Abstract
High resolution manometry (HRM) categorizes esophageal motor processes into specific Chicago Classification (CC) diagnoses, but the clinical impact of these motor diagnoses on symptom burden remain unclear.
Methods
211 subjects (56.8±1.0 yrs, 66.8% F) completed symptom questionnaires [GERDQ, Mayo dysphagia questionnaire (MDQ), visceral sensitivity index (VSI), short-form 36 (SF-36), dominant symptom index (DSI), and global symptom severity (GSS) on a 100-mm visual analog scale] prior to HRM. Subjects were stratified according to CC v3.0 and by dominant presenting symptom; contraction wave abnormalities (CWA) were evaluated within ‘normal’ CC. Symptom burden, impact of diagnoses, and HRQOL were compared within and between cohorts.
Results
Major motor disorders had highest global symptom burden (p=0.02), ‘normal’ had lowest (p<0.01). Dysphagia (MDQ) was highest with esophageal outflow obstruction (p=0.02), but reflux symptoms (GERDQ) were similar in CC cohorts (p=ns). Absent contractility aligned best with minor motor disorders. Consequently, pathophysiologic categorization into outflow obstruction, hypermotility, and hypomotility resulted in a gradient of decreasing dysphagia and increasing reflux burden (p<0.05 across groups); GSS (p=0.05) was highest with hypomotility and lowest with ‘normal’ (p=0.002). Within the ‘normal’ cohort, 33.3% had CWA; this subgroup had symptom burden similar to hypermotility. Upon stratification by symptoms, symptom burden (GSS, MDQ, HRQOL) was most profound with dysphagia.
Conclusions
CC v3.0 diagnoses identify subjects with highest symptom burden, but pathophysiologic categorization may allow better stratification by symptom type and burden. CWA are clinically relevant and different from true normal motor function. Transit symptoms have highest yield for a motor diagnosis.
Keywords: dysphagia, symptom burden, high resolution manometry, Chicago Classification
Graphical abstract
The interrelationship between esophageal symptom characteristics, symptom burden, and motor diagnoses (Chicago Classification v 3.0) were further studied by obtaining validated self-report questionnaires in 211 patients undergoing esophageal high resolution manometry (HRM). Chicago Classification diagnoses (outflow obstruction, major disorders) were associated with the highest symptom burden. Symptom characteristics were best characterized by pathophysiologic categorization of motor disorders into outflow obstruction, hypermotility disorders and hypomotility disorders. Contraction wave abnormalities in patients without a motor disorder (according to Chicago Classification) had distinct symptom characteristics and symptom burden that aligned best with hypermotility disorders.
High-resolution manometry (HRM) has improved interpretation of esophageal motor pathophysiology, and consequently, better decision-making prior to endoscopic or surgical interventions in achalasia spectrum disorders, esophageal outflow obstruction, and gastroesophageal reflux disease (GERD)1-3. Software metrics including integrated relaxation pressure (IRP), distal contractile integral (DCI), and distal latency (DL) have refined description of esophagogastric junction (EGJ) and esophageal body processes2. The Chicago classification (CC), originally developed in 2009 and revised in 2012 and 2015, uses these software metrics to categorize esophageal motor patterns, making nomenclature and reporting of motor diagnoses uniform2.
The health impact of motor diagnoses is most profound in the achalasia spectrum group. Patients with achalasia have symptoms that distinguish them from the general population, are extremely symptomatic at diagnosis, and symptom scores (i.e. Eckardt score) significantly improve after LES disruption4, 5. Beyond achalasia spectrum disorders, there is only limited data available addressing the symptomatic outcome of esophageal motor patterns, with one study suggesting that patients with normal patterns or minor motor abnormalities report minimal symptoms requiring only limited intervention over time6. Thus, it is unclear if CC is discriminative in terms of symptoms and quality of life among the various CC diagnoses beyond achalasia, and if there is correlation between symptom burden and findings on esophageal motor testing.
Therefore, the objectives of this study are two-fold: a) to assess the relationship between esophageal symptom burden and esophageal motor diagnoses, and b) to determine the influence of motor diagnoses on symptom burden as assessed by validated measures.
METHODS
Consecutive adult patients (>18 years) undergoing esophageal HRM using the Given-Medtronic system (Medtronic, Duluth, GA) over a one year period (2014-2015) at our open access tertiary care motility center were eligible. For inclusion, all subjects completed symptom assessment questionnaires immediately before HRM. Exclusion criteria consisted of incomplete esophageal HRM and incomplete symptom data. Informed consent was obtained from each subject to include review of clinical data and completion of survey questions related to the study. The study protocol including review of HRM studies and clinical records was approved by the Human Research Protection Office (Institutional Review Board) at Washington University in St. Louis.
