Abstract
Objective
To describe preschool neurodevelopmental outcomes of children with complex congenital heart disease (CHD), who were evaluated as part of a longitudinal cardiac neurodevelopmental follow-up program, as recommended by the American Heart Association and the American Academy of Pediatrics, and identify predictors of neurodevelopmental outcomes in these children.
Study design
Children with CHD meeting the American Heart Association/American Academy of Pediatrics high-risk criteria for neurodevelopmental delay were evaluated at 4–5 years of age. Testing included standardized neuropsychological measures. Parents completed measures of child functioning. Scores were compared by group (single ventricle [1V]; 2 ventricles [2V]; CHD plus known genetic condition) to test norms and classified as: normal (within 1 SD of mean); at risk (1–2 SD from mean); and impaired (>2 SD from mean).
Results
Data on 102 patients were analyzed. Neurodevelopmental scores did not differ based on cardiac anatomy (1V vs 2V); both groups scored lower than norms on fine motor and adaptive behavior skills, but were within 1 SD of norms. Patients with genetic conditions scored significantly worse than 1V and 2V groups and test norms on most measures.
Conclusions
Children with CHD and genetic conditions are at greatest neurodevelopmental risk. Deficits in children with CHD without genetic conditions were mild and may not be detected without formal longitudinal testing. Parents and providers need additional education regarding the importance of developmental follow-up for children with CHD.
Children with congenital heart disease (CHD) are at higher risk for neurodevelopmental problems than healthy children, across all time points in development, from infancy through adolescence.1 Although IQ is often in the low average/average range, a characteristic pattern of mild deficits in multiple other domains, including motor and visual spatial skills, adaptive behavior, executive functioning, language, and social cognition, is common, and found in children with a wide range of CHD diagnoses.2–7 Deficits are thought to be related to a number of factors including altered prenatal brain maturation,8 comorbid genetic conditions,9,10 perioperative and postoperative events,11 socioeconomic status,12 and parenting style.13 As a result of these deficits, children with CHD are more likely than healthy children to require special education services,14 resulting in a significant impact on them, their families and society.15
To promote early detection of delays and optimize outcomes, the American Heart Association (AHA) and the American Academy of Pediatrics (AAP) now recommend systematic evaluation of development in children with CHD throughout childhood.1 Cardiac centers have begun to incorporate developmental follow-up programs as part of routine cardiac care.16,17 We have previously reported developmental outcomes of children who were evaluated in our longitudinal developmental follow-up program over the first 3 years of life and found that 46% of patients were delayed in at least 1 domain (cognitive, language, or motor skills); feeding difficulty and medical and genetic comorbidities increased risk for delays.10,18 The aim of this study was to summarize and identify predictors of neurodevelopmental outcomes for preschoolers who were seen as part of a longitudinal developmental evaluation program for children with CHD.
Methods
Children with CHD believed to be at high risk for developmental delay as defined by the AHA/AAP guidelines,1 were recruited from the Herma Heart Center Developmental Follow-up Clinic at Children’s Hospital of Wisconsin. Eligibility criteria and operation of the Herma Heart Center Developmental Follow-Up Clinic have been previously described.10,16,18 Children were deemed to be at high risk for developmental delay and eligible for the clinic if they had any cardiac surgery as a neonate, surgery using cardiopulmonary bypass (CPB) in the first year of life, a cardiac defect resulting in cyanosis, or other comorbid conditions or complications such as prematurity, genetic syndromes, seizures, or cardiac arrest that placed them at higher risk for delay. Genetic testing was used to confirm a diagnosis when a genetic syndrome was suspected, but all patients did not routinely undergo genetic testing. All families whose children met the AHA/AAP high risk for delay criteria were contacted by letter and subsequently called to schedule a preschool evaluation. Children were seen for neurodevelopmental testing within the cardiology clinic; appointments lasted approximately 2–3 hours. Parents provided informed consent to have their child’s data included in a databank approved by the Institutional Review Board at Children’s Hospital of Wisconsin. No subjects were excluded based on race or other coexisting medical or genetic condition. Only children who spoke English were included, as tests were administered in English.
Children completed a variety of neurodevelopmental measures that were selected based on developmental challenges that are commonly seen in children with CHD. In addition, parents completed several measures of child functioning and behavior. All measures are validated and have normative values based on a healthy population. New editions of some measures were published during the 4 years in which evaluations were completed; the testing protocol was updated to include the most current version of all measures at the time of assessment.
