Abstract
Background & Aims
It is a challenge to deliver nucleic acids to gastrointestinal (GI) tissues due to their size and need for intracellular delivery. They are also extremely susceptible to degradation by nucleases, which are ubiquitous in the GI tract. We investigated whether ultrasound, which can permeabilize tissue through a phenomenon known as transient cavitation, can be used to deliver RNA to the colonic mucosa of living mice.
Methods
We investigated delivery of fluorescently labeled permeants to colon tissues of Yorkshire pigs ex vivo and mice in vivo. Colon tissues were collected and fluorescence was measured by confocal microscopy. We then evaluated whether ultrasound is effective in delivering small interfering (si)RNA to C57/Bl6 mice with dextran sodium sulfate-induced colitis. Some mice were given siRNAs against tumor necrosis factor (Tnf) mRNA for 6 days; colon tissues were collected and analyzed histologically and TNF protein levels measured by ELISA. Feces were collected and assessed for consistency and occult bleeding. We delivered mRNA encoding firefly luciferase to colons of healthy C57/Bl6 mice.
Results
Exposure of ex vivo pig colon tissues to 20 kHz ultrasound for 1 min increased the level of delivery of 3 kDa dextran 7-fold, compared with passive diffusion (P=.037); 40 kHz ultrasound application for 0.5 sec increased the delivery 3.3-fold in living mice (P = .041). Confocal microscopy analyses of colon tissues from pigs revealed regions of punctuated fluorescent dextran signal, indicating intracellular delivery of macromolecules. In mice with colitis, ultrasound delivery of unencapsulated siRNA against Tnf mRNA reduced protein levels of TNF in colon tissues, compared to mice with colitis given siRNA against Tnf mRNA without ultrasound (P≤.014), and reduced features of inflammation (P≤4.1×10–5). Colons of mice given ultrasound delivery of an mRNA encoding firefly luciferase and the D-luciferin substrate had levels of bioluminescence 11-fold greater than colons of mice given the mRNA alone (P=.0025). Ultrasound exposures of 40 kHz ultrasound for 0.5 seconds were well tolerated, even in mice with acute colitis.
Conclusions
Ultrasound can be used to deliver mRNAs and siRNAs to the colonic mucosa of mice and knock down expression of target mRNAs.
Keywords: Antisense Therapy, Ulcerative Colitis, Inflammatory Bowel Disease, Ultrasound-Mediated Gastrointestinal Drug Delivery
INTRODUCTION
The gastrointestinal (GI) tract presents a striking opportunity for the delivery of therapeutics. It is characterized by a large surface area and is designed to absorb material1. These two features in particular make the GI tract a logical site for drug administration. However, the ability to absorb nutrients is facilitated by a harsh environment well suited to breaking down complex nutrients. Specifically, the low pH and wealth of proteases and nucleases makes the delivery of biologics via the GI tract extremely challenging2. This has largely limited GI luminal drug delivery to small molecules3. Indeed, the efficient delivery of biologics via the GI tract might present a paradigm shift in clinical and research settings, allowing for the local administration of highly effective biologics in the clinic to treat diseases like inflammatory bowel disease (IBD)4,5.
More broadly, challenges in delivery have also hampered clinical development of new therapeutics for a host of diseases. The development of new therapeutics necessitates the targeting and validation of new therapeutic targets with an active molecule. However, drugging a target necessitates delivering the molecule to the biological target, the delivery of which, is non-trivial. In the case of IBD, for example, these challenges are highlighted by the development times of Alicaforsen and Mongersen, two new antisense therapies6,7. The development times of these molecules, with Alicaforsen having been under development for Crohn’s Disease as early as 19978, underscores the difficulties in identifying, validating, and drugging new therapeutic targets. Beyond the targets of these two new drugs, mainly intercellular adhesion molecule-1 and SMAD7, respectively, lie a myriad of potential targets that might be effectively modulated to treat the underlying disease9. However, properly identifying a potential target and its function requires drugging it with an active biologic, the delivery of which, is challenging. This is particularly true in ulcerative colitis research, where the optimal therapeutic target has yet to be identified despite decades of intense research10.
These limitations highlight the glaring need for drug-independent methods of delivery. A platform that might enable the delivery of biologics and nucleic acids without the need for extensive formulation and nucleic acid modification could represent a paradigm shift in drug delivery science and have broad clinical impact. A physical enhancer, such as ultrasound, may enable formulation-independent delivery of biologics.
Ultrassound is a pressure wave with frequencies above 20 kHz. Clinically, ultrasound is widely used for imaging, lithotripsy and tumor ablation. Ultrasound has been shown to reversibly permeabilize tissue through a phenomenon known as transient cavitation11. This phenomenon has been investigated extensively for facilitating permeabilization of the skin in the context of transdermal drug delivery and received FDA-approval for the topical delivery of lidocaine12.
Aside from its use in clinical settings, ultrasound has also recently been noted to facilitate reversible permeabilization of cellular membranes allowing for intracellular delivery of fluorescent permeants13. This has been noted to result from cavitation events creating small defects in the cell membrane, allowing diffusion of species into the cell13. However, few studies exist on the use of ultrasound to facilitate intracellular delivery in vivo in complex biological settings, such as the GI tract.
Building on these two observations, we sought to investigate the use of ultrasound to facilitate permeabilization of the GI tract and simultaneously porate individual cells, enabling intracellular delivery. We chose the delivery of RNAs given the need for these therapeutics to be delivered into cells and because of the recognized difficulty in delivering this type of molecule currently14.
MATERIALS AND METHODS
Chemicals
Phosphate buffered saline (PBS), lysine-fixable 3 kDa dextran labeled with Texas Red, murine siRNA targeting Tnf mRNA (Stealth siRNA MSS211991), and DEPC-treated water were purchased from Invitrogen (Carlsbad, California). 14C-labeled inulin was purchased from American Radiolabeled Chemicals (St. Louis, Missouri). Dextran sodium sulfate (DSS) was purchased from Affymetrix Inc. (Santa Clara, California). mRNA coding for firefly luciferase was purchased from TriLink Biotechnologies (San Diego, California). D-luciferin, Soluene-350, and Hionic-Fluor scintillation cocktail fluid were purchased from Perkin-Elmer (Waltham, Massachusetts).
