Abstract
Background
Percutaneous neurostimulation of the peripheral nervous system involves the insertion of a wire “lead” through an introducing needle to target a nerve/plexus or a motor point within a muscle. Electrical current may then be passed from an external generator through the skin via the lead for various therapeutic goals, including providing analgesia. With extended use of percutaneous leads sometimes greater than a month, infection is a concern. It was hypothesized that the infection rate of leads with a coiled design is lower than for leads with a noncoiled cylindrical design.
Methods
The literature was retrospectively reviewed for clinical studies of percutaneous neurostimulation of the peripheral nervous system of greater than 2 days that included explicit information on adverse events. The primary endpoint was the number of infections per 1,000 indwelling days.
Results
Forty-three studies were identified that met inclusion criteria involving coiled (n = 21) and noncoiled (n = 25) leads (3 studies involved both). The risk of infection with noncoiled leads was estimated to be 25 times greater than with coiled leads (95% confidence interval [CI] 2–407, P = 0.006). The infection rates were estimated to be 0.03 (95% CI 0.01–0.13) infections per 1,000 indwelling days for coiled leads and 0.83 (95% CI 0.16–4.33) infections per 1,000 indwelling days for noncoiled leads (P = 0.006).
Conclusions
Percutaneous leads used for neurostimulation of the peripheral nervous system have a much lower risk of infection with a coiled design compared with noncoiled leads: approximately 1 infection for every 30,000 vs. 1,200 indwelling days, respectively.
Keywords: neuromodulation, percutaneous peripheral nerve stimulation, peripheral nerve stimulator, helical lead, small-diameter open-coiled helical lead, postoperative pain
INTRODUCTION
Percutaneous neurostimulation within the peripheral nervous system involves the insertion of a wire “lead” through an introducing needle to target a nerve/plexus or a motor point within a muscle.1–3 Electrical current may then be passed from an external generator through the skin via the lead for various therapeutic goals, including providing analgesia when treating chronic pain states.4,5 The original percutaneous leads had a noncoiled cylindrical shape—essentially simply a straight wire—and the majority of leads retain that structure (Figure 1). However, an alternative lead design takes the form of an open-coiled helix (see Figure 1).5,6 Both designs have been used clinically for extended periods of time—over 12 months in some cases.7–10 Infection is a concern because the leads create a potential conduit for contamination as they traverse the skin, and other percutaneous devices (such as intravascular catheters) have historically had a relatively high risk of infection when left indwelling for extended periods of time.11,12
Figure 1.

Examples of (A) a coiled lead and (B) a noncoiled electrical lead used for percutaneous neurostimulation of the peripheral nervous system.
There is a theoretical reason to believe that infection risk might be associated with lead design. Transcutaneous contamination may be a consequence of small movements of the externalized leads relative to the exit site that cause a “pistoning” effect, transferring infectious agents from the externalized portion of the lead/catheter to the subcutaneous tissues. Pistoning might also increase susceptibility to infection by disrupting the cell layer that forms around the lead (ie, encapsulation), which serves as an infection barrier.13,14 When a noncoiled, cylindrical lead traverses the skin, movement of the extremities can cause the wire to piston at the exit site. In contrast, an open-coil design allows the lead to stretch and compress to theoretically minimize the piston effect, while also allowing rapid tissue fibrosis into the helix, possibly hastening/improving a bacteriostatic seal.7,13
However, the relative infection risks among these 2 designs remain unexamined for leads designed for percutaneous stimulation of the peripheral nervous system. We therefore conducted this retrospective investigation, hypothesizing that the infection rate of leads with open-coil design is lower than for leads with a noncoiled cylindrical design. The primary endpoint was the number of investigator-reported infections per 1,000 indwelling days, as per recommendations from the Centers for Disease Control and Prevention (CDC; Atlanta, Georgia, USA) for reporting catheter infection rates.15
METHODS
Literature Review
A literature review was conducted using PubMed to identify published articles involving percutaneous electrical stimulation of the peripheral nervous system with a mean indwelling time of more than 2 days. Studies were excluded if adverse events were not explicitly reported, if data were available only for nonhuman subjects, or if there were fewer than 8 subjects evaluated (eg, case reports and small series). Of interest were studies reporting data for stimulation targeting peripheral nerves and motor points within muscles (eg, sacral nerves, occipital nerves, upper and lower extremity nerves, etc.) and not studies reporting data for cardiac stimulation, cochlear stimulation, deep brain stimulation, transcutaneous electrical stimulation, or spinal cord stimulation. Studies that only reported leads from a permanent (fully implanted) system were excluded from the analysis. If a study reported leads from both the trial (percutaneous) phase and the permanent (fully implanted) phase, only the leads from the former were included in the analysis. Duplicate data among studies were excluded. The following search terms were inputted for the initial PubMed search: “(((((neuromodulation) OR neurostimulation) OR nerve stimulation) OR electric stimulation) AND peripheral) AND percutaneous.” The title, abstract, and manuscript were reviewed for all articles to determine if the reference was potentially appropriate for the current investigation. Among these, the articles listed in each reference section were also reviewed in a similar manner. This was performed iteratively until no additional original articles were found. Data were collected by 2 of the authors independently and subsequently cross-checked for accuracy. Conflicting data were reanalyzed by both authors and agreed upon by consensus, with a third author available to arbitrate any unresolved disagreements. No major disagreements occurred.
Infections were defined as purulent discharge, erythema, or cellulitis at the lead exit site treated with antibiotics. The number of infections in each study was recorded, with additional note taken of severe infections defined as requiring any intervention in addition to antibiotic administration (eg, abscesses, meningitis, surgical drainage, debridement). The time from insertion until infection was recorded, when available. The numbers of patients and leads used in each study were also recorded, along with the total indwelling duration. Duplicate data among publications were excluded.
When calculating the number of infections per 1,000 indwelling days, a range existed for each group’s infection rate because some studies reported a range of indwelling times without reporting the mean indwelling time. Therefore, the highest possible infection rate for open-coil leads was compared to the lowest possible rate for noncoiled leads, ensuring conservative estimates were used to test for significant differences.
