Abstract
Hospital readmission after lung transplantation negatively impacts quality of life and resource utilization. A secondary analysis of data collected prospectively was conducted to identify the pattern (incidence, count, cumulative duration), reasons and predictors of readmission for 201 lung transplant recipients (LTR) assessed at 2, 6, and 12 months post-discharge. The majority of LTRs (83.6%) were readmitted, and 64.2% had multiple readmissions. The median cumulative readmission duration was 19 days. The main reasons for readmission were: other than infection or rejection (55.5%), infection only (25.4%), rejection only (9.9%), and infection and rejection (0.7%). LTRs who required reintubation (odd ratio [OR]=1.92; p=.008) or discharged to care facilities (OR=2.78; p=.008) were at higher risk for readmission with a 95.7% cumulative incidence of readmission at 12 months. Thirty-day readmission (40.8%) was not significantly predicted by baseline characteristics. Predictors for higher readmission count were lower capacity to engage in self-care (incidence rate ratio [IRR]=0.99; p=.03) and discharge to care facilities (IRR=1.45; p=.01). Predictors for longer cumulative readmission duration were older age (arithmetic mean ratio [AMR]=1.02; p=.009), return to ICU (AMR=2.00; p=.01), and lower capacity to engage in self-care (AMR=0.99; p=.03). Identifying LTRs at risk may assist in optimizing pre-discharge care, discharge planning, and long-term follow-up.
INTRODUCTION
Hospital readmission is considered an important proxy measure for the quality of care and short-term effectiveness of hospital discharge processes (1, 2). In most cases, readmission has been found to be associated with poorer quality of inpatient care, remediable care deficiencies (3), illness burden, and social determinants of health (4). The Medicare Payment Advisory Commission reported that 75% of readmissions of Medicare beneficiaries were avoidable, resulting in extra cost of US$15 billion annually (5, 6) and negatively impacting patients quality of life. While the Centers for Medicare & Medicaid Services (CMS) have begun limiting reimbursement for higher standardized readmission rates for certain medical conditions (e.g., heart failure, acute myocardial infarction, and pneumonia) (7, 8), these penalties are anticipated to be broadened to include other medical and surgical conditions (9–11), including transplantation (11).
Lung transplantation is becoming a more prevalent treatment option for patients with end-stage lung disease (12–14). Patients who undergo transplantation are expected to have better physical quality of life (15) and longer life expectancy (16–18). Despite the advances and improved survival, lung transplant recipients (LTRs) often experience post-transplant complications (19, 20), and unplanned hospital readmissions. Although it is known that the incidence of readmission is the highest during the first year after lung transplantation (21, 22), there is lack of information about how these readmissions unfold over the course of the first year. Furthermore, factors associated with risk for readmission, such as pre-discharge characteristics (sociodemographic, clinical, self-care), and the reasons for readmission are largely unknown. It is crucial to understand these risk factors in order to allocate health care resources more efficiently, reduce readmission rates, and improve LTRs quality of life (23). Therefore, the purposes of this study were to: 1) describe the pattern (incidence, count, and cumulative duration) and reasons for readmission during first year after lung transplantation and 2) identify predictors of hospital readmission.
METHODS
Design, Setting, and Sample
The study was a secondary analysis of data from a randomized controlled trial (RCT) to evaluate the efficacy of a mobile health (mHealth) intervention for improving capacity for self-care, self-management, and health outcomes during the first year after lung transplantation (24) (ClinicalTrials.gov identifier: NCT00818025). The final sample consisted of all 201 LTRs who were randomized to one of two groups (intervention or usual care) in view of the fact that the original study did not find significant impact of the intervention on readmission. Included LTRs in the parent study were over 18 years old, able to read and speak English, had no history of previous organ or bone marrow transplant, and able to perform their personal care (24). All LTRs received a lung transplant between December 2008 and December 2011 at the University of Pittsburgh Medical Center (UPMC) Cardiothoracic Transplantation Program. All LTRs received clinical management according to the standard UPMC protocol for immunosuppression, infection prophylaxis, routine surveillance biopsies, intravenous steroids in case of acute rejection, and follow-up evaluations as needed. For most patients with acute rejection, readmission to the hospital was required due to logistical challenges with home care and comorbidities, particularly diabetes. However, some patients were treated with intravenous steroids at an outpatient infusion center or at home. The study was approved by the Institutional Review Board of the University of Pittsburgh.
Data Collection and Measures
Pattern of readmission
For the purpose of the present study, readmission was defined as a stay in an inpatient hospital setting for at least 24 hours following the patient’s medical discharge after index transplant hospitalization (22). Data related to readmission (incidence, count, cumulative duration, and reasons for readmission) were collected prospectively during each of the intervals 0–2, 2–6, and 6–12 months post-discharge. Medical records were used to extract data for patients readmitted to any hospital within the same healthcare network (UPMC) or from medical records regarding a readmission from outside hospitals (14.8% of total readmissions).
For each interval, readmission incidence was determined based on any readmission, and the count of readmission was the sum of readmissions. The cumulative duration of readmission was the sum of hospital stay days for each interval.
Reasons for readmission
Reasons for readmission were independently classified by two evaluators according to the four categories outlined by the International Society of Heart and Lung Transplantation (ISHLT) (21): 1) infection, 2) rejection, 3) infection and rejection, or 4) other than infection or rejection. The category “unable to determine” was used in cases of lack of sufficient data to make decision. In cases of disagreement between the two evaluators, readmission was discussed until consensus was reached for all admissions.
Predictors
Potential predictors of readmission were measured prior to index hospital discharge and included RCT group assignment, sociodemographic, clinical, and self-care characteristics.
Sociodemographic characteristics included gender (male v. female), age at transplant (years), marital status (married v. unmarried), race (white v. non-white), education (less than high school v. high school or higher) and employment (employed v. unemployed).
Clinical characteristics included underlying lung disease (obstructive v. non-obstructive), type of transplant procedure (single v. double), post-operative ventilation period (< 48 hours v. ≥ 48 hours), return to intensive care unit (ICU) (return v. no return), reintubation (reintubated v. not reintubated), length of hospital stay (days), and hospital discharge destination (home v. any care facility).
