Summary
T and B lymphocytes communicate by forming immunological synapses with antigen-presenting target cells. These highly dynamic contacts are characterized by continuous cytoskeletal remodeling events, which not only structure the interface but also exert a considerable amount of mechanical force. In recent years, it has become increasingly clear that synaptic forces influence information transfer both into and out of the lymphocyte. Here, we review our current understanding of synapse mechanics, focusing on its role as an avenue for intercellular communication.
Keywords: Immunology, mechanobiology, signal transduction, T cell, B cell
Immune synapses are mechanically active
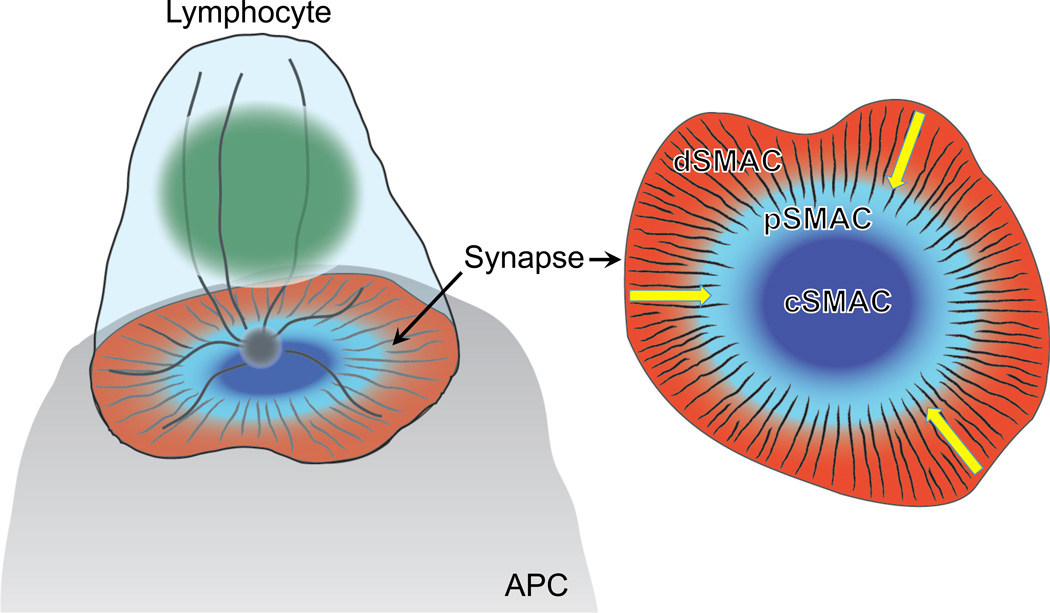
Information exchange between T and B lymphocytes and their antigen-presenting cells (APC) dictates the character and scope of immune responses. The immunological synapse (IS), a stereotyped cell-cell interaction that forms at the lymphocyte-APC interface (Dustin et al., 2010, Harwood and Batista, 2010), acts as a node through which this communication occurs. The ‘canonical’ IS displays a “bull’s eye” architecture made up of concentric Supra-Molecular Activation Clusters (SMACs), each containing distinct protein complexes and activities (Figure 1). At the center lies the cSMAC, containing ligand-bound antigen receptors and associated signaling proteins. Surrounding the cSMAC is the peripheral pSMAC, which is rich in adhesion molecules such as the αLβ2 integrin LFA-1. The pSMAC is itself enclosed by a dense ring of filamentous actin (F-actin), also called the distal SMAC (dSMAC), which forms the outer boundary of the IS.
Figure 1. Schematic diagram of the mature IS.
A side view is shown to the left and an en face view to the right. The lymphocyte nucleus is colored green, with F-actin and microtubules depicted as black and gray lines, respectively. Yellow arrows on the right indicate the direction of retrograde F-actin flow.
Immune cell-cell interactions in general, and the IS in particular, are roiling interfaces that undergo continuous architectural change. The F-actin within the dSMAC undergoes robust retrograde flow, driven both by actin polymerization at the leading edge and myosin-based contractility, which drives clusters of antigen receptors and integrins toward the center of the contact (Hammer and Burkhardt, 2013, Le Floc'h and Huse, 2015). Concurrently, bursts of anterograde flow originate at the IS and propagate to the back of the cell (Ritter et al., 2015). Lymphocytes also form protrusive pseudopodial structures that can dramatically indent the surface of the APC (Sage et al., 2012, Stinchcombe et al., 2001, Ueda et al., 2011). These micron-scale movements generate a substantial amount of mechanical force within the cell-cell interface. In recent years, it has become increasingly clear that many of the cell surface receptors that govern lymphocyte activation are mechanically sensitive proteins and that applied force derived from cytoskeletal remodeling events plays a crucial role in their regulation. There is also evidence that immune cells use mechanical force to transmit information across cell-cell interfaces. This review will focus on our current understanding of the mechanical aspects of signal transduction at the IS, focusing on both the cytoskeletal drivers of force generation and the cell surface proteins that mediate mechanotransduction. We will first cover mechanosensitive receptors that transmit afferent information flow into lymphocytes and then discuss the use of force in efferent information transfer from one cell to another, before closing with a survey of emerging topics in the field and intriguing future directions.
Mechanical control of immunoreceptor function
Integrins
Integrins are among the best-characterized mechanosensitive receptors. All family members are heterodimers comprising one α and one β chain, each containing a long, stalk-like extracellular domain, a transmembrane helix, and a short intracellular tail (Luo et al., 2007). Integrins bind either to components of the extracellular matrix or to adhesion molecules on the surfaces of other cells, and their affinity for these cognate ligands is intimately coupled to their conformation. In the resting state, integrins maintain a bent, low affinity architecture in which the ligand-binding site points toward the base of the integrin stalk. Activating signaling pathways from within the cell can relieve this inhibitory conformation in an “inside-out” fashion by assembling specific protein complexes on the cytoplasmic tails of the α and β chains (Kim et al., 2011). These complexes, which contain cytoskeletal adaptors such as talin and kindlin, drive the integrin tails apart, inducing a conformational change that promotes the extension of the extracellular domain. Although this extended conformation is capable of binding ligand, it does so at intermediate affinity only. To achieve full binding potential, pulling force must first be applied to the integrin-ligand contact (Astrof et al., 2006, Friedland et al., 2009, Woolf et al., 2007). This induces an additional conformational change into an “open” state that exhibits high affinity ligand binding (~100 fold over the extended state). Importantly, ligand bound integrins in the open state transduce powerful “outside-in” signals that organize the cytoskeleton into focal contacts (Legate et al., 2009). These signals also have profound effects on cell survival, proliferation, and differentiation.
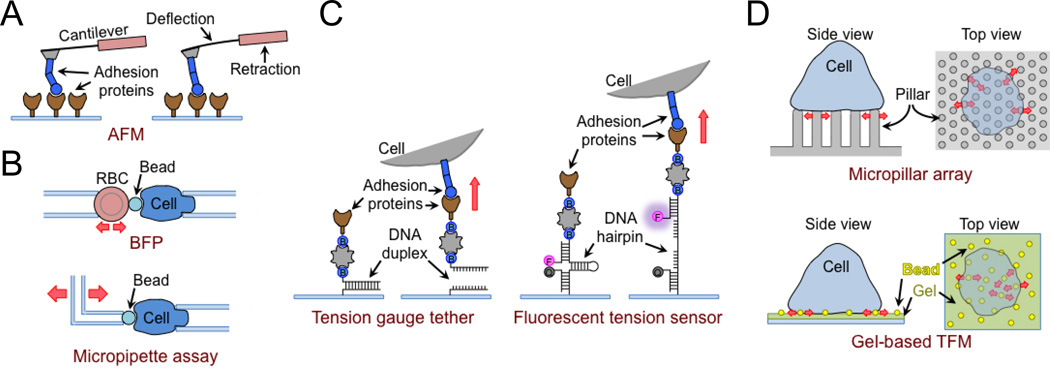
Enhanced affinity under load is the defining property of a “catch-bond” (Dembo et al., 1988), which is an unusual property for a bimolecular contact (Chen and Zhu, 2013). Indeed, most protein-protein interactions exhibit “slip-bond” behavior, becoming weaker as tensile strength increases. Integrin catch bonds were first observed using an atomic force microscopy (AFM)-based approach in which the dissociation rates of single bonds were measured as a function of AFM cantilever deflection (Box 1, Figure IA). In this manner, it was shown that the affinity of the interaction between the α5β1 integrin and its ligand, fibronectin, increased with applied force up to at least 30 pN (Kong et al., 2009). Subsequently, catch bond behavior was also observed for LFA-1, which binds to the adhesion protein ICAM1 (Chen et al., 2010). In this study, a biomembrane force probe (BFP) setup was used in which a red blood cell (RBC) bearing an ICAM1-coated bead was brought into contact with an isolated Jurkat T cell and then retracted (Box 1, Figure IB). The BFP approach enables precise bond lifetime analysis at lower force using intact cells. This proved to be particularly important for the characterization of LFA-1, which exhibited a catch bond to slip bond transition at < 15 pN. BFP has also been used to great effect in more recent studies of antigen receptors (see below).