Data Analysis
Leads were grouped according to design (coiled vs. noncoiled). The primary endpoint was designated the rate of infection per 1,000 indwelling days, as per recommendations from the CDC for reporting catheter infection rates.15 Secondary endpoints included analyses of the rate of infection in the first 30 and 60 days, necessarily restricted to the subgroup of studies, which report sufficient information to determine these rates. Potential confounding covariates included publication year, study design (eg, randomized, controlled trial vs. retrospective chart review), country of origin (within vs. outside of the United States), sex, aseptic technique (not described and none vs. sterile technique described), and pre-insertion antibiotic administration.
The primary analysis approach was a generalized linear model with the outcome modeled as Poisson with quasi-likelihood to account for study heterogeneity or “over-dispersion.”16 This approach models the rate of infection as a Poisson process by considering the infection counts and exposure, or the mean number of indwelling days, per study. If only a range was reported for indwelling days, we conservatively used the minimum number of indwelling days for coiled leads and the maximum for noncoiled leads. We assessed confounding factors by testing the association of each covariate with (1) infection rate and (2) coil type (using logistic regression for the latter). If a covariate was associated with both infection rate and coil type at the inclusive 10% level, it was deemed a potential confounder and included in the final analysis. The primary test of association between infection rate and coil type was the chi-squared test comparing models with vs. without coil type. A similar quasi-likelihood binomial (logistic) model was applied for the 2 secondary outcomes: infections within 30 and 60 days of lead insertion.
RESULTS
The initial search yielded a total of 235 references. Among these, 24 articles were deemed potentially appropriate for the study, 8 of which met inclusion criteria for the study. Articles listed in each article’s reference list were also reviewed, which led to a final count of 43 articles meeting inclusion criteria involving coiled (n = 21; Table 1) and noncoiled (n = 25; Table 2) leads (with 3 articles involving both designs).
Table 1.
Percutaneous Coiled Electrical Leads: Articles and Infection Rates
| Author [PMID] | Publication Year |
Target Tissue | Total Leads |
Total Infections |
Severe Infections |
Infections During First 30 Days |
Infections During First 60 Days |
Mean Indwelling Days (range) |
|---|---|---|---|---|---|---|---|---|
| Marsolais & Kobetic [3490566]7 |
1986 | Lower extremity |
1,025 | 8 | 0 | * | * | 180† (*–1,140) |
| Scheiner et al. [8070801]8 | 1994 | Upper extremity | 775 | 32 | 0 | * | * | 509 (*–1,643) |
| Shimada et al. [8857879]9 | 1996 | Upper extremity | 327 | 10 | 0 | * | * | 800 (365– 1,765) |
| Carey et al. [11121986]24 | 2001 | Sacral nerve | 12 | 0 | 0 | 0 | 0 | 7 (7–7) |
| Daly et al. [11732829]25 | 2001 | Lower extremity |
124 | 10 | 0 | * | * | 347 (*–*) |
| Knutson et al. [17943669]12 |
2002 | Upper extremity | 940 | 14 | 0 | 1 | 1 | 313† (*–*) |
| Matzel et al. [15094271]26 | 2004 | Sacral nerve | 8 | 0 | 0 | 0 | 0 | 19.4‡ (10–*) |
| Rasmussen et al. [15216409]27 |
2004 | Sacral nerve | 34§ | 0 | 0 | 0 | 0 | * (*–21) |
| Renzenbrink & IJzerman [15180118]28 |
2004 | Shoulder | 60 | 0 | 0 | 0 | 0 | 49 (49–49) |
| Yu et al. [15129391]29 | 2004 | Shoulder | 128 | 0 | 0 | 0 | 0 | 49 (49–49) |
| Shimada et al. [22151766]30 |
2006 | Peroneal nerve | 20 | 0 | 0 | 0 | 0 | 1935 (730– 2,283) |
| Dudding et al. [18581439]31 |
2008 | Sacral nerve | 70§ | 0 | 0 | 0 | 0 | 14 (14–14) |
| Goldman et al. [18092334]32 |
2008 | Dorsal genital nerve |
21 | 0 | 0 | 0 | 0 | 6.6 (*–7) |
| Gstaltner et al. [18317481]33 |
2008 | Sacral nerve | 15 | 0 | 0 | 0 | 0 | 6 (*–*) |
| Chae et al. [19155351]34 | 2009 | Shoulder | 77 | 1 | 0 | 1 | 1 | 49 (49–49) |
| Onders et al. [19067067]10 | 2009 | Diaphragm | 440 | 2 | 0 | * | * | 730 (73– 2,812) |
| Dudding & Vaizey [19508525]35 |
2010 | Sacral nerve | 13 | 0 | 0 | 0 | 0 | 21.6 (16–29) |
| Chae et al. [22448759]36 | 2013 | Deltoid | 8 | 0 | 0 | 0 | 0 | 28 (28–28) |
| Rauck et al. [23947830]5 | 2014 | Sciatic nerve | 19 | 0 | 0 | 0 | 0 | 10 (1–14) |
| Wilson et al. [24355994]37 | 2014 | Shoulder | 14 | 0 | 0 | 0 | 0 | 26 (*–28) |
| Wilson et al. [24512114]6 | 2014 | Shoulder | 10 | 0 | 0 | 0 | 0 | 28 (28–28) |
Data not reported and unable to be determined.
Median indwelling days used when mean not reported.
Manuscript reported both coiled and noncoiled leads; however, mean indwelling time was not reported for each. Mean of all the leads in study was used.
Number of subjects substituted for number of leads when not reported.
Table 2.