Self-care characteristics have a crucial role in promoting patients to control and reduce the physical and psychological consequences of the disease (25, 26), and thus they were hypothesized to predict readmission after lung transplantation. The original study used three measures to assess self-care attributes: 1) Perception of Self-Care Agency (PSCA) (27), a reliable and valid (28) self-report measure of one’s capacity for self-care (29). The PSCA includes 53 Likert-type items scored on a scale of 1 to 5 and summed for scores that range from 53 to 265, with higher scores indicating greater self-care agency. Internal consistency reliability (Cronbach’s alpha) was .94 in the current sample, well above the acceptable level of (.70) (30). 2) Dyadic Adjustment Scale (DAS) (31), a reliable, self-report measure of the quality of the relationship with patient’s primary lay caregiver. Stronger recipient-caregiver relationship was previously reported to be associated with higher capacity for self-care (32). The DAS includes 15 Likert-type items scored on a scale of 1 to 5 and summed for scores that range from 15 to 75, with higher scores indicating higher quality of caregiver relationship (33). Cronbach’s alpha was .83 in the current sample. 3) Health Locus of Control (HLOC), Internality subscale (34, 35), a self-report measure of how strongly individuals believe they are responsible for their own health outcomes (36). The internality measure is reliable (37) and includes six Likert-type items scored on a scale of 1 to 6 and summed for scores that range from 6 to 36 with high scores indicating higher internality. Cronbach’s alpha was .75 in this sample.
Statistical Analysis
Descriptive statistics were used to summarize categorical variables by frequencies and proportions, and continuous variables by medians and interquartile ranges (IQR). Distribution differences of LTRs by readmission status during the first year were compared using Wilcoxon rank sum test for continuous and Chi-square test or its exact counterpart (Fisher’s test) for categorical variables. The extent of missing data was assessed and only two values were incomplete for the DAS measure; unconditional mean imputation (38) was used to compute scores for these data. The degree of concordance on classification of reasons for readmission between two evaluators was examined by the percentage of agreement before reconciliation and Cohen’s kappa (39).
Regression analysis using Generalized Estimating Equations (GEE) models (40) was used to identify predictors of readmission pattern. GEE allows analysis of repeated measurement of readmission incidence for the three study intervals (0–2, 2–6, and 6–12 months); this repeated measures analysis is considered powerful relative to sample size (41) because it allows for studying variability within individuals over time (42, 43). Individual differences in follow-up time during each interval were adjusted using the loge-transformed of total participation days as offset. To account for attrition over time, the models included the days up to the event (19 deaths, 1 withdrawal) as offset for that time interval and omitted the subsequent intervals from analysis (i.e. censored). Binomial (logistic) and negative binomial distributions were adopted by the GEE regression models for the readmission incidence (dichotomous data) and the count of readmissions (count data), respectively. Another GEE model used gamma distribution to assess predictors of readmission duration in case of readmission (44). For all GEE analyses, univariate regression was first used to identify potential predictors at significance level of p < .10 (45). Retained variables were then entered into a multivariate model to identify the final significant predictors (p < .05). Effect sizes in form of odds ratios (OR), incident rate ratios (IRR), and arithmetic mean ratios (AMR) were reported with the 95% confidence intervals and p-values for the logistic, negative binomial, and gamma regression models, respectively.
To provide useful information about the likelihood of being readmitted over the course of the first year, competing risk regression analysis was used to calculate and create cumulative incidence plots for the final predictors of readmission incidence identified earlier by the logistic GEE analysis. This method accounts for death as a competing risk for readmission (46, 47). Time-to-event was calculated from the date of index discharge until the date of first readmission. In addition, another competing risk regression analysis was conducted to identify the predictors of 30-day readmission, which is broadly used by hospitals, insurers and payers, and regulatory agencies to measure hospital quality and as a benchmark for reimbursement (4, 48).
