Abstract
The impact of allograft injury time-of-onset on the risk of chronic lung allograft dysfunction (CLAD) remains unknown. We hypothesized that episodes of late-onset (≥6 months) allograft injury would produce an augmented CXCR3/ligand immune response, leading to increased CLAD. In a retrospective single-center study, 1894 transbronchial biopsies from 441 lung transplant recipients were reviewed for the presence of acute rejection (AR), lymphocytic bronchiolitis (LB), diffuse alveolar damage (DAD) and organizing pneumonia (OP). The association between the time-of-onset of each injury pattern and CLAD was assessed using multivariable Cox models with time-dependent covariates. BAL CXCR3 ligand concentrations were compared between early and late-onset injury patterns using linear mixed-effects models. Late-onset DAD and OP were strongly associated with CLAD: adjusted HRs 2.8 95%CI 1.5-5.3 and 2.0 95%CI 1.1-3.4, respectively. The early-onset form of these injury patterns did not increase CLAD risk. Late-onset LB and AR predicted CLAD in univariable models, but lost significance after multivariable adjustment for late DAD and OP. AR was the only early-onset injury pattern associated with CLAD development. Elevated BAL CXCR3 ligand concentrations during late-onset allograft injury parallel the increase in CLAD risk and support our hypothesis that late allograft injuries result in a more profound CXCR3/ligand immune response.
Introduction
Despite the severe impact of chronic lung allograft dysfunction (CLAD) on lung transplant survival (1), the pathogenesis is poorly understood. Since there are no effective treatments available for CLAD, the identification of risk factors is a key step towards both understanding CLAD pathogenesis and improving post-transplant outcomes. There are numerous “non-alloimmune” (e.g., respiratory infections (2-9), gastroesophageal reflux (10, 11), air pollution (12, 13), autoimmune reactivity (14, 15)) and “alloimmune” insults (e.g. acute cellular rejection (16-18)) which challenge the lung allograft. However, the histopathologic allograft response patterns are generally limited to four: diffuse alveolar damage (DAD), organizing pneumonia (OP), lymphocytic bronchiolitis (LB), and a vascular mononuclear cell infiltration consistent with acute cellular rejection (AR).
Prior studies have established AR (16-27), LB (21, 25, 28-31) and DAD (32, 33) as major risk factors for CLAD development. Several of these studies have suggested a possible propensity for higher CLAD risk after late-onset injury, but the results have been inconsistent.(18, 25, 27, 28, 32, 34) Importantly, these earlier studies evaluated only one or two injury patterns (e.g., AR and LB) at a time and were limited by lack of multivariable adjustment. Furthermore, the association between OP and CLAD development has not been well studied to date. In this study, we evaluated the effect of time-of-onset for all four allograft injury patterns concurrently using appropriate Cox models for CLAD with time-dependent covariates.
We previously showed in animal models and human studies that the association between allograft injury and CLAD may be mediated in part by aberrant CXCR3/ligand biology. (33, 35, 36) CXCL9 (MIG), CXCL10 (IP10), and CXCL11 (ITAC) are interfreron-γ inducible ELR-CXC chemokines (CXCR3 ligands) that signal through a shared receptor, CXCR3.(37, 38) During the first few months post-transplant, T-cells differentiate from the naïve to memory subclass depending on a number of factors (e.g., site of stimulation, antigen concentration, costimulation and cytokine milieu). (39-41) Importantly, CXCR3 ligand expression is augmented during the memory immune response and act as a potent chemoattractant for lymphocytes, particularly memory T-cells (CD4, CD8).(42-44)
Thus, we hypothesized that episodes of allograft injury occurring at later times post-transplant would produce a more profound and sustained Type I immune response, leading to increased fibroproliferation and CLAD. Given the role of CXCR3/ligand biology in perpetuating a Type I immune response (35, 36, 45), we further hypothesized that episodes of late allograft injury would be associated with increased bronchoalveolar lavage (BAL) CXCR3 ligand concentrations.
Methods
The study cohort consisted of all LTRs who received a first transplant at UCLA between January 1, 2000 and December 31, 2010. LTRs received bronchoscopy with bronchoalveolar lavage (BAL) and transbronchial biopsy (TBBX) at 1, 3, 6 and 12-months post-transplant, as well as during episodes of clinical deterioration. One of three pulmonary pathologists interpreted the biopsies according to International Society for Heart and Lung Transplantation criteria (AR and LB) (46, 47) and International Multidisciplinary Consensus Statement on Idiopathic Interstitial Pneumonias (DAD and OP) (48). LB was graded as present or absent until March 1, 2009, and thereafter graded according to the revised 2007 ISHLT criteria (0, B1R, B2R or ungradable). (46) Biopsy data were coded for the presence or absence of DAD, OP, LB and AR (grade A1 or greater). TBBXs with two concurrent histopathologic findings were coded for the presence of both injury patterns.
Immunosuppression, anti-microbial prophylaxis and treatment of acute rejection were administered in accordance with the UCLA lung transplant protocols as described previously. (7) Serial spirometry was performed at least quarterly. CLAD was defined as a sustained drop of at least 20% in the forced expiratory volume in 1 second (FEV1) from the average of the two best post-transplant FEV1 measurements. (20, 49) In a subset analysis of double lung transplant recipients, CLAD was further classified as restrictive allograft syndrome (RAS) or bronchiolitis obliterans syndrome (BOS) based on Sato and colleagues's 2013 definition utilizing spirometry (50) and chest computed tomography (CT). RAS was defined as: ΔFVC%/ΔFEV1% > 0.5 and chest CT showing ground glass opacification, interstitial reticulation or interlobular septal thickening. Recipients with CLAD who did not fulfill RAS criteria were considered to have the BOS phenotype. Those who did not have a chest CT within 3 months of CLAD diagnosis were excluded from this subset analysis.
To explore the effect of allograft injury time-of-onset on CLAD risk, univariable Cox models for CLAD were constructed with cumulative time-dependent counts for the injury patterns. For example, a recipient was initially coded 0 for acute rejection, 1 at the first episode of acute rejection, 2 at the second episode etc. The dataset contained one observation for each recipient with multiple columns for the time and value of TBBX results. The TBBX results were entered into the Cox model as cumulative time-dependent repeated measures. Thus, both the timing and recurrence of the allograft injuries were taken into account. We began by constructing univariable Cox models with cumulative time-dependent counts for the injury patterns using various time from transplant cutoffs: ≥1-month, ≥3-month, ≥6-month, ≥9-month and ≥12-months. In this exploratory analysis, an injury pattern only increased the cumulative count when it occurred after the specified cutoff time.
In keeping with prior studies, for all subsequent analysis an injury pattern was considered “late-onset” if the time from transplant was ≥ 6 months, otherwise it was considered “early-onset”. (18, 25, 28). Univariable Cox models for CLAD were constructed with time-dependent cumulative counts for the “early” and “late-onset” forms of each allograft injury patterns. For example, a recipient was initially coded 0 for early-AR and late-AR. At the first episode of “early-onset” AR, the early-AR variable increased from 0 to 1, the late-AR variable remained at 0. At the first episode of “late-onset” AR, the late-AR variable increased from 0 to 1, the early-AR variable remained at its prior value. The final multivariable model included all significant (p<0.05) variables from the univariable models.