HRM Procedure and Analysis
HRM studies were performed after an overnight fast using a 36-channel solid-state catheter system with high fidelity circumferential sensors at 1 cm intervals (Medtronic, Duluth, GA), as previously described7, 8. After calibration, the catheter was passed through an anesthetized nasal canal, and taped to the nose after adequate positioning. A 20 second swallow-free period was obtained with the subject resting quietly in the recumbent position (landmark period), from which basal LES pressures were obtained. Ten swallows were recorded using 4-5 mL of ambient temperature water spaced >20 s apart. Studies were acquired and analyzed using dedicated computerized HRM acquisition, display and analysis systems (ManoView; Medtronic, Duluth, GA).
For analysis, the landmark phase recording was first identified and confirmed to be separate from swallows and artifacts, obtained during a period of quiet rest after the patient settled down7. All studies were analyzed according to CC v3.02, using software metrics (IRP, DCI, DL)2. Motor abnormalities not meeting CC criteria were identified as contraction wave abnormalities (CWA, manifest as multi-peaked waves, broad contraction segments, distal shift in contraction vigor, DCI>5000 but <8000 mmHg.cm.s and rapid sequences with contraction front velocity >8 cm/s)9-11.
Symptom Assessment
Prior to HRM, all subjects completed symptom questionnaires to characterize their symptom burden and health related quality of life (HRQOL). Each subject identified a dominant symptom from the following presentations: difficulty swallowing liquids or solid foods, heartburn, regurgitation, chest pain, belching, cough, and wheezing. The dominant symptom was further grouped as dysphagia (difficulty swallowing liquids or solid foods), potentially reflux related (heartburn, regurgitation, or chest pain), or nonspecific (belching, cough, or wheezing). Reflux, transit symptoms, and overall symptomatic status were assessed to characterize symptom character, severity, and impact. Reflux severity was calculated using six questions comprising the GERDQ12, and a subset of these questions determined the impact score. Transit symptoms were assessed by the Mayo Dysphagia Questionnaire (MDQ)13, which quantitated dysphagia severity (scaled 1-5, with a score of 5 being the most severe) and dysphagia frequency (scaled 1-6, with a score of 6 being the most severe) when present. Overall global symptom severity (GSS) was recorded on a 100-mm visual analog scale (VAS)14. Visceral Sensitivity Index (VSI) and Short-form 36 (SF-36) assessed HRQOL15, 16. The sensitivity index was calculated by the addition of fifteen questions scaled 1-6, with a score of 1 being the most severe. Total SF-36 scores including mental and physical sub-scores were calculated.
Study Groups
The study cohorts were analyzed in two separate settings as described below:
1. Subjects were first categorized based on their CC motor diagnoses as follows a) esophageal outflow obstruction [achalasia spectrum or esophagogastric junction outflow obstruction (EGJOO)] b) major disorders of peristalsis [absent contractility, hypercontractile esophagus, or diffuse esophageal spasm (DES)] c) minor disorders of peristalsis [ineffective esophageal motility (IEM) or fragmented peristalsis] and d) normal2. Subjects designated normal by CC criteria were sub-divided into a) contraction wave abnormalities (CWA) b) true normal.
2. Subjects were then segregated by dominant symptom group, which comprised of dysphagia (difficulty swallowing liquids or solids), reflux-related (heartburn, regurgitation, or chest pain), or nonspecific (belching, cough, or wheezing).
Data Analysis
Continuous variables are reported as mean ± standard error of the mean (SEM) or median (interquartile range). Categorical data are reported using frequencies and proportions. Continuous data were compared using the 2-tailed Student’s t-test or ANOVA and categorical data were compared using the chi-squared test or Fisher’s exact test as appropriate. In all cases, p<0.05 was required for statistical significance. All statistical analyses were performed using IBM SPSS 23 (Chicago, IL).
RESULTS
During the study period, 211 subjects (56.8 ±1.0 years, 66.8% female) fulfilled all study inclusion criteria. On CC 3.0 analysis, normal manometry (i.e. not meeting any of the CC 3.0 motor diagnoses) was the most common pattern, in 120 subjects (55.9 ±1.4 years, 68.3% female, Table 1). The remaining 91 subjects met criteria for an abnormal CC 3.0 diagnosis, as follows: 33 (15.6%) with outflow obstruction, 19 (9.0%) with a major motor disorder, and 39 (18.4%) with a minor motor disorder. Among the 120 normal subjects, 40 (33.3%) had esophageal body CWA not meeting CC. The remaining 80 subjects were identified as “true normal.” 199 patients identified a dominant symptom prompting esophageal evaluation.
Table 1.