Percutaneous Noncoiled Cylindrical Electrical Leads: Articles and Infection Rates
| Author | Publication Year |
Target Tissue | Total Leads |
Total Infections |
Severe Infections |
Infections During First 30 Days |
Infections During First 60 Days |
Mean Indwelling Days (range) |
|---|---|---|---|---|---|---|---|---|
| Thon et al. [*]38 | 1991 | Sacral nerve | 1,500 | 0 | 0 | 0 | 0 | * (5–7) |
| Janknegt [9123698] | 1997 | Sacral nerve | 10 | 0 | 0 | 0 | 0 | * (5–14) |
| Siegel et al. [11114569]39 | 2000 | Sacral nerve | 914 | 3 | 0 | 3 | 3 | * (3–7) |
| Carey et al. [11121986]24 | 2001 | Sacral nerve | 12 | 0 | 0 | 0 | 0 | 7 (7–7) |
| Popeney & Alo [12656708]40 |
2003 | Occipital nerve | 50 | 0 | 0 | 0 | 0 | * (5–7) |
| Spinelli et al. [12507546]41 |
2003 | Sacral nerves | 32 | 0 | 0 | 0 | 0 | * (21–28) |
| Matzel et al. [15094271]26 | 2004 | Sacral nerve | 29 | 9 | 0 | 9 | 9 | 19.4† (10–*) |
| Rasmussen et al. [15216409]27 |
2004 | Sacral nerve | 15‡ | 3 | 0 | * | 3 | * (21–35) |
| Slavin et al. [17341049]42 | 2006 | Trigeminal nerve, occipital nerve |
49 | 0 | 0 | 0 | 0 | 6 (4–7) |
| Slavin [16385335] | 2006 | Occipital nerve | 23 | 0 | 0 | 0 | 0 | * (5–7) |
| Guralnick et al. [17572189]17 |
2007 | Sacral nerve | 117 | 9 | 1 | 9 | 9 | 23.5 (14– 41) |
| Schwedt et al. [17257236]43 |
2007 | Occipital nerve | 23 | 0 | 0 | 0 | 0 | * (5–7) |
| Thimineur & De Ridder [18028042]44 |
2007 | C2 scalp area | 24 | 0 | 0 | 0 | 0 | * (7–21) |
| Bannowsky et al. [18629503]45 |
2008 | Sacral nerve | 105 | 0 | 0 | 0 | 0 | * (5–7) |
| Kessler et al. [18073008]46 | 2008 | Sacral nerve | 85 | 0 | 0 | 0 | 0 | 30§ (14–*) |
| Huntoon & Burgher [20021597]4 |
2009 | Upper extremity | 9 | 0 | 0 | 0 | 0 | * (3–7) |
| Huwyler et al. [19338551]47 |
2009 | Sacral nerve | 37 | 1 | 0 | 0 | 1 | 30§ (21–62) |
| Verrills et al. [22151226]48 |
2009 | Back | 13 | 0 | 0 | 0 | 0 | * (5–7) |
| Paemeleire & Bartsch [20511204]49 |
2010 | Occipital nerve | 48 | 1 | 0 | 0 | 1 | * (30–*) |
| Yakovlev [20672555] | 2010 | Lower Extremity | 24 | 0 | 0 | 0 | 0 | 2 (2–2) |
| Yakovlev et al. [21854498]50 |
2011 | Back | 72 | 0 | 0 | 0 | 0 | 2 (2–2) |
| Serra & Marchioretto [22622909]51 |
2012 | Occipital nerve | 68 | 1 | 1 | * | * | 45 (12–122) |
| Amend et al. [22738331]52 | 2013 | Sacral nerve | 34 | 0 | 0 | 0 | 0 | 52.3 (27– 116) |
| Elneil et al. [23601054]53 | 2013 | Sacral nerve | 24 | 1 | 0 | 1 | 1 | * (*–56) |
| Plazier et al. [24118206]54 | 2013 | Occipital nerve | 11 | 1 | 0 | * | * | 77 (77–77) |
Data not reported and unable to be determined.
Manuscript reported both coiled and noncoiled leads; however, mean indwelling time was not reported for each. Mean of all the leads in study was used.
Number of subjects substituted for number of leads when not reported.
Median indwelling days used when mean not reported.
The percentage of male subjects met our criteria to be deemed a potential confounder (Table 3). The percentage of males was significantly associated with infection rate (for each percentage increase in males, the risk of infection dropped by a factor of 0.95; 95% CI 0.93–0.98; chi-squared test P = 0.003) and coil type (for each percentage increase in males, the odds that the study was of coiled leads increased by a factor of 1.05; 95% CI 1.01–1.09; P = 0.002). There was a significant association between coil type and overall infection rate with and without controlling for the percentage of males. No other covariate was determined to be a potential confounder.
Table 3.
Unadjusted Descriptive Statistics by Coil Type
| Coiled (n = 21) |
Noncoiled (n = 25) |
Combined (n = 43) |
|
|---|---|---|---|
| Study design | |||
| Randomized, controlled | 3 (14%) | 1 (4%) | 4 (9%) |
| Nonrandomized, prospective | 1 (5%) | 1 (4%) | 1 (2%) |
| Case series | 15 (71%) | 19 (76%) | 32 (74%) |
| Retrospective chart review | 2 (10%) | 4 (16%) | 6 (14%) |
| Operator type | |||
| Anesthesiology | 1 (5%) | 6 (24%) | 7 (16%) |
| General surgery | 5 (24%) | 2 (8%) | 7 (16%) |
| Neurology | 0 (0%) | 4 (16%) | 4 (9%) |
| Neurosurgery | 0 (0%) | 2 (8%) | 2 (4%) |
| Orthopedics | 12 (57%) | 0 (0%) | 12 (27%) |
| Thoracic surgery | 1 (5%) | 0 (0%) | 1 (2%) |
| Urology | 2 (10%) | 11 (44%) | 13 (29%) |
| Lead diameter (mm) | 0.203 (0.051) | 1.213 (0.262) | 0.795 (0.545) |
| Lead diameter ≥ 0.65 mm | 0 (0%) | 21 (84%) | 21 (49%) |
| Total leads per study | 197.1 (321.6) | 128.0 (329.4) | 158.9 (324.3) |
| Total subjects per study | 24.4 (22.1) | 46.0 (112.5) | 36.2 (84.2) |
| Total infections (%) | 0.918 (1.981) | 3.022 (7.398) | 2.082 (5.573) |
| Infections first 30 days (%) | 0.088 (0.324) | 1.879 (6.601) | 1.144 (5.105) |
| Infections first 60 days (%) | 0.088 (0.324) | 2.834 (7.398) | 1.735 (5.846) |
| Male (%) | 49.5 (27.9) | 24.9 (17.1) | 35.4 (25.3) |
| Aseptic technique described | 10 (48%) | 9 (36%) | 19 (44%) |
| Mean infections per 1,000 indwelling days | 0.036 (0.076) | 1.971 (4.756) | 0.722 (2.903) |
| Minimum infections per 1,000 indwelling days | 0.034 (0.075) | 1.144 (3.352) | 0.638 (2.511) |
| Maximum infections per 1,000 indwelling days | 0.036 (0.076) | 1.320 (3.639) | 0.749 (2.764) |
Values are means (SD) for continuous variables.