All statistical testes were 2-sided and conducted using the software packages of SPSS (version 22; IBM Corp, Armonk, NY) (49) and Stata (version 14; Stata Corp LP, College Station, TX) (50).
RESULTS
Baseline characteristics of the sample are presented in Table 1. Only age (p=.02) and reintubation status (p=.01) were different between the groups of patients who did vs. did not experience a readmission. The RCT group assignment was not significantly associated with readmission (p=.78).
Table 1.
Characteristics of the Sample by Readmission Status
| Readmission Status | ||||
|---|---|---|---|---|
| Characteristics | Total (N=201) a |
Not Readmitted (n=33) |
Readmitted (n=168) |
P |
| RCT intervention, % (n) mHealth Group | 49.3 (99) | 51.5 (17) | 48.8 (82) | .78 |
| Sociodemographic | ||||
| Gender, % (n) male | 55.2 (111) | 63.6 (21) | 53.6 (90) | .29 |
| Age, years, median (IQR) | 62 (51,67) | 57 (41,63) | 63 (52,68) | .02 |
| Marital status, % (n) married | 71.6 (144) | 78.8 (26) | 70.2 (118) | .32 |
| Race, % (n) white | 91.0 (183) | 90.9 (30) | 91.1 (153) | 1.00 |
| Education, % (n) ≥ high school | 94.0 (189) | 97.0 (32) | 93.5 (157) | .70 |
| Employment, % (n) yes | 11.4 (23) | 18.2 (6) | 10.1 (17) | .23 |
| Clinical Health Status | ||||
| Lung disease, % (n) obstructive | 49.8 (100) | 60.6 (20) | 47.6 (80) | .17 |
| Type of transplant, % (n) single | 18.4 (37) | 9.1 (3) | 20.2 (34) | .13 |
| Post-op ventilation need, % (n) <48 hours | 65.2 (131) | 60.6 (20) | 66.1 (111) | .55 |
| Return to ICU, % (n) yes | 20.9 (42) | 24.2 (8) | 20.2 (34) | .61 |
| Reintubated, % (n) yes | 23.4 (47) | 6.1 (2) | 26.8 (45) | .01 |
| Length of stay, days, median (IQR) | 27 (19,44) | 28 (20,42) | 27 (18,44) | .94 |
| Hospital discharge destination, % (n) home | 86.6 (174) | 93.9 (31) | 85.1 (143) | .26 |
| Self-Care Attributes | ||||
| Self-care agency, median (IQR) | 224 (207,241) | 221 (201,248) | 222 (207,241) | .97 |
| Caregiver relationship quality, median (IQR) | 67 (63,71) | 67 (63,71) | 68 (63,71) | .94 |
| Internal locus of control, median (IQR) | 24 (20,29) | 23 (21,26) | 24 (20,30) | .35 |
The number of LTRs who were alive at the start of the study
Reasons for readmission
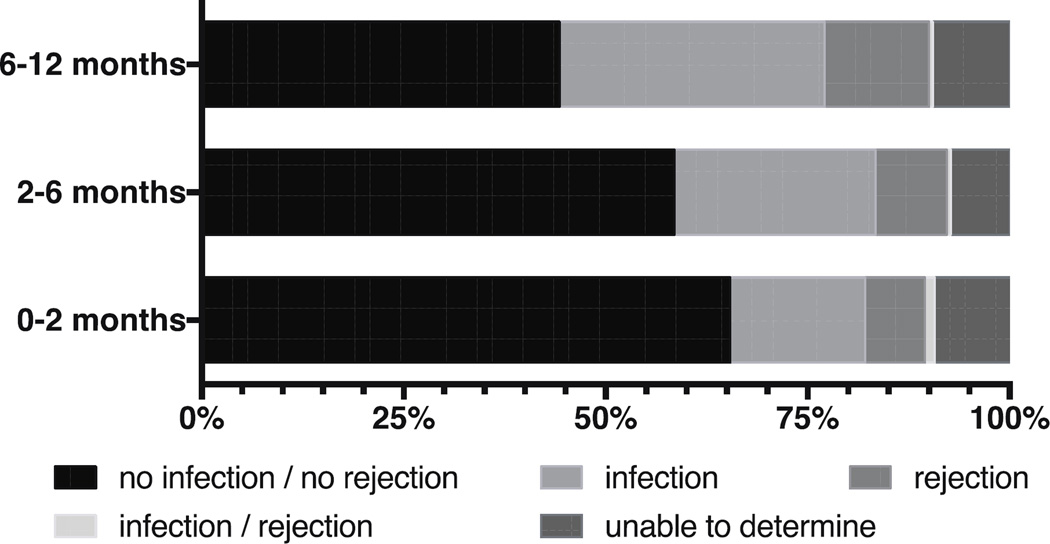
Of the 568 readmissions during the first year, the evaluators agreed on classification of 94.5% of the readmissions (kappa=.91). Figure 1 presents the percentages of reasons for readmission during each study interval. Reasons categorized as ‘other than infection or rejection’ (including, but not limited to, dehydration, nausea, vomiting, diarrhea and acute renal failure) accounted for more than half (55.5%) of readmissions during the first year after discharge; admissions in this category decreased over time (Figure 1). The percentages of readmissions categorized as ‘infection only’ and ‘rejection only’ were 25.4% and 9.9%, respectively; admissions in these categories increased with time. Finally, 0.7% of readmissions were categorized as ‘infection and rejection simultaneously’ and showed little change over time.
Figure 1.
Reasons for readmission per assessment time interval during the first year after lung transplantation discharge. Categorized as outlined by the International Society of Heart and Lung Transplantation (ISHLT) (21).
Readmission incidence
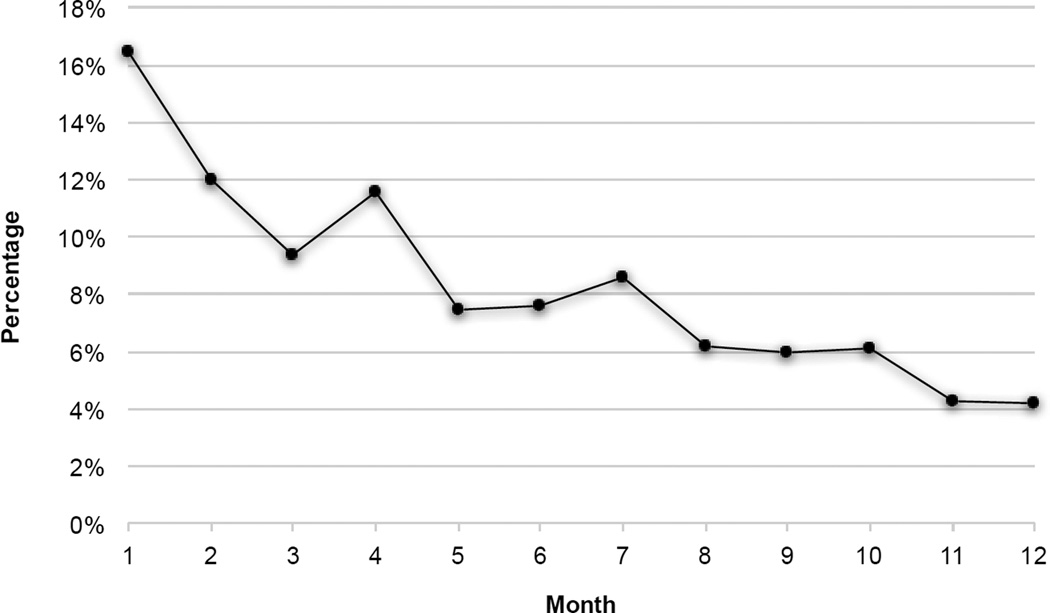
Of the 201 LTRs, 168 (83.6%) were readmitted to the hospital at least once during the first year. The percentages of readmission incidence are displayed in Table 2 for the study period, which was divided into two intervals of equal duration to facilitate comparison. Results from the logistic regression model examining predictors of readmission incidence are presented in Table 3. After controlling for other variables in the multivariate model, there was a significant decrease in readmission incidence over time (OR=0.88; 95% CI, 0.85–0.92; p<.001). In addition, reintubation during the index admission (OR=1.92; 95% CI, 1.18–3.12; p=.008) and discharge destination to care facilities (OR=2.78; 95% CI, 1.31–5.87; p=.008) were statistically significant risk factors for readmission. Furthermore, 82 (40.8%) of LTRs were readmitted during the first 30-day readmission after lung transplantation discharge. None of the baseline characteristics was identified as a significant predictor of 30-day readmission, and the findings from this analysis are not reported in this article in the interest of brevity. The percentage of total readmissions per each month during the first year after lung transplantation discharge are displayed in Figure 2, which shows a decline in the number of readmissions over time.