Box 1. Measuring forces at the immunological synapse.
Our understanding of IS mechanics relies heavily on methods designed to measure synaptic forces between single cells and even single molecules. The most prevalent approaches are listed here.
Atomic Force Microscopy (AFM)
In this approach, a cell or molecule of interest is attached to a flexible cantilever and then brought into contact with a glass surface coated with target cells or cognate ligands (Figure IA). Adhesive forces are then measured by monitoring the negative deflection of the cantilever as it is withdrawn away from the surface. AFM has been used to measure T cell-APC adhesion strength and also to profile integrin catch bond behavior (Kong et al., 2009, Lim et al., 2012).
The Biomembrane Force Probe (BFP)
This imaging-based approach makes use of a red blood cell (RBC) as a sensitive force transducer (Figure IB). A bead coated with stimulatory ligands (e.g. pMHC) is attached to the RBC, which is then immobilized by suction at the end of a micropipette. The bead is then brought into contact with a lymphocyte attached to another micropipette. Subsequent pushing and pulling of the bead by the lymphocyte results in squeezing and stretching, respectively, of the RBC. These distortions can be converted into force measurements using the spring constant of the RBC, which is easily derived from the suction pressure, the dimensions of the RBC, and the width of the pipette. Importantly, because RBC stiffness depends on the applied suction, investigators can sample a wide range of forces (0.01–1000 pN) simply by modulating suction pressure (Evans et al., 1995).
Micropipette-based force measurement
This approach is somewhat similar to the BFP, the major difference being that the stimulatory bead is attached directly to the micropipette by suction, leaving out the RBC force transducer (Figure IB). In addition, the bead-bearing pipette is pulled thin enough so that it can be deflected by nanonewton scale forces. Deflections of the bead after contact with the lymphocyte can be converted into forces based on the stiffness of the bead micropipette, which is determined using established calibration strategies (Basu et al., 2016). The micropipette approach accesses forces that are above the range covered by the BFP.
Traction Force Microscopy (TFM)
In this approach, small fluorescent beads are embedded on the top surface of a polyacrylamide hydrogel substrate bearing stimulatory ligands (Figure ID) (Dembo and Wang, 1999). As lymphocytes form synapses with the substrate, they distort the hydrogel, thereby moving the beads. Fluorescence videomicroscopy is used to measure bead displacements, which are then converted into traction stress maps using Fourier techniques. Stress maps reveal not only the magnitude of applied forces but also their spatial distribution and orientation within the IS.
PDMS micropillar arrays
This approach is a version of TFM in which the hydrogel substrate is replaced by a hexagonal array of PDMS micropillars coated with stimulatory ligands (Figure ID) (Bashour et al., 2014, Tan et al., 2003). Lymphocytes form IS-like contacts with the pillar tops and move them as they exert force against the surface. Each pillar deflection can be converted into a discrete force measurement based on the height, width, and composition of the pillars. The micropillar method provides enhanced spatial resolution relative to gel-based TFM because pillars 1) can be spaced within 1 µm of one another and 2) they move independently of their neighbors.
DNA-based tension gauge tether (TGT)
This approach relies on molecular tension gauges containing DNA duplexes linked to stimulatory ligands (Figure IC) (Wang and Ha, 2013). Suitable force exertion on the ligand will rupture the duplex, detaching the ligand from the surface. The strength of the duplex depends on its length, degree of base pairing, GC content, and junction point with the protein ligand (e.g. the center or the end of the strand). Because mechanosensitive receptors like integrins only signal effectively under tension, they will be activated only by TGTs that are strong enough to withstand the associated pulling forces. Hence, by measuring signaling responses on a panel of different TGT surfaces, one can establish the force threshold required for receptor activation (Wan et al., 2015, Wang and Ha, 2013). The TGT principle has also been applied to generate fluorescent tension probes (Figure IC) (Blakely et al., 2014, Zhang et al., 2014). These constructs typically comprise a stimulatory ligand attached to a DNA hairpin containing a fluorophore at one end and a quencher at the other. When the hairpin is wound, the quencher is in close proximity to the fluorophore and intrinsic fluorescence is low. Suitable force exertion on the probe, however, will unwind the hairpin, dramatically increasing its fluorescence. By modulating the sequence of the hairpin, one can generate probes that detect different levels of applied force. The obvious advantage of TGTs is that they enable rapid force measurements at a truly molecular level. Each probe, however, only reports when a given force threshold has been achieved, making it difficult to use any one probe to monitor a distribution of interfacial forces over time.
Figure I. Techniques to measure synaptic forces. (A) Atomic force microscopy (AFM) to measure bond strength as a function of applied force. (B) Micropipette-based approaches for force measurement. Above, an RBC is used as a biomembrane force probe (BFP) to detect piconewton scale forces. Below, a flexible micropipette can capture applied forces in the nanonewton range. (C) Left, DNA-based tension gauge tethers (TGTs) remain attached to the surface unless a sufficient force is applied to rupture Watson-Crick base pairing. Right, a DNA hairpin coupled to a fluorophore (F) and quencher (Q) is used to generate a tension probe that fluoresces upon application of a threshold force. (D) Traction force microscopy (TFM). Above, cells are imaged on an array of flexible micropillars, allowing applied forces to be calculated from pillar deflections. Below, in gel-based TFM, force exertion is determined from the movements of beads embedded in the gel matrix.