Primary Endpoint
The risk of infection with noncoiled leads was estimated to be 25 times greater than with coiled leads (95% CI 2–407; chi-squared test P = 0.006) controlling for the percentage of males. The infection rates were estimated to be 0.03 (95% CI 0.01–0.13) infections per 1,000 indwelling days for coiled leads, and 0.83 (95% CI 0.16–4.33) infections per 1,000 indwelling days for noncoiled leads. The effect of the percentage of males was not significant in this model. Without adjusting for the percentage of males, the risk of infection with noncoiled leads was estimated to be 21 times greater than with coiled leads (95% CI 8–54; chi-squared test P < 0.001).
Secondary Endpoints
The risk of infection over the first 30 days was 4 and 5 times higher for noncoiled compared with coiled leads after 30 days (chi-squared test P = 0.20) and 60 days (chi-squared test P = 0.14), respectively. The estimated 30-day infection rates were 0.1% for coiled and 0.5% for noncoiled leads. Similarly, the estimated 60-day infection rates were 0.1% for coiled and 0.7% for noncoiled. No covariates met the criteria to be deemed a confounder in our (subgroup) analysis of the infection rate over the first 30 and 60 indwelling days. Coiled leads had an average indwelling time of 375 (range 1–2,812) days with no severe infections reported. Noncoiled leads had an average indwelling time of 11 (range 2–122) days and included 2 reports of severe infection, 1 requiring open surgical drainage and debridement.17
DISCUSSION
The principal finding of this retrospective study involving electrical leads used for percutaneous neurostimulation of the peripheral nervous system was that the infection rate of noncoiled cylindrical leads is far higher—25 times higher—than the rate for coiled leads. To put this difference in perspective, 1 infection occurred approximately every 1,200 indwelling days for noncoiled leads compared with 1 infection for every 30,000 indwelling days for coiled leads. With many therapeutic goals requiring multiple weeks of stimulation, these findings could have significant implications in the choice of a lead design for percutaneous neurostimulation.
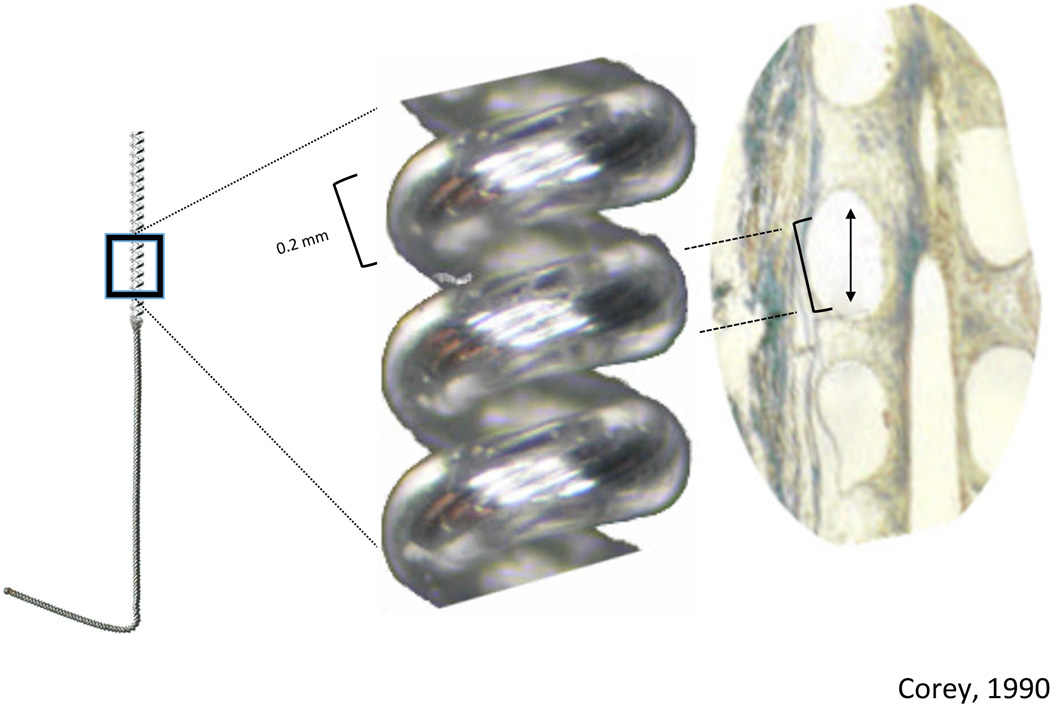
The reason(s) for the difference in infection rate between lead designs remain unknown, but there are theoretical explanations that deserve future study. The open-coil design might permit fibrosis at the insertion site, leading to both a superior bacteriostatic seal at the skin and a solid anchor preventing lead movement (see Figure 2).13 The coiled leads can also flex, bend, or “uncoil” inside muscle and soft tissue, which minimizes translation of the lead relative to the skin. Decreasing lead movement would theoretically decrease any “pistoning” effect that could draw pathogens subcutaneously.7,13 In addition, most of the coiled leads had a small diameter (0.2 mm) of the lead wire and even of the entire helix itself (<0.6 mm) relative to most of the cylindrical noncoiled leads (0.6–1.3 mm), and would therefore create a relatively smaller exit site. Insertion needles were presumably smaller for the smaller diameter leads as well—and did not require a surgical incision for placement—possibly further decreasing the infection risk. Unfortunately, this information was not available from all sources, and therefore any conclusions can only be inferred regarding this attribute that deserves further study.