Table 2.
Pattern of Readmission per Assessment Time Interval During the First Year After Lung Transplantation Discharge
| Time Interval | |||
|---|---|---|---|
| 0–6 months a (n=201) b |
6–12 months (n=190) b |
First Year (n=201) |
|
| Readmission, yes | 75.1% | 50.5% | 83.6% |
| Readmission count | |||
| 0 | 24.9% | 49.5% | 16.4% |
| 1 | 26.4% | 24.2% | 19.4% |
| 2–3 | 32.8% | 17.9% | 33.3% |
| >= 4 | 15.9% | 8.4% | 30.9% |
| Readmission duration c | Median= 13 days | Median= 10 days | Median= 19 days |
| <=10 days | 41.1% | 51.0% | 33.3% |
| 11–20 days | 20.5% | 20.8% | 19.0% |
| 21–30 days | 10.6% | 11.5% | 10.1% |
| >30 days | 27.8% | 16.7% | 37.5% |
The original first two time intervals (0–2 and 2–6 months) were combined to create intervals of equal duration
The number of LTRs who were alive and did not withdraw at the start of the time interval
Calculated based on the number of LTRs who were readmitted for each time interval
Table 3.
Predictors of Readmission Incidence During the First Year After Lung Transplantation Discharge
| Characteristics | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR a | 95% CI | P | OR b | 95% CI | P | |
| Time, per month increase | 0.88 | 0.85–0.92 | <.001 | 0.88 | 0.85–0.92 | <.001 |
| Sociodemographic | ||||||
| Gender, male | 0.90 | 0.60–1.36 | .62 | |||
| Age at transplant (years) | 1.01 | 1.00–1.03 | .26 | |||
| Marital status, unmarried | 1.61 | 1.00–2.61 | .05 | |||
| Race, white | 1.08 | 0.52–2.22 | .84 | |||
| Education, < high school | 1.79 | 0.77–4.17 | .18 | |||
| Employment, unemployed | 2.07 | 1.10–3.88 | .02 | 1.59 | 0.88–2.87 | .12 |
| Clinical | ||||||
| Underlying disease, obstructive | 1.10 | 0.73–1.66 | .64 | |||
| Type of transplant, single | 1.56 | 0.91–2.69 | .11 | |||
| Post-op vent days, < 48 hours | 1.16 | 0.76–1.79 | .49 | |||
| Length of stay days, per 1 day increase | 1.01 | 1.00–1.02 | .11 | |||
| Reintubated, yes | 2.71 | 1.67–4.42 | <.001 | 1.92 | 1.18–3.12 | .008 |
| Return to ICU, yes | 1.36 | 0.78–2.37 | .28 | |||
| Hospital discharge destination, facility | 3.97 | 1.91–8.25 | <.001 | 2.78 | 1.31–5.87 | .008 |
| Self-Care | ||||||
| Self-care agency (PSCA) c | 0.99 | 0.99–1.00 | .20 | |||
| Caregiver relationship quality (DAS) c | 1.00 | 0.98–1.03 | .74 | |||
| Internal locus of control (HLOC, internality) c | 1.01 | 0.98–1.04 | .56 | |||
OR= unadjusted odds ratio
OR= adjusted odds ratio
PSCA= Perceived Self-Care Agency score; DAS= Dyadic Adjustment Scale; HLOC= Health Locus of Control score.
Figure 2.
Percentage of total readmissions per each month during the first year after lung transplantation discharge.
Cumulative incidence of readmission
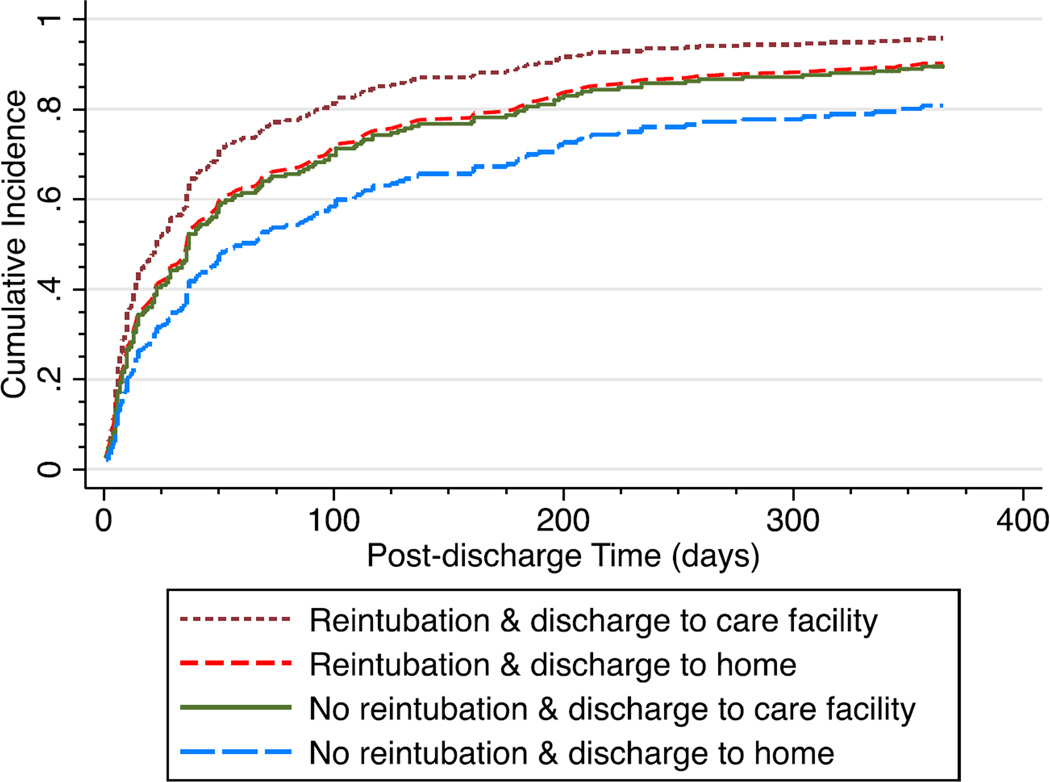
Results from the competing risks regression model shows that the cumulative incidences of readmission, after controlling for reintubation and discharge destination, were 54.5%, 73.7%, and 84.5% at time points 2, 6, and 12 months, respectively. Figure 3 depicts the cumulative incidences of readmission by the combinations of reintubation and discharge destination during the first year following index discharge. Cumulative incidences of readmission at 12 months ranged from 80.8% for non-reintubated LTRs discharged home to 95.7% for reintubated LTRs discharged to care facilities.