Ex Vivo Experiments
Porcine Tissue Preparation
The MIT Committee on Animal Care approved all animal-related research aspects of this study. Colon tissue was procured from Yorkshire pigs within 20 minutes of the animal being euthanized and stored at 4°C. The tissue was used within 6 hours of procurement. Tissue was washed with PBS, sectioned into pieces approximately 2 cm by 2 cm in size, and mounted in 15 mm-diameter Franz diffusion cells (PermeGear, Hellertown, Pennsylvania) or 29 mm-diameter custom-made diffusion cells to accommodate different sized ultrasound horns. with the luminal side exposed to the donor chamber. After mounting all tissue, Franz cells were randomly assigned to either ultrasound or control experimental groups.
Ultrasound Administration
Immediately before administration, the donor chamber was filled with 1.5 mL of permeant solution. 14C-labeled inulin was used as received at a concentration of 0.03 mg/mL and 3 kDa dextran tagged with Texas red at a concentration of 0.2 mg/mL in PBS. 20 kHz ultrasound was utilized to maximize transient cavitation events, which have previously been shown to be the primary mechanism of enhancement15. 20 kHz and 60 kHz ultrasound were generated with a 13 mm diameter VCX 500 and a 13 mm diameter custom-ordered probe, respectively (Sonics and Materials, Inc., Newtown, Connecticut). 1 MHz ultrasound was generated using a Dynatron D125 ultrasound probe (Dynatronics, Corporation, Salt Lake City, Utah). For all applications, the ultrasound probe tip was placed 3 mm away from the surface of the tissue. Ultrasound intensities were calibrated by calorimetry to 5 W/cm2, 9.6 W/cm2, and 1.5 W/cm2 for 20 kHz, 60 kHz, and 1 MHz ultrasound, respectively. For frequency comparison studies, total applied ultrasound power and permeant contact time were kept constant across all three frequencies. For 3 kDa dextran, ultrasound was applied for 1 minute using a 50% duty cycle (5 s on, 5 s off).
After administration, the permeant was removed and the tissue thoroughly washed with PBS. Delivery into the tissue using 14C-labeled inulin was quantified using a liquid scintillation counter (Perkin-Elmer, Waltham, Massachusetts) by solubilizing the tissue. Delivery of 3 kDa dextran was quantified by imaging the tissue with an IVIS fluorescence imager (Perkin-Elmer, Waltham, Massachusetts).
Multiphoton Microscopy
After delivery of 3 kDa dextran, tissue sections were fixed in 10% formalin overnight. They were then stained for 30 minutes with 4',6-diamidino-2-phenylindole (DAPI) nuclear stain. After staining, the tissue was washed and imaged by the microscopy core facility in the Swanson Biotechnology Center (MIT). Specifically, an Olympus FV1000 Multiphoton Laser Scanning Microscope was used. Z-stack images were acquired with a step size of 5 µm to a total depth of 125 µm. The second harmonic (to show tissue architecture), DAPI, and dextran channels were acquired.
In Vivo Experiments
Animals
15-week old, female C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, Massachusetts) for all studies. Each cage (group) of animals was used as received and assigned randomly to a particular experimental group by the researchers performing the work.
In Vivo Ultrasound Administration
For this study, a custom-designed 40 kHz ultrasound probe was manufactured to enable administration in the colon of mice (Sonics and Materials, Inc., Newtown, Connecticut). The shaft has a diameter of 2 mm and contains two, 3 mm diameter protrusions at half-wavelength intervals along the shaft to induce radial ultrasound activity. Ultrasound intensity was calibrated to 4.0 W by calorimetry. Ultrasound was administered by inserting the probe into the rectum and turning it on for 0.5 seconds.
3 kDa Dextran Delivery Study
In order to assess the potential for macromolecule delivery in mice in vivo, 3 kDa dextran tagged with Texas red was used as a model permeant. The dextran was diluted in PBS to a concentration of 0.33 mg/mL. Immediately before ultrasound administration, mice were sedated with isoflurane. 0.5 mL of the dextran solution was administered as an enema into the colon followed by ultrasound. The solution remained in the rectum for 2 minutes and then the colon was thoroughly lavaged with PBS and the animal returned to its cage. Immediately after, or two hours after ultrasound administration, animals were euthanized for the purpose of dextran quantification in the colon. Immediately after euthanization, the colon was carefully dissected out, placed on black backing, and imaged using an IVIS Fluorescent Imaging System (Perkin-Elmer, Waltham, Massachusetts). Settings utilized were an excitation of 570 nm, emission of 620 nm, binning value of 4, f-value of 2, and a field-of-view of 12.8. Exposure time was varied to ensure the total counts exceeded 6,000, according to the manufacturer’s recommendations.
Colon samples were then fixed in 10% formalin and mounted in paraffin blocks for histological sectioning. Two, 8 µm-thick sections separated by a 200 µm step were used for subsequent imaging. These sections were imaged by the microscopy core facility in the Swanson Biotechnology Center (MIT). Samples were imaged using an Olympus FV-1000MP multiphoton microscope with a 25×, 1.05 N.A. objective. Excitation at 860 nm was achieved using a Ti-Sapphire pulsed laser (Spectra-Physics, Santa Clara, California). The resulting emission was collected with a 607/70 nm band-pass filter. Additionally, collagen was imaged using second harmonic generation at 430 nm. Discrete image channels were further processed using ImageJ (National Institutes of Health, Bethesda, Maryland).
Dextran Sodium Sulfate Colitis
Colitis induction and administration of ultrasound with or without siRNA targeting Tnf mRNA (Stealth siRNA MSS211991) was performed following previously published protocols with slight modifications15,16. On day 1, mice were weighed and feces were collected. The animals’ drinking water was then spiked with 3% w/v DSS (40–50 kDa). The water was given ad libitum and replaced with a fresh DSS solution on days 3 and 5. On day 7, the DSS water was removed and the animals given normal drinking water. All DSS used in the study was from the same manufacturer batch number to reduce variability.
Experimental therapies were administered starting on day 1. Each experimental group received either an enema containing siRNA targeting Tnf mRNA, siRNA targeting Tnf mRNA administered in combination with ultrasound, or siRNA targeting Tnf mRNA followed by insertion of the ultrasound probe without turning it on to control for any potential injury as a result of insertion. Specifically, 100 ng of siRNA in 200 µL DEPC-treated water was instilled in the rectum of mice sedated with isoflurane. Ultrasound was administered as described above followed immediately by a second dose of siRNA. Enemas were administered daily for six consecutive days.