Symptom Burden and HRQOL Across Chicago Classification 3.0 Diagnoses
| All | EGJ outflow obstruction |
Major motor disorders |
Minor motor disorders |
Normal | p-value | |
|---|---|---|---|---|---|---|
| n=211 | n=33 | n=19 | n=39 | n=120 | ||
| Age | 56.8±1.0 | 61.2±2.4 | 59.7±3.4 | 54.8±2.3 | 55.9±1.4 | 0.18 |
| Gender (% F) | 66.8% | 60.6% | 68.4% | 66.7% | 68.3% | 0.87 |
| GERDQ | 9.4±0.2 | 9.2±0.4 | 9.0±0.8 | 9.5±0.6 | 9.5±0.3 | 0.89 |
| GERDQ Impact | 3.0±0.2 | 2.8±0.3 | 3.3±0.5 | 3.0±0.4 | 3.0±0.2 | 0.88 |
| MDQ (%) | 64.6% | 81.8% | 63.2% | 61.5% | 61.0% | 0.049 |
| MDQ severity | 2.4±0.1 | 2.8±0.2 | 2.6±0.3 | 2.1±0.2 | 2.3±0.1 | 0.05 |
| MDQ frequency | 2.3±0.1 | 2.9±0.2 | 2.2±0.3 | 1.9±0.2 | 2.3±0.1 | 0.01 |
| SF-36 | 56.6±1.6 | 52.2±3.9 | 53.2±3.8 | 57.8±4.1 | 58.0±2.2 | 0.55 |
| VSI | 59.5±1.5 | 60.4±3.8 | 58.8±4.7 | 62.2±3.6 | 58.5±2.0 | 0.82 |
| GSS | 61.7±1.8 | 66.1±4.2 | 75.2±5.6 | 66.1±4.3 | 57.0±2.4 | 0.01 |
SF-36: Short Form 36; MDQ: Mayo Dysphagia Questionnaire, VSI: Visceral Sensitivity Index; GSS: Global Symptom Severity
Symptom Burden and CC Motor Patterns
As expected, proportions with dysphagia, MDQ frequency and severity scores were highest with esophageal outflow obstruction compared to other CC 3.0 diagnostic categories (Table 1, Figure 1). Symptom burden measured by GSS was highest with major motor disorders, and lowest with normal studies (Table 1, p=0.01). Within patients with major motor disorders, there were significant differences between absent contractility on the one hand and hypermotility disorders (hypercontractile esophagus, DES) on the other hand. In particular, GERDQ impact scores demonstrated a gradient, with values higher in absent contractility compared to minor motor disorders and hypermotility disorders (Table 2, p<0.05). Consequently, absent contractility aligned better with minor motor disorders as part of the pathophysiologic category of hypomotility disorders (Figure 1). In comparing cohorts based on such pathophysiologic grouping, hypomotility subjects had highest GERD impact scores, proportions with reflux symptoms and symptom burden (GSS 68.5 ±3.9, p<0.05 compared to other groups, Table 3). In contrast, proportions with dysphagia symptoms were higher with outflow obstruction and hypermotility disorders in comparison to other groups, further supporting such pathophysiologic grouping.
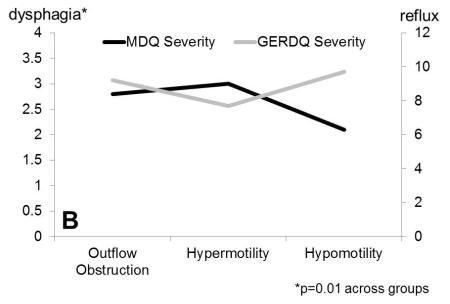
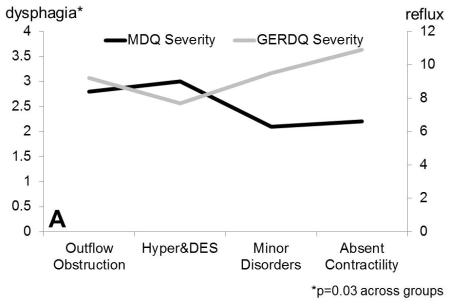
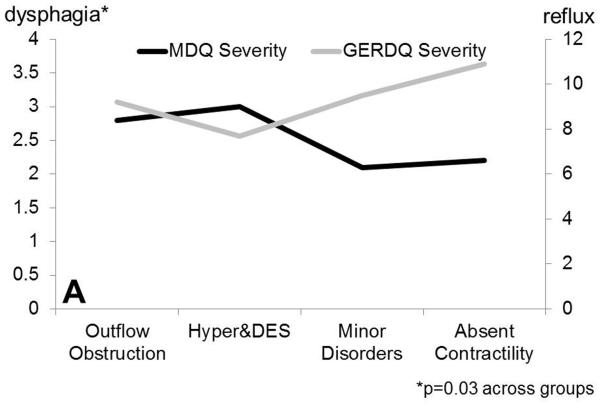
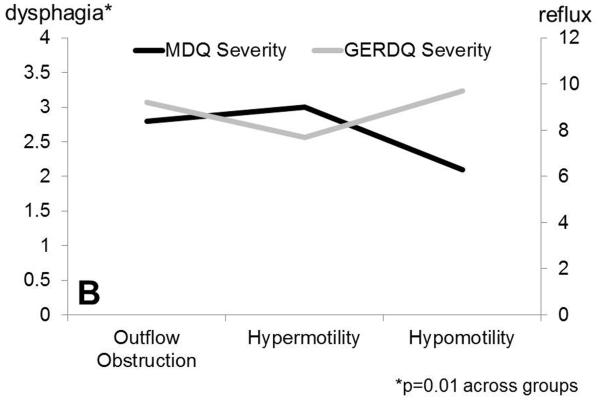
Figure 1.