Figure 2.
Example of a coiled fluoropolymer insulated lead and fibrosis at the insertion site, possibly leading to both a superior bacteriostatic seal at the skin and a solid anchor preventing lead movement. Exposed, finely coiled 0.2 mm diameter stainless steel wire makes up the active electrode, with the distal portion bent to facilitate anchoring in the tissue. (Reprinted with permission from Corey 1990.)
To help put the observed infection rates found in the current study in clinical perspective, it is useful to compare to other types of techniques with similar therapeutic goals. For example, percutaneous neurostimulation has been reported to treat both phantom limb pain5,18 and acute postoperative pain,19 as has ambulatory perineural local anesthetic infusion.20,21 The percutaneous perineural catheters used for continuous peripheral nerve blocks have an approximate diameter of 0.8–1.0 mm and a noncoiled cylindrical profile. Although they rarely remain in situ for longer than 6 days, the published infection risk is 1.5% (175 infections in 12,078 catheters),22 compared with 30-day infection rates of 0.1% and 0.5% for coiled and noncoiled percutaneous leads of the current study, respectively. Other temporary percutaneous therapies left indwelling more than 2 days such as spinal cord stimulators, continuous epidural blocks, and intrathecal treatments have also been reported to carry a higher risk of infection (mean of 1.0%–1.6%) during indwelling times of up to 30 days.23
LIMITATIONS
Infection rates could be overestimated in this analysis if prior studies did not explicitly mention the absence of infections and were thus excluded from our analysis. Similarly, infection rates could also be under-represented for the same reason, if infections occurred in prior investigations but were not reported. It is reassuring that a previous study from 2002 reviewing the infection rates of coiled leads reported a 0.1% infection rate during the first 60 indwelling days—similar to the present finding of 0.1% within 30 and 60 days.12 This previous uncontrolled investigation reported on 62 subjects implanted with 858 leads with solely the coil design, unlike the current investigation with 513 subjects and 4,140 leads from 21 trials with a comparison group of noncoiled leads.
In the current investigation, not all identified publications were included in every infection rate calculation because of unavailable data (eg, if the exact timing of infections were not reported). For example, 5 of 21 open-coil lead studies were not included in the calculation of the 30-day infection rate because the timing of infections was not adequately reported (see Table 1). The 5 excluded studies had an overall infection rate of 2.3%, compared to a rate of 1.0% in the other 16 included studies. However, the excluded studies had an average indwelling time of 448 days per lead, compared to 241 days per lead in the included studies. Calculation of the rate of infections per 1,000 indwelling days allowed many of the studies that did not report exact timing of infections to be included, and this again demonstrated that the rate of infection with open-coil leads (0.03 infections per 1,000 indwelling days) was an order of magnitude lower than the comparison group composed of noncoiled leads.
In addition, factors other than the design of the electrical leads could have influenced the calculated infection rates, but all variables could not be controlled in the current investigation due to lack of published information. For example, only 8 studies explicitly mentioned the utilization of prophylactic antibiotics; however, the use of prophylactic antibiotics in the other studies was unclear. Sterile technique may also play a role in infection rate; however, the available information was inadequate to draw any conclusions. Male gender was deemed a potential confounding factor, but further analysis found that the type of lead (coiled or noncoiled) and the risk of infection were still significantly associated even when controlling for the percentage of males. None of the other variables included in this analysis were determined to be confounding factors.
In conclusion, the infection rate during percutaneous neurostimulation of the peripheral nervous system differs by lead design: 0.03 vs. 0.83 per 1,000 indwelling days for coiled and noncoiled leads, respectively. With many therapeutic goals such as functional improvements and the relief of pain requiring multiple weeks of stimulation, these findings could have significant implications in the choice of a lead design and the potential for extending the use of percutaneous peripheral neurostimulation.
Figure 3.
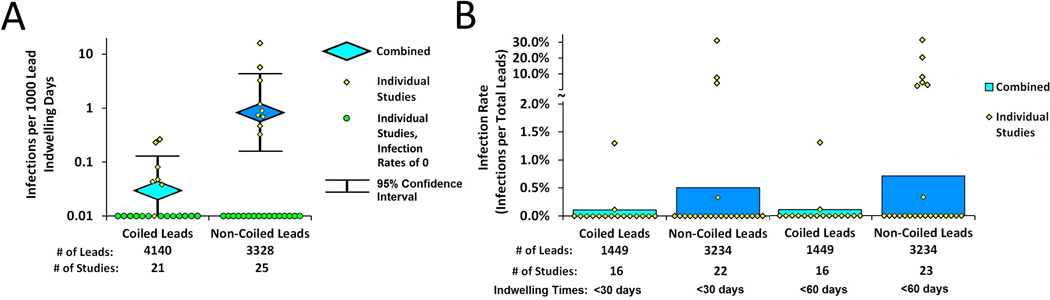
Infection rates for coiled and noncoiled leads used for percutaneous neurostimulation of the peripheral nervous system reported as (A) infections per 1,000 indwelling days (individual study infection rates represented with circles along the axis of the log scale identify infection rates of <0.01) and (B) infection rates during the first 30 and 60 indwelling days.
Acknowledgments
Financial Support
Several authors of this report are employees of SPR Therapeutics, LLC (Cleveland, Ohio); and the statistical analysis was paid for by SPR Therapeutics, LLC. This work was supported in part by the National Institutes of Health (R01HD075542, R44HD067094) and the Department of Defense (W81XWH-12-2-0132). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Defense.
Footnotes
Therapy of Interest
SPR Therapeutics, LLC, has developed a peripheral nerve stimulation therapy using a percutaneously inserted coiled/helical lead. The coiled/helical lead has been cleared in the United States for use in the treatment of chronic and acute pain, including postoperative and post-traumatic pain.