Animal weight, fecal consistency, and the presence of fecal blood was monitored daily. Fecal consistency and bleeding were scored based on previously published protocols15,17. Fecal consistency was scored as follows: (1) normal pellet, (2) stool easily crushed, (3) stool that is soft and watery with the presence of granules, or (4) diarrhea. Blood in the stool was confirmed by hemoccult testing. Negative hemoccult results were scored (1). Positive hemoccult results were further stratified: (2) feces with no visible blood at the time of collection, (3) feces with discrete blood speckles on the surface, or (4) feces covered with blood or observation of blood around the animal’s anus. The total fecal score was determined by summing the consistency and fecal occult blood scores. Therefore, the total fecal score ranges from 2 (normal) to 8 (severe disease).
On day 8, weight and fecal score were determined and then the animals were sacrificed. The colon was dissected and a small portion frozen for quantification of TNF protein by ELISA. The remainder of the colon was fixed in 10% formalin. Fixed tissue was sectioned into 2–6 pieces and mounted in paraffin blocks. Two, 8 µm-thick sections from each block separated by a 200 µm step were stained with hematoxylin and eosin and mounted to slides.
Histological Scoring
Histology was scored in a blinded fashion by a clinical pathologist at Massachusetts General Hospital. Scoring was performed according to previously published protocols15: Specifically, normal colonic mucosa with the preservation of normal crypt architecture was assigned a score of 0. Tissue showing signs of inflammation was further stratified as follows: Tissue with shortened crypts with moderate inflammatory infiltrate above the muscularis mucosae (1), tissue with the base of the mucosa eliminated but demonstrating residual surface epithelium with the upper portion of the crypts preserved (2), tissue lacking any mucosa and demonstrating chronic inflammation of the lamina propria with residual surface epithelium present (3), and tissue showing complete effacement and erosion of the mucosa combined with fibrinopurulent debris. Each tissue cross-section was scored 0–4 weighted by a percentage involvement. The percent involvement-weighted score for each tissue section from all animals in a given experimental group were averaged to give the histology score for that experimental group.
TNF Protein Quantification
To determine colonic TNF protein levels, frozen colon tissue was homogenized in protease inhibitor solution in PBS using a bead-based homogenizer (Precellys 24, Bertin Technologies, France). Cell debris was pelleted by centrifugation for 10 minutes and the resulting supernatant collected. TNF protein levels were quantified using an ELISA kit following the manufacturer’s protocol (ThermoFisher Scientific, Waltham, Massachusetts). TNF protein levels were normalized by total protein content, which was quantified using a BCA total protein assay kit following the manufacturer’s protocol (ThermoFisher Scientific, Waltham, Massachusetts).
mRNA Synthesis, Delivery, and Quantification
mRNA lipid nanoparticles (LNPs) were synthesized as previously described18. Briefly, the ethanol phase containing the lipids and the aqueous phase containing the mRNA were mixed in a microfluidic chip device. The aqueous phase contained 300 µg firefly luciferase mRNA (5-methylcytidine and pseudouridine substituted, 1mg/mL in 10mM TRIS-HCL), 150 µL of citrate (100 mM, pH 3), and 1050 µL of water. The ethanol phase contained the ionizable lipid ckk-E12 (3.0 mg, synthesized in our laboratory, as previously described19, with 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE, 4.1 mg, Avanti Polar Lipids, Alabaster, AL), cholesterol (3.3 mg, Sigma-Aldrich, St. Louis, Missouri), and 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (C14-PEG 2000, 1.2 mg, Avanti) in ethanol (504 µL, Sigma-Aldrich, St. Louis, Missouri). The resulting LNP solution was dialyzed against PBS (1×) in 20,000 MWCO cassettes at 20 °C for 2 hours. The size (123.1 nm, 100%) and polydispersity index (PDI = 0.153) of the LNP was measured using dynamic light scattering (ZetaPALS, Brookhaven Instruments, Holtsville, New York).
With the mice anesthetized, 300 µL of the LNP solution was administered in the colon and the ultrasound probe administered. Two, 0.5 second ultrasound exposures were administered over a 5-minute period. For control experiments, the probe was administered but not turned on. After administration the animals were returned to their cage. 24 hours post administration, mice were injected intraperitoneally with D-luciferin (0.2 mL; 10 mg/mL in PBS). 15 minutes after the injection, animals were euthanized and the colons dissected, opened, and bioluminescence measured using an IVIS fluorescence imager (Perkin-Elmer, Waltham, Massachusetts). The bioluminescence signal was quantified using Living Image software (Caliper Life Science, Hopkinton, Massachusetts).
Statistical Analysis
Statistical analysis of 3 kDa dextran delivery and mRNA delivery was performed using two-tailed Student’s t-tests to determine statistical significance. Statistical analysis for the in vivo mouse colitis work was performed using one-way analysis-of-variance (ANOVA) tests with multiple comparisons. Statistical significance was defined as P < 0.05. All calculations were performed in MatLab R2015b (MathWorks).
RESULTS
Ultrasound Enhances the Delivery of Macromolecules Ex Vivo
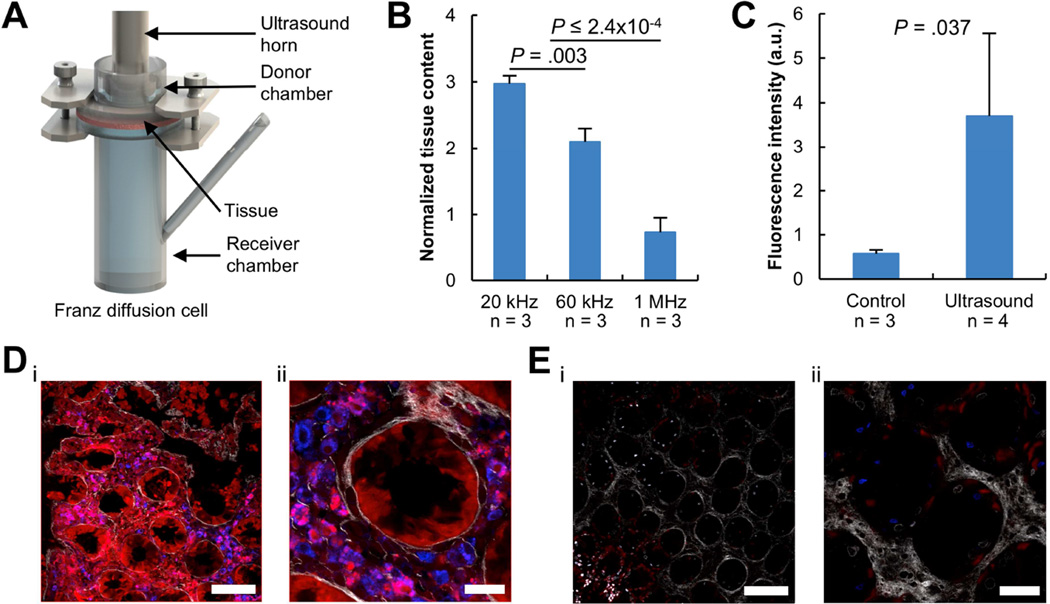
The delivery of macromolecules by ultrasound was investigated using fresh porcine tissue mounted in Franz diffusion cells (Figure 1A) using a range of ultrasound frequencies from 20 kHz to 1 MHz. Lower frequencies were hypothesized to result in greater enhancement because previous work has demonstrated transient cavitation, the predominant mechanism of enhancement, to inversely correlate with frequency15,20. 14C-labeled inulin (5 kDa) was used as a model permeant (Figure 1B). Additional ultrasound intensities were investigated to further characterize the impact on delivery. As expected, 20 kHz ultrasound provided the greatest level of enhancement over passive diffusion. 60 kHz ultrasound did enhance the delivery of inulin over passive diffusion but less so than that using 20 kHz ultrasound (P = .003; Figure 1B). 1 MHz ultrasound resulted in no enhancement in delivery over passive diffusion. As a result, further experiments focused on the use of frequencies < 60 kHz.
