifespan (Back and Volpe, 1997; Volpe, 1995). In this study, we adopted a change point model to characterize the impact of brain maturity at birth on early postnatal development, which may also be partially attributed to typical co-morbid conditions associated with preterm birth. The change point analysis has been applied previously in Alzheimer’s disease (Miller et al., 2015; Younes et al., 2014) to capture the critical age when the medial temporal lobe structures experience an altered morphological change during aging. Here, we adapted the model in the context of GAB-dependent MRI measures to determine the critical gestational age when a significant change occurs in the developmental trajectory, based on multi-modality MRI markers from preterm and term-born infants. The change point model adopted in this study approximates the development trajectory by two linear phases with different slopes, which is a relatively simple but well-supported pattern based on earlier studies (Brown et al., 2012; Dubois et al., 2008b; Huppi et al., 1998; Mukherjee et al., 2001; Neil et al., 2002; Oishi et al., 2011). It is possible that high-order or nonlinear models could better represent the developmental changes, but they may introduce model complexity and instability in fitting. While the best mathematical formulation of brain development is a question of debate, results from the bi-phase change point model characterized important features of the early postnatal MRI measurements.
In most of the structures with significant change point, the corrected FA was positively correlated with GAB until the change point, followed by no or less correlation with GAB. The positive correlation observed before the change point was due to the fact that RD reduced with GAB in a faster rate than that of AD. According to the “fibers myelination” hypothesis (Dubois et al., 2008b), myelination during development reduces RD more than AD. Therefore, the correlation between FA and GAB might be due to delayed myelination that occurred in the preterm infants with the GAB lower than the change point. We postulated that change points were associated with the critical GAB point that causes delay in myelination. If the preterm birth occurs later than this critical GAB, it may have little or no adverse impact on the myelination. The regional variation of the FA-based change points throughout the brain indicates a bottom-to-top and central-to-peripheral pattern of maturation. For instance, the brainstem structures, deep brain structures, and projection fiber tracts showed relatively early change points, whereas the association tracts and cortical regions showed relatively late change points. This temporal-spatial pattern generally agrees with existing knowledge about white matter development, with the commissural and projection fibers being the most developed structures at term-birth and the association fibers being the last to mature. A central-to-peripheral, bottom-to-top, and posterior-to-anterior developmental order was established from earlier studies (Dubois et al., 2006; Gilmore et al., 2007; Huang et al., 2006; Huppi et al., 1998; Yap et al., 2013). On the other hand, several structures that are located in central and posterior areas, such as the posterior limb of the internal capsule, the posterior corona radiata, and some gray matter structures, demonstrated a different pattern of GAB-dependency. Specifically, FA demonstrated little change with GAB before the change point but showed GAB-dependent decrease afterwards, caused by positive slope of RD with GAB. For the white matter structures of this type, we speculate that this pattern resulted from two counteracting factors: (i) delayed membrane proliferation (Dubois et al., 2008b) due to preterm birth, which relates to the increase of FA with GAB; and (ii) accelerated brain development after birth due to maturation of the oligodendrocyte (Pistollato et al., 2007) and input of external stimuli (Citri and Malenka, 2008; Morishita and Hensch, 2008; Tau and Peterson, 2010), which may cause FA to decrease with GAB (note that lower GAB infants were older in chronological age). (ii) is partially supported by the positive correlation between FA and chronological age in these structures. FA may decrease with GAB, as seen after the change point, when the second factor outweigh the first one; and when the effects of the two factors balance, FA may not change with GAB, as seen before the change point in Figure 1B. In addition to these two physiological influences, the FA values can also be affected by the occurrence of crossing fibers—a known limitation of the diffusion tensor approach (Tuch et al., 2002; Wedeen et al., 2008). For example, the crossing between the corona radiata and corpus callosum is commonly observed (Wiegell et al., 2000), which may lead to FA reduction in related areas as the crossing fibers develop.