Disclosures
Brian Ilfeld: Dr. Ilfeld’s institution has received funding for his research from SPR Therapeutics (for studies other than the current investigation), Baxter Healthcare, Smiths Medical, Summit Medical, Teleflex Medical, Myoscience, and Pacira Pharmaceuticals. In addition, Dr. Ilfeld has previously been a consultant to Pacira Pharmaceuticals. Dr. Ilfeld received no compensation of any kind related to this manuscript.
Rodney Gabriel: Dr. Gabriel’s institution has received funding for his research from SPR Therapeutics (for studies other than the current investigation). Dr. Gabriel received no compensation of any kind related to this manuscript.
Michael F. Saulino: Dr. Saulino is a paid consultant of SPR Therapeutics, Medtronic, and Jazz Pharmaceuticals. Dr. Saulino’s institution has received funding for his research from Medtronic, Mallinckrodt, and Jazz Pharmaceuticals.
John Chae: Dr. Chae is a consultant and Chief Medical Advisor to SPR Therapeutics. He has received research grants from and owns equity in SPR Therapeutics.
P. Hunter Peckham: Dr. Peckham owns equity in SPR Therapeutics and sits on an SPR Therapeutics advisory board.
Stuart Grant: Dr. Grant’s institution has received funding for his research from SPR Therapeutics (for studies other than the current investigation), Cara Therapeutics, and Durect. Dr. Grant also acts as a consultant to BBraun Medical. Dr. Grant received no compensation of any kind related to this manuscript.
Christopher Gilmore: Dr. Gilmore’s institution has received funding for his research from SPR Therapeutics, and he has acted as a consultant for SPR Therapeutics.
Michael Donohue: Dr. Donohue acted as a paid consultant of SPR Therapeutics for his work on this project in the capacity of statistician.
Matthew deBock: Mr. deBock is an employee of SPR Therapeutics.
Amorn Wongsarnpigoon: Dr. Wongsarnpigoon is an employee of SPR Therapeutics.
Joseph W. Boggs: Dr. Boggs is an employee of SPR Therapeutics and owns equity in the company.
This work was presented, in part, as a scientific abstract for the Annual Meeting of the North American Neuromodulation Society in Las Vegas, Nevada, December 11–14, 2014; presented, in part, as a scientific abstract for the International Neuromodulation Society in Montreal, Canada, June 6–11, 2015; and presented, in part, as a scientific abstract for the Annual Pain Medicine Meeting of the American Society of Regional Anesthesia in Miami, Florida, November 19–21, 2015.
References
- 1.Huntoon MA, Huntoon EA, Obray JB, Lamer TJ. Feasibility of ultrasound-guided percutaneous placement of peripheral nerve stimulation electrodes in a cadaver model: part one, lower extremity. Reg Anesth Pain Med. 2008;33:551–557. doi: 10.1016/j.rapm.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Huntoon MA, Hoelzer BC, Burgher AH, Hurdle MF, Huntoon EA. Feasibility of ultrasound-guided percutaneous placement of peripheral nerve stimulation electrodes and anchoring during simulated movement: part two, upper extremity. Reg Anesth Pain Med. 2008;33:558–565. doi: 10.1016/j.rapm.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Yu DT, Chae J, Walker ME, Fang ZP. Percutaneous intramuscular neuromuscular electric stimulation for the treatment of shoulder subluxation and pain in patients with chronic hemiplegia: a pilot study. Arch Phys Med Rehabil. 2001;82:20–25. doi: 10.1053/apmr.2001.18666. [DOI] [PubMed] [Google Scholar]
- 4.Huntoon MA, Burgher AH. Ultrasound-guided permanent implantation of peripheral nerve stimulation (PNS) system for neuropathic pain of the extremities: original cases and outcomes. Pain Med. 2009;10:1369–1377. doi: 10.1111/j.1526-4637.2009.00745.x. [DOI] [PubMed] [Google Scholar]
- 5.Rauck RL, Cohen SP, Gilmore CA, et al. Treatment of post-amputation pain with peripheral nerve stimulation. Neuromodulation. 2014;17:188–197. doi: 10.1111/ner.12102. [DOI] [PubMed] [Google Scholar]
- 6.Wilson RD, Harris MA, Gunzler DD, Bennett ME, Chae J. Percutaneous peripheral nerve stimulation for chronic pain in subacromial impingement syndrome: a case series. Neuromodulation. 2014;17:771–776. doi: 10.1111/ner.12152. discussion 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marsolais EB, Kobetic R. Implantation techniques and experience with percutaneous intramuscular electrodes in the lower extremities. J Rehabil Res Dev. 1986;23:1–8. [PubMed] [Google Scholar]
- 8.Scheiner A, Polando G, Marsolais EB. Design and clinical application of a double helix electrode for functional electrical stimulation. IEEE Trans Biomed Eng. 1994;41:425–431. doi: 10.1109/10.293216. [DOI] [PubMed] [Google Scholar]
- 9.Shimada Y, Sato K, Kagaya H, Konishi N, Miyamoto S, Matsunaga T. Clinical use of percutaneous intramuscular electrodes for functional electrical stimulation. Arch Phys Med Rehabil. 1996;77:1014–1018. doi: 10.1016/s0003-9993(96)90061-1. [DOI] [PubMed] [Google Scholar]
- 10.Onders RP, Elmo M, Khansarinia S, et al. Complete worldwide operative experience in laparoscopic diaphragm pacing: results and differences in spinal cord injured patients and amyotrophic lateral sclerosis patients. Surg Endosc. 2009;23:1433–1440. doi: 10.1007/s00464-008-0223-3. [DOI] [PubMed] [Google Scholar]