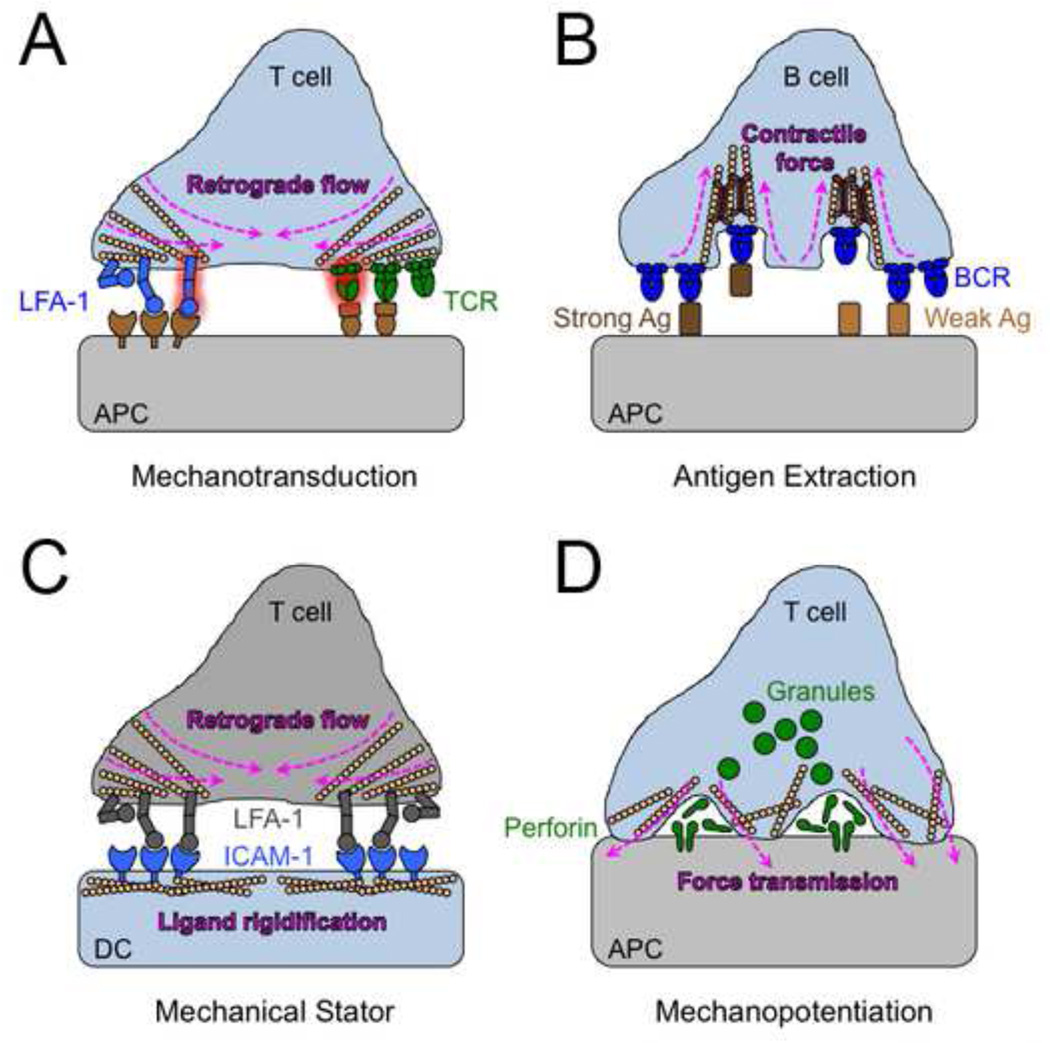
LFA-1 is the predominant integrin within the IS. It is critical both for strong adhesion to the APC and also for transducing costimulatory signals that contribute to lymphocyte activation (Pribila et al., 2004). LFA-1 engages ICAM-1 on the target cell surface during initial cell spreading. Ligand bound LFA-1 molecules are then trafficked toward the center of the IS by retrograde F-actin flow, where they coalesce into the annular pSMAC (Figure 1, 2A) (Grakoui et al., 1999, Yi et al., 2012). The importance of retrograde flow for promoting LFA-1 activation was recently addressed by using antibodies that differentiate between the conformational states of the integrin (Comrie et al., 2015a). It was found that different forms of the protein occupied distinct regions of the IS, with the highest affinity conformation residing more centrally. Importantly, global arrest of F-actin flow by combined treatment with the F-actin stabilizer jasplakinolide and the myosin II inhibitor blebbistatin markedly reduced the accumulation of open LFA-1. Thus, integrin activation at the IS depends on forces imposed by cytoskeletal dynamics (Figure 2A).
Figure 2. Mechanical information transfer at the IS.
(A) Pulling forces derived from retrograde F-actin flow within the lymphocyte promote the mechanical activation of LFA-1 and the TCR. (B) Contractile forces driven by actin and myosin generate membrane invaginations that facilitate the discrimination between strong and weak antigens by the BCR. (C) DCs use their cortical cytoskeleton to constrain the diffusion of ICAM-1, thereby promoting mechanical activation of LFA-1 on the T cell side of the IS. (D) CTLs use mechanical force to strain the surface of the target cell, which boosts killing responses by potentiating the pore forming activity of perforin.
T cells also express the α4β1 integrin VLA-4 and the αEβ7 integrin CD103, which both influence T cell activation in specialized contexts. Little is known about how the mechanical properties of these proteins and their respective ligands, VCAM-1 and E-cadherin, influence synaptic architecture and T cell effector responses. Although it is generally thought that all integrins undergo the same conformational transitions during their activation, differences in cytoskeletal coupling and ligand distribution could significantly alter their behavior at a cell biological level. In that regard, it is interesting to note that strong engagement of VLA-4 dramatically retards retrograde F-actin flow within the IS (Hui et al., 2014), leading to the arrest of signaling microclusters that would otherwise move centripetally into the cSMAC (Nguyen et al., 2008). Importantly, LFA-1 does not appear to alter F-actin dynamics in this manner, implying that integrin function in this context is specialized. The mechanistic basis for these and other effects is not well understood, and remains an interesting topic for future studies.
Antigen Receptors
Antigen recognition is in many ways the signature event in a lymphocyte’s life. In naïve T cells and B cells, it triggers the dramatic changes in metabolism, proliferation, and gene expression required for their expansion and differentiation into armed effectors. These effector cells will then continue to use antigen recognition as a touchstone to guide their immune responses. Activated B cells bind to antigen repeatedly during the antibody affinity maturation process, ensuring the development of plasma cells that secrete high affinity antibodies. CD4+ helper T cells and CD8+ cytotoxic T lymphocytes (CTLs), for their part, use antigen as a signal to mount communicative or cytotoxic secretory responses in the periphery.
It is generally thought that the vast majority of antigen recognition occurs within lymphocyte-APC interfaces. In the case of T cells, this feature is dictated by the requirements of the T cell antigen receptor (TCR), which can only bind to its peptide ligands (~10 amino acids) if they are presented by major histocompatibility complex (MHC) proteins on the surface of another cell. Even the B cell receptor (BCR), however, which recognizes whole protein antigen, often binds to it in the form of immune complexes presented on an APC surface (Harwood and Batista, 2010). Hence, ligand engagement by TCRs and BCRs occurs within the same intercellular context in which mechanosensitive integrins operate.
Over the past decade, it has become increasingly clear that antigen receptors are themselves mechanosensitive. The first hints of this came from experiments in which stimulatory ligands were presented to T cells on surfaces of varying stiffness. Using polyacrylamide hydrogel substrates coated with antibodies against the TCR, it was initially shown that stiffer surfaces (100–200 kPa) were more stimulatory to murine CD4+ T cells than softer ones, eliciting higher levels of intracellular signaling and subsequent cytokine secretion (Judokusumo et al., 2012). This effect disappeared in the presence of the myosin II inhibitor blebbistatin, indicating that cytoskeletal contractility was required for the tension sensing process. A subsequent study, however, found that ~100 kPa stimulatory surfaces, in this case fabricated using polydimethylsiloxane (PDMS) elastomer, were significantly more stimulatory to human CD4+ T cells than stiffer (~2 MPa) substrates (O'Connor et al., 2012). Taken together, these distinct activation trends suggest that the TCR may have a bell-shaped response to substrate stiffness, peaking at ~100 kPa. It is also possible, however, that PDMS surfaces elicit qualitatively different T cell activation than hydrogels, or that the murine T cells used in the first study behave in a different manner than the human T cells used in the second. Analogous substrate stiffness experiments have also been performed on B cells (Wan et al., 2013, Zeng et al., 2015). In general, stiffer antigen-bearing hydrogel and PDMS surfaces elicit stronger early BCR signaling and effector responses, while longer term proliferative responses seem to be optimal on softer PDMS. As with the T cell studies, the mechanistic basis for these differential effects remains unclear. Work in this area has left little doubt, however, that both T cells and B cells are sensitive to the physical disposition of antigen.
Recently, a BFP approach was employed to provide direct evidence that the TCR is indeed a mechanosensitive receptor (Liu et al., 2014). This study, which focused on the interaction between the OT-1 TCR and its cognate ligand, the ovalbumin257–264 peptide (OVA) presented by the class I MHC protein H2-Kb, revealed a peak in apparent affinity at 10 pN of applied force, indicative of catch bond behavior. Interestingly, mutations known to reduce or abolish the biological activity of the OVA peptide switched the interaction from a catch into a slip bond. Hence, while the wild type ligand exhibited increased affinity under load, the mutants bound less well with increasing force. It has been known for some time that T cells can distinguish bona fide antigenic ligands from self peptide-MHC complexes that differ in their TCR affinity by 3-fold or less (measured under no-force conditions) (Davis et al., 1998). The application of force would presumably enhance the discriminatory power of the TCR, providing an elegant mechanism for maintaining the specificity of T cell responses.