Dysphagia severity (Mayo Dysphagia Questionnaire) and GERDQ impact score across motor groups. A. Dysphagia severity and GERDQ impact scores follow gradients in opposite directions when absent contractility is extracted out of the major motor disorders category. B. Similar gradients are maintained when motor disorders are grouped by pathophysiologic mechanisms to outflow obstruction, hypermotility disorders and hypomotility disorders, where absent contractility is included with hypomotility disorders.
Table 2.
Absent Contractility Aligns Better with Hypomotility Disorders
| Hypercontractile esophagus & DES |
Minor motor Disorders |
Absent Contractility |
|
|---|---|---|---|
| n=10 | n=39 | n=9 | |
| GERDQ | 7.7±1.0 | 9.5±0.6 | 10.9±1.1* |
| GERDQ Impact | 2.4±0.7 | 3.0±0.4 | 4.6±0.6** |
| MDQ (%) | 70.0% | 61.5% | 55.6% |
| MDQ severity | 3.0±0.4 | 2.1±0.2 | 2.2±0.5 |
| MDQ frequency | 2.6±0.4 | 1.9±0.2 | 1.8±0.3 |
| SF-36 | 58.9±4.6 | 57.8±4.1 | 46.9±5.8 |
| VSI | 60.5±6.7 | 62.2±3.6 | 56.9±7.0 |
| GSS | 72.4±7.3 | 66.1±4.3 | 78.8±9.0 |
SF-36: Short Form 36; MDQ: Mayo Dysphagia Questionnaire, VSI: Visceral Sensitivity Index; GSS: Global Symptom Severity; DES: diffuse esophageal spasm.
Hypercontractile esophagus, DES and absent contractility constitute major disorders of peristalsis
p=0.05 compared to Hypercontractile & DES
p<0.05 compared to Hypercontractile & DES
Table 3.
Symptom Burden and HRQOL Across Pathophysiologic Motor Groups
| All | EGJ outflow obstruction |
Hypermotility | Hypomotility | Normal | p-value | |
|---|---|---|---|---|---|---|
| n=211 | n=33 | n=10 | n=48 | n=120 | ||
| Age | 56.8±1.0 | 61.2±2.4 | 58.1±5.1 | 56.1±2.1 | 55.9±1.4 | 0.31 |
| Gender (%F) | 66.8% | 60.6% | 70.0% | 66.7% | 68.3% | 0.86 |
| GERDQ | 9.4±0.2 | 9.2±0.4 | 7.7±1.0 | 9.7±0.5 | 9.5±0.3 | 0.27 |
| GERDQ Impact | 3.0±0.2 | 2.8±0.3 | 2.4±0.7 | 3.2±0.3 | 3.0±0.2 | 0.68 |
| MDQ (%) | 64.6% | 81.8% | 70.0% | 60.4% | 61.0% | 0.03 |
| MDQ severity | 2.4±0.1 | 2.8±0.2 | 3.0±0.4 | 2.1±0.2 | 2.3±0.1 | 0.02 |
| MDQ frequency | 2.3±0.1 | 2.9±0.2 | 2.6±0.4 | 1.9±0.1 | 2.3±0.1 | 0.004 |
| SF-36 | 56.6±1.6 | 52.2±3.9 | 58.9±4.6 | 55.8±3.6 | 58.0±2.2 | 0.63 |
| VSI | 59.5±1.5 | 60.4±3.8 | 60.5±6.7 | 61.2±3.2 | 58.5±2.0 | 0.89 |
| GSS | 61.7±1.8 | 66.1±4.2 | 72.4±7.3 | 68.5±3.9 | 57.0±2.4 | 0.02 |
SF-36: Short Form 36; MDQ: Mayo Dysphagia Questionnaire, VSI: Visceral Sensitivity Index; GSS: Global Symptom Severity
Hypermotility: CC v3.0 hypercontractile disorder and diffuse esophageal spasm
Hypomotility: CC v3.0 absent contractility, ineffective esophageal motility, and fragmented peristalsis
Symptom burden metrics were evaluated in the sub-analysis of the 120 CC normal subjects (Table 4). CWA had significantly lower GERDQ and impact scores (8.6±0.4, p=0.03 and 2.3±0.3, p=0.02) compared to true normal. 85.0% of subjects with CWA had dysphagia on the MDQ compared to only 48.7% in the true normal group (p<0.001). Further, these subjects also had higher dysphagia severity/frequency on the MDQ (2.7±0.2, p<0.01 and 2.7±0.2, p=0.01).
Table 4.
