Abstract
Background and Objective
Breast cancer is one of the most common cancers, and recognized as the third leading cause of mortality in women. Optical coherence tomography (OCT) enables three dimensional visualization of biological tissue with micrometer level resolution at high speed, and can play an important role in early diagnosis and treatment guidance of breast cancer. In particular, ultra-high resolution (UHR) OCT provides images with better histological correlation. This paper compared UHR OCT performance with standard OCT in breast cancer imaging qualitatively and quantitatively. Automatic tissue classification algorithms were used to automatically detect invasive ductal carcinoma in ex vivo human breast tissue.
Study Design/Materials and Methods
Human breast tissues, including non-neoplastic/normal tissues from breast reduction and tumor samples from mastectomy specimens, were excised from patients at Columbia University Medical Center. The tissue specimens were imaged by two spectral domain OCT systems at different wavelengths: a home-built ultra-high resolution (UHR) OCT system at 800nm (measured as 2.72 μm axial and 5.52 μm lateral) and a commercial OCT system at 1300nm with standard resolution (measured as 6.5 μm axial and 15 μm lateral), and their imaging performances were analyzed qualitatively. Using regional features derived from OCT images produced by the two systems, we developed an automated classification algorithm based on relevance vector machine (RVM) to differentiate hollow-structured adipose tissue against solid tissue. We further developed B-scan based features for RVM to classify invasive ductal carcinoma (IDC) against normal fibrous stroma tissue amongst OCT datasets produced by the two systems. For adipose classification, 32 UHR OCT B-scans from 9 normal specimens, and 28 standard OCT B-scans from 6 normal and 4 IDC specimens were employed. For IDC classification, 152 UHR OCT B-scans from 6 normal and 13 IDC specimens, and 104 standard OCT B-scans from 5 normal and 8 IDC specimens were employed.
Results
We have demonstrated that UHR OCT images can produce images with better feature delineation compared with images produced by 1300 nm OCT system. UHR OCT images of a variety of tissue types found in human breast tissue were presented. With a limited number of datasets, we showed that both OCT systems can achieve a good accuracy in identifying adipose tissue. Classification in UHR OCT images achieved higher sensitivity (94%) and specificity (93%) of adipose tissue than the sensitivity (91%) and specificity (76%) in 1300 nm OCT images. In IDC classification, similarly, we achieved better results with UHR OCT images, featured an overall accuracy of 84%, sensitivity of 89% and specificity of 71% in this preliminary study.
Conclusion
In this study, we provided UHR OCT images of different normal and malignant breast tissue types, and qualitatively and quantitatively studied the texture and optical features from OCT images of human breast tissue at different resolutions. We developed an automated approach to differentiate adipose tissue, fibrous stroma, and IDC within human breast tissues. Our work may open the door towards automatic intraoperative OCT evaluation of early-stage breast cancer.
INTRODUCTION
Breast cancer is one of the most common cancers and the third leading cause of mortality in women in 2015 (1). Early detection holds the key to successful treatment of breast cancer and also leads to more flexible treatment options, including breast conserving surgery and non-surgery approaches(2). Minimally invasive procedures in breast cancer management (3) require accurate detection and localization of the malignancy in breast tissue. There has been increased research interest in deploying high-resolution imaging modalities for delineation of tumor morphology in breast tissue. Previously, a variety of optical and non-optical techniques (4–10) were investigated to identify tumor margins in breast tissues based on optical properties of normal and cancerous tissues. Nonlinear microscopy (4) and fluorescence imaging techniques (5,6) showed encouraging results in offering histology-grade visualization of breast tissue sections in freshly excised tissues. However, these microscopy techniques usually suffer from the limited field of view and lack of depth information. Diffuse optical spectroscopy (7,8) or RF spectroscopy (9) made non-invasive full breast imaging possible, but the image resolution was insufficient for detection of micrometer level lesions.
Optical coherence tomography (OCT) is an emerging imaging modality that provides micrometer-resolution and three-dimensional (3D) images of tissue microstructure at high speed. OCT has higher spatial resolution than conventional medical imaging modalities used in breast cancer management such as mammogram and ultrasound, and can be miniaturized into a needle probe to locally diagnose and interrogate the cancerous region (11). Different tissue types possess different optical properties, such as scattering and absorption (12). Since the intensity of OCT signal is correlated with these optical properties of the tissue, it can be used to differentiate malignancy from normal breast tissue. OCT was first introduced to breast cancer management as a non-destructive high resolution imaging tool to evaluate tumor morphology in ex vivo breast tissue (13). Thanks to the high-speed and wide-field imaging capability, OCT has been implemented in intraoperative settings (14) as well as handheld probes and needle catheters (15–18) to enable ex vivo and in vivo assessment of tumor margins. In addition, efforts have been made to move towards computer-aided detection (CAD) of tumor tissue with OCT needle biopsy (15,19–22). Furthermore, functional OCT systems were also introduced to breast tumor margin assessment with enhanced imaging contrast. Physical properties of the tissue, such as elasticity (23–25) and optical birefringence (26–28) can be mathematically reconstructed from additional mechanical and optical detection channels, respectively. Although the additional contrast may help to better delineate malignant sites and further assist CAD, these systems were more complicated to construct, and the reconstruction of the functional information may require spatial averaging to reduce the speckle noise, which ultimately limits the image resolution.
On the other hand, in order to match the resolution provided by traditional histology, OCT systems with enhanced lateral resolution, such as optical coherence microscopy (OCM) (29) and full-field (FF) OCT (30), were developed to generate micro-meter resolution en face images of freshly excised breast tissue. These en face preferential OCT systems clearly provided images showing good correlations with histology. Nevertheless, they inherited some limitations from those microscopy techniques, including limited field of view and signal penetration. Although large field of view can be achieved by mosaicking and/or stitching multiple en face images, the process itself can be rather time consuming. The limited depth of focus may be mediated by wavefront correction, yet it may cause more complications in the system. Ultrahigh resolution (UHR) OCT generally categorizes OCT systems with axial resolution less than 5 μm enabled by a broadband light source. Just as the conventional OCT system, the depth of focus can be extended if the lateral resolution is compromised to a certain degree. The overall image quality is still improved due to a superior axial resolution. The improvement in signal penetration may be critical to some applications. For example, a larger margin width is usually more appreciated for ductal carcinoma in-situ (DCIS) (31,32). A recent study (33) demonstrated that UHR OCT may enable high resolution 3D visualization of a variety of tissue types in fresh excised breast tissue to aid in differentiation of malignancy with an appreciable imaging depth. Moreover, UHR OCT was also demonstrated in small diameter catheters (34,35), which would benefit not only surgical margin guidance during lumpectomy but also local biopsy of early cancerous sites during ductoscopy (36).