The full scale IQ score from the Wechsler Preschool and Primary Scale of Intelligence (WPPSI), Third or Fourth Edition19,20 (mean: 100 ± 15) was used as a measure of cognitive functioning. The WPPSI-Fourth Edition full scale IQ score correlates .86 with the WPPSI-Third Edition full scale IQ score. The Letter-Word Identification, Applied Problems, and Spelling subtests of the Woodcock Johnson III Tests of Achievement21 were used to assess prereading, premath, and prespelling skills, respectively (mean: 100 ± 15). The Developmental Test of Visual Motor Integration, Sixth Edition22 was used to assess visual motor integration ability (mean: 100 ± 15). The Pegboard subtest of the Wide Range Assessment of Visual Motor Abilities23 was used to assess fine motor skills (mean: 100 ± 15). The General Communication Composite score of the Children’s Communication Checklist-2 (completed by parent)24 was used as a measure of language skills (mean: 100 ± 15). The General Adaptive Composite score of the Adaptive Behavior Assessment System-Second Edition (completed by parent)25 was used as a measure of adaptive behavior (mean: 100 ± 15). The Global Executive Composite score of the Behavior Rating Inventory of Executive Function-Preschool Version (completed by parent)26 was used as a measure of executive functioning (mean T score: 50 ± 10; higher scores indicate more problems). The Total score of the Conners’ Parent Rating Scale-Revised-Short Form (completed by parent)27 was used as a measure of attention problems (mean T score: 50 ± 10; higher scores indicate more problems). The Total Problems score of the Child Behavior Checklist (completed by parent)28 was used as a measure of child behavior problems (mean T scores: 50 ± 10; higher scores indicate more problems). The Total score of the Social Responsiveness Scale, First or Second Edition (completed by parent)29,30 was used as a measure of child social problems (mean T scores: 50 ± 10; higher scores indicate more problems). The Total score is comparable across versions of this measure, as items are exactly the same. The second edition of this measure allows for administration across a wider age range, and 1 subscale name was changed.
For children who were too developmentally impaired to complete a task (n = 10), the lowest possible score for that test was assigned. Children who did not complete a task for other reasons (separation anxiety, n = 1; distractibility, n = 1; language delay, n = 1; fatigue, n = 4; oppositional behavior, n = 7) were excluded from analysis for tasks they did not complete.
Statistical Analyses
Sample characteristics and clinical variables are presented as medians with IQR (25th percentile-75th percentile) for continuous data and frequencies (%) for categorical data. Neurodevelopmental test scores were converted to standard z scores based on test norm means/SDs and compared with the population mean using a Wilcoxon signed rank test. Converting neurodevelopmental test scores to standard z scores, a common metric, allowed for comparison of scores across measures, as not all neurodevelopmental tests have the same scales. To adjust for multiple comparisons, a step-down Bonferroni procedure was used because it is less conservative than the Bonferroni in controlling for the family of hypotheses error rate.31 To perform this adjustment, raw P value needs to be a number. Therefore, a P value of <.0001 was treated as .0001. Scores were classified as: normal (within 1 SD of test mean); at risk (1–2 SDs from test mean); or impaired (>2 SDs from test mean). A 1-sample proportion test was used to examine whether the observed percentages were different from the expected percentages for impaired (2.5%) and at risk (13.5%) categories. A Kruskal-Wallis test or Mann-Whitney-Wilcoxon test was used to compare test scores by group (single ventricle [1V] without genetic condition; 2 ventricles [2V] without genetic condition; CHD with genetic condition). A Cochran-Armitage trend exact test was used to examine the trend in proportions of the number of domains that fell in the normal, at risk, or impaired range for the genetic vs 1V and 2V nongenetic groups. Univariable and multivariable logistic regression analyses were used to assess the impact of patient and clinical factors on binary neurodevelopmental test scores (at risk/impaired vs normal). Predictors with P value of < .1 from the univariate analyses were included in the multivariable logistic regression models (1 model for each neurodevelopmental test) using a forward selection method. The following variables were included as predictors based on our previous findings and a review of the literature: sex, race (White, non-Hispanic, vs others), maternal education (completed beyond high school vs completed high school or less), prenatal diagnosis, cardiac anatomy (1V vs 2V), comorbidities (none vs other medical vs genetic), age at first cardiac surgery, cumulative hospital length of stay (LOS), cumulative CPB time, cumulative deep hypothermic circulatory arrest (DHCA) time, history of early intervention (age 0–3 years). Statistical significance in the final model was defined as P value of < .05. All statistical analyses were performed using SAS v 9.4 (SAS Institute, Cary, North Carolina) software.
Results
From March 2011 through April 2015, 108 subjects with CHD completed preschool neurodevelopmental testing as part of the cardiac neurodevelopmental follow-up clinic. This represented 28% of the potentially eligible population. Of those who did not attend the clinic, 212 (80%) did not respond to letters or phone calls, so the reason they did not participate is unknown. Participants were compared with nonparticipants on demographic and treatment characteristics. There were no significant differences between groups on sex, presence of known genetic or noncardiac morbidity, insurance (public or private) at time of surgery, history of CPB, gestational age, age at first cardiac operation, or total surgical LOS. When compared with nonparticipants, participants were more likely to have 1V anatomy (35% vs 24%, P = .02); were more likely to have been seen in the 0- to 3-year-old clinic (74% vs 58%, P = .002); had more cardiac operations (3.8 vs 2.9, P = .01); had more minutes of CPB time (286 vs 235, P = .013); and had higher maximum Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery Congenital Heart Surgery Mortality Categories (3.8 vs 3.4, P = .002).