Given that the TCR is now known to be a catch-bond receptor, it is tempting to speculate that, like integrins, it might also undergo physically induced conformational change to promote signaling. Biophysical and imaging-based studies from multiple labs have indicated that pMHC engagement by the TCR induces a conformational change in its associated CD3 signaling chains (Gil et al., 2002, Lee et al., 2015, Swamy et al., 2016, Xu et al., 2008). In the unbound state, the cytoplasmic tails of several CD3 subunits associate with the inner leaflet of the plasma membrane, effectively shielding their immunotyrosine-based activation motifs (ITAMs) from kinases. Upon ligand recognition, the tails fall away from the membrane, enabling ITAM phosphorylation. Crystal structures of unbound αβ TCR ectodomains and their complexes with cognate pMHC have not revealed any large-scale conformational changes that propagate toward the membrane (Kuhns et al., 2006). Hence, release of the CD3 tails most likely results from rigid body displacements of the αβ TCR with respect to the plasma membrane and the CD3 ectodomains. Applied pulling force could conceivably generate such rigid body movements, and future studies will no doubt examine the link between physical stress and the conformation of the CD3 tails.
Although single molecule BFP studies of the BCR have not, as yet, been performed, there are strong indications that it, too, functions as a catch bond receptor. By coupling antigen to DNA-based tension gauge tethers (TGTs) (Box 1, Figure IC), it was shown that B cells pull against their ligands at a single molecule level and, importantly, that the sustained application of force is required for optimal signal transduction (Wan et al., 2015). Thus, the IgM BCR, which is expressed on naïve B cells, requires at least 50 pN of resistance per molecule of bound antigen to achieve full activation. Interestingly, the mechanical requirements for the IgG and IgE BCR variants, which are expressed on certain memory B cells, were found to be substantially less stringent (12 pN). Structure-function analysis mapped this differential dependence on force to the short cytoplasmic tail of the BCR. It is tempting to speculate that this component controls BCR mechanosensing by modulating interactions with the cortical cytoskeleton.
B cells also employ the BCR to internalize antigen in order to present it to helper T cells and thereby receive signals to expand and differentiate. Recent work has demonstrated that myosin dependent pulling forces are crucial for this process (Figure 2B) (Natkanski et al., 2013). By imaging B cells on immobilized plasma membrane sheets (which have more physiological viscoelastic properties compared to planar lipid bilayers) bearing fluorescently-labeled antigen, it was possible to dilineate the uptake process into a defined series of steps. First, the target membrane containing antigen was pulled by myosin dependent forces into an invagination within the IS. Then, clathrin assembled around the invagination to complete the internalization process. Most invaginations ruptured well before clathrin assembly; only those containing large clusters of antigen, and presumably more BCR binding events, persisted long enough (~20 s) for productive endocytosis. These results indicated that only high avidity antigen particles (i.e. large clusters) could maintain B cell contact in the face of cytoskeletal forces, and implied that B cells might use these forces to select for higher quality antigen. Consistent with this hypothesis, blocking myosin II was found to impair the ability of B cells to discriminate between high and low affinity antigen during endocytosis. Hence, as with the TCR, applied force plays a critical role in the regulation of BCR specificity.
Mechanical crosstalk between integrin and immunoreceptor signaling
Within bona fide cell-cell contacts, antigen receptors and integrins do not function in isolation but rather as cooperative components of an adhesive and stimulatory interface. Our current understanding of this crosstalk is largely limited to biochemical interactions. TCRs and BCRs promote multiple aspects of integrin function through inside-out signaling, while conversely, ligand bound integrins deliver costimulatory outside-in signals that lower the threshold for antigen dependent lymphocyte activation (Kinashi, 2005, Pribila et al., 2004). Recent studies, however, have begun to explore mechanical links between the two systems.
To analyze crosstalk between the TCR and LFA-1, T cells were imaged on micropatterned surfaces containing segregated domains of stimulatory anti-TCR antibody and ICAM-1 (Tabdanov et al., 2015). Regions of TCR stimulation were found to colocalize with the Arp2/3 complex, which induces the formation of branched F-actin arrays. By contrast, ICAM-1 domains were characterized by the accumulation of the formin protein FHOD1, which assembles linear F-actin bundles. Consistent with these mutually exclusive colocalization patterns, depletion or inhibition of FHOD1 blocked integrin dependent actin polymerization, while Arp2/3 was shown to be required for the TCR-induced response. Interestingly, inhibition of Arp2/3 also blunted actin polymerization in the ICAM-1 domains, despite little to no accumulation of Arp2/3 in these regions. This result suggested that Arp2/3 dependent actin polymerization downstream of the TCR plays an important role in “priming” the formation of integrin-based structures, consistent with previous studies implicating F-actin in the rapid stabilization of integrin mediated adhesion (Alon et al., 2005, Rullo et al., 2012). Myosin-based contractility, which is driven by integrin engagement at the IS, was also shown to enhance TCR-induced tyrosine phosphorylation and actin polymerization. Taken together, these data are consistent with a model in which the actin cytoskeleton couples the TCR and LFA-1 in a positive feedback loop that coordinates IS formation and growth.
Cortical F-actin appears to play an analogous coordinating role during phagocytic responses in macrophages. On surfaces coated with ligands for engulfment receptors, macrophages form “frustrated” phagosomes that are amenable to high-resolution total internal reflection fluorescence imaging. Recently, this approach was further elaborated to explore crosstalk between engulfment receptors, integrins, and the cytoskeleton (Freeman et al., 2016). Macrophages were allowed to land on surfaces containing 1) micron-scale spots of Fc fragment, which binds to the engulfment receptor FcγRIIA, and 2) a field of integrin-binding matrix proteins between the Fc spots. Both F-actin and integrins were found to be crucial for proper phagosome architecture on surfaces of this kind. Integrin engagement of the matrix between the Fc spots enabled outward propagation of the interface to incorporate additional Fc ligands, and it also facilitated the exclusion of the inhibitory phosphatase CD45. In addition, both interface stability and CD45 exclusion were compromised by actin depolymerization agents. The integrin-F-actin axis also promoted the recognition of multiple activated FcγRIIA domains as one object; blockade of either component converted the interface into a set of adjacent microphagosomes. Hence, F-actin dependent coordination between FcγRIIA and integrins enables macrophages to translate nanometer scale receptor activation events into a micron scale cellular response.
Taken together, these recent studies suggest that cortical F-actin functions as a biochemical and mechanical motherboard that integrates and modulates signals from different receptors in distinct membrane domains. This intriguing concept will no doubt receive more attention in future investigations.
Force in efferent transfer of information
The APC as a mechanical stator
It is now well established that the rigidity of a ligand-presenting surface can profoundly affect the signaling output of integrins and other activating immunoreceptors (Hui et al., 2015, Judokusumo et al., 2012, O'Connor et al., 2012, Pryshchep et al., 2014, Wan et al., 2013, Zeng et al., 2015). It follows that APCs and other target cells should be able to transmit information through the IS simply by modulating the mechanical stiffness of activating ligands on their surfaces. This possibility was directly addressed in a recent study of LFA1-ICAM-1 interactions in T cell-DC synapses (Comrie et al., 2015b). It was initially found that ICAM-1 drives much more extensive integrin extension when it is immobilized on plastic than when it is presented to T cells in soluble form. This raised the question of whether APCs might rigidify cell surface ICAM-1 in order to promote the mechanical activation of T cell LFA-1, essentially behaving as a stator within a mechanical system (Figure 2C). Using fluorescence recovery after photobleaching (FRAP) to analyze ICAM-1 mobility, they found that a substantial fraction of ICAM-1 molecules form clustered puncta on the DC surface that are essentially immobile. This clustering depended on the adaptor proteins α-actinin and moesin, which link the ICAM-1 tail domain to the actin cytoskeleton. Deletion of this tail abrogated the clustering and immobilization of ICAM-1 on the DC surface, further supporting a role for cortical F-actin in restraining the movement of ICAM-1. Importantly, tail-less ICAM-1 also failed to induce strong LFA-1 opening in T cell-APC conjugates, indicating that ligand rigidification can influence integrin activation on the opposite side of the IS. Furthermore, ICAM-1−/− DCs reconstituted with tail-less ICAM-1 induced suboptimal T cell proliferation responses. Hence, ligand rigidification can strongly influence both the biochemical state and the signaling potential of bound integrins.