A subset of patients consented to the collection of BAL fluid for research purposes at the time of their bronchoscopies. Three 60 ml aliquots of isotonic saline were instilled into the sub-segmental bronchus in the lingula, right middle lobe or area of interest and pooled. After centrifugation, the supernatant was collected and stored unconcentrated at -80° C. CXCR3 ligand concentrations were measured using CXCL9, CXCL10 and CXCL11 bead assays (Millipore, Billerica MA). The lower limit of detection for CXCL9, CXCL10 and CXCL11 were 19.2, 14.0 and 1.7 picogram/milliliter (pg/ml), respectively. Given the high correlation among the three CXCR3 chemokines, a principal component analysis was performed to assess them in aggregate. The first principal component (PC) of the three CXCR3 ligands was calculated as: PC = 0.357 logCXCL9 + 0.439 logCXCL10 + 0.386 logCXCL11. The PC accounted for 57% of the total chemokine variation. CXCR3 ligand concentrations were compared between early and late-onset injury patterns using linear mixed effects models to account for repeated measurements from the same individuals. Analyses were performed with SAS (v9.4).
Results
Histopathologic Findings
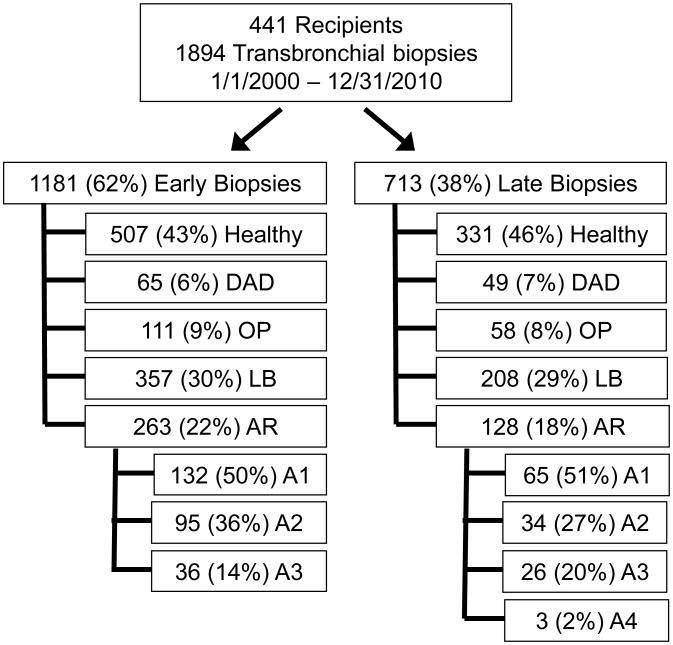
In total, 1894 bronchoscopies with TBBXs were evaluated from 441 lung transplant recipients. There were 114 (6%) biopsies with DAD, 169 (9%) biopsies with OP, 565 (30%) biopsies with LB and 391 (21%) biopsies with AR. The AR biopsies were graded as follows: 197 (50%) grade A1, 129 (33%) grade A2, 62 (16%) grade A3, and 3 (1%) grade A4. There were 303 TBBXs with concurrent injury patterns. AR and LB co-occurred most frequently (n=193). OP occurred with LB (n=79), AR (n=45) and DAD (n=30). DAD occurred with LB (n=49) and AR (n=25). Eight hundred and thirty-eight (44%) TBBXs from 441 (100%) recipients had no evidence of histopathology and were classified as “healthy biopsies”. Using a predetermined 6-month cutoff for late-onset, the number of early vs. late episodes of the “healthy”, DAD, OP, LB and AR biopsies were: 507 vs 331, 65 vs. 49, 111 vs 58, 357 vs 208 and 263 vs. 128, respectively (Figure 1). Table 1 shows demographic and clinical characteristics of recipients who had histopathologic allograft injury, stratified by whether they developed early vs. late-onset injury. Most of the demographic and clinical variables were evenly distributed between recipients who developed early vs late-onset injury. However, a higher proportion of biopsies in the early period was for surveillance purposes compared with the late period: 821 / 1181 (70%) vs. 246 / 713 (35%), respectively.
Figure 1.
Study profile. DAD = diffuse alveolar damage; OP = organizing pneumonia; LB = lymphocytic bronchiolitis; AR = Acute rejection (Grade A2 or higher); Early < 6 months, Late ≥ 6 months. B
Table 1. Baseline Patient Characteristics By Allograft Injury Status (Early vs. Late Onset).
| Early | Late | |||
|---|---|---|---|---|
|
|
|
|||
| n (%) | % | n (%) | % | |
|
|
|
|||
| Number of patients: | 316 | 72% | 169 | 38% |
| Median age: | 60 | 59 | ||
| Male gender: | 186 | 59% | 99 | 59% |
| Single lung transplant: | 134 | 42% | 69 | 41% |
| Diagnosis: | ||||
| Restrictive ILD | 186 | 59% | 93 | 55% |
| COPD / AAT | 84 | 26% | 52 | 31% |
| CF / bronchiectasis | 21 | 7% | 9 | 5% |
| Other | 25 | 8% | 15 | 9% |
| Induction: | ||||
| ATG | 165 | 52% | 104 | 61% |
| Basiliximab | 150 | 47% | 64 | 38% |
| None | 1 | 1% | 1 | 1% |
| Number of patients with: | ||||
| Any Injury | 316 | 72% | 169 | 38% |
| DAD | 61 | 14% | 40 | 9% |
| OP | 84 | 19% | 47 | 11% |
| LB | 239 | 54% | 126 | 29% |
| AR | 183 | 41% | 51 | 12% |
| Number of TBBX:† | 2.8 | (1.1) | 2.4 | (1.6) |
| Number of Surveillance TBBX:†† | 2.1 | (0.9) | 1.3 | (0.5) |
Definition of abbreviations: Early onset < 6 months from transplant; Late onset ≥ 6 months from transplant; ILD = interstitial lung disease; COPD = chronic obstructive pulmonary disease; AAT alpha=1 antitrypsin; CF = cystic fibrosis; ATG thymoglobulin; ALI = acute lung injury; OP = organizing pneumonia; LB = lymphocytic bronchiolitis; AR = acute cellular rejection. TBBX: transbronchial biopsies.
Average biopsies per patient (with standard deviation).
Average surveillance biopsies per patient (with standard deviation).
Risk of CLAD after Allograft Injury
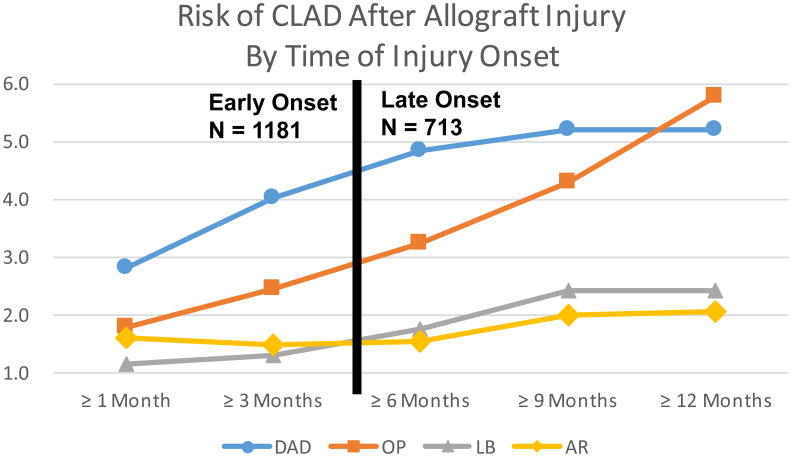
To explore the impact of the time-of-onset of specific allograft injury patterns on CLAD risk, univariable Cox models were constructed with cumulative time-dependent counts for the injury patterns using various time from transplant cutoffs (Figure 2). Overall, episodes of allograft injury occurring at later times post-transplant were associated with increased CLAD risk. This trend was most notable for DAD and OP. The HR for an episode of DAD occurring after 1, 3, 6, 9 and 12 months was: 2.8, 4.0, 4.9, 5.2 and 5.2, respectively. Similarly, the HR for an episode of OP occurring after 1, 3, 6, 9 and 12 months was: 1.8, 2.5, 3.3, 4.3 and 5.8, respectively. The HRs for LB increased from 1.1, 1.3, 1.8, 2.4 and 2.4 during these time intervals. Compared with the other injury patterns, the HRs for AR remained relatively constant over time: 1.6, 1.5, 1.5, 2.0 and 2.1, respectively.