Ninety-eight percent of the subjects consented to participation in our research databank. Four patients were excluded from this analysis because their primary diagnosis was acquired cardiomyopathy, resulting in a final sample of 102 subjects with structural CHD. Of these, 75 of 102 (74%) had been previously evaluated in our developmental follow-up program before 3 years of age. All patients who had cardiac surgery at less than 1 year of age were previously referred for early intervention services. Characteristics of the sample are presented in Table I. Median gestational age at birth was 39 weeks (37–40 weeks).Median age at assessment was 4.5 years (4.3–4.7 years). Anatomy was classified according to the child’s diagnosis at birth. Thirty-three percent of the subjects had anatomy that required surgical palliation resulting in a functional single ventricle (list of primary cardiac diagnoses in Table II; available at www.jpeds.com). Twenty-five percent (n = 26) of the subjects had a known medical comorbidity, in addition to their CHD, involving the following systems: airway (n = 10), gastrointestinal/genitourinary (n = 5), hearing (n = 3), neurologic (n = 3), chronic lung disease (n = 2), multisystem (n = 2), and orthopedic (n = 1). Seventeen percent (n = 17) of the subjects had a diagnosed genetic condition (confirmed by genetic testing): trisomy 21 (n = 7); 22q11 deletion (n = 3); chromosomal deletion (n = 3); Turner syndrome (n = 2);VACTERL syndrome (vertebral, anal, cardiac, tracheo-esophageal, renal, and limb defects) (n = 1); and Pierre Robin syndrome (n = 1). Of these, 16 of 17 children had 2V anatomy. One subject with double inlet left ventricle had Pierre Robin syndrome.
Table I.
Sample demographics (n = 102)
| Demographics | n (%) |
|---|---|
| Sex: male | 64 (63) |
| Race/ethnicity | |
| White, non-Hispanic | 72 (71) |
| Hispanic | 13 (13) |
| Black | 8 (8) |
| Other | 9 (9) |
| Maternal education | |
| Post-high school | 80 (78) |
| High school or less | 16 (16) |
| Missing | 6 (6) |
| Family constellation | |
| Married | 73 (72) |
| Single | 17 (17) |
| Other | 11 (11) |
| Missing | 1 (<1) |
| Prenatal diagnosis: yes | 54 (53) |
| Premature (GA <37 wk) | 18 (18) |
| Anatomy | |
| 2V | 68 (67) |
| 1V | 34 (33) |
| Comorbidities | |
| None | 59 (58) |
| Other medical | 26 (25) |
| Genetic | 17 (17) |
GA, gestational age.
Table II.
Primary cardiac diagnoses
| 2V congenital heart defects | n = 68 |
| Aortic arch hypoplasia or coarctation | 12 |
| Atrioventricular septal defect | 8 |
| Ventricular septal defect | 8 |
| Transposition of the great arteries with intact ventricular septum | 6 |
| Aortic valve stenosis | 4 |
| Pulmonary atresia + ventricular septal defect | 4 |
| Double outlet right ventricle | 3 |
| Interrupted aortic arch | 3 |
| Tetralogy of Fallot | 3 |
| Total anomalous pulmonary venous connection | 3 |
| Transposition of the great arteries with ventricular septal defect | 3 |
| Atrioventricular septal defect with tetralogy of Fallot | 2 |
| Pulmonary atresia with intact ventricular septal septum | 2 |
| Truncus arteriosus | 2 |
| Ebstein malformation of tricuspid valve | 1 |
| Double aortic arch | 1 |
| Mitral valve stenosis | 1 |
| Pulmonary valve stenosis | 1 |
| Congenitally corrected transposition of the great arteries with ventricular septal defect | 1 |
| 1V congenital heart defects | n = 34 |
| Hypoplastic left heart syndrome | 20 |
| Double inlet left ventricle | 6 |
| Double outlet right ventricle | 3 |
| Pulmonary atresia + intact ventricular septum | 3 |
| Congenitally corrected transposition of the great arteries | 1 |
| Tricuspid atresia | 1 |
The majority of subjects, 92 of 102 (90%) had undergone at least 1 heart surgery requiring CPB; median cumulative CPB time (to the time of preschool evaluation) was 221 minutes (141–362 minutes). Of the 37 subjects who had DHCA, median cumulative DHCA time (to the time of preschool evaluation) was 12 minutes (7–28 minutes).Median cumulative hospital LOS was 46 days (24–82 days). A majority of subjects, 85 of 102 (83%), had received early intervention services (eg, physical, occupational or speech therapy) previously; 42 of 102 (41%) were currently enrolled in early intervention services at the time of their preschool evaluation.
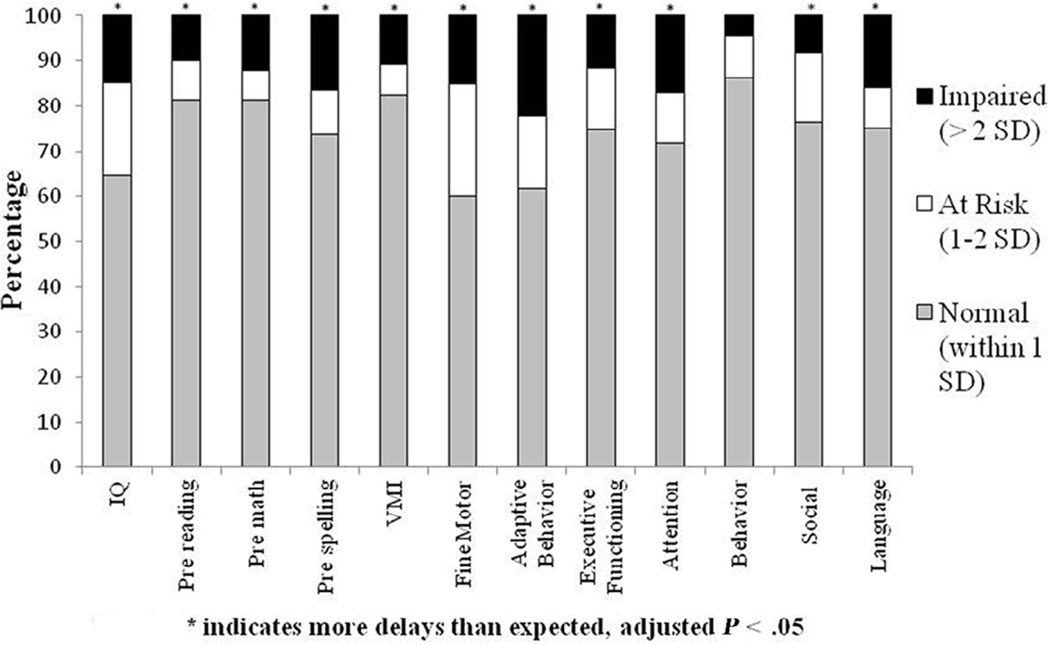
The percentages of the subjects for the entire cohort that fell within the normal (within 1SD of test norm), at risk (1–2 SD from test norm) and impaired (>2 SD from test norm) ranges are illustrated in Figure 1 (available at www.jpeds.com). In a normal distribution, 84% of a given sample should fall within the normal or above normal range, 13.5% within the at risk range, and approximately 2.5% within the impaired range. Assuming a normal distribution, this cohort of children with CHD had more scores in the impaired range for all domains except parent-reported behavior problems and more scores in the at risk range for fine motor skills than would be expected (all adjusted P < .05).