Figure 3.
Cumulative incidence of readmission by reintubation status and discharge destination during the first year after lung transplantation discharge.
Readmission count
Slightly less than two thirds of the sample (64.2%) had multiple readmissions during the first year. The numbers of readmissions for each time interval and for the total study period are displayed in Table 2. Results from the negative binomial regression indicate that LTRs had less readmissions over time (OR=0.92; 95% CI, 0.90–0.95; p<.001), after adjusting for other predictors in the multivariate model (Table 4). LTRs who were discharged to care facilities had 1.45 times higher count of readmissions than those who were discharged home (95% CI, 1.08–1.94; p=.01). Furthermore, for every one point increase in capacity for self-care (PSCA score), the count of readmissions would decrease by 1% (95% CI, 0.99–1.00, p=.03), while holding all other variables in the model constant.
Table 4.
Predictors of Readmission Count During the First Year After Lung Transplantation Discharge
| Characteristics | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| IRR a | 95% CI | P | IRR b | 95% CI | P | |
| Time, per month increase | 0.93 | 0.91–0.95 | <.001 | 0.92 | 0.90–0.95 | <.001 |
| Sociodemographic | ||||||
| Gender, male | 0.98 | 0.77–1.25 | .88 | |||
| Age at transplant (years) | 1.00 | 0.99–1.01 | .53 | |||
| Marital status, unmarried | 1.25 | 0.97–1.62 | .09 | 1.19 | 0.94–1.52 | .15 |
| Race, white | 1.01 | 0.65-1.57 | .96 | |||
| Education, < high school | 1.51 | 0.95–2.40 | .08 | 1.28 | 0.87–1.87 | .21 |
| Employment, unemployed | 1.33 | 0.87–2.05 | .19 | |||
| Clinical | ||||||
| Underlying disease, obstructive | 1.26 | 0.99–1.60 | .06 | 1.27 | 0.99–1.63 | .06 |
| Type of transplant, single | 1.17 | 0.86–1.57 | .31 | |||
| Post-op vent days, < 48 hours | 1.07 | 0.82–1.40 | .63 | |||
| Length of stay days, per 1 day increase | 1.00 | 1.00–1.01 | .25 | |||
| Reintubated, yes | 1.44 | 1.13–1.83 | .003 | 1.25 | 0.97–1.61 | .09 |
| Return to ICU, yes | 1.14 | 0.85–1.52 | .39 | |||
| Hospital discharge destination, facility | 1.68 | 1.28–2.21 | <.001 | 1.45 | 1.08–1.94 | .01 |
| Self-Care | ||||||
| Self-care agency (PSCA) c | 0.99 | 0.99–1.00 | .01 | 0.99 | 0.99–1.00 | .03 |
| Caregiver relationship quality (DAS) c | 0.99 | 0.98–1.01 | .46 | |||
| Internal locus of control (HLOC, internality) c | 1.00 | 0.98–1.02 | .94 | |||
IRR= unadjusted incidence rate ratio
IRR= adjusted incidence rate ratio
PSCA= Perceived Self-Care Agency score; DAS= Dyadic Adjustment Scale; HLOC= Health Locus of Control score.
Readmission cumulative duration
Cumulative number of readmission days for the LTRs who were readmitted are presented in Table 2. The median cumulative duration of all first year readmissions was 19 days, and more than one third of LTRs in the sample (37.5%) were readmitted for more than 30 days. Results from the gamma regression shows that cumulative readmission duration decreased over time (OR=0.91; 95% CI, 0.88–0.94; p<.001), after controlling for other predictors in the multivariate model (Table 5). In addition, for every one year increase in age, mean cumulative readmission duration would increase by 2% (95% CI, 1.00–1.03; p=.009). For LTRs who returned to the ICU, the mean cumulative readmission duration was twice the mean cumulative readmission duration for LTRs who did not return to the ICU (95% CI, 1.17–3.43; p=.01), after adjusting for other variables in the model.
Table 5.
Predictors of Cumulative Readmission Duration During the First Year After Lung Transplantation Discharge
| Characteristics | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| AMR a | 95% CI | P | AMR b | 95% CI | P | |
| Time, per month increase | 0.92 | 0.88–0.95 | <.001 | 0.91 | 0.88–0.94 | <.001 |
| Sociodemographic | ||||||
| Gender, male | 1.16 | 0.83–1.61 | .38 | |||
| Age at transplant (years) | 1.02 | 1.00–1.03 | .01 | 1.02 | 1.00–1.03 | .009 |
| Marital status, unmarried | 0.94 | 0.65–1.36 | .73 | |||
| Race, white | 0.80 | 0.41–1.57 | .52 | |||
| Education, < high school | 0.85 | 0.54–1.34 | .49 | |||
| Employment, unemployed | 0.96 | 0.56–1.65 | .87 | |||
| Clinical | ||||||
| Underlying disease, obstructive | 1.30 | 0.94–1.79 | .11 | |||
| Type of transplant, single | 1.45 | 1.01–2.07 | .04 | 0.97 | 0.65–1.44 | .86 |
| Post-op vent days, < 48 hours | 1.16 | 0.82–1.66 | .40 | |||
| Length of stay days, per 1 day increase | 1.01 | 1.00–1.01 | .04 | 0.99 | 0.99–1.00 | .24 |
| Reintubated, yes | 1.28 | 0.89–1.84 | .18 | |||
| Return to ICU, yes | 1.65 | 1.15–2.37 | .006 | 2.00 | 1.17–3.43 | .01 |
| Hospital discharge destination, facility | 1.41 | 0.98–2.04 | .07 | 1.19 | 0.73–1.92 | .49 |
| Self-Care | ||||||
| Self-care agency (PSCA) c | 0.99 | 0.99–1.00 | .06 | 0.99 | 0.99–1.00 | .03 |
| Caregiver relationship quality (DAS) c | 1.00 | 0.98–1.02 | .71 | |||
| Internal locus of control (HLOC, internality) c | 1.00 | 0.98–1.02 | .84 | |||
AMR = unadjusted arithmetic mean ratio
AMR = adjusted arithmetic mean ratio
PSCA= Perceived Self-Care Agency score; DAS= Dyadic Adjustment Scale; HLOC= Health Locus of Control score.