Figure 2.
Risk of CLAD after Allograft Injury by Time of Injury Onset. Early < 6 months, Late ≥ 6 months. DAD = diffuse alveolar damage; OP = organizing pneumonia; LB = lymphocytic bronchiolitis; AR = acute rejection.
We further evaluated this relationship using Cox models with time-dependent counts for the each of the early and late-onset injury patterns as well as plausible demographic variables associated with poor outcomes (Table 2). In the univariable models for time to CLAD, the late-onset allograft injuries DAD, OP and LB were strongly predictive of CLAD development, while early-onset forms of these injuries were not. The unadjusted HRs for CLAD for late vs early-onset DAD, OP and LB were: 4.9 (p<0.001) vs 1.2 (p=0.25), 3.3 (p<0.001) vs 1.2 (p=0.40) and 1.8 (p<0.001) vs 1.2 (p=0.18), respectively (Table 2). Late and early-onset AR were both associated with CLAD in univariable models with HRs: 1.5 (p=0.014) vs 1.4 (p=0.026), respectively. Other demographic variables including: age > 70, gender, single lung transplant, pre-transplant diagnosis of idiopathic pulmonary fibrosis, and induction immunosuppression was not associated with CLAD development (p<0.05). The injury patterns significant in the univariable models were entered into the multivariable model: late-DAD (HR 2.8 95% CI 1.5-5.3), late-OP (HR 2.0 95% CI 1.1-3.4), and early-AR (HR 1.4 95% CI 1.0-1.8) remained significant predictors of CLAD, whereas late-LB and late-AR both lost significance.
Table 2. Cox Model for CLAD with Time-Dependent Covariates.
| Univariable | Multivariable† | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
|
|
|
|
|
|
|
|
| DAD < 6 months | 1.2 | 0.9 - 1.8 | 0.2545 | |||
| DAD ≥ 6 months | 4.9 | 2.8 - 8.4 | 0.0001 | 2.8 | 1.5 - 5.3 | 0.0020 |
| OP < 6 months | 1.2 | 0.8 - 1.7 | 0.4037 | |||
| OP ≥ 6 months | 3.3 | 2.1 - 5.1 | 0.0001 | 2.0 | 1.1 - 3.4 | 0.0177 |
| LB < 6 months | 1.2 | 0.9 - 1.6 | 0.1814 | |||
| LB ≥ 6 months | 1.8 | 1.3 - 2.4 | 0.0004 | 1.3 | 0.9 - 1.9 | 0.1839 |
| AR < 6 months | 1.4 | 1.0 - 1.8 | 0.0260 | 1.4 | 1.0 - 1.8 | 0.0319 |
| AR ≥ 6 months | 1.5 | 1.1 - 2.2 | 0.0137 | 1.2 | 0.8 - 1.8 | 0.3754 |
| Age > 70 | 1.3 | 0.8 - 2.1 | 0.2965 | |||
| Single lung | 1.3 | 1.0 - 1.7 | 0.0500 | |||
| Diagnosis: IPF | 0.9 | 0.7 - 1.2 | 0.5075 | |||
| Induction: ATG | 0.9 | 0.7 - 1.2 | 0.3705 | |||
Definition of abbreviations: CLAD = chronic lung allograft dysfunction; HR = hazards ratio; CI = confidience interval; ALI = acute lung injury; OP = organizing pneumonia; LB = lymphocytic bronchiolitis; AR = acute rejection; Single lung = single lung transplant; IPF = idiopathic pulmonary fibrosis; ATG - thymoglobulin;
Multivariable model includes only the variables listed.
Kaplan-Meier curves for freedom from CLAD were constructed and stratified by recipients with never vs. late vs. early-onset allograft injury. Recipients who never had an episode of allograft injury had a median time to CLAD of 6.0 years. (Figure 3) Those with an episode of late-onset DAD had a median time of 2.1 years, while those who had an episode of early-DAD had a median time of 3.6 years. Similarly, the median time to CLAD for those with late and early-onset OP was 1.9 and 3.3 years, respectively. The median time to CLAD for recipients with late and early-onset LB was 2.7 and 4.4 years, while the median time for those with late and early-onset AR was 2.8 and 3.7 years, respectively.
Figure 3.
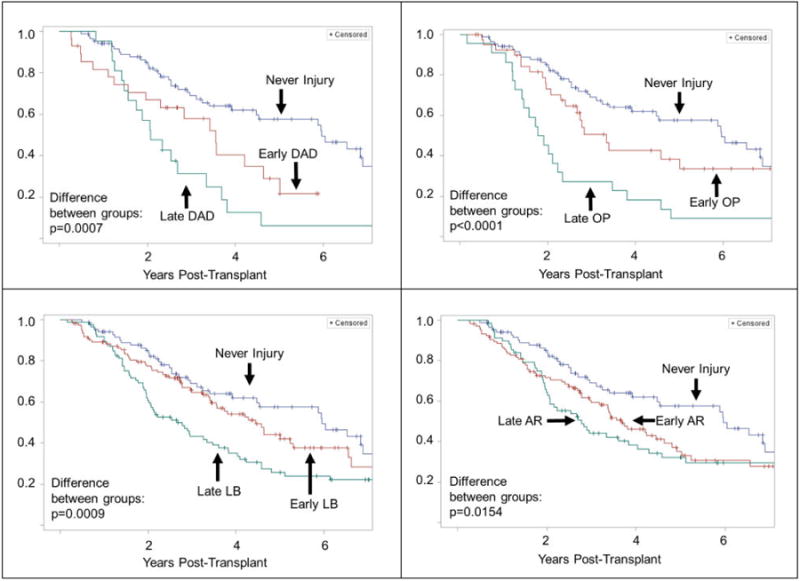
Kaplan-Meier plot for time to CLAD in lung transplant recipients with never vs early vs late-onset episodes of allograft injury. Early: < 6 months from transplant, late: ≥ 6 months post-transplant.