Figure 1.
Percentage of patients in the normal, at risk, and impaired ranges on neurodevelopmental measures.
Child neurodevelopmental scores and parent rating scale results are presented in Table III. To address concerns that scores for the patients with known genetic conditions would skew the results for the entire cohort, scores were compared with test norms based on patient diagnostic group (1V without genetic condition, 2V without genetic condition, or CHD with genetic condition). Scores for each diagnostic group were also compared with each other. Children with 1V or 2V anatomy without genetic conditions scored lower than test norms on fine motor and adaptive behavior skills (adjusted P < .05). Children with CHD and genetic conditions scored lower than test norms and lower than children with 1V anatomy without genetic conditions on most child completed neurodevelopmental measures; they also scored lower on adaptive behavior skills and had higher rates of parent-reported social problems (adjusted P < .05). Children with CHD and genetic conditions scored lower than children with 2V anatomy without genetic conditions on IQ, premath, and fine motor skills (adjusted P < .05). No significant differences in neurodevelopmental scores were found between children with 1V vs 2V anatomy without genetic conditions.
Table III.
Child completed neurodevelopmental measures and parent rating scale results
| Tests | Domains | Median (IQR: 25th–75th percentile) for cases |
Standardized median z (IQR: 25th–75th percentile) for cases |
Raw P (z score compared with 0) |
Step-down Bonferroni adjusted P |
|
|---|---|---|---|---|---|---|
| WPPSI-III/IV IQ* | Cognitive | 1V | 97.0 (80.0–105.0) | −0.2 (−1.3 to 0.3) | .066 | .59 |
| 2V | 102.0 (87.5–110.5) | 0.1 (−0.8 to 0.7) | .90 | >0.99 | ||
| Genetic | 68.00 (40.0–79.0) | −2.1 (−4.0 to −1.4) | .001 | .01 | ||
| WJTA-III Letter-Word Identification* |
Pre-reading | 1V | 104.0 (90.0–112.0) | 0.3 (−0.7 to 0.8) | .56 | >.99 |
| 2V | 104.0 (91.0–114.0) | 0.3 (−0.6 to 0.9) | .13 | .78 | ||
| Genetic | 79.0 (40.0–106.0) | −1.4 (−4.0 to 0.4) | .024 | .10 | ||
| WJTA-III Applied Problems* |
Pre-math | 1V | 106.0 (94.0–118.0) | 0.4 (−0.4 to 1.2) | .34 | >.99 |
| 2V | 109.0 (98.0–117.0) | 0.6 (−0.1 to 1.1) | .006 | .06 | ||
| Genetic | 81.0 (40.0–94.0) | −1.3 (−4.0 to −0.4) | .007 | .04 | ||
| WJTA-III Spelling* | Pre-spelling | 1V | 98.0 (86.0–110.0) | −0.1 (−0.9 to 0.7) | .53 | >.99 |
| 2V | 102.0 (87.0–111.0) | 0.1 (−0.9 to 0.7) | .88 | >.99 | ||
| Genetic | 61.0 (40.0–92.0) | −2.6 (−4.0 to −0.5) | .003 | .02 | ||
| VMI* | Visual motor | 1V | 95.0 (90.0–101.0) | −0.3 (−0.7 to 0.1) | .023 | .23 |
| 2V | 98.5 (93.0–106.5) | −0.1 (−0.5 to 0.4) | .31 | >.99 | ||
| Genetic | 87.0 (66.0–92.0) | −0.9 (−2.3 to −0.5) | .001 | .01 | ||
| WRAVMA* | Fine motor | 1V | 88.0 (80.0–100.0) | −0.8 (−1.3 to 0.0) | .0006 | .007 |