Symptom Burden and HRQOL in “Normal” Motor Patterns Compared to Hypermotility Disorders
| True Normal | CWA | Hypermotility | |
|---|---|---|---|
| n=80 | n=40 | n=10 | |
| GERDQ | 9.9±0.49.9± | 8.6±0.4* | 7.7±1.0 |
| GERDQ Impact | 3.4±0.3 | 2.3±0.3* | 2.4±0.7 |
| MDQ (%) | 48.7% | 85.0%* | 70.0% |
| MDQ severity | 2.1±0.1 | 2.7±0.2* | 3.0±0.4 |
| MDQ frequency | 2.1±0.2 | 2.7±0.2* | 2.6±0.4 |
| SF-36 | 54.3±2.7 | 55.4±4.0 | 58.9±4.6 |
| VSI | 58.9±2.5 | 59.5±3.4 | 60.5±6.7 |
| GSS | 58.9±5.3 | 53.3±4.3 | 72.4±7.3 |
p<0.03 compared to true normal; SF-36: Short Form 36; MDQ: Mayo Dysphagia Questionnaire, VSI: Visceral Sensitivity Index; GSS: Global Symptom Severity
CWA was most similar to CC hypermotility, demonstrating no significant differences in symptom burden or HRQOL when compared to this category (Table 4). When CWA was combined with CC diagnosis of hypermotility, the expanded hypermotility group had significantly higher proportions with dysphagia, higher dysphagia severity/frequency compared to hypomotility (p<0.02) on the MDQ. Hypomotility subjects had higher GERDQ, GERDQ impact, and GSS compared to expanded hypermotility (p≤0.05).
Dominant Symptom Analysis
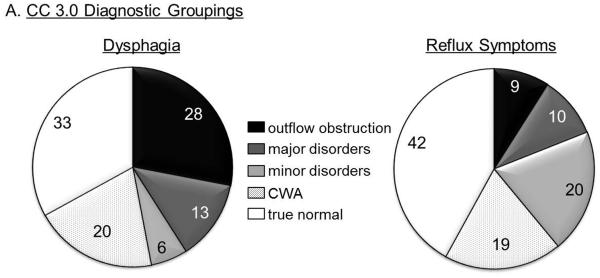
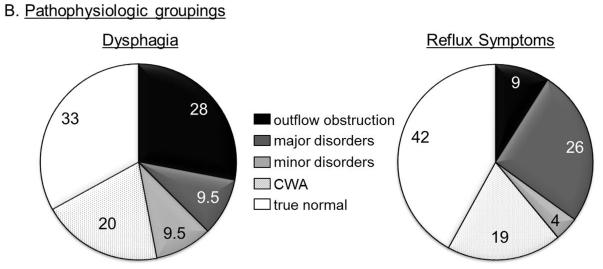
Subjects who identified dysphagia as a dominant symptom had the highest likelihood of being diagnosed with outflow obstruction (28%) or a major motor disorder (13%) on HRM (Figure 2). The likelihood of a normal study was lowest with a dysphagia predominant presentation, compared to reflux symptoms or nonspecific symptoms. In contrast, 26% of subjects complaining of primary reflux had a hypomotility disorder making this the most common abnormal motor pattern in those with a reflux predominant presentation (Figure 2). The nonspecific symptom group had the lowest GSS values (54.6±3.3, p=0.02 compared to other groups) among all the symptom groups.
Figure 2.
Proportions of motor diagnosis according to presenting symptom. A. Chicago Classification 3.0 diagnosis groups. The ‘normal’ group is further categorized into those with contraction wave abnormalities (CWA) and true normal. B. Pathophysiologic groups. The hypermotility group includes diffuse esophageal spasm and hypercontractile disorder (jackhammer esophagus), and the hypomotility group includes ineffective esophageal motility, fragmented peristalsis and absent contractility.
HRQOL
Averaged SF-36 and VSI raw scores for total subjects, those within CC diagnostic groups, CC normal subjects, and pathophysiologic groups are described in tables 1-3. SF-36 scores were the lowest in patients with a dominant symptom of dysphagia and this trended towards significance when compared to other groups (52.6±3.1, p=0.07). There were no other major differences in SF-36 or VSI scores among the study groups. Further, QOL did not correlate with individual HRM metrics.
DISCUSSION
In this study, we demonstrate that CC v3.0 identifies HRM motor patterns with the most profound symptom character, burden, and impact; outflow obstruction is associated with the highest proportion and burden of transit symptoms. Pathophysiologic groupings (outflow obstruction, hypermotility, hypomotility) demonstrate gradients of symptom character and burden better than current breakdown into major and minor motor disorders. In particular, hypomotility patterns are associated with more reflux symptoms and global symptom severity. We demonstrate that CWA are relevant from a symptom burden standpoint despite not identified as abnormal by CC; these align best with hypermotility disorders. Subjects who identified a transit dominant symptom (dysphagia) had higher symptom burden, lower HRQOL, and a higher likelihood of a motor abnormality identified on HRM. Based on these findings, esophageal motor testing has highest yield for a motor diagnosis in patients with transit symptoms, and use of CC designations appropriately segregate patients with most profound esophageal symptoms.