In this study, we employed a home-built UHR OCT system at 800nm (37) and a commercial 1300nm system to image human breast cancer specimens ex vivo. We first compared OCT images of human breast tissue specimens at different spatial resolutions. We showed qualitatively that UHR OCT images enabled better visualization of detailed features in different types of breast tissue. Moreover, UHR OCT images with corresponding histology analysis of different tumor types were shown and compared with previous findings (29,30,33). We contributed to the UHR OCT breast cancer imaging body of work with new tumor types that have not been reported before, including phyllodes tumor, fibrotic focus carcinoma and necrotic tumor. We quantitatively examined the texture and optical features from the OCT images of human breast tissue at different resolutions, and developed computational methods for differentiation of major tissue types found in OCT images, such as adipose, fibrous stroma and malignant lesions. Relevance vector machine (RVM), a Bayesian frame work of support vector machine, was used to perform classification on adipose tissue against solid type of tissue, and invasive ductal carcinoma (IDC) against normal stroma tissue. A total 60 B-scans (32 from UHR OCT, 28 from Thorlabs) from 15 normal and 4 IDC specimens were fed into the adipose classifier, and 256 B-scans (152 from UHR OCT, 104 from Thorlabs) from 10 normal and 19 IDC specimens for the IDC classifier. Leave-one-out test was performed to measure sensitivity and specificity. In this preliminary study, the tissue classification results indicated that UHR OCT images can lead to a better performance on differentiation of adipose and IDC in the breast tissue compared with the images produced by the 1300nm system. Especially, using UHR OCT images, we showed a sensitivity of 94% and specificity of 93% for adipose delineation and a sensitivity of 89% and specificity of 71% for identifying IDC against normal fibrous stroma.
MATERIAL AND METHODS
Sample Preparation
Breast tissue specimens were discarded tissue not required for diagnosis as defined by the department of pathology collected from patients undergoing surgical procedures at Columbia University Medical Center (CUMC), and include both non-neoplastic/normal tissue from breast reductions and malignant tissue from mastectomy. The protocol was considered as non-human subject research in accordance with 45CFR46, and was performed under Columbia University Tissue Bank IRB AAAB2667 as all tissue samples were de-identified. The specimens were placed in Rosewell Park Memorial Institute (RPMI) media and were imaged ex vivo within 24 hours after surgical excision. A total of 82 specimens from 49 human breast cases were collected and imaged, including normal tissue specimens derived from normal breast reduction (n = 40) and pathological tissue specimens from mastectomy (n = 42), with an average size of 1.2 cm2. UHR OCT imaging was performed 29 cases, including 23 normal specimens and 29 specimens with malignant lesions: phyllodes tumor (n = 2), fibrotic focus carcinoma (n = 1), mucinous carcinoma (n = 3), ductal carcinoma in situ (DCIS) (n = 3) and IDC (n = 20). In particular,10 out of 29 cases were also imaged by both systems for comparison. The other 20 cases, including 17 normal specimens and 13 pathological specimens (five IDC specimens), were solely imaged by Telesto system. Specimens were classified based on histological analysis by an experienced pathologist after imaging.
From 30 cases, including 18 normal and 23 IDC specimens, cross-sectional images (B-scans) of 3D OCT datasets imaged by two systems were incorporated for tissue classification analysis, focused on extracting adipose tissue ratio and differentiating IDC against normal fibrous stroma.
Imaging Protocol
OCT systems
Two SD OCT systems were used to acquire volumetric images from the excised breast tissue specimens: a commercial system (Thorlabs Telesto I) at 1300nm and a previous reported home-built ultrahigh resolution (UHR) OCT system at the optical window of 800nm (37) (schematic shown in Fig.1). The commercial system has an axial resolution of 6.5 μm and lateral resolution of 15 μm measured in air, with an imaging range of 2.52 mm. The UHR OCT system has an axial resolution of 2.7 μm and lateral resolution of 5.5 μm measured in air, with an extended imaging range measured as 1.8 mm and 6-dB sensitivity fall-off range as 0.89mm enabled by a supercontinuum source (NKT Extreme EXR-9) and a customized spectrometer. The customized spectrometer features a modified Cooke triplet lenses optimized for the focusing performance on a 2048-pixel line-scan camera within the wavelength range from 740 nm to 940 nm.
Fig.1.

Schematic of UHR SD OCT system (37). NBS: non-polarized beam splitter, DAQ: data acquisition board, SMF: single mode fiber.
Imaging protocol details
Multiple three-dimensional OCT volumetric images were acquired on both the top and bottom sides of the specimens, covering the entire surface area of the specimens. Within one specimen, different volumes represented different locations. For the UHR OCT system, OCT volumes were taken at 32kHz linerate. Each volume had 800-by-800-by-1024 pixels, covering 3mm-by-3mm-by-1.78mm in space, with an acquisition time of 20 s per volume and a measured sensitivity of 96 dB. For the 1300nm system, OCT volumes were taken at 28kHz linerate, with an acquisition time of 23.6 s for one single volume of 800-by-800-by-511 pixels covering 4mm-by-4mm-by-2.52 mm in space and a measured sensitivity of 100 dB. Specimens were all imaged fresh in free space. During the imaging process, PBS spray was applied to prevent the sample from drying. For the image comparison study, the specimens were manually transferred from one system to the other, located and orientated the same way with respect to the scanning beam using best effort. All OCT images were presented without scaling by tissue refractive index.
Feature extraction and classification
It remains a challenge in OCT image processing of breast tissue to differentiate normal stromal tissue from cancerous tissue. However, adipose tissue has a characteristic honeycomb texture. As a result, our approach is to decouple the automated classification procedure by first identifying large adipose regions, with intact honeycombing features, which normally corresponds to non-neoplastic areas, using an adipose classifier. Thereafter, regions not classified as adipose will be classified as normal stroma of fibroelastic origin or cancer. Detailed algorithm flowchart is presented in Fig.2.
Fig.2.

Tissue classification algorithm flow. OCT B-scans were first processed to identify adipose region based on regional texture features. B-scans with a small adipose ratio will then be classified into normal stroma and IDC based on frame-based features derived from tissue optical properties.
To differentiate adipose tissue at a specific region, we extracted region-based local features, including standard deviation, entropy, and homogeneity of the OCT intensity signal. Every frame was denoised and divided into small sized grids. The features of each grid were then input into a trained machine learning model, RVM (38–40), and the model will assign a tissue type to that grid. Frames that contained 30% or less adipose tissue in the regions where the intensity signal was above SNR threshold will be classified as solid tissue and sent to the IDC.