| 2V | 93.0 (83.0–101.5) | −0.5 (−1.1 to 0.1) | .0001 | .001 | ||
| Genetic | 63.0 (45.0–76.0) | −2.5 (−3.7 to −1.6) | <.0001 | .001 | ||
| ABAS-II* | Adaptive behavior | 1V | 88.0 (79.0–104.0) | −0.8 (−1.4 to 0.3) | .003 | .033 |
| 2V | 94.0 (77.0–101.0) | −0.4 (−1.5 to 0.1) | .0006 | .007 | ||
| Genetic | 66.0 (60.0–90.0) | −2.3 (−2.7 to −0.7) | .0002 | .002 | ||
| BRIEF-P† | Executive functioning | 1V | 50.5 (39.0–65.0) | 0.1 (−1.1 to 1.5) | .50 | >.99 |
| 2V | 48.0 (39.0–57.0) | −0.2 (−1.1 to 0.7) | .66 | >.99 | ||
| Genetic | 56.5 (46.0–69.5) | 0.7 (−0.4 to 2.0) | .81 | >.99 | ||
| CPRS† | Attention problems | 1V | 50.0 (43.0–64.0) | 0.0 (−0.7 to 1.4) | .28 | >.99 |
| 2V | 54.0 (44.0–64.0) | 0.4 (−0.6 to 1.4) | .06 | .48 | ||
| Genetic | 53.0 (47.0–79.0) | 0.3 (−0.3 to 2.9) | .16 | .48 | ||
| CBCL† | Behavior problems | 1V | 49.0 (38.5–58.5) | −0.1 (−1.2 to 0.9) | .54 | >.99 |
| 2V | 46.0 (39.5–56.0) | −0.4 (−1.1 to 0.6) | .10 | .70 | ||
| Genetic | 50.0 (45.0–56.0) | 0.0 (−0.5 to 0.6) | >.999 | >.99 | ||
| SRS/SRS-2† | Social problems | 1V | 51.0 (43.0–61.0) | 0.1 (−0.7 to 1.1) | .21 | >.99 |
| 2V | 50.5 (45.0–58.0) | 0.1 (−0.5 to 0.8) | .40 | >.99 | ||
| Genetic | 59.5 (54.0–66.0) | 1.0 (0.4 to 1.6) | .0039 | .03 | ||
| CCC-2* | Language | 1V | 102.0 (86.0–115.0) | 0.1 (−0.9 to 1.0) | .93 | >.99 |
| 2V | 109.0 (96.5–116.0) | 0.6 (−0.2 to 1.1) | .018 | .16 | ||
| Genetic | 72.0 (40.0–89.0) | −1.9 (−4.0 to −0.7) | .0037 | .03 | ||
ABAS-II, Adaptive Behavior Assessment System, Second Edition; BRIEF-P, Behavior Rating Inventory of Executive Function-Preschool; CBCL, Child Behavior Checklist; CCC-2, Children's Communication Checklist, Second Edition; CPRS, Conners' Parent Rating Scales; SRS/SRS-2, Social Responsiveness Scale, First or Second Edition; VMI, Visual Motor Integration; WJTA-III, Woodcock Johnson Tests of Achievement-Third Edition; WPPSI-III/IV, Wechsler Preschool and Primary Scales of Intelligence, Third or Fourth Edition; WRAVMA, Wide Range Assessment of Visual Motor Abilities.
Bold P values indicate statistically significant.
Test mean/SD 100 ± 15; higher scores indicate better functioning.
Test mean/SD 50 ± 10; higher scores indicate worse functioning.
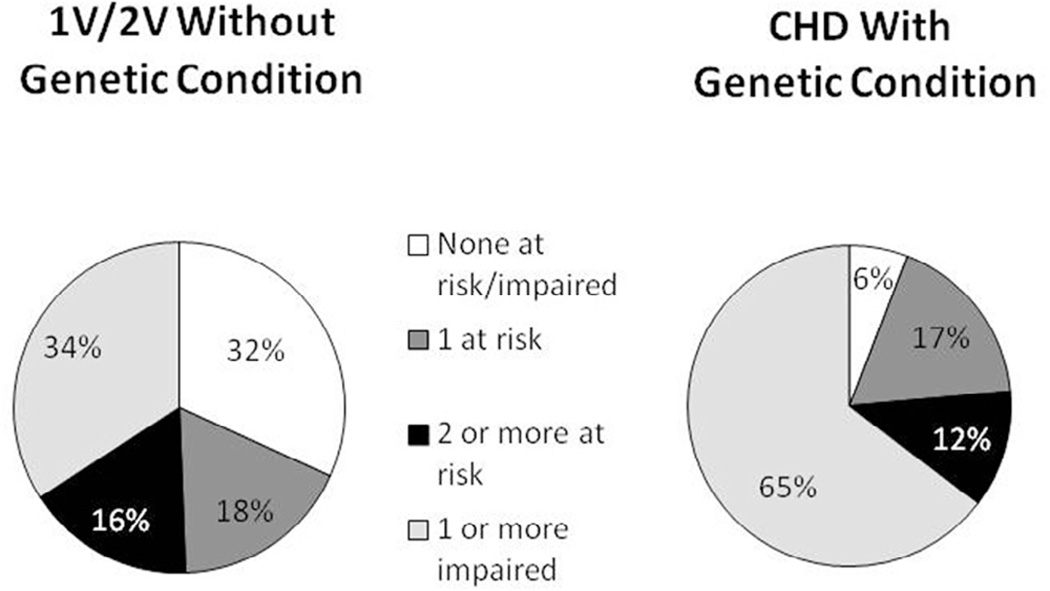
Children with 1V anatomy and 2V anatomy without genetic conditions were combined into 1 group and compared with children with CHD and genetic conditions on the percentage of children who fell into the following categories: (1) no domain scores in the at risk/impaired range (ie, all scores within the normal range); (2) 1 domain score in the at risk range; (3) ≥ 2 domain scores in the at risk range; or (4) ≥ 1 domain scores in the impaired range (Figure 2). Children with CHD without genetic conditions were less likely than children with CHD and genetic conditions to have ≥1 domain score in the impaired range (P = .011).