MD in a majority of the brain structures followed “an initial GAB-dependent decrease then plateau” pattern; therefore, the interpretation of MD-based change points for structural maturation are similar to those of the first pattern of GAB-dependent change in FA. Compared to FA results, fewer structures showed significant change points on MD (49 for FA versus 16 for MD). One possible cause for this difference is that water content measurement by MD may not be as sensitive to prematurity as the FA measurement, because FA reflects the complex microstructural organization, including the ‘premyelination’ changes (Wimberger et al., 1995) as well as the myelination process (Huppi and Amato, 2001; Nelson et al., 1998). MD values in the left fornix demonstrated a reversed relationship with GAB— MD was stable before the change point but showed GAB-dependent decreases after the change point. We postulate that, in this pattern of GAB-dependent change, the change point may be associated with the factors that affect initiation of membrane proliferation or myelination.
The T2 relaxation times generally demonstrated an initial GAB-dependent increase before the change point followed by GAB-dependent shortening after the change points. The initial T2 increase phase may result from external stimulation-associated postnatal development, similar to the second pattern observed in FA; whereas the T2 shortening phase after the change point may indicate microstructural development, including the myelination processes, with the change point points being the initiation points of these processes. However, the spatial pattern of T2-based change points was distinct from that for FA or MD. In particular, the majority of significant changes were located in the cortical regions with change points at 30–32 weeks of GAB. T2 relaxation time, being a general measure of spin-spin interaction and chemical composition (Nishimura, 2010), is sensitive to a broad range of biological events. The shortening of T2 relaxation time after the change point as observed in the cortical gray matter of the neonates may reflect the growth spurt due to intra-cortical myelination of the dendritic processes of pyramidal cells (MiotNoirault et al., 1997), as well as changes in chemical composition and iron deposition related to increased macromolecules and membranes (Baratti et al., 1999).
Findings from these MRI markers are in support of our hypotheses that the neurodevelopment trajectory is altered at a given threshold determined by the GAB of the infant; and each brain structure has its characteristic threshold for this change point. The clinical implications of the findings will require long-term neurological evaluations in these preterm and term-born infants.. Our ongoing longitudinal neonatal study would provide a better understanding of the consequences of the change points and change patterns observed from the multi-modality MRI data.
This study has several limitations. First, due to the low and continuously-changing gray and white matter contrast in neonatal brain MRI (Baratti et al., 1999; Barkovich et al., 1988; Huang et al., 2006), the cortical ROIs in our neonatal brain atlas (Oishi et al., 2011) included both cortical gray and subcortical white matter, which had the mixture of variable developmental changes that may confound the interpretations of the change points. Voxel-based analysis may be less susceptible to this limitation. Second, the imaging data may be affected by motion, which is common in pediatric imaging, despite our rigorous protocol for QC during data acquisition and analysis. Future use of prospective motion-correction techniques (Herbst et al., 2015; Jiang et al., 2007; Rousseau et al., 2006) are warranted. Third, the automated segmentation of both DTI and T2 maps using a single-brain atlas may not be sufficient; the segmentation accuracy could be further improved by employing more advanced image analysis tools, such as multi-atlas-based image segmentation (Tang et al., 2014). Fourth, relative small sample size (n=43) in the preterm infant range (GAB from 23.7 to 36.9 weeks) may affect the significance of the change point analysis. Despite the fact that we matched the number of term-born infants to that of the preterm infants, we still have a higher sample density in the high-GAB range (37.3—52.1 weeks) than those born earlier. Since the MRI measurements in high GAB infants are likely to have similar normative values, we expected that the relatively higher number of samples in this GAB range could reduce the measurement noise while imposing minimal bias in the change point model fitting. Lastly, the MRI markers investigated in this study (FA/MD/T2), are composite measures that reflect the contribution of multiple and heterogeneous microscopic tissue compartments. Advanced image acquisition and reconstruction techniques may provide more specific microstructural information. For example, advanced diffusion MRI models (Assaf et al., 2008; Zhang et al., 2012) and myelin water imaging (Whittall et al., 1997) could provide specific information about the various tissue components. These techniques, however, are more demanding with regard to image acquisition time and hardware and therefore difficult to perform in neonatal studies.
5. Conclusion
In summary, we performed a novel change point analysis to investigate the critical GAB that may lead to altered postnatal brain development, using DTI and T2 mapping data from preterm and term-born infants. The analysis from multi-modality MRI markers revealed structure specific GAB that are related to neuronal maturation, such as membrane proliferation or myelination. The findings from this study suggest differential regional response to the preterm birth that may impact postnatal brain development; however, long-term follow-up evaluation is required to investigate the consequent clinical implications of these findings.
Supplementary Material
Highlights.