Risk of BOS and RAS after Allograft Injury
In a subset analysis among double lung transplant recipients with chest CTs within 3 months of CLAD diagnosis, we explored the association between allograft injury and the CLAD phenotypes: BOS and RAS. Of the 202 double LTRs with sufficient spirometric and radiographic data, 106 (53%) developed CLAD. 51 (48%) of these recipients met RAS criteria, while the remaining 55 (42%) were categorized BOS. The univariable models for time to RAS showed a similar pattern as time to CLAD but with higher HRs for all injury patterns. The late-onset injury patterns DAD, OP and LB were strongly predictive of RAS development, while the early-onset forms of these injuries were not. The unadjusted HRs for RAS for late vs early-onset DAD, OP and LB were: 16.0 (p<0.001) vs 1.8 (p=0.087), 6.6 (p<0.001) vs 1.3 (p=0.41) and 2.0 (p=0.034) vs 1.5 (p=0.21), respectively (Table 3). Late and early-onset AR were both associated with RAS in univariable models with HRs: 2.0 (p=0.026) vs. 1.8 (p=0.040), respectively. In the multivariable model: late-DAD (HR 6.2 95% CI 2.0-18.8), late-OP (HR 3.2 95% CI 1.1-8.8), and early-AR (HR 1.8 95% CI 1.0-3.2) remained significant predictors of RAS, whereas late-LB and late-AR both lost significance. The univariable models for time to BOS showed a different pattern from the time to CLAD or RAS models: Late-LB was the only injury pattern associated with time to BOS with HR 2.1 (95% CI 1.1-3.9).
Table 3. Cox Model for RAS with Time-Dependent Covariates.
| Univariable | Multivariable † | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
|
|
|
|
|
|
|
|
| DAD < 6 months | 1.8 | 0.92 - 3.7 | 0.0867 | |||
| DAD ≥ 6 months | 16.0 | 6.8 - 37.5 | 0.0001 | 6.2 | 2.0 - 18.8 | 0.0014 |
| OP < 6 months | 1.3 | 0.7 - 2.6 | 0.4088 | |||
| OP ≥ 6 months | 6.6 | 3.2 - 13.4 | 0.0001 | 3.2 | 1.1 - 8.8 | 0.0275 |
| LB < 6 months | 1.5 | 0.8 - 2.6 | 0.2057 | |||
| LB ≥ 6 months | 2.0 | 1.1 - 3.7 | 0.0336 | 1.0 | 0.5 - 2.2 | 0.9992 |
| AR < 6 months | 1.8 | 1.0 - 3.1 | 0.0397 | 1.8 | 1.0 - 3.2 | 0.0395 |
| AR ≥ 6 months | 2.0 | 1.1 - 3.6 | 0.0262 | 1.6 | 0.8 - 3.3 | 0.1774 |
Definition of abbreviations: CLAD = chronic lung allograft dysfunction; HR = hazards ratio; CI = confidience interval; ALI = acute lung injury; OP = organizing pneumonia; LB = lymphocytic bronchiolitis; AR = acute rejection;
Multivariable model includes only the variables listed.
BAL Chemokines Concentrations during Early and Late Allograft Injury
We previously showed that the association between allograft injury and CLAD may be mediated in part by aberrant CXCR3/ligand biology causing persistent mononuclear cell infiltration and further allograft injury. In the current study, we hypothesized that late episodes of allograft injury would be associated with a stronger CXCR3/ligand immune response, as measured as BAL CXCR3 ligand concentrations, corresponding to their increased CLAD risk.
779 BAL CXCR3 concentrations from 251 recipients were available for evaluation. There was no difference in CXCL9, CXCL10 or CXCL11 concentrations between the late and early “healthy” biopsies: The median CXCL9, CXCL10 and CXCL11 concentrations were: 242 vs 291 pg/ml (p=0.80), 120 vs 122 pg/ml (p=0.43), and 81 vs 80 pg/ml (p=0.80), respectively (Table 5). In contrast, late-onset allograft injury was associated with significantly higher BAL CXCR ligand concentrations compared to early-onset injury. The median CXCL9, CXCL10 and CXCL11 concentrations for late and early-onset injuries were: 1703 vs 623 pg/ml (p=0.0006), 340 vs 207 pg/ml (p=0.022) and 95 vs 82 pg/ml (p=0.034), respectively. The PC analysis confirmed the increased CXCR3 ligand concentrations during late-onset allograft injury compared with early-onset. The PC for the late and early-onset allograft injury was: 0.258 vs -0.056 (p=0.0024). There was no difference in the PC between the late and early healthy biopsies.
Table 5. Median BAL CXCR3 Ligand Concentrations Stratified by Time of Injury Onset.
| Healthy | Allograft Injury | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Early pg/ml | Late pg/ml | p-value † | Early pg/ml | Late pg/ml | p-value † | |
|
|
|
|
|
|
|
|
| CXCL9 | 291 | 242 | 0.7978 | 623 | 1,703 | 0.0006 |
| CXCL10 | 122 | 120 | 0.4246 | 207 | 340 | 0.0227 |
| CXCL11 | 80 | 81 | 0.7951 | 82 | 95 | 0.0343 |
| PC†† | (0.435) | (0.496) | 0.4156 | (0.056) | 0.258 | 0.0024 |
Definition of abbreviations: BAL = bronchoalveolar lavage; Early < 6 months, late ≥ 6 months from transplant. Pg/ml = picogram/milliliter.
Mixed effects model comparing early vs late biopsies.
PC: First principal component of CXCL9, CXCL10 and CXCL11.
Discussion
Since the pathogenesis of CLAD remains poorly understood with no known effective therapies, the identification and avoidance of risk factors is critical. Previous studies have established AR, LB and DAD as strong risk factors for CLAD development (17, 18, 21, 32, 33). Several of these studies suggest a higher CLAD risk after late-onset injury, but the results have not been consistent (18, 25, 27, 28, 32, 34). These prior studies evaluated only one or two injury patterns (e.g., AR and LB) at a time, and the association between OP and CLAD has not been well described. In this study, using time-dependent multivariable analysis controlling for all four allograft injury patterns, we sought to evaluate the significance of time-of-onset when considering the association between histopathologic injury and CLAD. We demonstrate that late-onset (≥6 months from transplant) DAD and OP were strongly predictive of CLAD development, while the early-onset forms of these injuries have minimal effect on CLAD risk. Late-onset LB and AR were associated with CLAD in univariable models, but lost significance after multivariable adjustment for DAD and OP. AR was the only early injury pattern associated with CLAD in univariable and multivariable models.
DAD is the most severe form of acute lung allograft injury. However, the impact of DAD on CLAD risk has only been evaluated in a few studies. Fisher and colleagues found no difference in the incidence of CLAD among 291 LTRs after early-DAD (<7 days) compared to those without early-DAD. (34) In contrast, Sato and colleagues showed increased CLAD risk after both early (< 3 months) and late (≥ 3 months) onset DAD among 720 bilateral LTRs. (32) Specifically, they found that early-DAD increased the risk of bronchiolitis obliterans syndrome (BOS), while late-DAD increased the risk of restrictive allograft syndrome (RAS). We similarly found a strong association between late-DAD and RAS (HR 6.2 95% CI 2.0-18.8), but no association between early-DAD and CLAD or BOS. Several studies have reported an association between primary graft dysfunction (PGD) and subsequent CLAD.(51, 52) The histopathology of PGD is often DAD; However, in our dataset PGD associated DAD was likely not captured due to our surveillance protocol which began at 1-month.