Figure 1. Ultrasound-Mediated Delivery of 3 kDa Dextran Ex Vivo.
(A) Ex vivo experimental setup, the Franz diffusion cell. A section of fresh GI tissue is shown sandwiched between a donor and receiver chamber. (B) Enhancement in the delivery of 14C-labeled inulin into GI tissue using 20 kHz, 60 kHz, or 1 MHz ultrasound compared to passive diffusion without ultrasound. The P-values were determined by one-way ANOVA with multiple comparisons. (C) Total fluorescent intensity of porcine colon segments immediately after administration of 3 kDa dextran tagged with Texas red with ultrasound or without ultrasound (Control). The P-value was determined by a two-tailed Student’s t-test. Representative multiphoton microscopy images at a fixed z-position within porcine colonic tissue after delivery of dextran (red) with ultrasound (D) and without ultrasound (E) followed by nuclear staining (blue). The scale bars in (Di, Ei) represent 100 µm and the scale bars in (Dii, Eii) represent 30 µm. Data (in B and C) are averages + 1SD.
After determining an appropriate range of ultrasound frequencies, the distribution of permeant throughout tissue because of ultrasound-mediated delivery was determined. 3 kDa dextran tagged with Texas red was used as a model permeant to allow for visualization of the permeant after administration. Short, 1-minute 20 kHz ultrasound exposures were found to enhance the delivery of dextran almost 7-fold compared to passive diffusion in the same timeframe (P = .037; Figure 1C). Confocal imaging demonstrated the dextran to be relatively homogenously dispersed throughout the tissue (Figure 1D). Conversely, negligible dextran signal was detectable in tissue not treated with ultrasound (Figure 1E). Higher magnification imaging showed colocalization of red 3 kDa dextran signal with blue signal from 4',6-diamidino-2-phenylindole (DAPI) nuclear stain (Figure 1D). Additionally, the 3 kDa dextran signal at these sites appeared as punctated, discrete spots.
Colonic Macromolecule Delivery In Vivo mediated by Radial Ultrasound
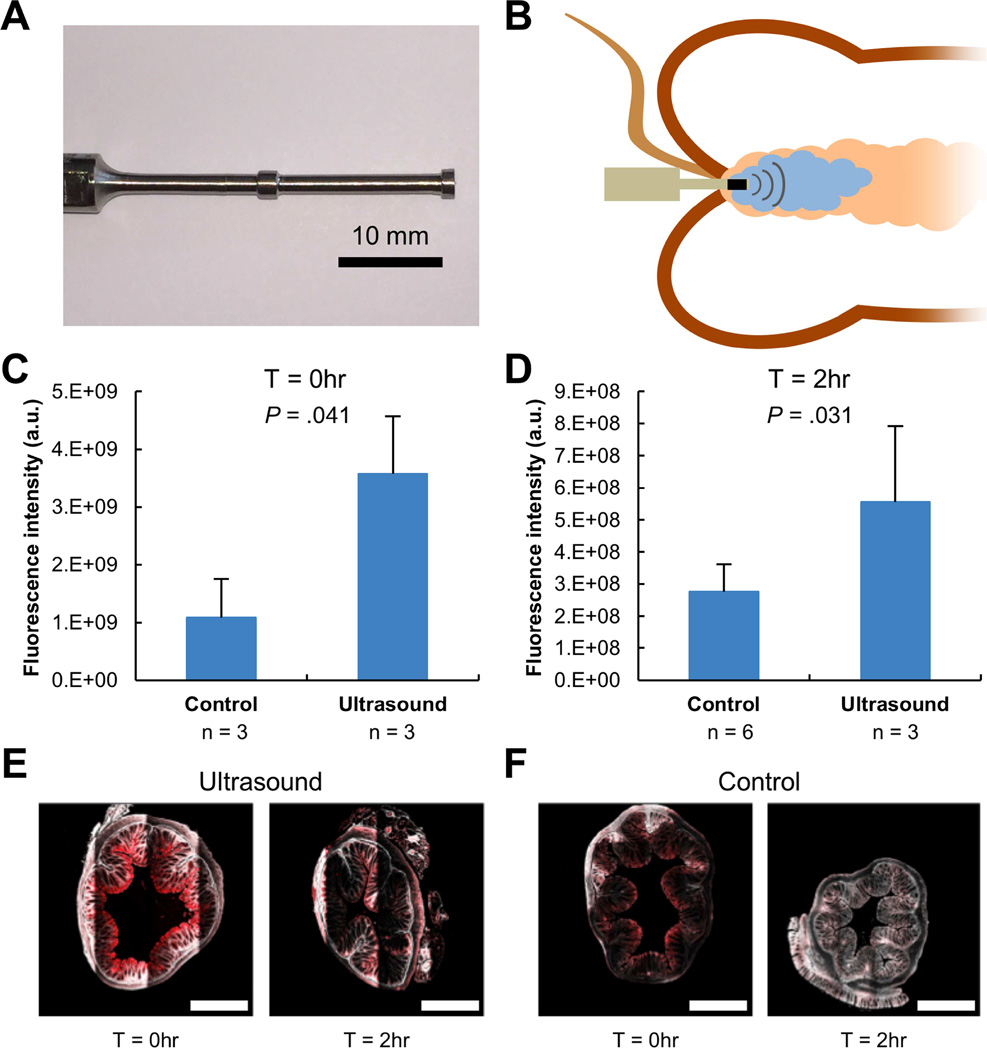
To assess the potential for macromolecule delivery in vivo, a miniature probe-based macromolecule delivery protocol was developed. This enables radial emission of 40 kHz ultrasound locally in the colon so as to direct cavitation towards the mucosa, maximizing colonic mucosal macromolecule delivery (Figure 2A). 3 kDa dextran was rectally administered into the colon followed by the placement of the ultrasound probe, as shown in Figure 2B.