Figure 2.
Number of domains at risk or impaired by group (1V/2V without genetic condition vs CHD with genetic condition).
Logistic regression was performed on the dichotomized neurodevelopmental test scores (at risk/impaired vs normal) for each neurodevelopmental test. The significant predictors of child neurodevelopmental outcomes in the multivariable models are presented in Table IV. An OR >1 represented an increased likelihood of having an “at risk/impaired” score. Presence of a genetic or other medical condition was associated with worse neurodevelopmental outcomes in most domains. Non-white race or Hispanic ethnicity was associated with worse outcomes in prereading, premath, visual motor, adaptive behavior, and language skills. Male sex was associated with worse outcomes in prespelling and fine motor skills. A longer cumulative hospital LOS was associated with worse outcomes in fine motor skills. Of note, maternal education, prenatal diagnosis, cardiac anatomy, age at first cardiac surgery, or minutes of CPB/DHCA were not significantly associated with any neurodevelopmental domains. None of the predictors in the regression model were associated with executive functioning, attention, behavior, or social skills.
Table IV.
Significant predictors (P < .05) of at risk/impaired neurodevelopmental outcomes in multivariable regression models
| Cognitive | Prereading | Premath | Prespelling | Visual motor | Fine motor | Adaptive behavior | Language | |
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | ||||||||
| Male vs female | 3.7 (1.04, 13.16) | 6.7 (1.69, 26.15) | ||||||
| White, non-Hispanic vs others |
0.3 (.08, 0.92) | 0.2 (.06, 0.64) | 0.2 (.07, 0.69) | 0.3 (0.10, 0.87) | 0.3 (0.10, 0.97) | |||
| Genetic condition vs none |
15.6 (3.55, 68.16) | 12.2 (2.85, 52.31) | 9.4 (2.14, 40.98) | 11.3 (2.63, 48.50) | 6.9 (1.75, 26.88) | 33.2 (5.46, 201.96) | 6.3 (1.61, 25.05) | 7.8 (2.10, 29.04) |
| Other medical condition vs none |
4.7 (1.59, 13.69) | 1.3 (0.27, 5.75) | 2.2 (0.69, 7.13) | 2.5 (0.66, 9.91) | 1.3 (0.42, 3.97) | 1.9 (0.66, 5.79) | 1.1 (0.28, 4.10) | |
| Hospital LOS* | 1.01 (1.00, 1.02) |
Cumulative to time of preschool assessment.
Discussion
In this cohort of preschool-age patients who underwent neurodevelopmental evaluation as part of a clinical follow-up program, findings indicate that most children with CHD without comorbid genetic conditions do not have severe delays. However, 50% of the children with CHD without known genetic conditions had multiple scores in the at risk range or at least 1 score in the impaired range, with adaptive behavior and fine motor skills being particular areas of concern.
Not surprisingly, children with a comorbid genetic condition had worse neurodevelopmental outcomes, which is consistent with previous findings.1,10,32 Although many studies have excluded children with CHD and comorbid genetic conditions,2,3,33 children with CHD and genetic conditions represent at least 20% of the CHD population, and this number is likely an underestimate, as not all children with CHD undergo formal genetic testing.10 Children with CHD and comorbid genetic conditions meet the AHA/AAP1 criteria regarding which children should be referred for serial neurodevelopmental assessment. Therefore, we believed that it was important to include these children in our results.10,16
Of note, neurodevelopmental outcomes did not differ based on cardiac anatomy (1V without genetic condition vs 2V without genetic condition), which is consistent with previous research on preschool neurodevelopmental outcomes in CHD.34 In contrast to the current preschool findings, research conducted with adolescents with CHD found worse executive functioning in subjects with single-ventricle anatomy compared with biventricular groups.2 It is possible that neurodevelopmental risks between 1V and 2V groups widen over the course of development, as societal demands for independent problem solving and executive functioning skills increase.
Presence of a comorbid medical or genetic condition was the strongest predictor of at risk/impaired neurodevelopmental outcomes across domains. In addition, minority race, male sex, and cumulative hospital LOS predicted adverse neurodevelopmental outcomes in some domains. Early patient or treatment characteristics, such as prenatal diagnosis, age at first cardiac surgery, or CPB/DHCA time, did not predict preschool neurodevelopmental outcomes. It appears that early treatment factors have less impact on development the farther the child is out from surgery; whereas intrinsic factors such as ongoing medical/genetic problems (which may contribute to longer hospital LOS) and minority race (which may be a proxy for socioeconomic disadvantage), may have more impact on development over time. It was surprising that males did worse on fine motor and prespelling skills, as sex was not found to be a risk factor in our previous studies.10,16,18 However, findings of the current study are consistent with recent reports that have shown similar differences by sex in motor and writing skills in healthy children.35,36 It is interesting to note that no factors examined in the present study predicted problems with executive functioning, attention, or behavior, deficits that are frequently seen in older children with CHD.2 In the present study, executive functioning, attention, and behavior were assessed exclusively via parent questionnaires, which may have influenced the results. Nonetheless, findings suggest that there is no clear profile of factors that consistently predicts all areas of development in patients with CHD over time.