We identified four parameters based on the signal penetration and backscattering strength in the OCT image as input to the RVM based IDC classifier. For feature extraction, a single scattering model (41–43) for homogenous media was incorporated to model the detected OCT signal from the sample arm as shown in Eq.1, where was the effective backscattering coefficient, IS the incident light intensity in the sample arm, lc the coherence length of the light source, and the 6-dB sensitivity fall-off induced by the spectrometer. The coupling efficiency was assumed to be constant along the A-line and over the B-scan to avoid the complexity. can be decoupled from the A-line profile by measuring the center of the scan as well as the 6-dB sensitivity fall-off range of the system (Zc,Zw) (42), as illustrated in Eq.2. The first two parameters were the mean and variance of the penetration depth across a single B-scan. The penetration depth was defined as the axial distance where the intensity drops to e−1 of its peak value from the tissue-air interface and was correlated with the attenuation coefficient μt of the tissue. The other two parameters were the mean and variance of “decay range” across a single B-scan. The decay range was defined as the axial distance from the tissue-air interface to the location where the magnitude of A-line intensity is 10 dB above the noise floor. It was correlated with both the effective backscattering coefficient μb and attenuation coefficient μt of the sample. Both penetration depth and decay range were extracted based on a smoothed A-line profile, which was done by averaging three consecutive A-lines and then applying a Savasky Golay filter with a 2nd order polynomial (22).
| (1) |
| (2) |
To evaluate both classifiers, we performed leave-one-out cross validation on the whole dataset. The classification results were validated against the correlated histology with pathological features reviewed and confirmed by a pathologist.
Histology
After imaging, tissue specimens first placed in 10% Formalin for 24 hours and were then transferred to 70% ethanol for histology process. Specimen blocks were embedded and sliced along the OCT B-scan direction. Multiple 5 μm-thick slices were taken from a single specimen block, with 100 μm discard between levels and each slide stained with Hematoxylin-eosin (H&E). The processed slides were digitalized at 40× magnification using Aperio system. The ImageScope (v12.1.0.5029) software was used to view and annotate H&E images.
Statistics
Details of the datasets used for classification were summarized in Table 1. One single OCT volume captured a small fractional region of the specimen. Based on the guidance of pathologist, the tissue type was identified locally in a sub-region of the histology slide comparable to the region of OCT b-scan. B-scans/frames used in the classification algorithm were selected based on the regional structural matching against corresponding histology with best effort, as well as image quality. An OCT frame was labeled as adipose if more than 70% of the effective area showed the adipose feature. The threshold of identifying a corresponding adipose-dominated histology slides was 80%. For IDC, a pathologist performed the classification of regions of interest within histology slides. In general, if the region of interest in an IDC slide contained a cluster of cancer cells, it will be regarded as IDC. Classification results were then compared with the pathologist-assigned tissue types for validation. The classification and validation were performed separately in UHR OCT dataset and standard OCT dataset. To evaluate the performance of the classification algorithm, we calculated specificity, sensitivity, and overall accuracy using the following definitions:
| (3) |
| (4) |
| (3) |
| (4) |
| (5) |
Table 1.
Summary of classification datasets(a)
| Adipose Classifier | IDC Classifier | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Specimens | Number of Frames | Number of Specimens | Number of Frames | |||||||||
| Normal | IDC | Total | Normal | IDC | Total | Normal | IDC | Total | Normal | IDC | Total | |
| UHR OCT system | 9 | 0 | 9 | 32 | 0 | 32 | 6 | 13 | 19 | 48 | 104 | 152 |
| Telesto system | 6 | 4 | 10 | 15 | 13 | 28 | 5 | 8 | 13 | 40 | 64 | 104 |
Datasets from 7 normal specimens were used for both classifiers. In IDC classification, 1 normal and 2 IDC specimens had both UHR OCT and Thorlabs Telesto datasets recorded.
RESULTS
UHR OCT Images of Breast Cancer
All OCT images presented have a corresponding histology slides, which were annotated with the help of an experienced pathologist. The aspect ratio of UHR OCT images was scaled to match the dimension of the actual cross-sectional field of view in air (3 mm by 1.78 mm), and Thorlabs OCT images were presented in their original scale. Fig.3 demonstrates the difference between OCT images taken from the UHR OCT system and Thorlabs system. Fig.3 (a)–(c) present single OCT B-scans and the corresponding histology of a normal breast specimen with terminal ductal lobule unit (TDLU) and adipose, and Fig.3 (d)–(e) present breast specimen with mucinous carcinoma. The signal penetration was more limited at the 800nm band compared with the 1300nm band. The insets in Fig.3 (a) (b) and (d) (e) provide zoom-in views of the adipose region and mucins region. Edges, which are high spatial frequency details, were better defined in UHR images due to an extended Nyquist limit in the spatial frequency domain (smaller spatial sampling size) and higher resolution. On the other hand, for the Thorlabs system, the pixel size in the axial direction is around 5 μm, very close to the actual axial resolution, which will ultimately limit the image resolution.
Fig.3.

Comparison of OCT B-scans taken from the commercial system at 1300mm (a) (d) and UHR OCT system at 800nm (b) (e), and their histopathological correlations (c) (f). (a)–(c) demonstrate an example of normal breast tissue, with adipose, stroma and TDLUs. (d)–(f) demonstrate a subtype of IDC, known as mucinous carcinoma. Insets: zoom-in view of adipose region, covering 0.3mm by 0.3mm area. Scale bar: 250 μm.
Fig.4 shows a range of different tumor types, including IDC, DCIS, phyllodes, fibrotic focus carcinoma, and necrotic tumor, as well as non-neoplastic breast tissue unveiled by UHR OCT images as Fig.4 (a)–(d) and (i)–(l), with correlated histology slides as Fig.4 (e)–(h) and (m)–(p). In UHR OCT images, signal penetration was deeper in normal specimens than in malignant specimens in general. Tumor cells appeared to be darker grey due to reduced scattering, while fibrous stroma were much brighter due to stronger backscattering of light. Normal duct structures in Fig.4 (a) and (b) featured clear epithelial lining and basal boundary as also reported by Hsiung et al (33). In Fig.4 (b), the wavy collagen fibrils of normal fibrous stroma tissue sometimes imposed a birefringence artifact in the image. Features of IDC, DCIS, and microcalcification in UHR OCT images were previously reported by several groups, and were confirmed in our observation (29,30,33). In addition, we presented UHR OCT images of phyllodes tumor, fibrotic focus carcinoma (a subtype of IDC) and necrotic tumor in Fig.4 (i)–(l), respectively. In OCT, phyllodes tumor appeared to have a cracked texture in Fig.4 (i), with reduced signal penetration and lower overall intensity. Fibrotic focus carcinoma appeared as dark islands surrounded by high-intensity necrotic regions in Fig.4 (j). Necrotic tumor mixture with fibrous tissue, picked up by only eosin stain in Fig.4 (p), showed similar features as necrosis such as high intensity and low penetration. Especially, a nodule of necrotic tumor was clearly delineated in UHR OCT image in Fig.4 (l), while it remained difficult to see in histology (red circle in Fig.4 (p)). The corresponding histology presented in Fig.4 (p) was intentionally zoomed in for better visualization of pathological details.