Figure 2. Ultrasound-Mediated GI Delivery of 3 kDa Dextran In Vivo.
(A) A custom-made 40 kHz ultrasound probe allowing for administration in the colon of mice. The protrusions enhance radial ultrasound activity. (B) Experimental setup depicting placement of an enema in the colon followed by ultrasound exposure with the custom probe shown in (A). Total fluorescent intensity (averages + 1SD) of the colon immediately after administration (C) of 3 kDa dextran tagged with Texas red with ultrasound or without ultrasound (Control) and two hours after administration (D). P-values determined by two-tailed Student’s t-tests. Representative multiphoton microscopy images of fixed murine colonic tissue after delivery of 3 kDa dextran tagged with Texas red with ultrasound (E) and without ultrasound (F) immediately and two hours after administration. The red channel and second harmonic are shown. The scale bars represent 500 µm.
Short, ~0.5 second ultrasound exposures in vivo in mice were safe and well tolerated based on histological examination of colon tissue post administration (Figure 3). The use of ultrasound was found to enhance the delivery of 3 kDa dextran 3-fold compared to passive diffusion (P = .041; Figure 2C). Further, the fluorescent signal from 3 kDa dextran was still significantly elevated in tissue samples two hours after administration using ultrasound (P = .031; Figure 2D). Ultrasound was found to enable deeper penetration of the 3 kDa dextran immediately after administration (Figure 2E) compared to that achieved with passive diffusion (Figure 2F), resulting in greater fluorescent intensity two hours after administration.
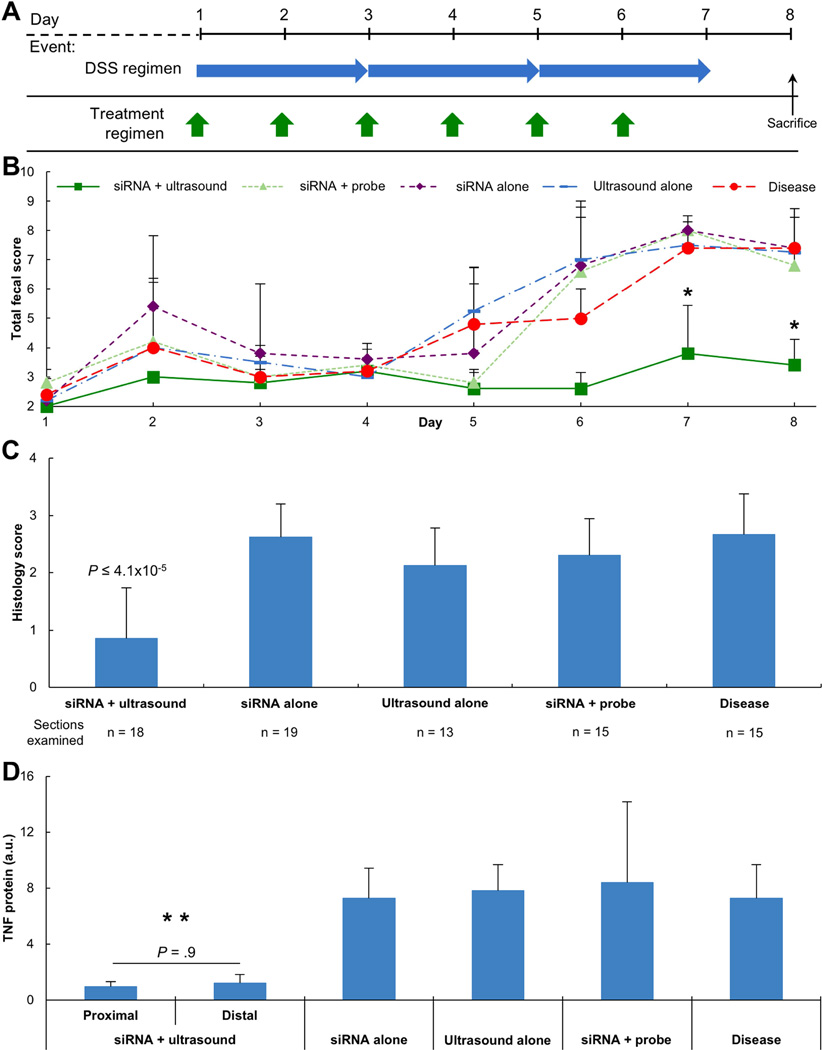
Figure 3. Ultrasound-Mediated Delivery of siRNA in DSS Colitis.
(A) DSS colitis induction and enema administration schedule. DSS was given for 7 consecutive days to induce acute colitis in mice. Concurrently, animals were administered enemas from day 1 through 6. Experimental groups consisted of either siRNA targeting Tnf mRNA in combination with ultrasound or siRNA targeting Tnf mRNA alone. (B) Total fecal score for animals with acute colitis receiving various enemas (n = 5 animals per group). * represents P < 0.021 for siRNA + Ultrasound compared to all other groups (determined by one-way ANOVA with multiple comparisons). (C) Histology scores of colonic tissue sections on Day 8. (D) TNF protein levels from colonic tissue biopsies normalized by total protein content on Day 8. ** represents P < 0.014 for both siRNA + Ultrasound groups compared to any other group (determined by one-way ANOVA with multiple comparisons). P-values (in C, D) determined by one-way ANOVA with multiple comparisons. Data (in B, C, D) are averages + 1SD.