- 11.Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81:1159–1171. doi: 10.4065/81.9.1159. [DOI] [PubMed] [Google Scholar]
- 12.Knutson JS, Naples GG, Peckham PH, Keith MW. Electrode fracture rates and occurrences of infection and granuloma associated with percutaneous intramuscular electrodes in upper-limb functional electrical stimulation applications. J Rehabil Res Dev. 2002;39:671–683. [PubMed] [Google Scholar]
- 13.Mortimer JT, Bhadra N. Peripheral nerve and muscle stimulation. In: Horch KW, Dhillon GS, editors. Neuroprosthetics: Theory and Practice. Singapore: World Scientific; 2004. [Google Scholar]
- 14.May MS, Banks C, Thomson SJ. A retrospective, long-term, third-party follow-up of patients considered for spinal cord stimulation. Neuromodulation. 2002;5:137–144. doi: 10.1046/j.1525-1403.2002.02023.x. [DOI] [PubMed] [Google Scholar]
- 15.Gerberding JL, McGowan JE, Jr, Tenover FC. Emerging nosocomial infections and antimicrobial resistance. Curr Clin Top Infect Dis. 1999;19:83–98. [PubMed] [Google Scholar]
- 16.McCullagh P, Nelder JA. Generalized Linear Models. 2nd. London: Chapman and Hall; 1989. [Google Scholar]
- 17.Guralnick ML, Benouni S, O’Connor RC, Edmiston C. Characteristics of infections in patients undergoing staged implantation for sacral nerve stimulation. Urology. 2007;69:1073–1076. doi: 10.1016/j.urology.2007.01.099. [DOI] [PubMed] [Google Scholar]
- 18.Rauck RL, Kapural L, Cohen SP, et al. Peripheral nerve stimulation for the treatment of postamputation pain—a case report. Pain Pract. 2012;12:649–655. doi: 10.1111/j.1533-2500.2012.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ilfeld BM, Wongsarnpigoon A. Percutaneous peripheral nerve stimulation for the treatment of postoperative pain following total knee arthroplasty: a case series feasibility study. North American Neuromodulation Society. doi: 10.1111/ner.12790. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilfeld BM, Moeller-Bertram T, Hanling SR, et al. Treating intractable phantom limb pain with ambulatory continuous peripheral nerve blocks: a pilot study. Pain Med. 2013;14:935–942. doi: 10.1111/pme.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ilfeld BM. Continuous peripheral nerve blocks: a review of the published evidence. Anesth Analg. 2011;113:904–925. doi: 10.1213/ANE.0b013e3182285e01. [DOI] [PubMed] [Google Scholar]
- 22.Capdevila X, Bringuier S, Borgeat A. Infectious risk of continuous peripheral nerve blocks. Anesthesiology. 2009;110:182–188. doi: 10.1097/ALN.0b013e318190bd5b. [DOI] [PubMed] [Google Scholar]
- 23.Ilfeld BM, Kapural L, Gilmore CA, et al. Percutaneous peripheral nerve stimulation with open-coil leads demonstrates low infection risk compared to conventional leads and catheters, and applicability in minimally-invasive, non-opioid management of pain, abstracted. Reg Anesth Pain Med. 2015;XX:A1089. [Google Scholar]
- 24.Carey M, Fynes M, Murray C, Maher C. Sacral nerve root stimulation for lower urinary tract dysfunction: overcoming the problem of lead migration. BJU Int. 2001;87:15–18. doi: 10.1046/j.1464-410x.2001.00024.x. [DOI] [PubMed] [Google Scholar]
- 25.Daly JJ, Kollar K, Debogorski AA, et al. Performance of an intramuscular electrode during functional neuromuscular stimulation for gait training post stroke. J Rehabil Res Dev. 2001;38:513–526. [PubMed] [Google Scholar]
- 26.Matzel KE, Kamm MA, Stosser M, et al. Sacral spinal nerve stimulation for faecal incontinence: multicentre study. Lancet. 2004;363:1270–1276. doi: 10.1016/S0140-6736(04)15999-0. [DOI] [PubMed] [Google Scholar]
- 27.Rasmussen OO, Buntzen S, Sorensen M, Laurberg S, Christiansen J. Sacral nerve stimulation in fecal incontinence. Dis Colon Rectum. 2004;47:1158–1162. doi: 10.1007/s10350-004-0553-8. discussion 1162–1163. [DOI] [PubMed] [Google Scholar]
- 28.Renzenbrink GJ, IJzerman MJ. Percutaneous neuromuscular electrical stimulation (P-NMES) for treating shoulder pain in chronic hemiplegia Effects on shoulder pain and quality of life. Clin Rehabil. 2004;18:359–365. doi: 10.1191/0269215504cr759oa. [DOI] [PubMed] [Google Scholar]
- 29.Yu DT, Chae J, Walker ME, et al. Intramuscular neuromuscular electric stimulation for poststroke shoulder pain: a multicenter randomized clinical trial. Arch Phys Med Rehabil. 2004;85:695–704. doi: 10.1016/j.apmr.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Shimada Y, Matsunaga T, Misawa A, Ando S, Itoi E, Konishi N. Clinical application of peroneal nerve stimulator system using percutaneous intramuscular electrodes for correction of foot drop in hemiplegic patients. Neuromodulation. 2006;9:320–327. doi: 10.1111/j.1525-1403.2006.00074.x. [DOI] [PubMed] [Google Scholar]
- 31.Dudding TC, Meng Lee E, Faiz O, et al. Economic evaluation of sacral nerve stimulation for faecal incontinence. Br J Surg. 2008;95:1155–1163. doi: 10.1002/bjs.6237. [DOI] [PubMed] [Google Scholar]
- 32.Goldman HB, Amundsen CL, Mangel J, et al. Dorsal genital nerve stimulation for the treatment of overactive bladder symptoms. Neurourol Urodyn. 2008;27:499–503. doi: 10.1002/nau.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gstaltner K, Rosen H, Hufgard J, Mark R, Schrei K. Sacral nerve stimulation as an option for the treatment of faecal incontinence in patients suffering from cauda equina syndrome. Spinal Cord. 2008;46:644–647. doi: 10.1038/sc.2008.6. [DOI] [PubMed] [Google Scholar]
