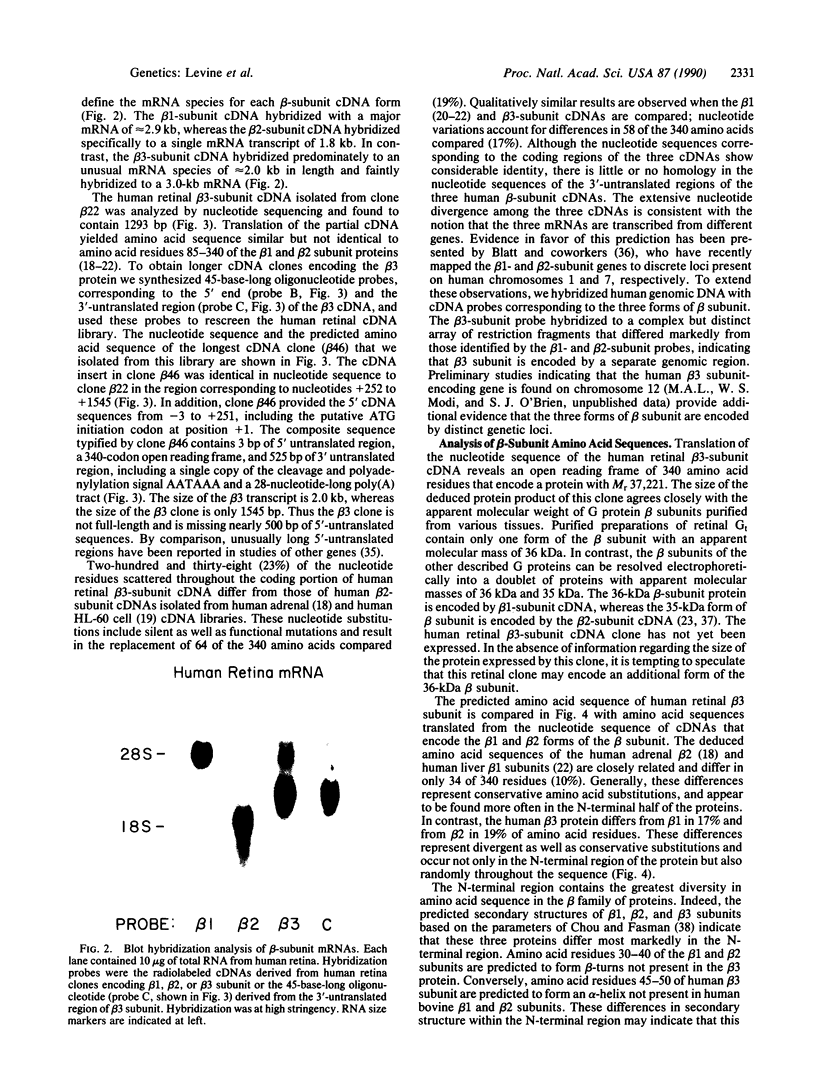
Abstract
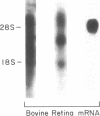
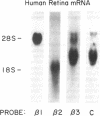
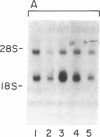
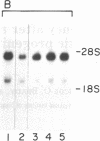
The signal-transducing guanine nucleotide-binding regulatory (G) proteins are heterotrimers composed of three subunits--alpha, beta, and gamma. Although multiple distinctive forms of the alpha subunit have been described, only two forms of the beta subunits of the G proteins have been identified. To investigate further the structural diversity of the beta subunits, we screened bovine and human retina cDNA libraries and isolated clones encoding three distinct types of G protein beta subunit. One form was identical to previously isolated beta 1-subunit cDNA clones that encode the 36-kDa form of the beta subunit, whereas a second form was identical to previously described beta 2 cDNAs that encode the 35-kDa beta isoform. In addition, we identified another species, designated beta 3 subunit, which encodes a third distinct form of the beta subunit. The beta 3-subunit cDNA corresponds to a 2.0-kilobase mRNA expressed in all tissues and clonal cell lines examined. Nucleotide sequence analysis indicates that the encoded peptide consists of 340-amino acid residues with a Mr of 37,221. The amino acid sequences of the three beta subunits are closely related: 83% identity between beta 1 and beta 3 subunits and 81% identity between beta 2 and beta 3 subunits. By contrast, the 3'-untranslated regions of the three cDNAs show no significant homology. Our data support the hypothesis that a family of beta-subunit polypeptides exists and extend understanding of beta-subunit structure.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahn T. G., Antonarakis S. E., Kronenberg H. M., Igarashi T., Levine M. A. Familial isolated hypoparathyroidism: a molecular genetic analysis of 8 families with 23 affected persons. Medicine (Baltimore) 1986 Mar;65(2):73–81. [PubMed] [Google Scholar]
- Ahn T. G., Cohn D. V., Gorr S. U., Ornstein D. L., Kashdan M. A., Levine M. A. Primary structure of bovine pituitary secretory protein I (chromogranin A) deduced from the cDNA sequence. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5043–5047. doi: 10.1073/pnas.84.14.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatruda T. T., 3rd, Gautam N., Fong H. K., Northup J. K., Simon M. I. The 35- and 36-kDa beta subunits of GTP-binding regulatory proteins are products of separate genes. J Biol Chem. 1988 Apr 15;263(11):5008–5011. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt C., Eversole-Cire P., Cohn V. H., Zollman S., Fournier R. E., Mohandas L. T., Nesbitt M., Lugo T., Jones D. T., Reed R. R. Chromosomal localization of genes encoding guanine nucleotide-binding protein subunits in mouse and human. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7642–7646. doi: 10.1073/pnas.85.20.7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Codina J., Stengel D., Woo S. L., Birnbaumer L. Beta-subunits of the human liver Gs/Gi signal-transducing proteins and those of bovine retinal rod cell transducin are identical. FEBS Lett. 1986 Oct 27;207(2):187–192. doi: 10.1016/0014-5793(86)81486-7. [DOI] [PubMed] [Google Scholar]