Fig.4.
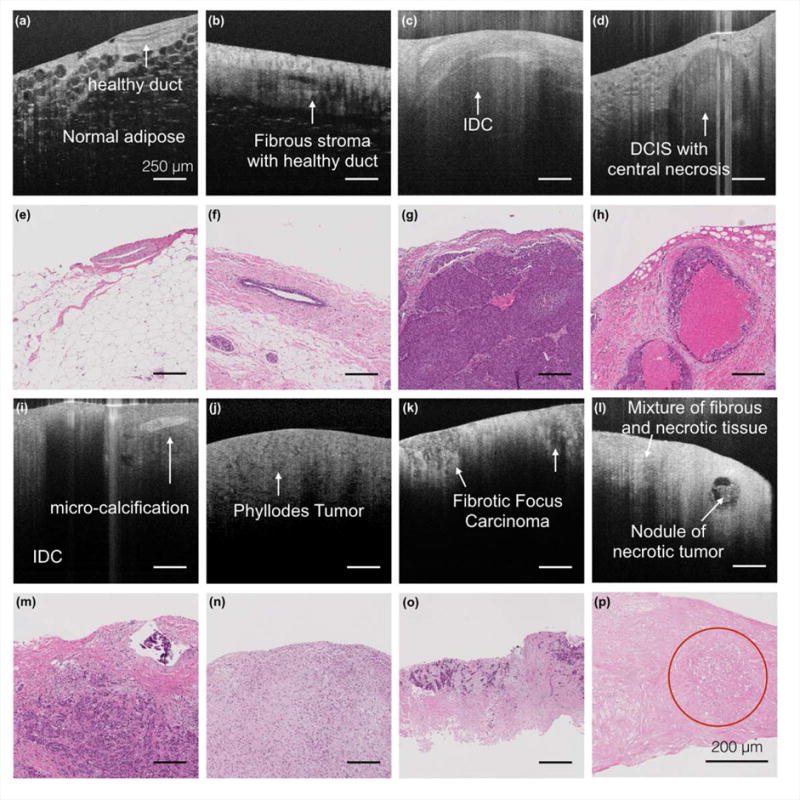
UHR OCT images and the corresponding histology slides of normal breast tissue and different cancer types. (a) – (o) Scale bar: 250 μm. (p) Scale bar: 200 μm.
Fig.5 illustrates wide field imaging and 3D rendering capability of UHR OCT. In Fig.5 (a), one stitched UHR OCT B-scan image of mucinous carcinoma specimen with a field of view greater than 7 mm was presented. Two en face images of mucinous carcinoma and IDC were presented in Fig.5 (c) and (e), respectively. All OCT images were shown in accord with corresponding histology slides in Fig. 5 (b), (d) and (f). Especially, for Fig.5 (d) and (f), the histology slides were produced in the en face plane on purpose to correlate with en face UHR OCT images.
Fig.5.

Wide field and 3D rendering capability of UHR OCT. (a)–(b) Stitched UHR OCT images and corresponding histology of mucinous carcinoma. (c)–(d) en face OCT and corresponding histology for mucinous carcinoma and (e)–(f) for IDC. Scaler bar: 500 μm.
Tissue Classification
Adipose Classifier
We compared the sensitivity and specificity of the adipose classification results based on OCT images (details in Table 1) from the Thorlabs system and the UHR OCT system in Fig.6 (a). In general, classification of UHR OCT images achieved higher sensitivity (94%) and specificity (93%) than the sensitivity (91%) and specificity (76%) of the Thorlabs images. Furthermore, we applied our algorithm on volumetric OCT images and obtained the corresponding color-coded 3D adipose maps using the regional adipose classification results. In specific, Fig.6 (b) and (c) present Thorlabs OCT volumes from normal and IDC specimens respectively, with corresponding color-coded 3D adipose maps shown in Fig.6 (e) and (f). And UHR OCT intensity data along with the color-coded 3D adipose map were presented in Fig.6 (d) and Fig.6 (g). We found that in UHR OCT images, it was possible capture more isolated adipose structure. Moreover, we calculated an adipose ratio within normal and IDC specimens and found the adipose ratio (52.3 ± 29.4%, n = 6) in normal tissue was higher than that in cancerous tissue (12.4 ± 10.1%, n = 4). This observation was further confirmed from the corresponding histology.
Fig.6.
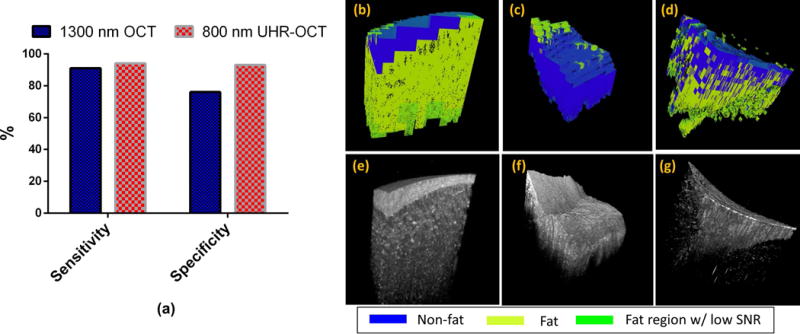
Classification results of fat region. (a) Comparison of sensitivity and specificity of tissue classification performed on Thorlabs datasets and UHR OCT datasets. (b),(e) and (c),(f) shows color-coded 3D fat maps with corresponding OCT volumes from Thorlabs system in normal and IDC specimens, respectively. (d),(g) shows color-coded 3D fat map with corresponding OCT volume from UHR OCT system.