-
▪
Diffusion tensor and T2-mapping MRI was measured from preterm and term-born infants.
-
▪
Change point analysis detects critical gestational age at birth (GAB) in 126 brain regions.
-
▪
Change-points maps revealed spatiotemporal order of structural development.
-
▪
GAB-dependent MRI change reflected brain prematurity and postnatal development.
Acknowledgments
This work was made possible by grants R21NS098018, R01HD065955, 2K24DA16170, U54NS056883, G12MD007601-26, and P41EB015909 from the National Institutes of Health, and grant 46039500 from the Central Norway Regional Health Authority. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official view of NIH or the Central Norway Regional Health Authority. The authors are grateful to the families of our research participants, the pediatricians/neonatologists who referred the participants (Dr. Lillian Fujimoto, Dr. Lois Chiu, and Dr. Joseph Hudak), and our dedicated research staff (Steven Buchthal, Eric Cunningham, Daniel Alicata, Heather Johansen, Antonette Hernandez, Robyn Yamakawa, Sara Hayama, Tamara Andres), who assisted with the data collection. We also thank our board-certified neuroradiologist, Dr. Doris Lin, for her radiological reading of the scans. Lastly, we appreciate the instructive discussion with Dr. Laurent Younes about the development of mathematical model and statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akazawa K, Chang L, Yamakawa R, Hayama S, Buchthal S, Alicata D, Andres T, Castillo D, Oishi K, Skranes J, Ernst T, Oishi K. Probabilistic maps of the white matter tracts with known associated functions on the neonatal brain atlas: Application to evaluate longitudinal developmental trajectories in term-born and preterm-born infants. Neuroimage. 2016;128:167–179. doi: 10.1016/j.neuroimage.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrou G, Martensson G, Skiold B, Blennow M, Aden U, Vollmer B. White matter microstructure is influenced by extremely preterm birth and neonatal respiratory factors. Acta Paediatr. 2014;103:48–56. doi: 10.1111/apa.12445. [DOI] [PubMed] [Google Scholar]
- Anjari M, Srinivasan L, Allsop JM, Hajnal JV, Rutherford MA, Edwards AD, Counsell SJ. Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. Neuroimage. 2007;35:1021–1027. doi: 10.1016/j.neuroimage.2007.01.035. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Blumenfeld-Katzir T, Yovel Y, Basser PJ. AxCaliber: a method for measuring axon diameter distribution from diffusion MRI. Magnetic Resonance in Medicine. 2008;59:1347–1354. doi: 10.1002/mrm.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J, Braddick O. Visual and visuocognitive development in children born very prematurely. Prog Brain Res. 2007;164:123–149. doi: 10.1016/S0079-6123(07)64007-2. [DOI] [PubMed] [Google Scholar]
- Back SA, Volpe JJ. Cellular and molecular pathogenesis of periventricular white matter injury. Mental Retardation and Developmental Disabilities Research Reviews. 1997;3:96–107. [Google Scholar]
- Ball G, Counsell SJ, Anjari M, Merchant N, Arichi T, Doria V, Rutherford MA, Edwards AD, Rueckert D, Boardman JP. An optimised tract-based spatial statistics protocol for neonates: applications to prematurity and chronic lung disease. Neuroimage. 2010;53:94–102. doi: 10.1016/j.neuroimage.2010.05.055. [DOI] [PubMed] [Google Scholar]
- Ball G, Srinivasan L, Aljabar P, Counsell SJ, Durighel G, Hajnal JV, Rutherford MA, Edwards AD. Development of cortical microstructure in the preterm human brain. Proc Natl Acad Sci U S A. 2013;110:9541–9546. doi: 10.1073/pnas.1301652110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baratti C, Barnett AS, Pierpaoli C. Comparative MR imaging study of brain maturation in kittens with T1, T2, and the trace of the diffusion tensor. Radiology. 1999;210:133–142. doi: 10.1148/radiology.210.1.r99ja09133. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ. Pediatric neuroimaging. New York, NY: Raven; 1995. [Google Scholar]