OP is arguably the second most severe form of acute allograft injury. However, there is a paucity of studies evaluating the association between OP and the development of CLAD. A study of 74 LTRs reported an association between OP and CLAD in univariable analysis, but found no association after multivariable adjustment. (31) A larger study involving 230 LTRs similarly did not find OP to be an independent predictor of CLAD. (28) We previously demonstrated that when time-of-onset is ignored, OP does not increase the risk of CLAD development. (33) However, when OP is stratified by time-of-onset, it is one of the strongest predictors of CLAD with HRs rivaling DAD. To our knowledge, this is the first study to show an association between OP and CLAD. The high HR of late-onset OP for CLAD development underscores the need for more studies to confirm this novel finding and determine treatment options to minimize the subsequent development of CLAD.
Several studies have suggested increased CLAD risk after episodes of late-onset AR and LB. A retrospective study of 259 LTRs by Hachem and colleagues reported slightly higher HRs for CLAD after late-onset (>6 months) minimal (A1) rejection compared with early rejection: HRs 2.97 vs. 2.28, respectively.(18) Similarly, a study involving 132 LTRs by Kroshus and colleagues found increased CLAD risk among recipients with late-onset AR (>3 months), but not early AR. (27) Husain and colleagues evaluated both AR and LB in a study of 134 LTRs and reported an association between both late (> 6 months) AR and late LB with CLAD. (25) In contrast to these prior studies which only evaluated one or two injury patterns at a time, we used time-dependent multivariable analysis adjusting concurrently for all four allograft injury patterns. While late-LB and late-AR predict CLAD in univariable models, they lost significance after multivariable adjustment for two risk factors which were significantly stronger, late-DAD and late-OP. Of note, the HRs for CLAD correlate with the severity of the allograft injury pattern with the HRs for DAD > OP > AR/LB.
The mechanism responsible for this association between time of injury onset and CLAD remains unclear. We previously demonstrated that Type I immune responses mediate in part the progression from allograft injury to CLAD.(33, 35, 36, 53) The allograft injury patterns likely represent a deleterious cycle of cell damage, Type I immune response mediated in part by CXCR3/ligands, recruitment of injurious mononuclear cells, particularly memory T-cells, into the allograft causing further cell damage, CXCR3 ligand release and eventual fibroproliferation. (33, 35, 36) Clinical studies by our group and others have shown increased BAL concentrations of the CXCR3 ligands during AR (35, 54), LB (33), DAD (33) and CLAD (33, 35, 55).
In the current study, we hypothesized that BAL CXCR3 ligand concentrations would be higher during episodes of late allograft injury compared with early injury, reflecting the increased risk of CLAD development. We evaluated 779 BAL CXCR3 ligand concentrations from 251 recipients and found significant elevations of all three CXCR3 ligands during the allograft injury patterns compared to healthy biopsies. Furthermore, late-onset injuries had markedly higher CXCR3 ligand concentrations compared to early-onset injuries supporting our hypothesis that late allograft injuries result in a more profound CXCR3/ligand immune response.
Several factors may be contributing to an augmented CXCR3/ligand response after late allograft injury. At the time of surgery, our lung transplant recipients receive induction immunosuppression with thymoglobulin or basiliximab which may lower CXCR3 ligand levels, both agents with a duration of action between 3-6 months. (56, 57) Alternatively, during the first few months post-transplant, lymphocytes differentiate from the naïve to memory subclass depending on a number of factors (e.g., site of stimulation, antigen concentration, costimulation and cytokine milieu). (39-41) The recruitment and stimulation of memory T-cells, as opposed to naïve T-cells, during late allograft injury may be producing a more profound and sustained Type I immune response, leading to increased fibroproliferation and CLAD. The more vigorous immune response generated by the memory T-cell recall response, compared with naïve T-cells, has been well established. (58, 59) Future studies characterizing the population of T-cells (memory vs naive) involved during late and early allograft injury are warranted.
AR was the only early-onset allograft injury which was associated with CLAD development. The pathogenesis of AR however, is fundamentally different than the other injury patterns. While many cases of DAD, OP and LB are initiated by exposure related insults (e.g., aspiration, infection, pollution (13, 33, 60)), AR represents an allo-immune response. Transplant recipients with early AR may have had the early presence of circulating allo-responsive memory T-cells. The development of pretransplant allo-specific memory T-cells may occur due to allosensitization from prior pregnancies, blood transfusions or infection. In fact, several studies in renal transplantation have demonstrated a strong association between pre-transplant donor-specific memory T-cells and the development of early AR. (42-44, 61, 62) Further research studying the mechanism responsible for the association between acute rejection and CLAD is clearly needed.
The major limitation of this study is the potential for confounding inherent to retrospective single center studies. Patients with clinical deterioration may have received more frequent biopsies leading to a higher incidence of histopathologic findings. Our study design accounted only for allograft injuries that were captured by TBBX. Undoubtedly, there were episodes that were missed due to the infrequency of TBBX sampling and poor sensitivity for detecting the more subtle injury patterns (i.e., AR and LB). Treatments received for the allograft injury were also not taken into account. At our institution, patients routinely received augmented immunosuppression for AR but not for DAD, OP or LB. Our study design included TBBXs performed for regular surveillance as well as clinical deterioration. Since there were more non-surveillance bronchoscopies occurring later, allograft injuries occurring in the late-onset group were likely to be more severe and symptomatic, compared to the asymptomatic injuries observed on surveillance bronchoscopies. Unfortunately, stratification by clinical indication of the bronchoscopy was limited due to sample size. Finally, multivariable adjustment for all known risk factors for CLAD (e.g., primary graft dysfunction, donor specific antibodies) was beyond the scope of this analysis.
This study extends our understanding of the association between CLAD and allograft injury, one of the most important risk factors associated with its development. We evaluated 1894 transbronchial biopsies from 441 lung transplant recipients and to our knowledge is the largest study to evaluate all four allograft injury patterns concurrently using appropriate Cox models for CLAD with time-dependent covariates. We demonstrate for the first time that OP is a strong predictor of CLAD when time-of-onset is taken into account, with HRs surpassing both LB and AR. Furthermore, this is one of the first studies to systematically evaluate pathophysiologic risk factors for the CLAD phenotypes: RAS and BOS. We show that the parenchymal and vascular injury patterns DAD, OP and AR predict RAS development, while the airway-centric injury pattern LB predicts BOS. Finally, this study evaluated 779 BAL CXCL9 concentrations from 251 lung transplant recipients and is the largest study to evaluate chemokine expression patterns during allograft injury.
In conclusion, we demonstrate the importance of time-of-onset when considering the association between allograft injury and CLAD. Late-onset DAD and OP markedly increase CLAD risk, specifically the restrictive phenotype RAS, whereas the early-onset form of these injury patterns do not. Late-onset LB increases the risk of BOS, the obstructive phenotype of CLAD, whereas early-onset LB does not. AR was the only early-onset injury pattern which predicted CLAD / RAS development. Elevated BAL CXCR3 ligand concentrations during late-onset allograft injury parallel the increase in CLAD risk and support our hypothesis that late allograft injuries result in a more profound CXCR3/ligand immune response. However, the mechanism responsible for this augmented immune response during late allograft injury requires further study. The identification of key events which increase CLAD risk represent unique opportunities to better understand the immunologic processes responsible for CLAD development. Given the potential importance of time-of-onset when considering the association between allograft injury and CLAD, this finding should be validated in a larger multi-center study.