IDC Classifier
Selected UHR OCT images of normal fibrous stroma and IDC were presented in Fig.7 (a) and (b) as a qualitative comparison. OCT features, including mean/standard deviation of penetration depth, mean/standard deviation of decay range were plot in 2D space in Fig.7 (c) and (d). It appeared that 800nm light can penetrate deeper in normal stroma than in IDC, and larger variation in those two optical properties existed in normal stroma than in IDC. And the lateral heterogeneity in OCT images of normal fibrous stroma tissue resulted in a larger variation, especially in the decay range. 152 UHR OCT B-scans from 6 normal and 13 IDC specimens, and 104 standard OCT B-scans from 5 normal and 8 IDC specimens were separately employed for IDC classification, and the validation results were listed in Table 2. In particular, for a limited number of samples within our dataset, we achieved an overall accuracy of 84%, sensitivity of 89% and specificity of 71% for classification based on UHR OCT images, which were better than the results (overall accuracy of 71%, sensitivity of 83% and specificity of 53%) based on the Thorlabs images. In Thorlabs system, the variation of decay range and penetration depth for normal stroma were lower than that in UHR OCT system, making the differentiation between normal tissue and adipose tissue more difficult. Therefore, the detection accuracy in Thorlab system in general was lower than UHR OCT system. In addition, we further applied our algorithm within a 3D dataset. We extracted the features in each B-scan and the RVM returned a probability of how likely this B-scan belonged to IDC tissue type. The color-coded probability bar was shown in Fig.8.
Fig.7.
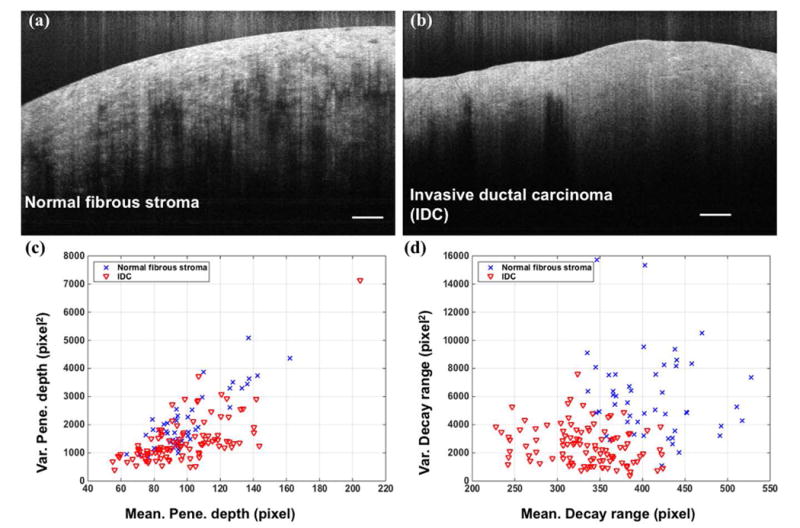
(a),(b) UHR OCT image examples of normal fibrous stroma and invasive ductal carcinoma. Scale bar: 250 μm. (c),(d) Extracted optical features on normal stroma and IDC tissue type based on 152 UHR OCT images.
Table 2.
Classification results of invasive ductal carcinoma
| UHR OCT system | Telesto system | |||||
|---|---|---|---|---|---|---|
| Histology (positive) | Histology (negative) | Total | Histology (positive) | Histology (negative) | Total | |
| OCT (positive) | 93 | 14 | 107 | 53 | 19 | 72 |
| OCT (negative) | 11 | 34 | 45 | 11 | 21 | 32 |
| Total | 104 | 48 | 64 | 40 | ||
| Sensitivity = 89% | Specificity = 71% | Overall accuracy =84% | Sensitivity = 83% | Specificity = 53% | Overall accuracy =71% | |
Fig. 8.
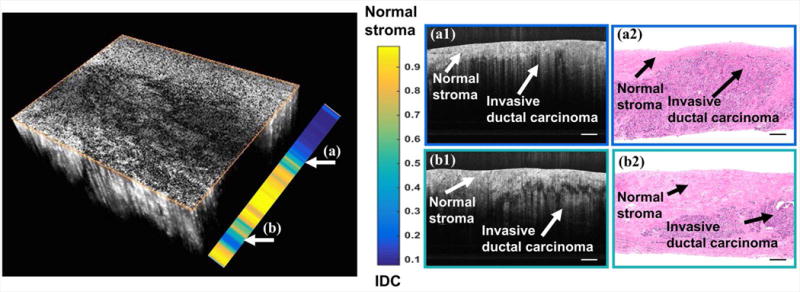
Volumetric UHR-OCT images and classification results between IDC and fibrous stroma. Two individual B-scans were selected to compare with the corresponding histology. Scale bar: 250 μm.
DISCUSSION
We showed that UHR OCT at 800 nm can provide better image quality compared with OCT at 1300 nm qualitatively and quantitatively. Detailed structures of basic units found in breast tissue, such as TDLUs, ducts, adipose and fibrous stroma, can be better delineated by UHR OCT. UHR OCT images of different pathological features in breast cancer specimens were also provided, including IDC, DCIS, microcalcification, phyllodes tumor, fibrotic focus carcinoma, mucinous carcinoma, and necrotic tumor. We compared our observations with previous reports and can reach similar conclusion on OCT feature descriptions of different tissue types, such as TDLU, ducts, adipose, fibrous stroma, IDC, DCIS, microcalcification and mucinous carcinoma. In addition, we were able to add phyllodes, fibrotic focus and necrotic tumor to the UHR OCT image library. Furthermore, automated tissue classification algorithms, including an adipose classifier and an IDC classifier, were proposed to identify IDC against normal breast tissue. The classification was done on both UHR OCT images and standard OCT images. For the adipose classifier, 32 UHR OCT B-scans from 9 normal specimens, as well as 28 standard OCT B-scans from 6 normal and 4 IDC specimens were included. For IDC classifier, 152 UHR OCT B-scans from 6 normal and 13 IDC specimens, as well as 104 B-scans from 5 normal and 8 IDC specimens were included. The preliminary results showed encouraging results indicating that features derived from UHR OCT images can lead to a better performance on differentiation of fat against non-fat regions, as well as IDC against normal fibrous stroma, compared with the results based on standard OCT images. We also showed that it was possible to apply the classifications on volumetric datasets.