- Dull T. J., Gray A., Hayflick J. S., Ullrich A. Insulin-like growth factor II precursor gene organization in relation to insulin gene family. 1984 Aug 30-Sep 5Nature. 310(5980):777–781. doi: 10.1038/310777a0. [DOI] [PubMed] [Google Scholar]
- Evans T., Fawzi A., Fraser E. D., Brown M. L., Northup J. K. Purification of a beta 35 form of the beta gamma complex common to G-proteins from human placental membranes. J Biol Chem. 1987 Jan 5;262(1):176–181. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fong H. K., Amatruda T. T., 3rd, Birren B. W., Simon M. I. Distinct forms of the beta subunit of GTP-binding regulatory proteins identified by molecular cloning. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3792–3796. doi: 10.1073/pnas.84.11.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong H. K., Hurley J. B., Hopkins R. S., Miake-Lye R., Johnson M. S., Doolittle R. F., Simon M. I. Repetitive segmental structure of the transducin beta subunit: homology with the CDC4 gene and identification of related mRNAs. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2162–2166. doi: 10.1073/pnas.83.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong H. K., Yoshimoto K. K., Eversole-Cire P., Simon M. I. Identification of a GTP-binding protein alpha subunit that lacks an apparent ADP-ribosylation site for pertussis toxin. Proc Natl Acad Sci U S A. 1988 May;85(9):3066–3070. doi: 10.1073/pnas.85.9.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B., Gilman A. G., Robishaw J. D. A second form of the beta subunit of signal-transducing G proteins. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6122–6125. doi: 10.1073/pnas.84.17.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B., Mumby S., Gilman A. G. The G protein beta 2 complementary DNA encodes the beta 35 subunit. J Biol Chem. 1987 Dec 25;262(36):17254–17257. [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hildebrandt J. D., Codina J., Rosenthal W., Birnbaumer L., Neer E. J., Yamazaki A., Bitensky M. W. Characterization by two-dimensional peptide mapping of the gamma subunits of Ns and Ni, the regulatory proteins of adenylyl cyclase, and of transducin, the guanine nucleotide-binding protein of rod outer segments of the eye. J Biol Chem. 1985 Nov 25;260(27):14867–14872. [PubMed] [Google Scholar]
- Hunkapiller M., Kent S., Caruthers M., Dreyer W., Firca J., Giffin C., Horvath S., Hunkapiller T., Tempst P., Hood L. A microchemical facility for the analysis and synthesis of genes and proteins. Nature. 1984 Jul 12;310(5973):105–111. doi: 10.1038/310105a0. [DOI] [PubMed] [Google Scholar]