In contrast to the behavior of ICAM-1, cell surface MHC was found to diffuse readily through the DC membrane, (Comrie et al., 2015b). Although this result argues against a role for the APC cytoskeleton in modulating TCR mechanosensing, it is important to keep in mind that only one DC subset was analyzed in this study. Furthermore, it is also possible that the MHC could become anchored to the cytoskeleton within the IS. Previous work strongly suggests that DCs and other professional APCs play an active role in IS formation, altering cytoskeletal architecture and intracellular trafficking at the interface (Benvenuti, 2016). Hence, the extent to which APCs function as stators for other mechanosensitive receptors remains to be seen.
Force exertion across the IS
Forces exerted by the lymphocyte affect not only the mechanosensitive receptors on its own surface, but also propagate across the IS to the target cell. In recent years, biophysical studies have shed light on both the magnitude and the dynamics of these forces, providing a quantitative basis for understanding their role in T cell activation.
Force exertion through the IS can be monitored over time using beads that are coated with stimulatory ligands and then attached to either RBCs or calibrated micropipettes (Box 1, Figure IB) (Basu et al., 2016, Husson et al., 2011). TCR engagement initially induces a protrusive response from the T cell that is coupled to its spreading over the bead surface. Having established its interface with the bead (often in less than a minute), the T cell then begins to pull, exerting nanonewton scale forces in what appears to be a frustrated phagocytic response. This overall pattern of protrusive spreading followed by pseudo-engulfment is reminiscent of antigen gathering and internalization by B cells, although the extent to which synaptic forces are coupled to endocytosis in T cells has not been examined. Not surprisingly, both the pushing and the pulling phases of IS formation were found to be associated with pronounced F-actin accumulation at the interface (Husson et al., 2011), consistent with a central role for actin dynamics in this process.
Although highly informative, micropipette-based studies only reveal force exertion perpendicular to the IS. To measure forces in the plane of the IS, an imaging-based approach has been developed in which T cells are applied to hexagonally packed arrays of PDMS micropillars coated with stimulatory ligands and spaced at 2 µm intervals (Box 1, Figure ID) (Bashour et al., 2014, Tan et al., 2003). The cells form IS-like contacts with the pillar tops and exert force against them, which is measured by the degree of pillar deflection. Cell spreading on the array is typically accompanied by outwardly oriented deflections. Within minutes, this initial centrifugal pattern reverses polarity as the cells begin an extended period in which they squeeze the pillars inward. This progression from centrifugal to centripetal force exertion likely corresponds to the pushing and then pulling observed in micropipette experiments. During both the spreading and squeezing phases, pillar deflections are enriched in the periphery of the contact, in a region overlapping with the pSMAC and dSMAC. This makes sense, given the presumed importance of F-actin dynamics and integrin-mediated adhesion for mechanosensing and force exertion.
Tangential forces within the IS have also been examined using a traction force microscopy (TFM) setup in which Jurkat T cells are imaged on polyacrylamide hydrogels coated with anti-TCR antibodies and embedded with fluorescent beads (Box 1, Figure ID) (Hui et al., 2015). The beads function as fiduciary markers to track distortions in the gel exerted by the T cells, which can be converted into force measurements using the known stiffness of the matrix. Synaptic forces were found to peak at 2–5 nanonewton per cell and also to concentrate in an annular band at the periphery of the contact. Taken together with the results of micropillar studies, these observations suggested that actin cytoskeletal dynamics and adhesive interactions in the dSMAC and pSMAC might drive synaptic force generation. Consistent with this hypothesis, pharmacological agents that either disrupted F-actin assembly or depolymerized existing filaments profoundly reduced force exertion.
The importance of myosin contractility has also been examined using both the TFM and micropillar systems. When applied before or in the early phases of IS formation, myosin inhibitors significantly decreased force exertion (Basu et al., 2016, Hui et al., 2015). However, blocking myosin activity in the mature IS (~ 15 minutes after contact formation) had little to no affect on the maintenance of force over time (Hui et al., 2015). Collectively, these results suggest that, despite ongoing F-actin dynamics, force bearing structures are established during initial IS assembly, and are relatively stable after that.
Mechanopotentiation of secreted factors
Lymphocytes release a wide variety of soluble effector molecules, ranging from cytokines to cytolytic factors, directionally into the IS (Huse et al., 2008). Perhaps the best-known practitioner of directional secretion is the CTL, which secretes the pore forming protein perforin and several granzyme proteases into the synaptic space in order to induce target cell apoptosis. It is thought that the role of the IS in this context is to limit the diffusion of these toxic molecules, thereby maintaining the specificity of the killing response (Stinchcombe and Griffiths, 2007). It was recently shown, however, that the IS also enhances the potency of cytolytic secretion by exerting force against the APC (Figure 2D).
Synaptic F-actin architecture is controlled by phosphatidylinositol 3,4,5 trisphosphate (PIP3), a lipid second messenger generated by phosphoinositide 3-kinases (PI3Ks). PIP3 accumulates in an annular gradient within the dSMAC, where it stimulates actin polymerization by recruiting Dock2, an exchange factor for the small GTPase Rac, which is the master regulator of lamellipodial growth (Jaffe and Hall, 2005, Le Floc'h et al., 2013, Nishikimi et al., 2013). T cells lacking Dock2 form miniaturized synapses with thin F-actin rings (Le Floc'h et al., 2013). Conversely, depletion of PTEN, a lipid phosphatase that antagonizes PI3K signaling, leads to a robust increase in IS size. These architectural changes are associated with striking mechanical phenotypes. Dock2 deficient CTLs impart less force across the IS, while PTEN deficient CTLs exert substantially more (Basu et al., 2016). Importantly, both perturbations also affect CTL function. Loss of Dock2 inhibits target cell killing, whereas depletion of PTEN dramatically enhances it (Le Floc'h et al., 2013). Taken together, these results suggest a link between IS mechanics and CTL effector function.
CTLs store perforin and granzymes in secretory lysosomes called lytic granules (Stinchcombe and Griffiths, 2007). Target cell recognition induces the trafficking of these granules to IS, where they fuse with the plasma membrane and release their contents into the synaptic space (Figure 2D). Perforin initiates the killing cascade by forming pores in the target cell membrane. This activates a membrane repair response that leads to the uptake of granzymes into the target cell cytoplasm, where they cleave a number of key substrates to trigger apoptosis (Keefe et al., 2005, Thiery et al., 2011). Single cell imaging experiments demonstrated that depletion of PTEN in CTLs significantly increased the speed of perforin pore formation on target cells (Basu et al., 2016). This phenotype could not be attributed to increased lytic granule release or perforin expression, raising the possibility that the IS might enhance perforin function mechanically. This hypothesis was particularly intriguing in light of previous work indicating that membrane tension could modulate the activity of pore forming peptides (Huang et al., 2004, Lee et al., 2008, Polozov et al., 2001). Consistent with these reports, increasing membrane tension either pharmacologically or osmotically markedly enhanced pore formation by perforin, while decreasing membrane tension had the opposite effect (Basu et al., 2016).