Symptom scores have been utilized in assessing transit symptoms in settings with extreme motor dysfunction, particularly achalasia, where the Eckardt score has been a standard. Higher Eckardt scores indicate greater symptomatic burden, and significantly lower Eckardt scores are reported following successful LES disruption compared to those with a persistent achalasia pattern4, 5. GERDQ scores have been used in the assessment of symptomatic reflux following achalasia management, with higher scores indicating greater reflux burden. While there are studies illustrating the use of symptom questionnaires to assess achalasia outcomes, there is no unified score that assesses symptoms in other motor diagnoses. This has necessitated the use of symptom specific scores in assessing symptom burden, such as MDQ for dysphagia, and GERDQ for reflux symptoms. In this study, we identified dominant symptoms using questionnaire designation. Similarly, global esophageal symptoms over the previous two weeks were characterized on VAS using GSS14, 17. The dominant symptom identification and GSS allowed assessment of both dominant and global symptoms, and facilitated follow-up of esophageal symptoms over time in our study cohort. Other assessment tools that are not specific to the esophagus focus on the impact of gastrointestinal symptoms on overall quality of life and functionality, e.g. the short form 36, which has both physical and mental components, and the VSI12,13.
In analyzing our data, it is evident that higher symptomatic scores are associated with a greater yield of a CC diagnosis. We demonstrate that high burden of dysphagia assessed by MDQ suggests patterns such as esophageal outflow obstruction and hypermotility. High reflux symptom burden specifically assessed by the GERDQ portends a higher likelihood of having a hypomotility pattern on HRM, which are known to be associated with GERD18. We report that subjects with dominant specific symptoms particularly dysphagia or reflux tend to have the most profound CC diagnoses, particularly when GSS is >70, and dysphagia is rated at least moderate or occurring daily. In contrast, GSS in the lower half of the spectrum, nonspecific dominant symptoms, and dysphagia rated as mild and infrequent was less likely to be associated with a CC diagnosis. These findings further support careful history taking and symptom evaluation in planning esophageal physiologic testing.
Our symptom burden data supports the current algorithms utilized by CC 3.0 in identifying profound motor disorders, with highest symptom burden in esophageal outflow obstruction. Therefore, the current hierarchical approach of evaluating first for esophageal outflow obstruction is validated by these findings. We demonstrate that absent contractility clusters better with hypomotility disorders, as this disorder was associated with higher reflux burden compared to transit symptom burden. Such clustering may also have pathophysiologic implications, as hypomotility disorders are associated with higher reflux symptoms, while the remaining major motor disorders (hypercontractile esophagus, DES) are associated with predominantly transit symptoms. Thus, pathophysiologic categorization of motor disorders may have clinical value from a symptom presentation standpoint, and potentially, therapeutic value, since management options vary according to pathophysiologic designation.
While the majority of subjects presenting with reflux symptoms could be expected to have a normal study, it is striking that over half of patients presenting with transit symptoms such as dysphagia are considered normal by CC v 3.0. In particular, some of the ‘normal’ CC diagnostic cohort had CWA, discussed in detail below. The remainder 33% of dysphagia patients had a true normal study, suggesting that either alternate evaluation for transit abnormalities was inadequate, or these patients had true ‘perceptive’ functional dysphagia19. As newer technologies (e.g. functional luminal imaging probe) are utilized in assessing esophageal motor function, it is anticipated that the proportion of dysphagia unexplained by esophageal function testing will decline20.
CWA consist of abnormal peristaltic contour, timing and vigor patterns that do not fulfil criteria for a CC diagnosis9-11. CWA had significantly greater prevalence, severity, and frequency of dysphagia while having less reflux symptoms compared to true normal. Our findings also suggest that patients with dysphagia rated as moderate and occurring on daily basis along with a lower reflux burden (GERDQ scores less than 9) have a greater chance of having a CWA motor pattern on HRM despite not meeting a CC diagnosis. Reflux patients with CWA have been noted to have a higher likelihood of persisting perceptive symptoms despite successful management of reflux disease with antireflux surgery21. Patients with higher contraction amplitudes and CWA have been noted to have lower pain perception thresholds on balloon distension studies22, higher reactivity and reduced compliance in the esophagus on impedance planimetry when presenting with chest pain23, and associated with esophageal acid sensitivity on ambulatory pH monitoring10. Further, cohorts with non-obstructive dysphagia and CWA have been demonstrated to have lower esophageal perception thresholds, higher reproduction of symptoms, as well as reproduction of CWA during balloon distension studies24, 25. Limited electroencephalographic evidence suggests abnormal cortical control of swallowing favoring hypervigilance, and potentially impacting downstream motor pathways26. Based on this data, CWA could be a marker of esophageal hypervigilance 21-23, 27, and could represent a unique motor entity.