DISCUSSION
Little is known about the pattern and predictors of hospital readmissions during the first year following lung transplantation. Here, we described the incidence, count, cumulative duration, and common reasons for readmission and identified certain sociodemographic, clinical and self-care characteristics at the time of transplant as risk factors for readmission within the first year after lung transplantation.
Complications such as gastrointestinal problems (diarrhea (51–53), nausea and vomiting (54, 55)) and renal failure (56–58) are common after lung transplantation, often requiring hospital readmission. Many of these complications (e.g., nausea) are likely side effects of immunosuppressants and anti-viral medications (55). Such complications decrease over time and with symptomatic treatments (20), which is consistent with our findings. On the other hand, there was an increase in infections as a reason for readmission after two months. This is likely attributable to changes in the nature of infectious diseases after two months including the emergence of opportunistic infections (59) and multidrug-resistant bacterial infections (60) due to combination of prolonged immunosuppression treatment and viral infections (61).
Reintubation during the index transplant hospitalization was a significant predictor of readmission incidence, which was expected given that reintubation is considered a prognostic factor for lung transplantation survival (62), an indicator for severe graft dysfunction (63), and associated with further complications such as nosocomial pneumonia (64). Novel methods to treat post-transplant respiratory failure and decrease the need for reintubation, such as rapid extubation and noninvasive positive pressure ventilation (NIPPV) (63), may improve health outcomes and reduce the risk for further complications, leading ultimately to lower readmission risk.
We found an association between discharge destination and both readmission incidence and count in the lung transplant population. This result is consistent with findings from other studies reporting that older community-dwelling adults (48, 65), vascular (66) and colorectal (67) surgery patients, and liver transplant recipients (68) who were discharged to care facilities were at significantly higher risk for readmission than those discharged home. This finding likely reflects the lower functional status of such patients (69) and thus higher risk for readmission. Coordination of care between the transplant center and care facilities such as rehabilitation and other skilled residential settings may improve future health outcomes and reduce risk for frequent readmissions.
Of all self-care characteristics, capacity for self-care was the only factor associated with fewer and shorter hospital readmissions, suggesting the importance of LTRs’ active involvement in the self-care process. Perceiving oneself to have greater capacity for self-care was previously found to be associated with actual self-care behaviors and thus improved health outcomes (26). This study adds to previous reports that found self-care to be associated fewer hospital readmissions in general (70–73). Employment of evidence-based protocols for transitional care, effective discharge planning, and education about self-care (74) are needed to promote self-care behaviors and reduce readmission count and duration.
Older age and readmission to the ICU were found to place LTRs are at risk for longer readmission duration. Identifying these LTRs at the time of hospital discharge may help modify discharge planning to meet their special needs. For those who require readmission to the hospital, mobilization of resources and targeted interventions, particularly those of older age, may help reduce their duration of the hospital stay.
This study has several limitations. The sample included LTRs from only one transplant center and thus the results may not be generalizable. Although the percentage of single lung transplant procedures in our sample was lower than the respective national average (about 31%), the sociodemographic characteristics of our sample were generally equivalent to US national lung transplant populations during the same period (75). In addition, the study is a secondary analysis, so there was limited control over the previously collected variables and data for some variables of interest were not available, such as characteristics of donors, comorbidities, and medication usage to provide more comprehensive models predicting readmissions. Several of the risk factors for readmission, such as age and need for reintubation, may not be surprising given that patients with higher acuity are likely to have worse health outcomes including readmission profiles. Yet this study is the first to provide empirical evidence of how these factors affect LTRs readmission. Furthermore, while several predictors in this study were interdependent, non-modifiable, they help identify LTR who at greater risk for readmission and thus may benefit from early supportive interventions. Finally, our data were collected for the first year after discharge, limiting our ability to investigate the long-term readmission patterns.
In conclusion, hospital readmission after lung transplantation remains one of the major complications of the procedure. In this study, we described and identified the pattern (incidence, count, duration), reasons, and predictors of readmission during first-year after lung transplantation. While the parent study did not find impact of the mHealth intervention on readmission, the knowledge presented in this study may impact clinical practice as discussed earlier. That is, recognition of the risks for readmission (i.e., the need for reintubation, re-admission to the ICU, discharge to an extended care facility, older patients and those who report of limited capacity for self-care) at the time of discharge may help mobilize resources to vulnerable subpopulations of patients and enhance the discharge process (76, 77), an endeavor that may reduce the cost and effort associated with readmission, and guide the utilization of healthcare resources (78).