Table 4. Cox Model for BOS with Time-Dependent Covariates.
| Univariable † | |||
|---|---|---|---|
|
|
|||
| HR | 95% CI | p-value | |
|
|
|
|
|
| DAD < 6 months | 1.2 | 0.5 - 2.6 | 0.7033 |
| DAD ≥ 6 months | 2.6 | 0.6 - 11.1 | 0.2012 |
| OP < 6 months | 0.6 | 0.3 - 1.5 | 0.2784 |
| OP ≥ 6 months | 1.4 | 0.5 - 4.2 | 0.5276 |
| LB < 6 months | 1.1 | 0.6 - 1.9 | 0.7887 |
| LB ≥ 6 months | 2.1 | 1.1 - 3.9 | 0.0203 |
| AR < 6 months | 1.0 | 0.6 - 1.7 | 0.9846 |
| AR ≥ 6 months | 1.4 | 0.8 - 2.7 | 0.2739 |
Definition of abbreviations: CLAD = chronic lung allograft dysfunction; HR = hazards ratio; CI = confidience interval; ALI = acute lung injury; OP = organizing pneumonia; LB = lymphocytic bronchiolitis; AR = acute rejection;
Univariable model since only LB was significant.
Acknowledgments
The research described was supported by NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR000124 (MYS.), 5U01AI113315-02 (SSW) and R01 HL112990–01, 5U01AI113315-02 (JAB).
Abbreviations
- DAD
diffuse alveolar damage
- AR
acute rejection
- BAL
bronchoalveolar lavage
- BOS
bronchiolitis obliterans syndrome
- CLAD
chronic lung allograft dysfunction
- CT
chest tomography
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- ISHLT
International Society of Heart and Lung Transplantation
- LB
lymphocytic bronchiolitis
- LTR
lung transplant recipients
- OP
organizing pneumonia
- PC
principal component
- pg/ml
pictogram per milliliter
- RAS
restrictive allograft syndrome
- SAS
Statistical Analysis Software
- TBBX
transbronchial biopsy
- UCLA
University of California Los Angeles
Footnotes
Disclosure: The authors of this study have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dobbels F, Kirk R, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Lung and Heart-Lung Transplant Report--2011. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2011 Oct;30(10):1104–22. doi: 10.1016/j.healun.2011.08.004. Epub 2011/10/04. eng. [DOI] [PubMed] [Google Scholar]
- 2.Billings JL, Hertz MI, Savik K, Wendt CH. Respiratory viruses and chronic rejection in lung transplant recipients. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2002 May;21(5):559–66. doi: 10.1016/s1053-2498(01)00405-3. Epub 2002/05/02. eng. [DOI] [PubMed] [Google Scholar]
- 3.Botha P, Archer L, Anderson RL, Lordan J, Dark JH, Corris PA, et al. Pseudomonas aeruginosa colonization of the allograft after lung transplantation and the risk of bronchiolitis obliterans syndrome. Transplantation. 2008 Mar 15;85(5):771–4. doi: 10.1097/TP.0b013e31816651de. Epub 2008/03/14. eng. [DOI] [PubMed] [Google Scholar]
- 4.Khalifah AP, Hachem RR, Chakinala MM, Schechtman KB, Patterson GA, Schuster DP, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. American journal of respiratory and critical care medicine. 2004 Jul 15;170(2):181–7. doi: 10.1164/rccm.200310-1359OC. Epub 2004/05/08. eng. [DOI] [PubMed] [Google Scholar]
- 5.Ruttmann E, Geltner C, Bucher B, Ulmer H, Hofer D, Hangler HB, et al. Combined CMV prophylaxis improves outcome and reduces the risk for bronchiolitis obliterans syndrome (BOS) after lung transplantation. Transplantation. 2006 May 27;81(10):1415–20. doi: 10.1097/01.tp.0000209439.27719.ed. Epub 2006/05/30. eng. [DOI] [PubMed] [Google Scholar]
- 6.Weigt SS, Derhovanessian A, Liao E, Hu S, Gregson AL, Kubak BM, et al. CXCR3 chemokine ligands during respiratory viral infections predict lung allograft dysfunction. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012 Feb;12(2):477–84. doi: 10.1111/j.1600-6143.2011.03859.x. Epub 2011/12/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weigt SS, Elashoff RM, Huang C, Ardehali A, Gregson AL, Kubak B, et al. Aspergillus colonization of the lung allograft is a risk factor for bronchiolitis obliterans syndrome. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009 Aug;9(8):1903–11. doi: 10.1111/j.1600-6143.2009.02635.x. Epub 2009/05/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weigt SS, Elashoff RM, Keane MP, Strieter RM, Gomperts BN, Xue YY, et al. Altered levels of CC chemokines during pulmonary CMV predict BOS and mortality post-lung transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008 Jul;8(7):1512–22. doi: 10.1111/j.1600-6143.2008.02280.x. Epub 2008/06/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregson AL, Wang X, Weigt SS, Palchevskiy V, Lynch JP, 3rd, Ross DJ, et al. Interaction between Pseudomonas and CXC chemokines increases risk of bronchiolitis obliterans syndrome and death in lung transplantation. American journal of respiratory and critical care medicine. 2013 Mar 1;187(5):518–26. doi: 10.1164/rccm.201207-1228OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blondeau K, Mertens V, Vanaudenaerde BA, Verleden GM, Van Raemdonck DE, Sifrim D, et al. Gastro-oesophageal reflux and gastric aspiration in lung transplant patients with or without chronic rejection. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2008 Apr;31(4):707–13. doi: 10.1183/09031936.00064807. Epub 2007/12/07. eng. [DOI] [PubMed] [Google Scholar]
- 11.D'Ovidio F, Mura M, Tsang M, Waddell TK, Hutcheon MA, Singer LG, et al. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. The Journal of thoracic and cardiovascular surgery. 2005 May;129(5):1144–52. doi: 10.1016/j.jtcvs.2004.10.035. Epub 2005/05/04. eng. [DOI] [PubMed] [Google Scholar]
- 12.Nawrot TS, Vos R, Jacobs L, Verleden SE, Wauters S, Mertens V, et al. The impact of traffic air pollution on bronchiolitis obliterans syndrome and mortality after lung transplantation. Thorax. 2011 Sep;66(9):748–54. doi: 10.1136/thx.2010.155192. Epub 2011/03/25. eng. [DOI] [PubMed] [Google Scholar]
- 13.Verleden SE, Scheers H, Nawrot TS, Vos R, Fierens F, Geenens R, et al. Lymphocytic bronchiolitis after lung transplantation is associated with daily changes in air pollution. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012 Jul;12(7):1831–8. doi: 10.1111/j.1600-6143.2012.04134.x. Epub 2012/06/12. eng. [DOI] [PubMed] [Google Scholar]
- 14.Sumpter TL, Wilkes DS. Role of autoimmunity in organ allograft rejection: a focus on immunity to type V collagen in the pathogenesis of lung transplant rejection. American journal of physiology Lung cellular and molecular physiology. 2004 Jun;286(6):L1129–39. doi: 10.1152/ajplung.00330.2003. Epub 2004/05/12. eng. [DOI] [PubMed] [Google Scholar]
- 15.Wilkes DS, Heidler KM, Yasufuku K, Devito-Haynes L, Jankowska-Gan E, Meyer KC, et al. Cell-mediated immunity to collagen V in lung transplant recipients: correlation with collagen V release into BAL fluid. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2001 Feb;20(2):167. doi: 10.1016/s1053-2498(00)00308-9. Epub 2001/03/16. Eng. [DOI] [PubMed] [Google Scholar]