There have been tremendous efforts on realization of computer-aid detection in order to improve the efficacy of diagnostic process in other image modalities (44). Zysk et al (19) pioneered in automated detection of tissue types in OCT images, and they proposed a tissue classification model using depth-resolved Fourier domain classification with periodicity analysis. The A-line based approach showed promising results in differentiating tumor against normal stroma tissue with a sensitivity of 99% and specificity of 58%. Similar A-line based classification was performed based on fractal analysis (45), with a sensitivity of 88% and specificity of 82% for cancer classification. Goldberg et al (20) also reported an automatic tissue classification algorithm based on spatial frequency analysis of A-line feature, and they reported a sensitivity of 98% and specificity of 82% for adipose classification. These studies demonstrated the possibility of implementing CAD with OCT imaging for breast cancer management. Furthermore, Savastru et al (22) pioneered in implementation of depth-resolved tissue classification for automated tumor margin assessment on ex vivo mice breast tumor tissue from animal models. They reported a sensitivity of 81% and specificity of 89% on 20 samples, which was very encouraging.
In this report, we developed an adipose classifier to differentiate adipose tissue against solid types of tissue, and an IDC classifier to identify IDC tissue from normal fibrous stroma tissue. The ultimate goal of this algorithm was to identify cancerous regions and the algorithm was implemented by a two-level classifier. The first level of the algorithm is the adipose tissue classifier, developed to quickly exclude the frames with a majority of adipose tissue. The second level classified image frames with adipose regions less than a specified threshold as stroma or IDC. The classification was tested with both UHR OCT images and standard OCT images of ex vivo human breast tissue. Rather than focusing on A-line classification, we focused on classification of B-scans/frames. The features were simplified so that no fitting was needed in the algorithm in order to accelerate the feature extraction process. Due to a limited amount of specimens, leave-one-out validation was carried out to evaluate the classifier performance. We showed that UHR OCT images resulted better classification results in general. In our preliminary study, we achieved a sensitivity of 94% and specificity of 93% for adipose classifier and a sensitivity of 89% and specificity of 71% for IDC classifier. For adipose classification, the classifier was based on the texture features within OCT intensity images. The decrease in SNR at larger depth was the major cause of false positives/negatives in frames which had layered tissue types. Since the tissue types were determined in gridded blocks, we found that the size of the grid will also affect the classification results. In general, we observed that the classification accuracy dropped at the boundary of an adipose region where a grid contains a mixture of adipose and stroma/IDC tissue. This, however, is not expected to cause major problems and could even help to identify the adipose boundary as well. For IDC classifier, the false positives were mainly caused by heterogeneity in the tissue, the birefringent artifact and irregular surface curvature in the OCT images. Especially, it was known that tissue birefringence had impact on the signal penetration, and it may be found prominent in UHR OCT images of fibrous stroma tissue which contains well organized collagen fibers. In some cases, the signal penetration was limited in stromal tissue when the incident beam was locally aligned with the optical axis of the organized collagen. Furthermore, the features for IDC tissue may vary depending on the type and grade of IDC. And the tissue immersing time in the RPMI solution, while less than 24 hours, was not controlled in our study, we expected the optical clearing effect may cause some variations in the signal penetration as well. These aspects contribute to the false negatives of IDC classifier.
It should be noted that there were limitations in our study. First, only IDC classification was studied. Because the mastectomy-produced tissue yielded a variety in cancer types, the numbers of specimens in each cancer type were limited. While these results are promising, this study is very preliminary and leave-one-out validation may result in overestimated performance evaluation. Therefore, in the future a full statistical evaluation must still be performed on a larger sample size, with the inclusion of multiple cancer types and grades. Detection of other tumor types must also be studied in order to fully assess the efficacy of the CAD technique. Second, while UHR OCT system may produce better delineations on different tissue types, it exhibits higher noise floor because of the supercontinuum light source. This will have an impact on texture-based feature extraction at larger depth. Moreover, the penetration depth for 800 nm light was more limited compared with 1300 nm light. In some of the breast tissue specimens, it can reach up to 1 mm, which may be due to the optical clearing effect by the RPMI media. On the other hand, the SLD-based 1300 nm OCT system provides shot noise limited imaging performance and larger signal penetration in general. Therefore, it will be more suitable if the imaging range was required to be above 1 mm into the tissue. Third, here we only presented off-line frame-based classification. Volume-based or specimen-based classification should be possible if a proper threshold can be identified to differentiate tumor tissue against normal tissue. For real-time automatic detection, the current computational speed was the main problem. The main cost was from the adipose classifier, which took around 8 second per frame in MATLAB using CPU. However, we expect this process can be greatly accelerated if GPU based parallel computing is utilized.
In the future, we will work on the IDC classifier to improve the performance, and optimize a volumetric classification algorithm. With an increase in the size of our datasets, we will continue to improve upon the algorithm, incorporating additional features that can help to improve the accuracy, resulting in an algorithm that can differentiate multiple types of cancerous regions. Next steps also include conducting the OCT imaging session in an intra-operative setting to reduce the time between surgery and imaging. Lastly, additional efforts will be put towards real-time processing, such as improvement in the hardware, the application of distributed computing, and corresponding graphical user interface, to speed up the current runtime.
CONCLUSION
In this preliminary study, we have showed qualitatively and quantitatively that UHR OCT images may enable better visualization of detailed features in different types of breast tissue. UHR OCT images of new breast cancer types such as phyllodes tumor, necrotic tumor and fibrotic focus carcinoma were provided for future references. RVM based stochastic tissue classification methods presented here shows great promise for automated classification of different tissue types in human breast tissue.
Acknowledgments
The authors would like to thank Syed Bin Amir for technical assistance on image acquisition, and the Columbia University Medical Center Histology Service for histology processing. The study was funded in part by Research Initiatives for Science and Engineering (RISE) grant (CPH) at Columbia University and National Institute of Health (NIH) 1DP2HL127776-01 (CPH).