There are some important limitations to the current study. Results are based on a single center experience, and not all patients who were eligible for the clinic participated; thus, findings may not be generalizable to the CHD population as a whole. It is possible that results represent a “best-case scenario,” as the majority of the sample (74%) had been previously evaluated in our neurodevelopmental follow-up program, and the majority (83%) had received early intervention services. Thus, results may underestimate the level of neurodevelopmental deficits in this age group, particularly for patients with CHD who are not receiving systematic neurodevelopmental evaluation or early intervention services. As evaluations were conducted as part of a clinical program, and not as part of a research study, it was not possible for us to recruit a healthy control sample, which is an additional limitation. As patients were evaluated in the clinic over a period of several years, it is possible that outcomes could have been influenced by changes in surgical or clinical management; however, this is unlikely, as our comprehensive neuroprotective strategy remained stable throughout the course of this study. Finally, although multiple neurodevelopmental domains were assessed as part of the preschool evaluation, it is not known whether preschool neurodevelopmental outcomes will predict neurodevelopmental outcomes at school-age and beyond.
Cardiology providers, primary care providers/pediatricians, and other members of the child’s medical team should counsel parents that formal developmental testing can identify the child’s strengths and weaknesses, which often change as the child gets older. The information obtained can also help parents advocate for their child’s unique needs as they enter the school system. As more cardiac centers incorporate developmental follow-up programs into their clinical care,17 quality improvement initiatives designed to increase family participation will be beneficial.
Acknowledgments
We acknowledge the support of Mara Koffarnus, Kristen Andersen, and Katie Wilde for patient scheduling and data management support. We thank the children and parents who attended developmental follow-up and were willing to participate in this research.
Supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (NIH; 8UL1TR000055). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Glossary
- 1V
Single ventricle
- 2V
Two ventricles
- AAP
American Academy of Pediatrics
- AHA
American Heart Association
- CPB
Cardiopulmonary bypass
- DHCA
Deep hypothermic circulatory arrest
- LOS
Length of stay
- WPPSI
Wechsler Preschool and Primary Scales of Intelligence
Footnotes
The authors declare no conflicts of interest.
References
- 1.Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126:1143–1172. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- 2.Cassidy AR, White MT, DeMaso DR, Newburger JW, Bellinger DC. Executive function in children and adolescents with critical cyanotic congenital heart disease. J Int Neuropsychol Soc. 2015;21:34–49. doi: 10.1017/S1355617714001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellinger DC, Wypij D, Rivkin MJ, DeMaso DR, Robertson RL, Jr, Dunbar-Masterson C, et al. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. 2011;124:1361–1369. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sananes R, Manlhiot C, Kelly E, Hornberger LK, Williams WG, MacGregor D, et al. Neurodevelopmental outcomes after open heart operations before 3 months of age. Ann Thorac Surg. 2012;93:1577–1583. doi: 10.1016/j.athoracsur.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Alton G, Taghados S, Joffe A, Robertson C, Dinu I The Western Canadian Pediatric Therapies Follow-Up Group. Prediction of preschool functional abilities after early complex cardiac surgery. Cardiol Young. 2015;25:655–662. doi: 10.1017/S1047951114000535. [DOI] [PubMed] [Google Scholar]
- 6.Calderon J, Angeard N, Pinabiaux C, Bonnet D, Jambaqué I. Facial expression recognition and emotion understanding in children after neonatal open-heart surgery for transposition of the great arteries. Dev Med Child Neurol. 2014;56:564–571. doi: 10.1111/dmcn.12381. [DOI] [PubMed] [Google Scholar]
- 7.Gerstle M, Beebe D, Drotar D, Cassedy A, Marino B. Executive functioning and school performance among pediatric survivors of complex congenital heart disease. J Pediatr. 2016;173:154–159. doi: 10.1016/j.jpeds.2016.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Licht DJ, Shera DM, Clancy RR, Wernovsky G, Montenegro LM, Nicolson SC, et al. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–536. doi: 10.1016/j.jtcvs.2008.10.025. discussion 536–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahed AC, Gelb BD, Seidman JG, Seidman CE. Genetics of congenital heart disease: the glass half empty. Circ Res. 2013;112:707–720. doi: 10.1161/CIRCRESAHA.112.300853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mussatto KA, Hoffmann RG, Hoffman GM, Tweddell JS, Bear L, Cao Y, et al. Risk and prevalence of developmental delay in young children with congenital heart disease. Pediatrics. 2014;133:e570–e577. doi: 10.1542/peds.2013-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahle WT, Clancy RR, McGaurn SP, Goin JE, Clark BJ. Impact of prenatal diagnosis on survival and early neurologic morbidity in neonates with the hypoplastic left heart syndrome. Pediatrics. 2001;107:1277–1282. doi: 10.1542/peds.107.6.1277. [DOI] [PubMed] [Google Scholar]