- 34.Chae J, Harley MY, Hisel TZ, et al. Intramuscular electrical stimulation for upper limb recovery in chronic hemiparesis: an exploratory randomized clinical trial. Neurorehabil Neural Repair. 2009;23:569–578. doi: 10.1177/1545968308328729. [DOI] [PubMed] [Google Scholar]
- 35.Dudding T, Vaizey C. Bacterial colonization of stimulation electrode wires in patients undergoing temporary sacral nerve stimulation. Colorectal Dis. 2010;12:141–143. doi: 10.1111/j.1463-1318.2009.01896.x. [DOI] [PubMed] [Google Scholar]
- 36.Chae J, Wilson RD, Bennett ME, Lechman TE, Stager KW. Single-lead percutaneous peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: a case series. Pain Pract. 2013;13:59–67. doi: 10.1111/j.1533-2500.2012.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson RD, Gunzler DD, Bennett ME, Chae J. Peripheral nerve stimulation compared with usual care for pain relief of hemiplegic shoulder pain: a randomized controlled trial. Am J Phys Med Rehabil. 2014;93:17–28. doi: 10.1097/PHM.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thon WF, Baskin LS, Jonas U, Tanagho EA, Schmidt RA. Neuromodulation of voiding dysfunction and pelvic pain. World J Urol. 1991;9:138–141. [Google Scholar]
- 39.Siegel SW, Catanzaro F, Dijkema HE, et al. Long-term results of a multicenter study on sacral nerve stimulation for treatment of urinary urge incontinence, urgency-frequency, and retention. Urology. 2000;56:87–91. doi: 10.1016/s0090-4295(00)00597-5. [DOI] [PubMed] [Google Scholar]
- 40.Popeney CA, Alo KM. Peripheral neurostimulation for the treatment of chronic, disabling transformed migraine. Headache. 2003;43:369–375. doi: 10.1046/j.1526-4610.2003.03072.x. [DOI] [PubMed] [Google Scholar]
- 41.Spinelli M, Giardiello G, Arduini A, van den Hombergh U. New percutaneous technique of sacral nerve stimulation has high initial success rate: preliminary results. Eur Urol. 2003;43:70–74. doi: 10.1016/s0302-2838(02)00442-6. [DOI] [PubMed] [Google Scholar]
- 42.Slavin KV, Colpan ME, Munawar N, Wess C, Nersesyan H. Trigeminal and occipital peripheral nerve stimulation for craniofacial pain: a single-institution experience and review of the literature. Neurosurg Focus. 2006;21:E5. doi: 10.3171/foc.2006.21.6.8. [DOI] [PubMed] [Google Scholar]
- 43.Schwedt TJ, Dodick DW, Hentz J, Trentman TL, Zimmerman RS. Occipital nerve stimulation for chronic headache—long-term safety and efficacy. Cephalalgia. 2007;27:153–157. doi: 10.1111/j.1468-2982.2007.01272.x. [DOI] [PubMed] [Google Scholar]
- 44.Thimineur M, De Ridder D. Chi-squared area neurostimulation: a surgical treatment for fibromyalgia. Pain Med. 2007;8:639–646. doi: 10.1111/j.1526-4637.2007.00365.x. [DOI] [PubMed] [Google Scholar]
- 45.Bannowsky A, Wefer B, Braun PM, Junemann KP. Urodynamic changes and response rates in patients treated with permanent electrodes compared to conventional wire electrodes in the peripheral nerve evaluation test. World J Urol. 2008;26:623–626. doi: 10.1007/s00345-008-0307-7. [DOI] [PubMed] [Google Scholar]
- 46.Kessler TM, Burkhard FC, Madersbacher H, Kofler A, Poewe W, Kiss G. Safety of prolonged sacral neuromodulation tined lead testing. Curr Med Res Opin. 2008;24:343–347. doi: 10.1185/030079908x253555. [DOI] [PubMed] [Google Scholar]
- 47.Huwyler M, Kiss G, Burkhard FC, Madersbacher H, Kessler TM. Microbiological tined-lead examination: does prolonged sacral neuromodulation testing induce infection? BJU Int. 2009;104:646–650. doi: 10.1111/j.1464-410X.2009.08501.x. discussion 650. [DOI] [PubMed] [Google Scholar]
- 48.Verrills P, Mitchell B, Vivian D, Sinclair C. Peripheral nerve stimulation: a treatment for chronic low back pain and failed back surgery syndrome? Neuromodulation. 2009;12:68–75. doi: 10.1111/j.1525-1403.2009.00191.x. [DOI] [PubMed] [Google Scholar]
- 49.Paemeleire K, Bartsch T. Occipital nerve stimulation for headache disorders. Neurotherapeutics. 2010;7:213–219. doi: 10.1016/j.nurt.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yakovlev AE, Resch BE, Yakovleva VE. Peripheral nerve field stimulation in the treatment of postlaminectomy syndrome after multilevel spinal surgeries. Neuromodulation. 2011;14:534–538. doi: 10.1111/j.1525-1403.2011.00387.x. discussion 538. [DOI] [PubMed] [Google Scholar]
- 51.Serra G, Marchioretto F. Occipital nerve stimulation for chronic migraine: a randomized trial. Pain Physician. 2012;15:245–253. [PubMed] [Google Scholar]
- 52.Amend B, Bedke J, Khalil M, Stenzl A, Sievert KD. Prolonged percutaneous SNM testing does not cause infection-related explanation. BJU Int. 2013;111:485–491. doi: 10.1111/j.1464-410X.2012.11263.x. [DOI] [PubMed] [Google Scholar]
- 53.Elneil S, Abtahi B, Helal M, Digesu A, Gonzales G. Optimizing the duration of assessment of stage-1 sacral neuromodulation in nonobstructive chronic urinary retention. Neuromodulation. 2014;17:66–70. doi: 10.1111/ner.12017. discussion 70–71. [DOI] [PubMed] [Google Scholar]
- 54.Plazier M, Dekelver I, Vanneste S, et al. Occipital nerve stimulation in fibromyalgia: a double-blind placebo-controlled pilot study with a six-month follow-up. Neuromodulation. 2014;17:256–263. doi: 10.1111/ner.12121. discussion 263–264. [DOI] [PubMed] [Google Scholar]