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
References
- 1.Society AC. Cancer Facts & Figures. 2015 [Google Scholar]
- 2.Society AC. Breast Cancer Facts & Figures. 2015–2016 [Google Scholar]
- 3.Vlastos G, Verkooijen HM. Minimally Invasive Approaches for Diagnosis and Treatment of Early-Stage Breast Cancer. The Oncologist. 2007;12(1):1–10. doi: 10.1634/theoncologist.12-1-1. [DOI] [PubMed] [Google Scholar]
- 4.Tao YK, Shen D, Sheikine Y, Ahsen OO, Wang HH, Schmolze DB, Johnson NB, Brooker JS, Cable AE, Connolly JL, Fujimoto JG. Assessment of breast pathologies using nonlinear microscopy. Proceedings of the National Academy of Sciences. 2014;111(43):15304–15309. doi: 10.1073/pnas.1416955111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tadrous PJ, Siegel J, French PMW, Shousha S, Lalani E-N, Stamp GWH. Fluorescence lifetime imaging of unstained tissues: early results in human breast cancer. The Journal of Pathology. 2003;199(3):309–317. doi: 10.1002/path.1286. [DOI] [PubMed] [Google Scholar]
- 6.Dobbs JL, Ding H, Benveniste AP, Kuerer HM, Krishnamurthy S, Yang W, Richards-Kortum R. Feasibility of confocal fluorescence microscopy for real-time evaluation of neoplasia in fresh human breast tissue. Journal of Biomedical Optics. 2013;18(10):106016–106016. doi: 10.1117/1.JBO.18.10.106016. [DOI] [PubMed] [Google Scholar]
- 7.Bydlon TM, Kennedy SA, Richards LM, Brown JQ, Yu B, Junker MK, Gallagher J, Geradts J, Wilke LG, Ramanujam N. Performance metrics of an optical spectral imaging system for intra-operative assessment of breast tumor margins. Optics Express. 2010;18(8):8058–8076. doi: 10.1364/OE.18.008058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma V, Shivalingaiah S, Peng Y, Euhus D, Gryczynski Z, Liu H. Auto-fluorescence lifetime and light reflectance spectroscopy for breast cancer diagnosis: potential tools for intraoperative margin detection. Biomed Opt Express. 2012;3(8):1825–1840. doi: 10.1364/BOE.3.001825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnabel F, Boolbol SK, Gittleman M, Karni T, Tafra L, Feldman S, Police A, Friedman NB, Karlan S, Holmes D, Willey SC, Carmon M, Fernandez K, Akbari S, Harness J, Guerra L, Frazier T, Lane K, Simmons RM, Estabrook A, Allweis T. A Randomized Prospective Study of Lumpectomy Margin Assessment with Use of MarginProbe in Patients with Nonpalpable Breast Malignancies. Annals of Surgical Oncology. 2014;21(5):1589–1595. doi: 10.1245/s10434-014-3602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregory WD, Marx JJ, Gregory CW, Mikkelson WM, Tjoe JA, Shell J. The Cole relaxation frequency as a parameter to identify cancer in breast tissue. Medical Physics. 2012;39(7):4167–4174. doi: 10.1118/1.4725172. [DOI] [PubMed] [Google Scholar]
- 11.Scolaro L, McLaughlin Robert A, Kennedy Brendan F, Saunders Christobel M, Sampson David D. A review of optical coherence tomography in breast cancer. Photonics & Lasers in Medicine. 2014;3:225. [Google Scholar]
- 12.Alfano RR, Pradhan A, Tang GC, Wahl SJ. Optical spectroscopic diagnosis of cancer and normal breast tissues. J Opt Soc Am B. 1989;6(5):1015–1023. [Google Scholar]
- 13.Boppart S, Luo W, Marks D, Singletary K. Optical Coherence Tomography: Feasibility for Basic Research and Image-guided Surgery of Breast Cancer. Breast Cancer Res Treat. 2004;84(2):85–97. doi: 10.1023/B:BREA.0000018401.13609.54. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen FT, Zysk AM, Chaney EJ, Kotynek JG, Oliphant UJ, Bellafiore FJ, Rowland KM, Johnson PA, Boppart SA. Intraoperative Evaluation of Breast Tumor Margins with Optical Coherence Tomography. Cancer Research. 2009;69(22):8790–8796. doi: 10.1158/0008-5472.CAN-08-4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zysk AM, Nguyen FT, Chaney EJ, Kotynek JG, Oliphant UJ, Bellafiore FJ, Johnson PA, Rowland KM, Boppart SA. Clinical Feasibility of Microscopically-Guided Breast Needle Biopsy Using a Fiber-Optic Probe with Computer-Aided Detection. Technology in cancer research & treatment. 2009;8(5):315–321. doi: 10.1177/153303460900800501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zysk AM, Chen K, Gabrielson E, Tafra L, May Gonzalez EA, Canner JK, Schneider EB, Cittadine AJ, Scott Carney P, Boppart SA, Tsuchiya K, Sawyer K, Jacobs LK. Intraoperative Assessment of Final Margins with a Handheld Optical Imaging Probe During Breast-Conserving Surgery May Reduce the Reoperation Rate: Results of a Multicenter Study. Annals of Surgical Oncology. 2015;22(10):3356–3362. doi: 10.1245/s10434-015-4665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erickson-Bhatt SJ, Nolan RM, Shemonski ND, Adie SG, Putney J, Darga D, McCormick DT, Cittadine AJ, Zysk AM, Marjanovic M, Chaney EJ, Monroy GL, South FA, Cradock KA, Liu ZG, Sundaram M, Ray PS, Boppart SA. Real-time imaging of the resection bed using a handheld probe to reduce incidence of microscopic positive margins in cancer surgery. Cancer research. 2015;75(18):3706–3712. doi: 10.1158/0008-5472.CAN-15-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaughlin RA, Quirk BC, Curatolo A, Kirk RW, Scolaro L, Lorenser D, Robbins PD, Wood BA, Saunders CM, Sampson DD. Imaging of Breast Cancer With Optical Coherence Tomography Needle Probes: Feasibility and Initial Results. IEEE Journal of Selected Topics in Quantum Electronics. 2012;18(3):1184–1191. [Google Scholar]
- 19.Zysk AM, Boppart SA. Computational methods for analysis of human breast tumor tissue in optical coherence tomography images. J Biomed Optics. 2006;11(5) doi: 10.1117/1.2358964. 054015-054015-054017. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg BD, Iftimia NV, Bressner JE, Pitman MB, Halpern E, Bouma BE, Tearney GJ. Automated algorithm for differentiation of human breast tissue using low coherence interferometry for fine needle aspiration biopsy guidance. BIOMEDO. 2008;13(1) doi: 10.1117/1.2837433. 014014-014014-014018. [DOI] [PubMed] [Google Scholar]