- 12.Cassedy A, Drotar D, Ittenbach R, Hottinger S, Wray J, Wernovsky G, et al. The impact of socio-economic status on health related quality of life for children and adolescents with heart disease. Health Qual Life Outcomes. 2013;11:99. doi: 10.1186/1477-7525-11-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rempel GR, Harrison MJ. Safeguarding precarious survival: parenting children who have life-threatening heart disease. Qual Health Res. 2007;17:824–837. doi: 10.1177/1049732307303164. [DOI] [PubMed] [Google Scholar]
- 14.Riehle-Colarusso T, Autry A, Razzaghi H, Boyle CA, Mahle WT, Van Naarden Braun K, et al. Congenital heart defects and receipt of special education services. Pediatrics. 2015;136:496–504. doi: 10.1542/peds.2015-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Individuals with Disabilities Education Act Reauthorization 2004. Pub L No. 108-446, 118 Stat 2647. [Google Scholar]
- 16.Soto CB, Olude O, Hoffmann RG, Bear L, Chin A, Dasgupta M, et al. Implementation of a routine developmental follow-up program for children with congenital heart disease: early results. Congenit Heart Dis. 2011;6:451–460. doi: 10.1111/j.1747-0803.2011.00546.x. [DOI] [PubMed] [Google Scholar]
- 17.Brosig CL, Butcher J, Butler S, Ilardi DL, Sananes R, Sanz JH, et al. Monitoring developmental risk and promoting success for children with congenital heart disease: recommendations for cardiac neurodevelopmental follow-up programs. Clin Pract Pediatr Psychol. 2014;2:153–165. [Google Scholar]
- 18.Mussatto KA, Hoffmann R, Hoffman G, Tweddell JS, Bear L, Cao Y, et al. Risk factors for abnormal developmental trajectories in young children with congenital heart disease. Circulation. 2015;132:755–761. doi: 10.1161/CIRCULATIONAHA.114.014521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence, Administration and Scoring Manual. 3rd. San Antonio (TX): Psychological Corporation; 2002. [Google Scholar]
- 20.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence, Adminstration and Scoring Manual. 4th. Bloomington, (MN): NCS Pearson, Inc; 2012. [Google Scholar]
- 21.Woodcock R, McGrew K, Mather N. Woodcock Johnson III Tests of Achievement. Itsaca (IL): Riverside Publishing; 2001. [Google Scholar]
- 22.Beery K, Beery N. Beery-Buktenica Developmental Test of Visual- Motor Integration Administration, Scoring and Teaching Manual. 6th. Bloomington (MN): NCS Pearson, Inc; 2010. [Google Scholar]
- 23.Adams W, Sheslow D. Wide Range Assessment of Visual Motor Abilities Manual. Lutz (FL): Psychological Assessment Resources, Inc; 1995. [Google Scholar]
- 24.Bishop D. Children’s Communication Checklist—2 Manual. San Antonio (TX): Pearson Clinical Assessment; 2003. [Google Scholar]
- 25.Harrison P, Oakland T. Adaptive Behavior Assessment System-Manual. 2nd. San Antonio (TX): Harcourt Assessment, Inc; 2003. [Google Scholar]
- 26.Gioia G, Epsy K, Isquith P. BRIEF-P Behavior Rating Inventory of Executive Function-Preschool Version. Lutz (FL): Psychological Assessment Resources, Inc; 2003. [Google Scholar]
- 27.Conners C. Conners’ Rating Scales-Revised User’s Manual. North Tonawanda (NY): Multi-Health Systems Inc; 1997. [Google Scholar]
- 28.Achenbach T, Rescorla L. Manual for the ASEBA Preschool Forms & Profiles. Burlington (VT): University of Vermont, Research Center for Children, Youth & Families; 2000. [Google Scholar]
- 29.Constantino J. Social Responsiveness Scale (SRS) Manual. Los Angeles (CA): Western Psychological Services; 2005. [Google Scholar]
- 30.Constantino J, Gruber C. Social Responsiveness Scale (SRS-2) 2nd. Torrance (CA): Western Psychological Services; 2012. [Google Scholar]
- 31.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 32.Atallah J, Joffe A, Robertson C, Leonard N, Blakely P, Nettel-Aguirre A, et al. Two-year general and neurodevelopmental outcome after neonatal complex cardiac surgery in patients with deletion 22q11.2: A comparative study. J Thorac Cardiovasc Surg. 2007;134:772–779. doi: 10.1016/j.jtcvs.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Mackie AS, Vatanpour S, Alton GY, Dinu IA, Ryerson L, Moddemann DM, et al. Clinical outcome score predicts adverse neurodevelopmental outcome after infant heart surgery. Ann Thorac Surg. 2015;99:2124–2132. doi: 10.1016/j.athoracsur.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 34.Gaynor JW, Ittenbach RF, Gerdes M, Bernbaum J, Clancy RR, McDonald-McGinn DM, et al. Neurodevelopmental outcomes in preschool survivors of the Fontan procedure. J Thorac Cardiovasc Surg. 2014;147:1276–1282. doi: 10.1016/j.jtcvs.2013.12.019. discussion 1282-83, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynolds MR, Scheiber C, Hajovsky DB, Schwartz B, Kaufman AS. Gender differences in academic achievement: is writing an exception to the gender similarities hypothesis? J Genet Psychol. 2015;176:211–234. doi: 10.1080/00221325.2015.1036833. [DOI] [PubMed] [Google Scholar]
- 36.Morley D, Till K, Ogilvie P, Turner G. Influences of gender and socioeconomic status on the motor proficiency of children in the UK. Hum Mov Sci. 2015;44:150–156. doi: 10.1016/j.humov.2015.08.022. [DOI] [PubMed] [Google Scholar]