The relationship between cellular rigidity and perforin function was further examined using polyacrylamide hydrogels. Adherent cells attached to stiff hydrogels adopt a spread, stellate configuration containing numerous actin stress fibers, while cells on soft hydrogels display a more collapsed morphology and lack stress fibers (Yeung et al., 2005). Consistent with these differences in cell shape, higher substrate stiffness has been documented to increase the surface tension of attached cells and the membrane tension of attached vesicles (Hui et al., 2015, Lo et al., 2000, Murrell et al., 2014, Oakes et al., 2009). Remarkably, cells cultured on stiff hydrogels were significantly more sensitive to purified perforin than cells grown on soft ones. Stiffer matrices also made adherent targets more sensitive to killing by CTLs, implying that cell tension affects perforin function in the context of the IS.
If CTLs use synaptic force to facilitate perforin pore formation, one would expect that force exertion would be correlated with perforin secretion in space and time (Figure 2D). To explore this possibility, a fluorescent reporter was employed that localizes to lytic granules but only becomes visible when these granules fuse with the plasma membrane (Rak et al., 2011). Imaging experiments of CTLs expressing this reporter on micropillar arrays revealed that granule release (also called degranulation) tends to occur close to force exertion hotspots, which are small (4–6 µm2) regions of strong pillar deflection that develop in many synapses (Basu et al., 2016). Notably, degranulation events were remarkably enriched in an annular zone halfway between the center of the IS and the outer edge. It has been proposed that lytic granules fuse with the plasma membrane at the cSMAC, which lacks the cortical F-actin prevalent in more peripheral domains (Ritter et al., 2015, Stinchcombe et al., 2006). In light of our results, however, we favor the model in which degranulation occurs more peripherally, where it can leverage the physical effects of the IS on the target cell membrane. It is tempting to speculate that there might be specific synaptic structures that mediate the coordinated release of perforin as well as the mechanopotentiation of its function.
Concluding Remarks
Despite recent advances, our understanding of how cellular mechanics influences intercellular communication remains quite rudimentary, and several important questions remain unanswered (see Outstanding Questions). Most cell surface receptors interact at some level with the cortical cytoskeleton, and many of their ligands are either transmembrane proteins themselves or noncovalently associate with membranes or extracellular matrix. Hence, mechanical regulation of cell surface receptor signaling is likely to be the rule rather than the exception. This concept will be tested rigorously as biophysical approaches are extended to analyze previously unexplored signaling systems. Notably, it was recently reported that matrix stiffness modulates signaling through CD40 (Zeng et al., 2015), a TNF receptor family member that mediates costimulatory activation of B cells and DCs. It will be interesting to see if and how interfacial forces influence other functionally relevant cell surface receptors.
To date, studies of mechanotransduction in the immune system have focused almost exclusively on T cells and B cells. Innate immune cells like DCs and macrophages are as cytoskeletally dynamic as lymphocytes and also assemble close cell-cell contacts to mediate critical information transfer. Biophysical studies focusing on these and other cell-cell interactions will likely be a fruitful research area in coming years. We also anticipate the application of mechanobiological approaches for the comparative analysis of distinct lymphocyte subsets. For example, single cell imaging and force measurements were recently combined to reveal that germinal center (GC) B cells extract antigen in a different manner than other B cells (Nowosad et al., 2016). Whereas naïve and memory B cells were found to gather antigen in large clusters within the cSMAC, GC B cells formed smaller clusters that were trafficked outward from the pSMAC prior to endocytosis. B cells enter the germinal center to undergo affinity maturation, an iterative, mutagenic process designed to generate modified BCRs with increased antigen affinity (Victora and Nussenzweig, 2012). After each round of mutagenesis, the highest affinity B cell clones are identified by their ability to internalize and present more antigen to follicular helper T cells. That GC B cells internalize antigen in small clusters makes sense in light of this process. To surmount the mechanical threshold required for internalization, a small cluster must exhibit increased affinity on a per molecule basis. Comparative studies of this kind have the potential to contribute key functional insights, and should become more common as cell isolation capabilities and biophysical technologies improve.
Efforts to explore the mechanical role of synaptic F-actin dynamics have largely focused on retrograde flow within the dSMAC, which drives integrin activation and contributes to receptor internalization and antigen uptake. There are other F-actin based structures at the IS, however, which are less well understood. It has been known for some time that lymphocytes form synaptic protrusions that can create micron-scale invaginations in the target cell membrane (Sage et al., 2012, Stinchcombe et al., 2001, Ueda et al., 2011). These structures resemble podosomes, and they appear to be required for efficient lymphocyte trafficking across endothelial monolayers. Potential roles for synaptic protrusions in other aspects of lymphocyte activation and effector responses remain to be investigated. Recent lattice light sheet imaging of live CTL-target cell conjugates revealed bursts of F-actin that originate at the IS and proceed toward the back of the cell (Ritter et al., 2015). These explosive anterograde flows could be involved in the exclusion of cell surface molecules from the IS or in the priming of Factin polymerization at the rear during CTL retraction from dying targets. Perturbation approaches that selectively block specific F-actin based structures will be required to resolve these issues.
If integrins and antigen receptors are indeed activated by piconewton scale forces, one would expect that mechanical noise from the environment could potentially disturb their regulation. To guard against this possibility, lymphocytes have presumably developed mechanisms that buffer cell surface receptors against the larger forces that could arise from bulk tissue movements, shear flow, and other extrinsic sources. The IS contains a thick band of F-actin at the periphery, which could potentially protect the interface from external forces, thereby providing a mechanical “oasis” within which receptor ligand interactions are shaped only by the lymphocyte and the target cell. The extent to which the IS functions as a mechanical insulator of this kind remains to be seen.
Our current understanding of IS mechanics is fundamentally limited by what we can detect. Higher resolution imaging modalities, such as lattice light sheet microscopy, will certainly help the field move forward, but they must be combined with biophysical strategies that enable precise quantification of forces at the submicron scale. Although gel-based TFM and PDMS micropillars have enabled investigators to measure force generation with high spatiotemporal resolution, they cannot be used to explore the effects of those forces on target cells. Further progress in this area will require the incorporation of genetically encoded probes akin to the Förster resonance energy transfer-based reporters recently developed to detect intracellular tension (Borghi et al., 2012, Conway et al., 2013, Grashoff et al., 2010). These tools and others like them could, in principle, be used to explore the effects of IS mechanics on the physical properties of both the lymphocyte and target cell, which would be a necessary step toward understanding the ramifications of these mechanics for immune function.
Trends.
Dynamic cell-cell interfaces mediate a large fraction of intercellular communication in the immune system. It is becoming increasingly clear that mechanical forces are critical for the proper function of these interactions.
The T cell immunological synapse (IS) generates forces that mechanically activate ligand bound integrins and antigen receptors, which behave like “catch bonds”. For antigen receptors, binding under physical load enables better discrimination between strong and weak ligands.
T cells also exert force across the IS. Recent work indicates that this force can potentiate the activity of secreted proteins, which implies that lymphocytes combine physical and chemical output to enhance the efficiency of intercellular communication.
Outstanding questions.
Do distinct cytoskeletal structures mediate different types of synaptic force exertion, and if so, how?
Is mechanosensing a general feature of cell surface receptors? What information can it provide beyond ligand/substrate stiffness?
Does the IS suppress mechanical noise by acting as a force insulator?
Do the mechanical properties of lymphocyte subsets differ, and are these differences functionally important?
What are the molecular mechanisms that couple cytoskeletal remodeling on one side of the IS to signal transduction on the other side?
Does IS formation alter the physical properties of the target cell, and if so, to what end?