- Barkovich AJ, Kjos BO, Jackson DE, Jr, Norman D. Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology. 1988;166:173–180. doi: 10.1148/radiology.166.1.3336675. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B-Methodological. 1995;57:289–300. [Google Scholar]
- Brown TT, Kuperman JM, Chung Y, Erhart M, McCabe C, Hagler DJ, Jr, Venkatraman VK, Akshoomoff N, Amaral DG, Bloss CS, Casey BJ, Chang L, Ernst TM, Frazier JA, Gruen JR, Kaufmann WE, Kenet T, Kennedy DN, Murray SS, Sowell ER, Jernigan TL, Dale AM. Neuroanatomical assessment of biological maturity. Curr Biol. 2012;22:1693–1698. doi: 10.1016/j.cub.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Akazawa K, Yamakawa R, Hayama S, Buchthal S, Alicata D, Andres T, Castillo D, Oishi K, Skranes J, Ernst T, Oishi K. Delayed early developmental trajectories of white matter tracts of functional pathways in preterm-born infants: Longitudinal diffusion tensor imaging data. Data Brief. 2016;6:1007–1015. doi: 10.1016/j.dib.2016.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon KA, Kim YS, Oh SH, Park SY, Yoon HW, Herrington J, Nair A, Koh YJ, Jang DP, Kim YB, Leventhal BL, Cho ZH, Castellanos FX, Schultz RT. Involvement of the anterior thalamic radiation in boys with high functioning autism spectrum disorders: a Diffusion Tensor Imaging study. Brain Res. 2011;1417:77–86. doi: 10.1016/j.brainres.2011.08.020. [DOI] [PubMed] [Google Scholar]
- Christensen GE, Rabbitt RD, Miller MI. Deformable templates using large deformation kinematics. Ieee Transactions on Image Processing. 1996;5:1435–1447. doi: 10.1109/83.536892. [DOI] [PubMed] [Google Scholar]
- Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- Ding XQ, Kucinski T, Wittkugel O, Goebell E, Grzyska U, Gorg M, Kohlschutter A, Zeumer H. Normal brain maturation characterized with age-related T2 relaxation times: an attempt to develop a quantitative imaging measure for clinical use. Invest Radiol. 2004;39:740–746. doi: 10.1097/00004424-200412000-00005. [DOI] [PubMed] [Google Scholar]
- Dubois J, Benders M, Cachia A, Lazeyras F, Ha-Vinh Leuchter R, Sizonenko SV, Borradori-Tolsa C, Mangin JF, Huppi PS. Mapping the early cortical folding process in the preterm newborn brain. Cereb Cortex. 2008a;18:1444–1454. doi: 10.1093/cercor/bhm180. [DOI] [PubMed] [Google Scholar]
- Dubois J, Dehaene-Lambertz G, Perrin M, Mangin JF, Cointepas Y, Duchesnay E, Le Bihan D, Hertz-Pannier L. Asynchrony of the early maturation of white matter bundles in healthy infants: quantitative landmarks revealed noninvasively by diffusion tensor imaging. Hum Brain Mapp. 2008b;29:14–27. doi: 10.1002/hbm.20363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Hertz-Pannier L, Dehaene-Lambertz G, Cointepas Y, Le Bihan D. Assessment of the early organization and maturation of infants’ cerebral white matter fiber bundles: a feasibility study using quantitative diffusion tensor imaging and tractography. Neuroimage. 2006;30:1121–1132. doi: 10.1016/j.neuroimage.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Duncan JS, Bartlett P, Barker GJ. Technique for measuring hippocampal T2 relaxation time. American Journal of Neuroradiology. 1996;17:1805–1810. [PMC free article] [PubMed] [Google Scholar]
- Fryer SL, Frank LR, Spadoni AD, Theilmann RJ, Nagel BJ, Schweinsburg AD, Tapert SF. Microstructural integrity of the corpus callosum linked with neuropsychological performance in adolescents. Brain Cogn. 2008;67:225–233. doi: 10.1016/j.bandc.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Li X, Hou X, Ding A, Chan KC, Sun Q, Wu EX, Yang J. Tract-based spatial statistics (TBSS): application to detecting white matter tract variation in mild hypoxic-ischemic neonates. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:432–435. doi: 10.1109/EMBC.2012.6345960. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Lin W, Corouge I, Vetsa YS, Smith JK, Kang C, Gu H, Hamer RM, Lieberman JA, Gerig G. Early postnatal development of corpus callosum and corticospinal white matter assessed with quantitative tractography. AJNR Am J Neuroradiol. 2007;28:1789–1795. doi: 10.3174/ajnr.A0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst M, Zahneisen B, Knowles B, Zaitsev M, Ernst T. Prospective Motion Correction of Segmented Diffusion Weighted EPI. Magnetic Resonance in Medicine. 2015;74:1675–1681. doi: 10.1002/mrm.25547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland BA, Haas DK, Norman D, Brant-Zawadzki M, Newton TH. MRI of normal brain maturation. AJNR Am J Neuroradiol. 1986;7:201–208. [PMC free article] [PubMed] [Google Scholar]
- Huang H, Ceritoglu C, Li X, Qiu A, Miller MI, van Zijl PC, Mori S. Correction of B0 susceptibility induced distortion in diffusion-weighted images using large-deformation diffeomorphic metric mapping. Magn Reson Imaging. 2008;26:1294–1302. doi: 10.1016/j.mri.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Zhang J, Wakana S, Zhang W, Ren T, Richards LJ, Yarowsky P, Donohue P, Graham E, van Zijl PC, Mori S. White and gray matter development in human fetal, newborn and pediatric brains. Neuroimage. 2006;33:27–38. doi: 10.1016/j.neuroimage.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Huppi PS, Amato M. Advanced magnetic resonance imaging techniques in perinatal brain injury. Biol Neonate. 2001;80:7–14. doi: 10.1159/000047112. [DOI] [PubMed] [Google Scholar]
- Huppi PS, Maier SE, Peled S, Zientara GP, Barnes PD, Jolesz FA, Volpe JJ. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res. 1998;44:584–590. doi: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Jiang SZ, Xue H, Glover A, Rutherford M, Rueckert D, Hajnal JV. MRI of moving subjects using multislice snapshot images with volume reconstruction (SVR): Application to fetal, neonatal, and adult brain studies. Ieee Transactions on Medical Imaging. 2007;26:967–980. doi: 10.1109/TMI.2007.895456. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Pennock JM, Bydder GM, Steiner RE, Thomas DJ, Hayward R, Bryant DR, Payne JA, Levene MI, Whitelaw A, et al. Clinical NMR imaging of the brain in children: normal and neurologic disease. AJR Am J Roentgenol. 1983;141:1005–1018. doi: 10.2214/ajr.141.5.1005. [DOI] [PubMed] [Google Scholar]
- Lee BC, Lipper E, Nass R, Ehrlich ME, de Ciccio-Bloom E, Auld PA. MRI of the central nervous system in neonates and young children. AJNR Am J Neuroradiol. 1986;7:605–616. [PMC free article] [PubMed] [Google Scholar]
- Lepomaki V, Matomaki J, Lapinleimu H, Lehtonen L, Haataja L, Komu M, Parkkola R. Effect of antenatal growth on brain white matter maturation in preterm infants at term using tract-based spatial statistics. Pediatr Radiol. 2013;43:80–85. doi: 10.1007/s00247-012-2509-9. [DOI] [PubMed] [Google Scholar]
- Li Y, Shea SM, Lorenz CH, Jiang H, Chou MC, Mori S. Image corruption detection in diffusion tensor imaging for post-processing and real-time monitoring. PLoS One. 2013;8:e49764. doi: 10.1371/journal.pone.0049764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RJ, Fanaroff AA, Walsh MC. Fanaroff and Martin’s Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant. Elsevier Health Sciences; 2014. [Google Scholar]
- Melbourne A, Kendall GS, Cardoso MJ, Gunny R, Robertson NJ, Marlow N, Ourselin S. Preterm birth affects the developmental synergy between cortical folding and cortical connectivity observed on multimodal MRI. Neuroimage. 2014;89:23–34. doi: 10.1016/j.neuroimage.2013.11.048. [DOI] [PubMed] [Google Scholar]
- Ment LR, Hirtz D, Huppi PS. Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol. 2009;8:1042–1055. doi: 10.1016/S1474-4422(09)70257-1. [DOI] [PubMed] [Google Scholar]