The strengths of our study are that we evaluated consecutive patients presenting for esophageal HRM, and used validated tools to assess symptom data. However, this could also be a limitation, and a data set limited to non-obstructive dysphagia could have provided better description of metrics correlating with dysphagia. Self-report questionnaires can also introduce subjectivity and can be a limitation, as they are vulnerable to a variety of confounders. While we used several instruments and analyses, some patient groups including outflow obstruction and hypermotility had relatively small samples sizes, which limited comparisons between CC and pathophysiologic categories. This reflects the low prevalence of certain categories of motor disorders, even at high volume tertiary centers. Since our motility facility is open access, the study investigators could not control the quality of prior esophageal evaluation prior to referral for manometry – this could have impacted how well structural or mucosal esophageal processes were excluded prior to evaluation for motor mechanisms for persisting symptoms. Our study did not evaluate other physiological factors that could contribute to esophageal symptoms and quality of life such as bolus flow measured by impedance, esophageal hypersensitivity, esophageal wall compliance, or EGJ distensibility. Management outcome following CC diagnoses was not available in this study, and could have provided additional insight into change in symptom burden metrics with time. Nevertheless, we feel our study provides validation for diagnostic categories under CC v3.0, describes categories within normal CC cohorts (i.e. CWA) that deserve further evaluation, and helps generate hypotheses for future research.
In conclusion, CC v3.0 isolates HRM motor patterns and diagnoses in terms of symptoms character, burden, and impact. The presence of specific dominant symptoms and greater symptom burden increases the yield of discovering motor abnormalities on esophageal motor testing. Global and specific symptom scores can be used alone or in combination to increase the yield of discovering clinically relevant motor abnormalities, and aid in selecting patients to undergo further esophageal motor testing. This is particularly useful at centers where advanced esophageal motor testing modalities and esophageal experts are not readily available. Further prospective research may help define changes in symptom burden over time, and help refine our understanding of how motor disorders generate symptoms.
Key Points.
High resolution manometry (HRM) categorizes motor patterns according to the Chicago Classification, but correlation between symptoms and motor diagnoses, and the health impact of these diagnoses remain unclear. We used validated questionnaires assessing symptom burden and characteristics in patients undergoing esophageal HRM for investigation of esophageal symptoms.
Chicago Classification designations (outflow obstruction, major disorders) identify patients with the highest symptom burden. Symptom characteristics are better stratified by pathophysiologic categorization into outflow obstruction, hypermotility disorders and hypomotility disorders.
Contraction wave abnormalities in patients with a ‘normal’ Chicago Classification designation have symptom burden and characteristics different from normal HRM, and these align best with the hypermotility disorders.
ACKNOWLEDGMENTS
This study was partially funded through NIH/NIDDK (T32 DK007130–AP), the Washington University Department of Medicine Mentors in Medicine (MIM), and Clinical Science Training and Research (CSTAR) programs.
Footnotes
Author roles: CR: study design, data collection, manuscript preparation and review; AP: data analysis, critical review of manuscript; CPG: study concept and design, data analysis, manuscript preparation, critical review and final approval of manuscript
CPG is a consultant and in the speaker bureau for Medtronic, Inc.; he also has research funding from Medtronic. None of the other authors have any relevant conflicts to declare.
REFERENCES
- 1.Gyawali CP, Bredenoord AJ, Conklin JL, et al. Evaluation of esophageal motor function in clinical practice. Neurogastroenterol Motil. 2013;25:99–133. doi: 10.1111/nmo.12071. [DOI] [PubMed] [Google Scholar]
- 2.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160–74. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan WW, Haroian LR, Gyawali CP. Value of preoperative esophageal function studies before laparoscopic antireflux surgery. Surg Endosc. 2011;25:2943–9. doi: 10.1007/s00464-011-1646-9. [DOI] [PubMed] [Google Scholar]
- 4.Teitelbaum EN, Soper NJ, Santos BF, et al. Symptomatic and physiologic outcomes one year after peroral esophageal myotomy (POEM) for treatment of achalasia. Surg Endosc. 2014;28:3359–65. doi: 10.1007/s00464-014-3628-1. [DOI] [PubMed] [Google Scholar]