Colonic Anti-Tnf mRNA Delivery by Radial Ultrasound in Mice
Having demonstrated the capacity for radial ultrasound to successfully deliver macromolecules in vivo in mice, the capacity to deliver unencapsulated antisense therapies was investigated. siRNA targeting Tnf mRNA (16.1 kDa) was chosen given the recognized enhancement in TNF protein levels in colonic tissue as a result of dextran sodium sulfate (DSS) administration16,21. Disease induction followed previously published protocols (Figure 3A)16. A 6-day administration regimen was chosen to allow for comparison to previously published work utilizing novel formulations of siRNA16. Experimental groups investigated included siRNA administered with simultaneous ultrasound, siRNA administered with simultaneous insertion of the ultrasound probe (Figure 2A) without turning it on, siRNA alone, and ultrasound alone. Disease induction was confirmed through the use of an experimental group receiving no ultrasound or siRNA. The total fecal score (a combination of fecal consistency and occult bleeding) was quantified daily (Figure 3B). The total fecal score increased in all animals not receiving ultrasound, demonstrating disease induction and progression. In animals receiving siRNA in combination with ultrasound, however, the total fecal score never exceeded 4 (Figure 3B). By day 7 and beyond, animals receiving siRNA in combination with ultrasound had significantly better fecal scores than any other experimental group (P < .021). This also supports the safety and tolerability of ultrasound administration, underscored by those experimental groups receiving either ultrasound alone and those receiving insertion of the probe with siRNA having a total fecal score no more elevated than the disease only group.
After assessing the total fecal score on day 8, animals were sacrificed and the colon dissected to allow for histological evaluation and quantification of TNF protein by ELISA. Histology scores comprising the degree of inflammation and architectural distortion were determined in a blinded fashion (Figure 3C). Animals receiving siRNA in combination with ultrasound were found to have statistically lower histology scores than any other experimental group (P ≤ 4.1×10−5), in agreement with the significantly better total fecal scores. Tissue from animals receiving siRNA with ultrasound showed minimal erosion of the epithelium and preservation of crypts. No evidence of macroscopic injury due to ultrasound administration was noted. The other experimental groups demonstrated greater erosion and significant shortening of the crypts. The improved total fecal score and histological score noting less inflammation were hypothesized to be a result of efficient suppression of TNF protein. Indeed, TNF protein levels normalized by total protein content of colon tissue samples were found to be 7 to 8-fold lower in animals receiving siRNA in combination with ultrasound than any other experimental group (Figure 3D, P ≤ .014). This knockdown was found to be a diffuse phenomenon, with statistically similar levels of TNF protein in tissue taken from proximal and distal locations (P = .9). This confirmed successful knockdown of Tnf mRNA.
Colonic mRNA Delivery in Mice using Radial Ultrasound
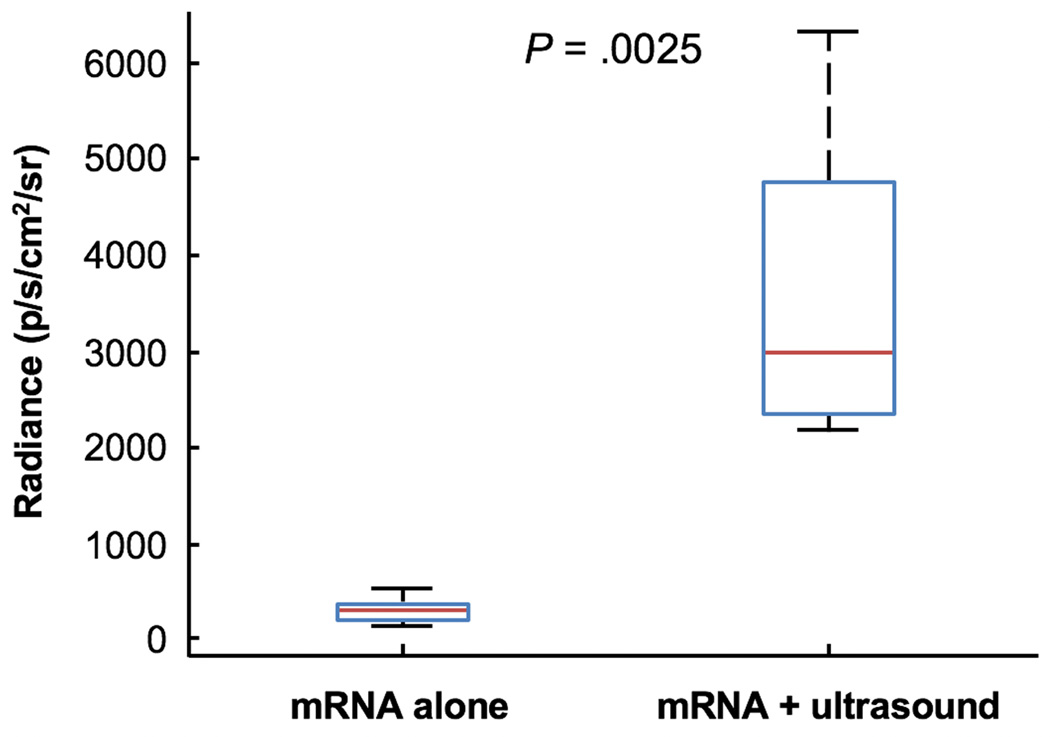
Having demonstrated the ability to deliver siRNA, we investigated the potential for localized delivery of mRNA directly to the colon. mRNA (~950 kDa) encoding the firefly luciferase was administered with ultrasound and found to be successfully translated locally in the colon, based on measured bioluminescent signal after administration of D-luciferin substrate (Figure 4). The measured bioluminescent signal from animals administered mRNA in combination with ultrasound was an order-of-magnitude higher than that from animals administered mRNA alone (P = .0025).
Figure 4. Ultrasound-Mediated Delivery of RNA in the Colon.
Radiance of dissected mouse colons (n = 5) 24 hours after rectal administration of mRNA alone or in combination with ultrasound in vivo. The median, 25th, and 75th percentiles are shown. The whiskers indicate the most extreme data points. The P-value was determined by a two-tailed Student’s t-tests.
DISCUSSION
Here we present an investigation on the delivery of macromolecules locally in the GI tract using low-frequency ultrasound. Specifically we focused on the delivery of RNA, a molecule recognized for its significant susceptibility to rapid nuclease breakdown. The delivery of macromolecules through topical approaches in the GI tract in sufficient loads to exert a therapeutic effect presents a significant challenge given the barrier presented by GI tissue, limiting passive delivery of molecules greater than 500 Da22. Low frequency ultrasound (< 60 kHz) was found to enable significant enhancement in delivery of macromolecules. High-frequency ultrasound (1 MHz) had no effect on delivery. Initial ex vivo evaluation of low frequency ultrasound-mediated delivery with a model macromolecule, Texas red-labeled 3 kDa dextran, was notable for evenly distributed signal throughout fresh porcine colonic tissue. Interestingly, areas in which the fluorescent 3 kDa dextran signal appeared to localize with the DAPI nuclear stain were observed (Figure 1D). This, in combination with the punctated appearance of the signal in certain regions, might suggest intracellular delivery of the 3 kDa dextran23,24. This is thought to be a result of the transient cavitation events induced by the ultrasound impinging upon individual cells, creating pores in the cellular membrane through which dextran could diffuse. This has been observed in previous studies on ultrasound-mediated delivery to cell cultures, noting the capacity for ultrasound to facilitate cellular delivery25.