Acknowledgments
Funding support: NIH, NINR (R01NR010711-DeVito Dabbs, PI)
Abbreviations
- AMR
arithmetic mean ratio
- CMS
Centers for Medicare & Medicaid Services
- CI
confidence interval
- DAS
Dyadic Adjustment Scale
- GEE
Generalized Estimating Equations
- HLOC
Health Locus of Control
- ICU
intensive care unit
- IRR
incidence rate ratio
- ISHLT
International Society of Heart and Lung Transplantation
- IQR
interquartile range
- LTR
lung transplant recipient
- mHealth
mobile health
- NIPPV
noninvasive positive pressure ventilation
- OR
odd ratio
- PSCA
Perception of Self-Care Agency
- RCT
randomized controlled trial
- UPMC
University of Pittsburgh Medical Center
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
REFERENCES
- 1.Axon RN, Williams MV. Hospital Readmission as an Accountability Measure. Jama. 2011;305(5):504. doi: 10.1001/jama.2011.72. [DOI] [PubMed] [Google Scholar]
- 2.Chen P, Wang W, Yan L, Yang J, Wen T, Li B, et al. Risk factors for first-year hospital readmission after liver transplantation. Eur J Gastroenterol Hepatol. 2015;27(5):600–606. doi: 10.1097/MEG.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 3.Ashton CM, Kuykendall DH, Johnson ML, Wray NP, Wu L. The association between the quality of inpatient care and early readmission. Ann Intern Med. 1995;122(6):415–421. doi: 10.7326/0003-4819-122-6-199503150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Graham KL, Wilker EH, Howell MD, Davis RB, Marcantonio ER. Differences between early and late readmissions among patients: a cohort study. Ann Intern Med. 2015;162(11):741–749. doi: 10.7326/AITC201506020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans KA. Reducing readmission rates. Adv NPs PAs. 2013;4(2):12. [PubMed] [Google Scholar]
- 6.McHugh MD, Carthon JM, Kang XL. Medicare readmissions policies and racial and ethnic health disparities: a cautionary tale. Policy Polit Nurs Pract. 2010;11(4):309–316. doi: 10.1177/1527154411398490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.United States. Compilation of Patient Protection and Affordable Care Act : as amended through November 1, 2010 including Patient Protection and Affordable Care Act health-related portions of the Health Care and Education Reconciliation Act of 2010. Washington: U.S. Government Printing Office; 2010. United States. Congress. House. Office of the Legislative Counsel, United States. Congress. House. Committee on Ways and Means., United States. Congress. House. Committee on Energy and Commerce., United States. Congress. House. Committee on Education and Labor; p. 267. [Google Scholar]
- 8.Joynt KE, Orav EJ, Jha AK. Thirty-day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305(7):675–681. doi: 10.1001/jama.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaduganathan M, Bonow RO, Gheorghiade M. Thirty-Day Readmissions. Jama. 2013;309(4):345. doi: 10.1001/jama.2012.205110. [DOI] [PubMed] [Google Scholar]
- 10.Paterno F, Wilson GC, Wima K, Quillin RC, Abbott DE, Cuffy MC, et al. Hospital utilization and consequences of readmissions after liver transplantation. Surgery. 2014;156(4):871–879. doi: 10.1016/j.surg.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Ladner DP, Skaro AI, Abecassis MM. Are all readmissions the same? Liver Transpl. 2012;18(9):1007–1008. doi: 10.1002/lt.23517. [DOI] [PubMed] [Google Scholar]
- 12.Tomaszek SC, Fibla JJ, Dierkhising RA, Scott JP, Shen K-HR, Wigle DA, et al. Outcome of lung transplantation in elderly recipients. European Journal of Cardio-Thoracic Surgery. 2011;39(5):726–731. doi: 10.1016/j.ejcts.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 13.Taylor DO, Stehlik J, Edwards LB, Aurora P, Christie JD, Dobbels F, et al. Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Heart Transplant Report—2009. Journal of Heart and Lung Transplantation. 2009;28(10):1007–1022. doi: 10.1016/j.healun.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Hartert M, Senbaklavacin O, Gohrbandt B, Fischer BM, Buhl R, Vahld CF. Lung transplantation: a treatment option in end-stage lung disease. Dtsch Arztebl Int. 2014;111(7):107–116. doi: 10.3238/arztebl.2014.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finlen Copeland CA, Vock DM, Pieper K, Mark DB, Palmer SM. Impact of lung transplantation on recipient quality of life: a serial, prospective, multicenter analysis through the first posttransplant year. Chest. 2013;143(3):744–750. doi: 10.1378/chest.12-0971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.NIH. What Is a Lung Transplant? 2011 Available from: https://www.nhlbi.nih.gov/health/health-topics/topics/lungtxp. [Google Scholar]
- 17.Kugler C, Gottlieb J, Warnecke G, Schwarz A, Weissenborn K, Barg-Hock H, et al. Health-Related Quality of Life After Solid Organ Transplantation. Transplantation. 2013;96(3):316–323. doi: 10.1097/TP.0b013e31829853eb. [DOI] [PubMed] [Google Scholar]
- 18.Singer JP, Chen J, Blanc PD, Leard LE, Kukreja J, Chen H. A Thematic Analysis of Quality of Life in Lung Transplant: The Existing Evidence and Implications for Future Directions. American Journal of Transplantation. 2013;13(4):839–850. doi: 10.1111/ajt.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen J, Singer P, Raviv Y, Bakal I, Shitrit D, Lev S, et al. Outcome of lung transplant recipients requiring readmission to the intensive care unit. J Heart Lung Transplant. 2011;30(1):54–58. doi: 10.1016/j.healun.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Arcasoy S, Wilt J. Medical Complications after Lung Transplantation. Seminars in Respiratory and Critical Care Medicine. 2006;27(5):508–520. doi: 10.1055/s-2006-954611. [DOI] [PubMed] [Google Scholar]
- 21.International Society for Hearth and Lung Transplantation (ISHLT) Registry Slides: Overall Lung and Adult Lung Transplantation Statistics. 2012 [Google Scholar]
- 22.Dezfouli AA, Najafizadeh K, Parsa T, Shadmehr MB, Dabir S, Mohammadi F, et al. Postlung transplant rehospitalization: a study of causes, health care burden, and outcomes. Exp Clin Transplant. 2009;7(3):192–196. [PubMed] [Google Scholar]
- 23.Jweinat JJ. Hospital readmissions under the spotlight. J Healthc Manag. 2010;55(4):252–264. [PubMed] [Google Scholar]