- 21.Mujat M, Ferguson RD, Hammer DX, Gittins C, Iftimia N. Automated algorithm for breast tissue differentiation in optical coherence tomography. J Biomed Opt. 2009;14(3) doi: 10.1117/1.3156821. 034040-034040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savastru D, Chang EW, Miclos S, Pitman MB, Patel A, Iftimia N. Detection of breast surgical margins with optical coherence tomography imaging: a concept evaluation study. Journal of Biomedical Optics. 2014;19(5) doi: 10.1117/1.JBO.19.5.056001. 056001-056001. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy KM, McLaughlin RA, Kennedy BF, Tien A, Latham B, Saunders CM, Sampson DD. Needle optical coherence elastography for the measurement of microscale mechanical contrast deep within human breast tissues. J Biomed Optics. 2013;18(12) doi: 10.1117/1.JBO.18.12.121510. 121510-121510. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy BF, Kennedy KM, Sampson DD. A Review of Optical Coherence Elastography: Fundamentals, Techniques and Prospects. IEEE Journal of Selected Topics in Quantum Electronics. 2014;20(2):272–288. [Google Scholar]
- 25.Chin L, Wijesinghe P, Latham B, Saunders CM, Sampson DD, Kennedy BF. OSA Technical Digest (online); 2016 2016/04/25. Fort Lauderdale, Florida: Optical Society of America; Mapping the mechanical heterogeneity of human breast tissue using optical coherence elastography; p. JM2A.3. OSA Technical Digest (online) [Google Scholar]
- 26.South FA, Chaney EJ, Marjanovic M, Adie SG, Boppart SA. Differentiation of ex vivo human breast tissue using polarization-sensitive optical coherence tomography. Biomed Opt Express. 2014;5(10):3417–3426. doi: 10.1364/BOE.5.003417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel R, Khan A, Quinlan R, Yaroslavsky AN. Polarization-Sensitive Multimodal Imaging for Detecting Breast Cancer. Cancer Research. 2014;74(17):4685–4693. doi: 10.1158/0008-5472.CAN-13-2411. [DOI] [PubMed] [Google Scholar]
- 28.Villiger M, Lorenser D, McLaughlin RA, Quirk BC, Kirk RW, Bouma BE, Sampson DD. Deep tissue volume imaging of birefringence through fibre-optic needle probes for the delineation of breast tumour. Scientific Reports. 2016;6:28771. doi: 10.1038/srep28771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou C, Cohen DW, Wang Y, Lee H-C, Mondelblatt AE, Tsai T-H, Aguirre AD, Fujimoto JG, Connolly JL. Integrated Optical Coherence Tomography and Microscopy for Ex Vivo Multiscale Evaluation of Human Breast Tissues. Cancer research. 2010;70(24):10071–10079. doi: 10.1158/0008-5472.CAN-10-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Assayag O, Antoine M, Sigal-Zafrani B, Riben M, Harms F, Burcheri A, Grieve K, Dalimier E, Le Conte de Poly B, Boccara C. Large field, high resolution full-field optical coherence tomography: a pre-clinical study of human breast tissue and cancer assessment. Technol Cancer Res Treat. 2014;13(5):455–468. doi: 10.7785/tcrtexpress.2013.600254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feldman SM. Surgical Margins in Breast Conservation. International journal of surgical oncology. 2013:2013. doi: 10.1155/2013/136387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiappa C, Rovera F, Corben AD, Fachinetti A, De Berardinis V, Marchionini V, Rausei S, Boni L, Dionigi G, Dionigi R. Surgical margins in breast conservation. International Journal of Surgery. 2013;11(Supplement 1):S69–S72. doi: 10.1016/S1743-9191(13)60021-7. [DOI] [PubMed] [Google Scholar]
- 33.Hsiung P-L, Phatak DR, Chen Y, Aguirre AD, Fujimoto JG, Connolly JL. Benign and Malignant Lesions in the Human Breast Depicted with Ultrahigh Resolution and Three-dimensional Optical Coherence Tomography. Radiology. 2007;244(3):865–874. doi: 10.1148/radiol.2443061536. [DOI] [PubMed] [Google Scholar]
- 34.Chu KK, Ughi GJ, Liu L, Tearney GJ. Toward Clinical μOCT—A Review of Resolution-Enhancing Technical Advances. Current Cardiovascular Imaging Reports. 2014;7(12):9308. [Google Scholar]
- 35.Yin B, Chu KK, Liang C-P, Singh K, Reddy R, Tearney GJ. μOCT imaging using depth of focus extension by self-imaging wavefront division in a common-path fiber optic probe. Optics Express. 2016;24(5):5555–5564. doi: 10.1364/OE.24.005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balci FL, Feldman SM. Exploring breast with therapeutic ductoscopy. Gland Surgery. 2014;3(2):136–141. doi: 10.3978/j.issn.2227-684X.2014.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao X, Gan Y, Marboe CC, Hendon CP. Myocardial imaging using ultrahigh-resolution spectral domain optical coherence tomography. Journal of Biomedical Optics. 2016;21(6):061006–061006. doi: 10.1117/1.JBO.21.6.061006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majumder SK, Ghosh N, Gupta PK. Relevance vector machine for optical diagnosis of cancer. Lasers in Surgery and Medicine. 2005;36(4):323–333. doi: 10.1002/lsm.20160. [DOI] [PubMed] [Google Scholar]
- 39.Barber D. Bayesian Reasoning and Machine Learning. Cambridge University Press; 2012. [Google Scholar]
- 40.Gan Y, Tsay D, Amir SB, Marboe CC, Hendon CP. Automated classification of optical coherence tomography images of human atrial tissue. Journal of Biomedical Optics. 2016;21(10):101407–101407. doi: 10.1117/1.JBO.21.10.101407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fleming CP, Eckert J, Halpern EF, Gardecki JA, Tearney GJ. Depth resolved detection of lipid using spectroscopic optical coherence tomography. Biomed Opt Express. 2013;4(8):1269–1284. doi: 10.1364/BOE.4.001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Soest G, Goderie T, Regar E, Koljenović S, van Leenders GLJH, Gonzalo N, van Noorden S, Okamura T, Bouma BE, Tearney GJ, Oosterhuis JW, Serruys PW, van der Steen AFW. Atherosclerotic tissue characterization in vivo by optical coherence tomography attenuation imaging. Journal of Biomedical Optics. 2010;15(1) doi: 10.1117/1.3280271. 011105-011105-011109. [DOI] [PubMed] [Google Scholar]
- 43.Xu C, Schmitt JM, Carlier SG, Virmani R. Characterization of atherosclerosis plaques by measuring both backscattering and attenuation coefficients in optical coherence tomography. Journal of Biomedical Optics. 2008;13(3) doi: 10.1117/1.2927464. 034003-034003-034008. [DOI] [PubMed] [Google Scholar]
- 44.Jalalian A, Mashohor SBT, Mahmud HR, Saripan MIB, Ramli ARB, Karasfi B. Computer-aided detection/diagnosis of breast cancer in mammography and ultrasound: a review. Clinical Imaging. 2013;37(3):420–426. doi: 10.1016/j.clinimag.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 45.Sullivan AC, Hunt JP, Oldenburg AL. Fractal analysis for classification of breast carcinoma in optical coherence tomography. Journal of Biomedical Optics. 2011;16(6) doi: 10.1117/1.3590746. 066010-066010-066016. [DOI] [PubMed] [Google Scholar]