Given the apparent capacity to achieve intracellular delivery locally, we investigated the potential for delivering therapeutically-relevant molecules that require intracellular delivery. We chose siRNA targeting Tnf mRNA given the recent clinical developments in antisense therapies for IBD6,26. Daily administration of ultrasound was found to be safe and well tolerated, even in the setting of a compromised mucosa due to colitis. Short, 0.5 second pulses of ultrasound were found to successfully enable the delivery of unencapsulated siRNA, suppressing Tnf mRNA and subsequent TNF protein production in a diffuse manner, resulting in improvements in the total fecal score and histological disease activity of colonic tissue in mice with DSS-induced colitis. Given the susceptibility of these molecules to degradation, it is surprising that such a robust response was achieved using naked siRNA and highlights the rapidity of ultrasound-mediated delivery. This represents, to the best of our knowledge, the first delivery of unencapsulated siRNA in vivo with therapeutic benefit. Even more surprising was the efficacy achieved considering the minimal dose administered. Indeed, the dose utilized was 30-fold lower than other published studies employing novel formulations encapsulating the siRNA administered via gavage and resulted in comparable efficacy16. The present dose was also lower than that utilized in other published studies evaluating intrarectal administration of formulated nanoparticles containing siRNA, and achieved superior suppression of TNF protein27. Considering the use of siRNA for gene silencing more broadly, even the best chemical formulations, gleaned from screens of thousands of compounds, in which the antisense molecule is encapsulated and protected, only show activity at doses almost 3× higher than that reported here28. These studies also administer the antisense through intravenous injection, avoiding the inhospitable environment of the GI tract. This significant dose-sparing is an especially important feature given that one of the significant hurdles to clinical adoption of antisense therapies, beyond the delivery of these species, is the cost of a potential dose, given their typically low bioavailability29.
In addition to clinical settings, the capacity to deliver naked, unformulated antisense molecules could present a major advance for drug target screening and validation. One particular challenge in the field of drug development is the ability to validate a molecule’s target and action. This necessitates the successful formulation and delivery of a molecule to confirm its activity. However, formulation work required to enable the delivery of these complex molecules can significantly delay the development and validation of these drugs. The laborious nature of this work is underscored by the development time of Mongersen, for example, with the identification of SMAD7 occurring almost two decades ago30 with subsequent elucidation of its relevance to chronic IBD31,32. A technology that can enable the rapid delivery of new drug entities without the need for any formulation would present a major shift in drug discovery and development.
Finally, having demonstrated the capacity to deliver unformulated antisense therapies with significant therapeutic benefit, we investigated the potential for the delivery of mRNA locally to the colon. This would therefore demonstrate the capacity of ultrasound to enable both silencing of specific proteins, as well as the preferential production of other proteins. The latter capability could have benefits for the expression of complex epitopes locally to aid in the stimulation and modulation of immunologic responses for novel vaccines, for example33. mRNA encoding firefly luciferase was successfully delivered to the colon, resulting in significant production of the protein, assessed through bioluminescent measurement after administration of the substrate. To the best of our knowledge, there has been no report to date demonstrating the capacity to deliver mRNA locally in the colon. Indeed, this represents a major advance towards the eventual clinical use of mRNA34.
In conclusion, we demonstrated the capacity of ultrasound to mediate the efficient and rapid delivery of macromolecules ex vivo and in vivo with significant benefit. Naked siRNA targeting Tnf mRNA was successfully administered locally in the colon of mice with DSS-induced colitis, leading to a 7-fold reduction in TNF protein levels in colonic tissue compared to other experimental groups, resulting in superior control of colitis. Further, mRNA was successfully delivered locally in the colon, resulting in translation of firefly luciferase protein. Future studies will investigate the cell types taking up the oligos and whether certain populations can be targeted. This technology could prove invaluable in clinical settings for treating local GI-based diseases, as well as in research settings to expedite the identification, validation, and eventual translation of new therapeutics.
Acknowledgments
Grant Support:
This work was supported in part by the National Institutes of Health (Grant# EB-000244), and Max Planck Research Award Ltr Dtd. 2/11/08, Alexander von Humboldt-Stiftung Foundation. C.M.S. was supported in part by a Koch Institute Quinquennial Cancer Research Fellowship. G. T. was supported in part by the Division of Gastroenterology, Brigham and Women’s Hospital.
C.M.S., R.L., and G.T. are inventors of U.S. Provisional Patent Application No. 62/144,842 filed on April 8, 2015 covering the application of ultrasound for delivery of macromolecules in the gastrointestinal tract.
We thank the Hope Babette Tang (1983) Histology Core Facility and J. Wyckoff of the microscopy core facility in the Swanson Biotechnology Center for imaging colon samples. We thank Sarah Saxton for help in procuring the fresh porcine gastrointestinal tissue. We are grateful for all members of the Langer and Traverso laboratories for helpful methodological suggestions.
Abbreviations
- DAPI
4',6-diamidino-2-phenylindole
- GI
Gastrointestinal
- IBD
Inflammatory Bowel Disease
- mRNA
Messenger Ribonucleic acid
- siRNA
Small Interfering Ribonucleic acid
- TNF
Tumor Necrosis Factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
These authors declare no other conflicts of interest. All other authors declare no conflicts of interest.
Author Contributions:
C.M.S., R.L., and G.T. conceived and designed the research. C.M.S., C.C., J.P., D.M., and Y.C. performed the ex vivo experiments. C.M.S., M.A.O., and C.C. performed the in vivo experiments. G.Y.L analyzed and scored the histology. C.M.S. and G.T. performed the statistical analysis. C.M.S., D.G.A., A.J., S.B.S., J.A.G., R.L., and G.T. analyzed the data. C.M.S., R.L., and G.T. wrote the manuscript.