- Hurley J. B., Fong H. K., Teplow D. B., Dreyer W. J., Simon M. I. Isolation and characterization of a cDNA clone for the gamma subunit of bovine retinal transducin. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6948–6952. doi: 10.1073/pnas.81.22.6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H., Kozasa T., Nagata S., Nakamura S., Katada T., Ui M., Iwai S., Ohtsuka E., Kawasaki H., Suzuki K. Molecular cloning and sequence determination of cDNAs for alpha subunits of the guanine nucleotide-binding proteins Gs, Gi, and Go from rat brain. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3776–3780. doi: 10.1073/pnas.83.11.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. Golf: an olfactory neuron specific-G protein involved in odorant signal transduction. Science. 1989 May 19;244(4906):790–795. doi: 10.1126/science.2499043. [DOI] [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem. 1987 Oct 15;262(29):14241–14249. [PubMed] [Google Scholar]
- Katada T., Bokoch G. M., Northup J. K., Ui M., Gilman A. G. The inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. Properties and function of the purified protein. J Biol Chem. 1984 Mar 25;259(6):3568–3577. [PubMed] [Google Scholar]
- Lerea C. L., Somers D. E., Hurley J. B., Klock I. B., Bunt-Milam A. H. Identification of specific transducin alpha subunits in retinal rod and cone photoreceptors. Science. 1986 Oct 3;234(4772):77–80. doi: 10.1126/science.3529395. [DOI] [PubMed] [Google Scholar]
- Matsuoka M., Itoh H., Kozasa T., Kaziro Y. Sequence analysis of cDNA and genomic DNA for a putative pertussis toxin-insensitive guanine nucleotide-binding regulatory protein alpha subunit. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5384–5388. doi: 10.1073/pnas.85.15.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby S. M., Kahn R. A., Manning D. R., Gilman A. G. Antisera of designed specificity for subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1986 Jan;83(2):265–269. doi: 10.1073/pnas.83.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neer E. J., Lok J. M., Wolf L. G. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984 Nov 25;259(22):14222–14229. [PubMed] [Google Scholar]
- Northup J. K., Sternweis P. C., Gilman A. G. The subunits of the stimulatory regulatory component of adenylate cyclase. Resolution, activity, and properties of the 35,000-dalton (beta) subunit. J Biol Chem. 1983 Sep 25;258(18):11361–11368. [PubMed] [Google Scholar]
- Roof D. J., Applebury M. L., Sternweis P. C. Relationships within the family of GTP-binding proteins isolated from bovine central nervous system. J Biol Chem. 1985 Dec 25;260(30):16242–16249. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Nukada T., Tanabe T., Takahashi H., Noda M., Minamino N., Kangawa K., Matsuo H., Hirose T., Inayama S. Primary structure of the beta-subunit of bovine transducin deduced from the cDNA sequence. FEBS Lett. 1985 Oct 28;191(2):235–240. doi: 10.1016/0014-5793(85)80015-6. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]