- 16.Burton CM, Iversen M, Carlsen J, Mortensen J, Andersen CB, Steinbruchel D, et al. Acute cellular rejection is a risk factor for bronchiolitis obliterans syndrome independent of post-transplant baseline FEV1. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2009 Sep;28(9):888–93. doi: 10.1016/j.healun.2009.04.022. Epub 2009/09/01. eng. [DOI] [PubMed] [Google Scholar]
- 17.Davis WA, Finlen Copeland CA, Todd JL, Snyder LD, Martissa JA, Palmer SM. Spirometrically Significant Acute Rejection Increases the Risk for BOS and Death After Lung Transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012 Mar;12(3):745–52. doi: 10.1111/j.1600-6143.2011.03849.x. Epub 2011/11/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hachem RR, Khalifah AP, Chakinala MM, Yusen RD, Aloush AA, Mohanakumar T, et al. The significance of a single episode of minimal acute rejection after lung transplantation. Transplantation. 2005 Nov 27;80(10):1406–13. doi: 10.1097/01.tp.0000181161.60638.fa. Epub 2005/12/13. eng. [DOI] [PubMed] [Google Scholar]
- 19.Burton CM, Iversen M, Carlsen J, Andersen CB. Interstitial inflammatory lesions of the pulmonary allograft: a retrospective analysis of 2697 transbronchial biopsies. Transplantation. 2008 Sep 27;86(6):811–9. doi: 10.1097/TP.0b013e3181852f02. Epub 2008/09/25. eng. [DOI] [PubMed] [Google Scholar]
- 20.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2002 Mar;21(3):297–310. doi: 10.1016/s1053-2498(02)00398-4. Epub 2002/03/19. eng. [DOI] [PubMed] [Google Scholar]
- 21.Glanville AR, Aboyoun CL, Havryk A, Plit M, Rainer S, Malouf MA. Severity of lymphocytic bronchiolitis predicts long-term outcome after lung transplantation. American journal of respiratory and critical care medicine. 2008 May 1;177(9):1033–40. doi: 10.1164/rccm.200706-951OC. Epub 2008/02/12. eng. [DOI] [PubMed] [Google Scholar]
- 22.Sharples LD, McNeil K, Stewart S, Wallwork J. Risk factors for bronchiolitis obliterans: a systematic review of recent publications. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2002 Feb;21(2):271–81. doi: 10.1016/s1053-2498(01)00360-6. Epub 2002/02/09. eng. [DOI] [PubMed] [Google Scholar]
- 23.Burton CM, Iversen M, Scheike T, Carlsen J, Andersen CB. Minimal acute cellular rejection remains prevalent up to 2 years after lung transplantation: a retrospective analysis of 2697 transbronchial biopsies. Transplantation. 2008 Feb 27;85(4):547–53. doi: 10.1097/TP.0b013e3181641df9. Epub 2008/03/19. eng. [DOI] [PubMed] [Google Scholar]
- 24.Hopkins PM, Aboyoun CL, Chhajed PN, Malouf MA, Plit ML, Rainer SP, et al. Association of minimal rejection in lung transplant recipients with obliterative bronchiolitis. American journal of respiratory and critical care medicine. 2004 Nov 1;170(9):1022–6. doi: 10.1164/rccm.200302-165OC. Epub 2004/08/07. eng. [DOI] [PubMed] [Google Scholar]
- 25.Husain AN, Siddiqui MT, Holmes EW, Chandrasekhar AJ, McCabe M, Radvany R, et al. Analysis of risk factors for the development of bronchiolitis obliterans syndrome. American journal of respiratory and critical care medicine. 1999 Mar;159(3):829–33. doi: 10.1164/ajrccm.159.3.9607099. Epub 1999/03/02. eng. [DOI] [PubMed] [Google Scholar]
- 26.Khalifah AP, Hachem RR, Chakinala MM, Yusen RD, Aloush A, Patterson GA, et al. Minimal acute rejection after lung transplantation: a risk for bronchiolitis obliterans syndrome. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005 Aug;5(8):2022–30. doi: 10.1111/j.1600-6143.2005.00953.x. Epub 2005/07/06. eng. [DOI] [PubMed] [Google Scholar]
- 27.Kroshus TJ, Kshettry VR, Savik K, John R, Hertz MI, Bolman RM., 3rd Risk factors for the development of bronchiolitis obliterans syndrome after lung transplantation. The Journal of thoracic and cardiovascular surgery. 1997 Aug;114(2):195–202. doi: 10.1016/S0022-5223(97)70144-2. Epub 1997/08/01. eng. [DOI] [PubMed] [Google Scholar]
- 28.Girgis RE, Tu I, Berry GJ, Reichenspurner H, Valentine VG, Conte JV, et al. Risk factors for the development of obliterative bronchiolitis after lung transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 1996 Dec;15(12):1200–8. Epub 1996/12/01. eng. [PubMed] [Google Scholar]
- 29.Yousem SA. Lymphocytic bronchitis/bronchiolitis in lung allograft recipients. The American journal of surgical pathology. 1993 May;17(5):491–6. doi: 10.1097/00000478-199305000-00008. Epub 1993/05/01. eng. [DOI] [PubMed] [Google Scholar]
- 30.El-Gamel A, Sim E, Hasleton P, Hutchinson J, Yonan N, Egan J, et al. Transforming growth factor beta (TGF-beta) and obliterative bronchiolitis following pulmonary transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 1999 Sep;18(9):828–37. doi: 10.1016/s1053-2498(99)00047-9. Epub 1999/10/21. eng. [DOI] [PubMed] [Google Scholar]
- 31.Heng D, Sharples LD, McNeil K, Stewart S, Wreghitt T, Wallwork J. Bronchiolitis obliterans syndrome: incidence, natural history, prognosis, and risk factors. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 1998 Dec;17(12):1255–63. Epub 1999/01/12. eng. [PubMed] [Google Scholar]
- 32.Sato M, Hwang DM, Ohmori-Matsuda K, Chaparro C, Waddell TK, Singer LG, et al. Revisiting the pathologic finding of diffuse alveolar damage after lung transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2012 Feb 11; doi: 10.1016/j.healun.2011.12.015. Epub 2012/02/15. Eng. [DOI] [PubMed] [Google Scholar]
- 33.Shino MY, Weigt SS, Li N, Palchevskiy V, Derhovanessian A, Saggar R, et al. CXCR3 ligands are associated with the continuum of diffuse alveolar damage to chronic lung allograft dysfunction. American journal of respiratory and critical care medicine. 2013 Nov 1;188(9):1117–25. doi: 10.1164/rccm.201305-0861OC. Epub 2013/09/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher AJ, Wardle J, Dark JH, Corris PA. Non-immune acute graft injury after lung transplantation and the risk of subsequent bronchiolitis obliterans syndrome (BOS) The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2002 Nov;21(11):1206–12. doi: 10.1016/s1053-2498(02)00450-3. Epub 2002/11/15. eng. [DOI] [PubMed] [Google Scholar]
- 35.Belperio JA, Keane MP, Burdick MD, Lynch JP, 3rd, Xue YY, Li K, et al. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J Immunol. 2002 Jul 15;169(2):1037–49. doi: 10.4049/jimmunol.169.2.1037. Epub 2002/07/05. eng. [DOI] [PubMed] [Google Scholar]
- 36.Belperio JA, Keane MP, Burdick MD, Lynch JP, 3rd, Zisman DA, Xue YY, et al. Role of CXCL9/CXCR3 chemokine biology during pathogenesis of acute lung allograft rejection. J Immunol. 2003 Nov 1;171(9):4844–52. doi: 10.4049/jimmunol.171.9.4844. Epub 2003/10/22. eng. [DOI] [PubMed] [Google Scholar]