- Miller MI, Christensen GE, Amit Y, Grenander U. Mathematical textbook of deformable neuroanatomies. Proc Natl Acad Sci U S A. 1993;90:11944–11948. doi: 10.1073/pnas.90.24.11944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MI, Ratnanather JT, Tward DJ, Brown T, Lee DS, Ketcha M, Mori K, Wang MC, Mori S, Albert MS, Younes L, Team BR. Network Neurodegeneration in Alzheimer’s Disease via MRI Based Shape Diffeomorphometry and High-Field Atlasing. Front Bioeng Biotechnol. 2015;3:54. doi: 10.3389/fbioe.2015.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, Karl T, Azakie A, Ferriero DM, Barkovich AJ, Vigneron DB. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357:1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- MiotNoirault E, Barantin L, Akoka S, LePape A. T2 relaxation time as a marker of brain myelination: Experimental MR study in two neonatal animal models. Journal of Neuroscience Methods. 1997;72:5–14. doi: 10.1016/s0165-0270(96)00148-3. [DOI] [PubMed] [Google Scholar]
- Morishita H, Hensch TK. Critical period revisited: impact on vision. Curr Opin Neurobiol. 2008;18:101–107. doi: 10.1016/j.conb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Mourmans J, Majoie CB, Barth PG, Duran M, Akkerman EM, Poll-The BT. Sequential MR imaging changes in nonketotic hyperglycinemia. AJNR Am J Neuroradiol. 2006;27:208–211. [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P, Miller JH, Shimony JS, Conturo TE, Lee BC, Almli CR, McKinstry RC. Normal brain maturation during childhood: developmental trends characterized with diffusion-tensor MR imaging. Radiology. 2001;221:349–358. doi: 10.1148/radiol.2212001702. [DOI] [PubMed] [Google Scholar]
- Neil J, Miller J, Mukherjee P, Huppi PS. Diffusion tensor imaging of normal and injured developing human brain - a technical review. NMR Biomed. 2002;15:543–552. doi: 10.1002/nbm.784. [DOI] [PubMed] [Google Scholar]
- Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665–675. doi: 10.1002/ana.410440413. [DOI] [PubMed] [Google Scholar]
- Nichols T, Hayasaka S. Controlling the familywise error rate in functional neuroimaging: a comparative review. Stat Methods Med Res. 2003;12:419–446. doi: 10.1191/0962280203sm341ra. [DOI] [PubMed] [Google Scholar]
- Nijman J, Gunkel J, de Vries LS, van Kooij BJ, van Haastert IC, Benders MJ, Kersbergen KJ, Verboon-Maciolek MA, Groenendaal F. Reduced occipital fractional anisotropy on cerebral diffusion tensor imaging in preterm infants with postnatally acquired cytomegalovirus infection. Neonatology. 2013;104:143–150. doi: 10.1159/000351017. [DOI] [PubMed] [Google Scholar]
- Nishimura DG. Principles of Magnetic Resonance Imaging. Stanford, California: Stanford University; 2010. [Google Scholar]
- Oishi K, Faria AV, Mori S. Advanced neonatal NeuroMRI. Magn Reson Imaging Clin N Am. 2012;20:81–91. doi: 10.1016/j.mric.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Faria AV, Yoshida S, Chang L, Mori S. Quantitative evaluation of brain development using anatomical MRI and diffusion tensor imaging. Int J Dev Neurosci. 2013;31:512–524. doi: 10.1016/j.ijdevneu.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Mori S, Donohue PK, Ernst T, Anderson L, Buchthal S, Faria A, Jiang H, Li X, Miller MI, van Zijl PC, Chang L. Multi-contrast human neonatal brain atlas: application to normal neonate development analysis. Neuroimage. 2011;56:8–20. doi: 10.1016/j.neuroimage.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura A, Hayakawa M, Tsuji T, Naganawa S, Watanabe K. Diffusion tensor imaging in infants with basal ganglia-thalamic lesions. Eur J Paediatr Neurol. 2008;12:412–416. doi: 10.1016/j.ejpn.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Ortibus E, Verhoeven J, Sunaert S, Casteels I, de Cock P, Lagae L. Integrity of the inferior longitudinal fasciculus and impaired object recognition in children: a diffusion tensor imaging study. Dev Med Child Neurol. 2012;54:38–43. doi: 10.1111/j.1469-8749.2011.04147.x. [DOI] [PubMed] [Google Scholar]