- 5.Nicodeme F, de Ruigh A, Xiao Y, et al. A comparison of symptom severity and bolus retention with Chicago classification esophageal pressure topography metrics in patients with achalasia. Clin Gastroenterol Hepatol. 2013;11:131–7. doi: 10.1016/j.cgh.2012.10.015. quiz e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravi K, Friesen L, Issaka R, et al. Long-term Outcomes of Patients With Normal or Minor Motor Function Abnormalities Detected by High-resolution Esophageal Manometry. Clin Gastroenterol Hepatol. 2015;13:1416–23. doi: 10.1016/j.cgh.2015.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel A, Ding A, Mirza F, et al. Optimizing the high-resolution manometry (HRM) study protocol. Neurogastroenterol Motil. 2015;27:300–4. doi: 10.1111/nmo.12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Price LH, Li Y, Patel A, et al. Reproducibility patterns of multiple rapid swallows during high resolution esophageal manometry provide insights into esophageal pathophysiology. Neurogastroenterol Motil. 2014;26:646–53. doi: 10.1111/nmo.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gyawali CP, Kushnir VM. High-resolution manometric characteristics help differentiate types of distal esophageal obstruction in patients with peristalsis. Neurogastroenterol Motil. 2011;23:502–e197. doi: 10.1111/j.1365-2982.2011.01672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kushnir VM, Prakash Gyawali C. High resolution manometry patterns distinguish acid sensitivity in non-cardiac chest pain. Neurogastroenterol Motil. 2011;23:1066–72. doi: 10.1111/j.1365-2982.2011.01787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clouse RE, Staiano A. Manometric patterns using esophageal body and lower sphincter characteristics. Findings in 1013 patients. Dig Dis Sci. 1992;37:289–96. doi: 10.1007/BF01308186. [DOI] [PubMed] [Google Scholar]
- 12.Jonasson C, Wernersson B, Hoff DA, et al. Validation of the GerdQ questionnaire for the diagnosis of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2013;37:564–72. doi: 10.1111/apt.12204. [DOI] [PubMed] [Google Scholar]
- 13.McElhiney J, Lohse MR, Arora AS, et al. The Mayo Dysphagia Questionnaire-30: documentation of reliability and validity of a tool for interventional trials in adults with esophageal disease. Dysphagia. 2010;25:221–30. doi: 10.1007/s00455-009-9246-8. [DOI] [PubMed] [Google Scholar]
- 14.Patel A, Sayuk GS, Gyawali CP. Parameters on Esophageal pH-Impedance Monitoring That Predict Outcomes of Patients With Gastroesophageal Reflux Disease. Clin Gastroenterol Hepatol. 2015;13:884–91. doi: 10.1016/j.cgh.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labus JS, Bolus R, Chang L, et al. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. 2004;20:89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 16.Jenkinson C, Coulter A, Wright L. Short form 36 (SF36) health survey questionnaire: normative data for adults of working age. BMJ. 1993;306:1437–40. doi: 10.1136/bmj.306.6890.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel A, Patel A, Mirza FA, et al. Achalasia symptom response after Heller myotomy segregated by high-resolution manometry subtypes. J Gastroenterol. 2016;51:112–8. doi: 10.1007/s00535-015-1088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porter RF, Kumar N, Drapekin JE, et al. Fragmented esophageal smooth muscle contraction segments on high resolution manometry: a marker of esophageal hypomotility. Neurogastroenterol Motil. 2012;24:763–8. doi: 10.1111/j.1365-2982.2012.01930.x. e353. [DOI] [PubMed] [Google Scholar]
- 19.Aziz Q, Fass R, Gyawali CP, et al. Functional Esophageal Disorders. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Carlson DA, Lin Z, Kahrilas PJ, et al. The Functional Lumen Imaging Probe Detects Esophageal Contractility Not Observed With Manometry in Patients With Achalasia. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winslow ER, Clouse RE, Desai KM, et al. Influence of spastic motor disorders of the esophageal body on outcomes from laparoscopic antireflux surgery. Surg Endosc. 2003;17:738–45. doi: 10.1007/s00464-002-8538-y. [DOI] [PubMed] [Google Scholar]
- 22.Borjesson M, Pilhall M, Eliasson T, et al. Esophageal visceral pain sensitivity: effects of TENS and correlation with manometric findings. Dig Dis Sci. 1998;43:1621–8. doi: 10.1023/a:1018886309364. [DOI] [PubMed] [Google Scholar]
- 23.Rao SS, Gregersen H, Hayek B, et al. Unexplained chest pain: the hypersensitive, hyperreactive, and poorly compliant esophagus. Ann Intern Med. 1996;124:950–8. doi: 10.7326/0003-4819-124-11-199606010-00002. [DOI] [PubMed] [Google Scholar]
- 24.Bohn B, Bonaz B, Gueddah N, et al. Oesophageal motor and sensitivity abnormalities in non-obstructive dysphagia. Eur J Gastroenterol Hepatol. 2002;14:271–7. doi: 10.1097/00042737-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Deschner WK, Maher KA, Cattau EL, Jr., et al. Manometric responses to balloon distention in patients with nonobstructive dysphagia. Gastroenterology. 1989;97:1181–5. doi: 10.1016/0016-5085(89)91688-0. [DOI] [PubMed] [Google Scholar]
- 26.Suntrup S, Teismann I, Wollbrink A, et al. Altered cortical swallowing processing in patients with functional dysphagia: a preliminary study. PLoS One. 2014;9:e89665. doi: 10.1371/journal.pone.0089665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richter JE, Barish CF, Castell DO. Abnormal sensory perception in patients with esophageal chest pain. Gastroenterology. 1986;91:845–52. doi: 10.1016/0016-5085(86)90685-2. [DOI] [PubMed] [Google Scholar]