- 37.Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, et al. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. The Journal of experimental medicine. 1998 Jun 15;187(12):2009–21. doi: 10.1084/jem.187.12.2009. Epub 1998/06/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, et al. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. The Journal of experimental medicine. 1996 Sep 1;184(3):963–9. doi: 10.1084/jem.184.3.963. Epub 1996/09/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006 May 11;441(7090):235–8. doi: 10.1038/nature04753. Epub 2006/05/02. eng. [DOI] [PubMed] [Google Scholar]
- 40.Neurath MF, Finotto S, Glimcher LH. The role of Th1/Th2 polarization in mucosal immunity. Nature medicine. 2002 Jun;8(6):567–73. doi: 10.1038/nm0602-567. Epub 2002/06/04. eng. [DOI] [PubMed] [Google Scholar]
- 41.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–57. Epub 1986/04/01. eng. [PubMed] [Google Scholar]
- 42.Ma T, Xu J, Zhuang J, Zhou X, Lin L, Shan Z, et al. Combination of C-X-C motif chemokine 9 and C-X-C motif chemokine 10 antibodies with FTY720 prolongs the survival of cardiac retransplantation allografts in a mouse model. Experimental and therapeutic medicine. 2015 Mar;9(3):1006–12. doi: 10.3892/etm.2015.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014 Oct 3;346(6205):98–101. doi: 10.1126/science.1254536. Epub 2014/08/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhuang J, Shan Z, Ma T, Li C, Qiu S, Zhou X, et al. CXCL9 and CXCL10 accelerate acute transplant rejection mediated by alloreactive memory T cells in a mouse retransplantation model. Experimental and therapeutic medicine. 2014 Jul;8(1):237–42. doi: 10.3892/etm.2014.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Busuttil A, Weigt SS, Keane MP, Xue YY, Palchevskiy V, Burdick MD, et al. CXCR3 ligands are augmented during the pathogenesis of pulmonary sarcoidosis. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2009 Sep;34(3):676–86. doi: 10.1183/09031936.00157508. Epub 2009/04/24. eng. [DOI] [PubMed] [Google Scholar]
- 46.Stewart S, Fishbein MC, Snell GI, Berry GJ, Boehler A, Burke MM, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2007 Dec;26(12):1229–42. doi: 10.1016/j.healun.2007.10.017. Epub 2007/12/22. eng. [DOI] [PubMed] [Google Scholar]
- 47.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 1996 Jan;15(1 Pt 1):1–15. Epub 1996/01/01. eng. [PubMed] [Google Scholar]
- 48.American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. American journal of respiratory and critical care medicine. 2002 Jan 15;165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01. Epub 2002/01/16. eng. [DOI] [PubMed] [Google Scholar]
- 49.Cooper JD, Billingham M, Egan T, Hertz MI, Higenbottam T, Lynch J, et al. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. International Society for Heart and Lung Transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 1993 Sep-Oct;12(5):713–6. Epub 1993/09/01. eng. [PubMed] [Google Scholar]
- 50.Sato M, S T, Waddell TK, Singer LG, Keshavjee S. Diagnosis of Restrictive Allograft Syndrome (RAS) without Using Total Lung Capacity. ISHLT 33rd Annual Meeting and Scientific Sessions. 2013 Abstract. [Google Scholar]
- 51.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. American journal of respiratory and critical care medicine. 2007 Mar 1;175(5):507–13. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 52.DerHovanessian A, Weigt SS, Palchevskiy V, Shino MY, Sayah DM, Gregson AL, et al. The Role of TGF-beta in the Association Between Primary Graft Dysfunction and Bronchiolitis Obliterans Syndrome. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016 Feb;16(2):640–9. doi: 10.1111/ajt.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Belperio JA, Burdick MD, Keane MP, Xue YY, Lynch JP, 3rd, Daugherty BL, et al. The role of the CC chemokine, RANTES, in acute lung allograft rejection. J Immunol. 2000 Jul 1;165(1):461–72. doi: 10.4049/jimmunol.165.1.461. Epub 2000/06/22. eng. [DOI] [PubMed] [Google Scholar]
- 54.Agostini C, Calabrese F, Rea F, Facco M, Tosoni A, Loy M, et al. Cxcr3 and its ligand CXCL10 are expressed by inflammatory cells infiltrating lung allografts and mediate chemotaxis of T cells at sites of rejection. The American journal of pathology. 2001 May;158(5):1703–11. doi: 10.1016/S0002-9440(10)64126-0. Epub 2001/05/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neujahr DC, Perez SD, Mohammed A, Ulukpo O, Lawrence EC, Fernandez F, et al. Cumulative exposure to gamma interferon-dependent chemokines CXCL9 and CXCL10 correlates with worse outcome after lung transplant. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012 Feb;12(2):438–46. doi: 10.1111/j.1600-6143.2011.03857.x. Epub 2011/12/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foster CE, 3rd, Weng RR, Piper M, Laugenou K, Ichii H, Lakey J, et al. Induction therapy by anti-thymocyte globulin (rabbit) versus basiliximab in deceased donor renal transplants and the effect on delayed graft function and outcomes. Transplantation proceedings. 2012 Jan;44(1):164–6. doi: 10.1016/j.transproceed.2011.12.055. Epub 2012/02/09. eng. [DOI] [PubMed] [Google Scholar]
- 57.Flaman F, Zieroth S, Rao V, Ross H, Delgado DH. Basiliximab versus rabbit anti-thymocyte globulin for induction therapy in patients after heart transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2006 Nov;25(11):1358–62. doi: 10.1016/j.healun.2006.09.002. Epub 2006/11/14. eng. [DOI] [PubMed] [Google Scholar]
- 58.Garcia S, DiSanto J, Stockinger B. Following the development of a CD4 T cell response in vivo: from activation to memory formation. Immunity. 1999 Aug;11(2):163–71. doi: 10.1016/s1074-7613(00)80091-6. Epub 1999/09/15. eng. [DOI] [PubMed] [Google Scholar]
- 59.Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nature immunology. 2000 Jul;1(1):47–53. doi: 10.1038/76907. Epub 2001/03/23. eng. [DOI] [PubMed] [Google Scholar]
- 60.Chaparro C, Chamberlain D, Maurer J, Winton T, Dehoyos A, Kesten S. Bronchiolitis obliterans organizing pneumonia (BOOP) in lung transplant recipients. Chest. 1996 Nov;110(5):1150–4. doi: 10.1378/chest.110.5.1150. Epub 1996/11/01. eng. [DOI] [PubMed] [Google Scholar]
- 61.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, et al. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999 Aug 15;163(4):2267–75. Epub 1999/08/10. eng. [PubMed] [Google Scholar]
- 62.Crespo E, Lucia M, Cruzado JM, Luque S, Melilli E, Manonelles A, et al. Pre-transplant donor-specific T-cell alloreactivity is strongly associated with early acute cellular rejection in kidney transplant recipients not receiving T-cell depleting induction therapy. PloS one. 2015;10(2):e0117618. doi: 10.1371/journal.pone.0117618. Epub 2015/02/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]