- Padilla N, Alexandrou G, Blennow M, Lagercrantz H, Aden U. Brain Growth Gains and Losses in Extremely Preterm Infants at Term. Cereb Cortex. 2015;25:1897–1905. doi: 10.1093/cercor/bht431. [DOI] [PubMed] [Google Scholar]
- Padilla N, Junque C, Figueras F, Sanz-Cortes M, Bargallo N, Arranz A, Donaire A, Figueras J, Gratacos E. Differential vulnerability of gray matter and white matter to intrauterine growth restriction in preterm infants at 12 months corrected age. Brain Res. 2014;1545:1–11. doi: 10.1016/j.brainres.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Panigrahy A, Bluml S. Advances in magnetic resonance neuroimaging techniques in the evaluation of neonatal encephalopathy. Top Magn Reson Imaging. 2007;18:3–29. doi: 10.1097/RMR.0b013e318093e6c7. [DOI] [PubMed] [Google Scholar]
- Paquette LB, Wisnowski JL, Ceschin R, Pruetz JD, Detterich JA, Del Castillo S, Nagasunder AC, Kim R, Painter MJ, Gilles FH, Nelson MD, Williams RG, Bluml S, Panigrahy A. Abnormal cerebral microstructure in premature neonates with congenital heart disease. AJNR Am J Neuroradiol. 2013;34:2026–2033. doi: 10.3174/ajnr.A3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar H, Sitoh YY, Ho L. Maple syrup urine disease: diffusion-weighted and diffusion-tensor magnetic resonance imaging findings. J Comput Assist Tomogr. 2004;28:93–97. doi: 10.1097/00004728-200401000-00015. [DOI] [PubMed] [Google Scholar]
- Partridge SC, Mukherjee P, Henry RG, Miller SP, Berman JI, Jin H, Lu Y, Glenn OA, Ferriero DM, Barkovich AJ, Vigneron DB. Diffusion tensor imaging: serial quantitation of white matter tract maturity in premature newborns. Neuroimage. 2004;22:1302–1314. doi: 10.1016/j.neuroimage.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Pistollato F, Chen HL, Panchision DM, Basso G. Effects of hypoxia on the proliferation of cells derived from cerebral tumors and oxygen-dependant modulation-depending on bmp and notch indirect signals. Haematologica-the Hematology Journal. 2007;92:9–10. [Google Scholar]
- Pogribna U, Yu X, Burson K, Zhou Y, Lasky RE, Narayana PA, Parikh NA. Perinatal clinical antecedents of white matter microstructural abnormalities on diffusion tensor imaging in extremely preterm infants. PLoS One. 2013;8:e72974. doi: 10.1371/journal.pone.0072974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter EJ, Counsell SJ, Edwards AD, Allsop J, Azzopardi D. Tract-based spatial statistics of magnetic resonance images to assess disease and treatment effects in perinatal asphyxial encephalopathy. Pediatr Res. 2010;68:205–209. doi: 10.1203/PDR.0b013e3181e9f1ba. [DOI] [PubMed] [Google Scholar]
- Rose SE, Hatzigeorgiou X, Strudwick MW, Durbridge G, Davies PS, Colditz PB. Altered white matter diffusion anisotropy in normal and preterm infants at term-equivalent age. Magnetic Resonance in Medicine. 2008;60:761–767. doi: 10.1002/mrm.21689. [DOI] [PubMed] [Google Scholar]
- Rousseau F, Glenn OA, Iordanova B, Rodriguez-Carranza C, Vigneron DB, Barkovich JA, Studholme C. Registration-based approach for reconstruction of high-resolution in utero fetal MR brain images. Academic Radiology. 2006;13:1072–1081. doi: 10.1016/j.acra.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Saksena S, Husain N, Malik GK, Trivedi R, Sarma M, Rathore RS, Pandey CM, Gupta RK. Comparative evaluation of the cerebral and cerebellar white matter development in pediatric age group using quantitative diffusion tensor imaging. Cerebellum. 2008;7:392–400. doi: 10.1007/s12311-008-0041-0. [DOI] [PubMed] [Google Scholar]