Acknowledgments
We acknowledge support from the National Institutes of Health (R01-AI087644) and the National Science Foundation (1562905).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alon R, Feigelson SW, Manevich E, Rose DM, Schmitz J, Overby DR, Ginsberg MH. Alpha4beta1-dependent adhesion strengthening under mechanical strain is regulated by paxillin association with the alpha4-cytoplasmic domain. J Cell Biol. 2005;171:1073–1084. doi: 10.1083/jcb.200503155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astrof NS, Salas A, Shimaoka M, Chen J, Springer TA. Importance of force linkage in mechanochemistry of adhesion receptors. Biochemistry. 2006;45:15020–15028. doi: 10.1021/bi061566o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bashour KT, Gondarenko A, Chen H, Shen K, Liu X, Huse M, Kam LC. CD28 and CD3 have complementary roles in T-cell traction forces. Proc Natl Acad Sci U S A. 2014;111:2241–2246. doi: 10.1073/pnas.1315606111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basu R, Whitlock BM, Husson J, Le Floc'h A, Jin W, Oyler-Yaniv A, Huse M. Cytotoxic T Cells Use Mechanical Force to Potentiate Target Cell Killing. Cell. 2016 doi: 10.1016/j.cell.2016.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benvenuti F. The Dendritic Cell Synapse: A Life Dedicated to T Cell Activation. Front Immunol. 2016;7:70. doi: 10.3389/fimmu.2016.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blakely BL, Dumelin CE, Trappmann B, McGregor LM, Choi CK, Anthony PC, Chen CS. A DNA-based molecular probe for optically reporting cellular traction forces. Nature methods. 2014;11:1229–1232. doi: 10.1038/nmeth.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borghi N, Sorokina M, Shcherbakova OG, Weis WI, Pruitt BL, Nelson WJ, Dunn AR. E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch. Proc Natl Acad Sci U S A. 2012;109:12568–12573. doi: 10.1073/pnas.1204390109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen W, Lou J, Zhu C. Forcing switch from short- to intermediate- and long-lived states of the alphaA domain generates LFA-1/ICAM-1 catch bonds. J Biol Chem. 2010;285:35967–35978. doi: 10.1074/jbc.M110.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Zhu C. Mechanical regulation of T-cell functions. Immunol Rev. 2013;256:160–176. doi: 10.1111/imr.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Comrie WA, Babich A, Burkhardt JK. F-actin flow drives affinity maturation and spatial organization of LFA-1 at the immunological synapse. J Cell Biol. 2015a;208:475–491. doi: 10.1083/jcb.201406121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comrie WA, Li S, Boyle S, Burkhardt JK. The dendritic cell cytoskeleton promotes T cell adhesion and activation by constraining ICAM-1 mobility. J Cell Biol. 2015b;208:457–473. doi: 10.1083/jcb.201406120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr Biol. 2013;23:1024–1030. doi: 10.1016/j.cub.2013.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 14.Dembo M, Torney DC, Saxman K, Hammer D. The reaction-limited kinetics of membrane-to-surface adhesion and detachment. Proceedings of the Royal Society of London. Series. B, Containing papers of a Biological character. Royal Society. 1988;234:55–83. doi: 10.1098/rspb.1988.0038. [DOI] [PubMed] [Google Scholar]
- 15.Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophysical journal. 1999;76:2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dustin ML, Chakraborty AK, Shaw AS. Understanding the structure and function of the immunological synapse. Cold Spring Harb Perspect Biol. 2010;2:a002311. doi: 10.1101/cshperspect.a002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans E, Ritchie K, Merkel R. Sensitive force technique to probe molecular adhesion and structural linkages at biological interfaces. Biophysical journal. 1995;68:2580–2587. doi: 10.1016/S0006-3495(95)80441-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman SA, Goyette J, Furuya W, Woods EC, Bertozzi CR, Bergmeier W, Grinstein S. Integrins Form an Expanding Diffusional Barrier that Coordinates Phagocytosis. Cell. 2016;164:128–140. doi: 10.1016/j.cell.2015.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- 20.Gil D, Schamel WW, Montoya M, Sanchez-Madrid F, Alarcon B. Recruitment of Nck by CD3 epsilon reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell. 2002;109:901–912. doi: 10.1016/s0092-8674(02)00799-7. [DOI] [PubMed] [Google Scholar]
- 21.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 22.Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, Schwartz MA. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–266. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammer JA, 3rd, Burkhardt JK. Controversy and consensus regarding myosin II function at the immunological synapse. Curr Opin Immunol. 2013 doi: 10.1016/j.coi.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harwood NE, Batista FD. Early events in B cell activation. Annu Rev Immunol. 2010;28:185–210. doi: 10.1146/annurev-immunol-030409-101216. [DOI] [PubMed] [Google Scholar]
- 25.Huang HW, Chen FY, Lee MT. Molecular mechanism of Peptide-induced pores in membranes. Phys Rev Lett. 2004;92:198304. doi: 10.1103/PhysRevLett.92.198304. [DOI] [PubMed] [Google Scholar]
- 26.Hui KL, Balagopalan L, Samelson LE, Upadhyaya A. Cytoskeletal forces during signaling activation in Jurkat T-cells. Mol Biol Cell. 2015;26:685–695. doi: 10.1091/mbc.E14-03-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hui KL, Kwak SI, Upadhyaya A. Adhesion-dependent modulation of actin dynamics in Jurkat T cells. Cytoskeleton. 2014;71:119–135. doi: 10.1002/cm.21156. [DOI] [PubMed] [Google Scholar]
- 28.Huse M, Quann EJ, Davis MM. Shouts, whispers, and the kiss of death: directional secretion in T cells. Nat Immunol. 2008;9:1105–1111. doi: 10.1038/ni.f.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Husson J, Chemin K, Bohineust A, Hivroz C, Henry N. Force generation upon T cell receptor engagement. PloS one. 2011;6:e19680. doi: 10.1371/journal.pone.0019680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annual review of cell and developmental biology. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 31.Judokusumo E, Tabdanov E, Kumari S, Dustin ML, Kam LC. Mechanosensing in T lymphocyte activation. Biophysical journal. 2012;102:L5–L7. doi: 10.1016/j.bpj.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keefe D, Shi L, Feske S, Massol R, Navarro F, Kirchhausen T, Lieberman J. Perforin triggers a plasma membrane-repair response that facilitates CTL induction of apoptosis. Immunity. 2005;23:249–262. doi: 10.1016/j.immuni.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Kim C, Ye F, Ginsberg MH. Regulation of integrin activation. Annual review of cell and developmental biology. 2011;27:321–345. doi: 10.1146/annurev-cellbio-100109-104104. [DOI] [PubMed] [Google Scholar]
- 34.Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nature reviews. 2005;5:546–559. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 35.Kong F, Garcia AJ, Mould AP, Humphries MJ, Zhu C. Demonstration of catch bonds between an integrin and its ligand. J Cell Biol. 2009;185:1275–1284. doi: 10.1083/jcb.200810002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhns MS, Davis MM, Garcia KC. Deconstructing the form and function of the TCR/CD3 complex. Immunity. 2006;24:133–139. doi: 10.1016/j.immuni.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Le Floc'h A, Huse M. Molecular mechanisms and functional implications of polarized actin remodeling at the T cell immunological synapse. Cellular and molecular life sciences : CMLS. 2015;72:537–556. doi: 10.1007/s00018-014-1760-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Floc'h A, Tanaka Y, Bantilan NS, Voisinne G, Altan-Bonnet G, Fukui Y, Huse M. Annular PIP3 accumulation controls actin architecture and modulates cytotoxicity at the immunological synapse. J Exp Med. 2013;210:2721–2737. doi: 10.1084/jem.20131324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee MS, Glassman CR, Deshpande NR, Badgandi HB, Parrish HL, Uttamapinant C, Kuhns MS. A Mechanical Switch Couples T Cell Receptor Triggering to the Cytoplasmic Juxtamembrane Regions of CD3zetazeta. Immunity. 2015;43:227–239. doi: 10.1016/j.immuni.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee MT, Hung WC, Chen FY, Huang HW. Mechanism and kinetics of pore formation in membranes by water-soluble amphipathic peptides. Proc Natl Acad Sci U S A. 2008;105:5087–5092. doi: 10.1073/pnas.0710625105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Legate KR, Wickstrom SA, Fassler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23:397–418. doi: 10.1101/gad.1758709. [DOI] [PubMed] [Google Scholar]