REFERENCES
- 1.Helander HF, Fandriks L. Surface area of the digestive tract - revisited. Scandinavian Journal of Gastroenterology. 2014;49:681–689. doi: 10.3109/00365521.2014.898326. [DOI] [PubMed] [Google Scholar]
- 2.Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nature Reviews Drug Discovery. 2014;13:655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schoellhammer CM, Traverso G. Low-frequency ultrasound for drug delivery in the gastrointestinal tract. Expert Opin. Drug Deliv. 2016 doi: 10.1517/17425247.2016.1171841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 1991;325:928–937. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- 5.Neurath MF. Cytokines in inflammatory bowel disease. Nature Reviews Immunology. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 6.Monteleone G, Neurath MF, Ardizzone S, et al. Mongersen, an Oral SMAD7 Antisense Oligonucleotide, and Crohn’s Disease. N Engl J Med. 2015;372:1104–1113. doi: 10.1056/NEJMoa1407250. [DOI] [PubMed] [Google Scholar]
- 7.Miner PB, Geary RS, Matson J, et al. Bioavailability and therapeutic activity of alicaforsen (ISIS 2302) administered as a rectal retention enema to subjects with active ulcerative colitis. Alimentary Pharmacology & Therapeutics. 2006;23:1427–1434. doi: 10.1111/j.1365-2036.2006.02909.x. [DOI] [PubMed] [Google Scholar]
- 8.Gewirtz AT, Gewirtz AT, Sitaraman S, et al. Alicaforsen. Isis Pharmaceuticals. Curr Opin Investig Drugs. 2001;2:1401–1406. [PubMed] [Google Scholar]
- 9.Danese S, Fiocchi C. Ulcerative Colitis. N Engl J Med. 2011;365:1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 10.Peyrin-Biroulet L, Peyrin-Biroulet L, Sandborn W, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol. 2015;110:1324–1338. doi: 10.1038/ajg.2015.233. [DOI] [PubMed] [Google Scholar]
- 11.Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polat BE, Blankschtein D, Langer R. Low-frequency sonophoresis: application to the transdermal delivery of macromolecules and hydrophilic drugs. Expert Opin. Drug Deliv. 2010;7:1415–1432. doi: 10.1517/17425247.2010.538679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlicher RK, Radhakrishna H, Tolentino TP, et al. Mechanism of intracellular delivery by acoustic cavitation. Ultrasound in Medicine & Biology. 32:915–924. doi: 10.1016/j.ultrasmedbio.2006.02.1416. [DOI] [PubMed] [Google Scholar]
- 14.Yin H, Kanasty RL, Eltoukhy AA, et al. Non-viral vectors for gene-based therapy. Nature Reviews Genetics. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 15.Schoellhammer CM, Schroeder A, Maa R, et al. Ultrasound-mediated gastrointestinal drug delivery. Science Translational Medicine. 2015;7:310ra168–310ra168. doi: 10.1126/scitranslmed.aaa5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson DS, Dalmasso G, Wang L, et al. Orally delivered thioketal nanoparticles loaded with TNF-α–siRNA target inflammation and inhibit gene expression in the intestines. Nature Materials. 2010;9:923–928. doi: 10.1038/nmat2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper HS, Murthy SN, Shah RS, et al. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 18.Chen D, Love KT, Chen Y, et al. Rapid Discovery of Potent siRNA-Containing Lipid Nanoparticles Enabled by Controlled Microfluidic Formulation. J. Am. Chem. Soc. 2012;134:6948–6951. doi: 10.1021/ja301621z. [DOI] [PubMed] [Google Scholar]
- 19.Dong Y, Love KT, Dorkin JR, et al. Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proceedings of the National Academy of Sciences. 2014;111:3955–3960. doi: 10.1073/pnas.1322937111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holland CK, Apfel RE. Thresholds for transient cavitation produced by pulsed ultrasound in a controlled nuclei environment. J. Acoust. Soc. Am. 1990;88:2059–2069. doi: 10.1121/1.400102. [DOI] [PubMed] [Google Scholar]
- 21.Hale LP, Cianciolo G. Treatment of experimental colitis in mice with LMP-420, an inhibitor of TNF transcription. Journal of Inflammation (London, England) 2008;5:4–4. doi: 10.1186/1476-9255-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ensign LM, Ensign LM, Cone R, et al. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Advanced drug delivery reviews. 2012;64:557–570. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh P, Yang X, Arvizo R, et al. Intracellular Delivery of a Membrane-Impermeable Enzyme in Active Form using Functionalized Gold Nanoparticles. J. Am. Chem. Soc. 2010;132:2642–2645. doi: 10.1021/ja907887z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharei A, Sharei A, Zoldan J, et al. A vector-free microfluidic platform for intracellular delivery. Proceedings of the National Academy of Sciences. 2013;110:2082–2087. doi: 10.1073/pnas.1218705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehier-Humbert S, Bettinger T, Yan F, et al. Plasma membrane poration induced by ultrasound exposure: Implication for drug delivery. J Control Release. 104:213–222. doi: 10.1016/j.jconrel.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 26.van Deventer SJH. A randomised, controlled, double blind, escalating dose study of alicaforsen enema in active ulcerative colitis. Gut. 2004;53:1646–1651. doi: 10.1136/gut.2003.036160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frede A, Neuhaus B, Klopfleisch R, et al. Colonic gene silencing using siRNA-loaded calcium phosphate/PLGA nanoparticles ameliorates intestinal inflammation in vivo. J Control Release. 222:86–96. doi: 10.1016/j.jconrel.2015.12.021. IS - [DOI] [PubMed] [Google Scholar]
- 28.Semple SC, Akinc A, Chen J, et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 29.Devi GR. siRNA-based approaches in cancer therapy. Cancer Gene Ther. 2006;13:819–829. doi: 10.1038/sj.cgt.7700931. [DOI] [PubMed] [Google Scholar]
- 30.Nakao A, Afrakhte M, Morn A, et al. Identification of Smad7, a TGF[beta]-inducible antagonist of TGF-[beta] signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 31.Fiocchi C. TGF-&bgr;/Smad signaling defects in inflammatory bowel disease: mechanisms and possible novel therapies for chronic inflammation. J Clin Invest. 2001;108:523–526. doi: 10.1172/JCI13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monteleone G, Kumberova A, Croft NM, et al. Blocking Smad7 restores TGF-β1 signaling in chronic inflammatory bowel disease. J Clin Invest. 2001;108:601–609. doi: 10.1172/JCI12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petsch B, Schnee M, Vogel AB, et al. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat Biotechnol. 2012;30:1210–1216. doi: 10.1038/nbt.2436. [DOI] [PubMed] [Google Scholar]
- 34.Islam MA, Reesor EKG, Xu Y, et al. Biomaterials for mRNA delivery. Biomater. Sci. 2015;3:1519–1533. doi: 10.1039/c5bm00198f. [DOI] [PMC free article] [PubMed] [Google Scholar]