- Shim SY, Jeong HJ, Son DW, Jeong JS, Oh SH, Park SY, Ryu TH, Kim YB, Cho ZH. Altered microstructure of white matter except the corpus callosum is independent of prematurity. Neonatology. 2012;102:309–315. doi: 10.1159/000341867. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Yoshida S, Hsu J, Huisman TA, Faria AV, Oishi K, Kutten K, Poretti A, Li Y, Miller MI, Mori S. Multi-contrast multi-atlas parcellation of diffusion tensor imaging of the human brain. PLoS One. 2014;9:e96985. doi: 10.1371/journal.pone.0096985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35:147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DK, Inder TE, Faggian N, Johnston L, Warfield SK, Anderson PJ, Doyle LW, Egan GF. Characterization of the corpus callosum in very preterm and full-term infants utilizing MRI. Neuroimage. 2011;55:479–490. doi: 10.1016/j.neuroimage.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi R, Agarwal S, Rathore RK, Saksena S, Tripathi RP, Malik GK, Pandey CM, Gupta RK. Understanding development and lateralization of major cerebral fiber bundles in pediatric population through quantitative diffusion tensor tractography. Pediatr Res. 2009;66:636–641. doi: 10.1203/PDR.0b013e3181bbc6b5. [DOI] [PubMed] [Google Scholar]
- Tuch DS, Reese TG, Wiegell MR, Makris N, Belliveau JW, Wedeen VJ. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magnetic Resonance in Medicine. 2002;48:577–582. doi: 10.1002/mrm.10268. [DOI] [PubMed] [Google Scholar]
- Van Braeckel K, Butcher PR, Geuze RH, van Duijn MA, Bos AF, Bouma A. Less efficient elementary visuomotor processes in 7- to 10-year-old preterm-born children without cerebral palsy: an indication of impaired dorsal stream processes. Neuropsychology. 2008;22:755–764. doi: 10.1037/a0013212. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neurology of the Newborn. 3rd. Philadelphia, PA: WB Saunder; 1995. [Google Scholar]
- Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY, Dai G, Pandya DN, Hagmann P, D’Arceuil H, de Crespigny AJ. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage. 2008;41:1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Whittall KP, MacKay AL, Graeb DA, Nugent RA, Li DK, Paty DW. In vivo measurement of T2 distributions and water contents in normal human brain. Magnetic Resonance in Medicine. 1997;37:34–43. doi: 10.1002/mrm.1910370107. [DOI] [PubMed] [Google Scholar]
- Wiegell MR, Larsson HB, Wedeen VJ. Fiber crossing in human brain depicted with diffusion tensor MR imaging. Radiology. 2000;217:897–903. doi: 10.1148/radiology.217.3.r00nv43897. [DOI] [PubMed] [Google Scholar]
- Wimberger DM, Roberts TP, Barkovich AJ, Prayer LM, Moseley ME, Kucharczyk J. Identification of “premyelination” by diffusion-weighted MRI. J Comput Assist Tomogr. 1995;19:28–33. doi: 10.1097/00004728-199501000-00005. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- Wu D, Chang L, Akazawa K, Oishi K, Skranes J, Ernst T, Oishi K. Change-point analysis of neonatal diffusion tensor MRI in preterm and term-born infants. Data Brief Submitted. 2017 doi: 10.1016/j.dib.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap QJ, Teh I, Fusar-Poli P, Sum MY, Kuswanto C, Sim K. Tracking cerebral white matter changes across the lifespan: insights from diffusion tensor imaging studies. J Neural Transm (Vienna) 2013;120:1369–1395. doi: 10.1007/s00702-013-0971-7. [DOI] [PubMed] [Google Scholar]
- Yoo SS, Park HJ, Soul JS, Mamata H, Park H, Westin CF, Bassan H, Du Plessis AJ, Robertson RL, Jr, Maier SE, Ringer SA, Volpe JJ, Zientara GP. In vivo visualization of white matter fiber tracts of preterm- and term-infant brains with diffusion tensor magnetic resonance imaging. Invest Radiol. 2005;40:110–115. doi: 10.1097/01.rli.0000149491.69201.cb. [DOI] [PubMed] [Google Scholar]
- Younes L, Albert M, Miller MI, Team BR. Inferring changepoint times of medial temporal lobe morphometric change in preclinical Alzheimer’s disease. Neuroimage Clin. 2014;5:178–187. doi: 10.1016/j.nicl.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage. 2012;61:1000–1016. doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]
- Zhang L, Thomas KM, Davidson MC, Casey BJ, Heier LA, Ulug AM. MR quantitation of volume and diffusion changes in the developing brain. AJNR Am J Neuroradiol. 2005;26:45–49. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.