- 42.Lim TS, Goh JK, Mortellaro A, Lim CT, Hammerling GJ, Ricciardi-Castagnoli P. CD80 and CD86 differentially regulate mechanical interactions of T-cells with antigen-presenting dendritic cells and B-cells. PloS one. 2012;7:e45185. doi: 10.1371/journal.pone.0045185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu B, Chen W, Evavold BD, Zhu C. Accumulation of dynamic catch bonds between TCR and agonist peptide-MHC triggers T cell signaling. Cell. 2014;157:357–368. doi: 10.1016/j.cell.2014.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophysical journal. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murrell MP, Voituriez R, Joanny JF, Nassoy P, Sykes C, Gardel ML. Liposome adhesion generates traction stress. Nat Phys. 2014;10:163–169. [Google Scholar]
- 47.Natkanski E, Lee WY, Mistry B, Casal A, Molloy JE, Tolar P. B cells use mechanical energy to discriminate antigen affinities. Science. 2013;340:1587–1590. doi: 10.1126/science.1237572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen K, Sylvain NR, Bunnell SC. T cell costimulation via the integrin VLA-4 inhibits the actin-dependent centralization of signaling microclusters containing the adaptor SLP-76. Immunity. 2008;28:810–821. doi: 10.1016/j.immuni.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 49.Nishikimi A, Kukimoto-Niino M, Yokoyama S, Fukui Y. Immune regulatory functions of DOCK family proteins in health and disease. Experimental cell research. 2013;319:2343–2349. doi: 10.1016/j.yexcr.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 50.Nowosad CR, Spillane KM, Tolar P. Germinal center B cells recognize antigen through a specialized immune synapse architecture. Nat Immunol. 2016;17:870–877. doi: 10.1038/ni.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Connor RS, Hao X, Shen K, Bashour K, Akimova T, Hancock WW, Milone MC. Substrate rigidity regulates human T cell activation and proliferation. J Immunol. 2012;189:1330–1339. doi: 10.4049/jimmunol.1102757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oakes PW, Patel DC, Morin NA, Zitterbart DP, Fabry B, Reichner JS, Tang JX. Neutrophil morphology and migration are affected by substrate elasticity. Blood. 2009;114:1387–1395. doi: 10.1182/blood-2008-11-191445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polozov IV, Anantharamaiah GM, Segrest JP, Epand RM. Osmotically induced membrane tension modulates membrane permeabilization by class L amphipathic helical peptides: nucleation model of defect formation. Biophysical journal. 2001;81:949–959. doi: 10.1016/S0006-3495(01)75753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pribila JT, Quale AC, Mueller KL, Shimizu Y. Integrins and T cell-mediated immunity. Annu Rev Immunol. 2004;22:157–180. doi: 10.1146/annurev.immunol.22.012703.104649. [DOI] [PubMed] [Google Scholar]
- 55.Pryshchep S, Zarnitsyna VI, Hong J, Evavold BD, Zhu C. Accumulation of serial forces on TCR and CD8 frequently applied by agonist antigenic peptides embedded in MHC molecules triggers calcium in T cells. J Immunol. 2014;193:68–76. doi: 10.4049/jimmunol.1303436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rak GD, Mace EM, Banerjee PP, Svitkina T, Orange JS. Natural killer cell lytic granule secretion occurs through a pervasive actin network at the immune synapse. PLoS Biol. 2011;9:e1001151. doi: 10.1371/journal.pbio.1001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ritter AT, Asano Y, Stinchcombe JC, Dieckmann NM, Chen BC, Gawden-Bone C, Griffiths GM. Actin depletion initiates events leading to granule secretion at the immunological synapse. Immunity. 2015;42:864–876. doi: 10.1016/j.immuni.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rullo J, Becker H, Hyduk SJ, Wong JC, Digby G, Arora PD, Cybulsky MI. Actin polymerization stabilizes alpha4beta1 integrin anchors that mediate monocyte adhesion. J Cell Biol. 2012;197:115–129. doi: 10.1083/jcb.201107140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sage PT, Varghese LM, Martinelli R, Sciuto TE, Kamei M, Dvorak AM, Carman CV. Antigen recognition is facilitated by invadosome-like protrusions formed by memory/effector T cells. J Immunol. 2012;188:3686–3699. doi: 10.4049/jimmunol.1102594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 61.Stinchcombe JC, Griffiths GM. Secretory mechanisms in cellmediated cytotoxicity. Annual review of cell and developmental biology. 2007;23:495–517. doi: 10.1146/annurev.cellbio.23.090506.123521. [DOI] [PubMed] [Google Scholar]
- 62.Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- 63.Swamy M, Beck-Garcia K, Beck-Garcia E, Hartl FA, Morath A, Yousefi OS, Schamel WW. A Cholesterol-Based Allostery Model of T Cell Receptor Phosphorylation. Immunity. 2016;44:1091–1101. doi: 10.1016/j.immuni.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 64.Tabdanov E, Gondarenko S, Kumari S, Liapis A, Dustin ML, Sheetz MP, Iskratsch T. Micropatterning of TCR and LFA-1 ligands reveals complementary effects on cytoskeleton mechanics in T cells. Integr Biol (Camb) 2015;7:1272–1284. doi: 10.1039/c5ib00032g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc Natl Acad Sci U S A. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thiery J, Keefe D, Boulant S, Boucrot E, Walch M, Martinvalet D, Lieberman J. Perforin pores in the endosomal membrane trigger the release of endocytosed granzyme B into the cytosol of target cells. Nat Immunol. 2011;12:770–777. doi: 10.1038/ni.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ueda H, Morphew MK, McIntosh JR, Davis MM. CD4+ T-cell synapses involve multiple distinct stages. Proc Natl Acad Sci U S A. 2011;108:17099–17104. doi: 10.1073/pnas.1113703108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 69.Wan Z, Chen X, Chen H, Ji Q, Chen Y, Wang J, Liu W. The activation of IgM- or isotype-switched IgG- and IgE-BCR exhibits distinct mechanical force sensitivity and threshold. eLife. 2015;4 doi: 10.7554/eLife.06925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wan Z, Zhang S, Fan Y, Liu K, Du F, Davey AM, Liu W. B cell activation is regulated by the stiffness properties of the substrate presenting the antigens. J Immunol. 2013;190:4661–4675. doi: 10.4049/jimmunol.1202976. [DOI] [PubMed] [Google Scholar]
- 71.Wang X, Ha T. Defining single molecular forces required to activate integrin and notch signaling. Science. 2013;340:991–994. doi: 10.1126/science.1231041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Woolf E, Grigorova I, Sagiv A, Grabovsky V, Feigelson SW, Shulman Z, Alon R. Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nat Immunol. 2007;8:1076–1085. doi: 10.1038/ni1499. [DOI] [PubMed] [Google Scholar]
- 73.Xu C, Gagnon E, Call ME, Schnell JR, Schwieters CD, Carman CV, Wucherpfennig KW. Regulation of T cell receptor activation by dynamic membrane binding of the CD3epsilon cytoplasmic tyrosine-based motif. Cell. 2008;135:702–713. doi: 10.1016/j.cell.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell motility and the cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 75.Yi J, Wu XS, Crites T, Hammer JA., 3rd Actin retrograde flow and actomyosin II arc contraction drive receptor cluster dynamics at the immunological synapse in Jurkat T cells. Mol Biol Cell. 2012;23:834–852. doi: 10.1091/mbc.E11-08-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeng Y, Yi J, Wan Z, Liu K, Song P, Chau A, Liu W. Substrate stiffness regulates B-cell activation, proliferation, class switch, and T-cell-independent antibody responses in vivo. Eur J Immunol. 2015;45:1621–1634. doi: 10.1002/eji.201444777. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y, Ge C, Zhu C, Salaita K. DNA-based digital tension probes reveal integrin forces during early cell adhesion. Nat Commun. 2014;5:5167. doi: 10.1038/ncomms6167. [DOI] [PMC free article] [PubMed] [Google Scholar]