- 24.DeVito Dabbs A, Song MK, Myers BA, Li R, Hawkins RP, Pilewski JM, et al. A Randomized Controlled Trial of a Mobile Health Intervention to Promote Self-Management after Lung Transplantation. Am J Transplant. 2016 doi: 10.1111/ajt.13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions: a review. Patient Education and Counseling. 2002;48(2):177–187. doi: 10.1016/s0738-3991(02)00032-0. [DOI] [PubMed] [Google Scholar]
- 26.DeVito Dabbs A, Dew MA, Myers B, Begey A, Hawkins R, Ren D, et al. Evaluation of a hand-held, computer-based intervention to promote early self-care behaviors after lung transplant. Clinical transplantation. 2009;23(4):537–545. doi: 10.1111/j.1399-0012.2009.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeVito Dabbs A, Song M-K, Myers B, Hawkins RP, Aubrecht J, Begey A, et al. Clinical trials of health information technology interventions intended for patient use: unique issues and considerations. Clinical trials (London, England) 2013;10:896–906. doi: 10.1177/1740774513493149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanson B, Bickel L. Development and testing of the questionnaire on perception of self-care agency. In: Riehl-Sisca J, editor. The science and art of self-care. Norwalk, CT: Appleton-Century-Crofts; 1985. pp. 271–278. [Google Scholar]
- 29.Hartweg DL. Dorothea Orem: self-care deficit theory. Newbury Park, Calif.: Sage Publications; 1991. [Google Scholar]
- 30.Nunnally JC. Psychometric Theory. 2nd. New York: McGraw-Hill; 1978. [Google Scholar]
- 31.Spanier GB. Measuring Dyadic Adjustment: New Scales for Assessing the Quality of Marriage and Similar Dyads. Journal of Marriage and the Family. 1976;38:15. [Google Scholar]
- 32.DeVito Dabbs A, Terhorst L, Song MK, Shellmer DA, Aubrecht J, Connolly M, et al. Quality of recipient-caregiver relationship and psychological distress are correlates of self-care agency after lung transplantation. Clinical transplantation. 2013;27(1):113–120. doi: 10.1111/ctr.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dew MA, Kormos RL, Roth LH, Murali S, DiMartini A, Griffith BP. Early post-transplant medical compliance and mental health predict physical morbidity and mortality one to three years after heart transplantation. J Heart Lung Transplant. 1999;18(6):549–562. doi: 10.1016/s1053-2498(98)00044-8. [DOI] [PubMed] [Google Scholar]
- 34.Wallston BD, Wallston KA. Locus of control and health: a review of the literature. Health education monographs. 1978;6:107–117. doi: 10.1177/109019817800600102. [DOI] [PubMed] [Google Scholar]
- 35.Wallston Ka, Wallston BS, DeVellis R, Strudler Wallston B. Development of the Multidimensional Health Locus of Control (MHLC) Scales. Health Education & Behavior. 1978;6:160–170. doi: 10.1177/109019817800600107. [DOI] [PubMed] [Google Scholar]
- 36.Thakral S, Bhatia T, Gettig EA, Nimgaonkar VL, Deshpande SN. A comparative study of health locus of control in patients with schizophrenia and their first degree relatives. Asian journal of psychiatry. 2014;7:34–37. doi: 10.1016/j.ajp.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rongen A, Robroek SJW, Burdorf A. The importance of internal health beliefs for employees' participation in health promotion programs. Preventive medicine. 2014;67:330–334. doi: 10.1016/j.ypmed.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 38.van der Heijden GJMG, Donders aRT, Stijnen T, Moons KGM. Imputation of missing values is superior to complete case analysis and the missing-indicator method in multivariable diagnostic research: a clinical example. Journal of clinical epidemiology. 2006;59:1102–1109. doi: 10.1016/j.jclinepi.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Sim J, Wright CC. The kappa statistic in reliability studies: Use, interpretation, and sample size requirements. Physical Therapy. 2005;85(3):257–268. [PubMed] [Google Scholar]
- 40.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 41.Minke A. Annual meeting of the Southwest Educational Research Association. Austin, TX: ERIC; 1997. Conducting Repeated Measures Analyses: Experimental Design Considerations. 1997. [Google Scholar]
- 42.Burton P, Gurrin L, Sly P. Extending the simple linear regression model to account for correlated responses: an introduction to generalized estimating equations and multi-level mixed modelling. Stat Med. 1998;17(11):1261–1291. doi: 10.1002/(sici)1097-0258(19980615)17:11<1261::aid-sim846>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 43.Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Second. New York: Springer; 2012. Regression methods in biostatistics: linear, logistic, survival, and repeated measures models. [Google Scholar]
- 44.Lee AH, Xiang L, Hirayama F. Modeling physical activity outcomes: a two-part generalized-estimating-equations approach. Epidemiology. 2010;21(5):626–630. doi: 10.1097/EDE.0b013e3181e9428b. [DOI] [PubMed] [Google Scholar]
- 45.Bejanyan N, Bolwell BJ, Lazaryan A, Rybicki L, Tench S, Duong H, et al. Risk factors for 30-day hospital readmission following myeloablative allogeneic hematopoietic cell transplantation (allo-HCT) Biol Blood Marrow Transplant. 2012;18(6):874–880. doi: 10.1016/j.bbmt.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 46.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith MA, Liou J-I, Frytak JR, Finch MD. 30-day survival and rehospitalization for stroke patients according to physician specialty. Cerebrovascular diseases (Basel, Switzerland) 2006;22:21–26. doi: 10.1159/000092333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bogaisky M, Dezieck L. Early hospital readmission of nursing home residents and community-dwelling elderly adults discharged from the geriatrics service of an urban teaching hospital: patterns and risk factors. J Am Geriatr Soc. 2015;63(3):548–552. doi: 10.1111/jgs.13317. [DOI] [PubMed] [Google Scholar]
- 49.Corp I. IBM SPSS Statistics for Macintosh, Version 22.0. Armonk, NY: IBM Corp; 2013. [Google Scholar]
- 50.StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 51.Pant C, Deshpande A, Larson A, O'Connor J, Rolston DD, Sferra TJ. Diarrhea in solid-organ transplant recipients: a review of the evidence. Curr Med Res Opin. 2013;29(10):1315–1328. doi: 10.1185/03007995.2013.816278. [DOI] [PubMed] [Google Scholar]
- 52.Pescovitz MD, Navarro MT. Immunosuppressive therapy and post-transplantation diarrhea. Clinical transplantation. 2001;15(Suppl 4):23–28. doi: 10.1111/j.1399-0012.2001.00023.x. [DOI] [PubMed] [Google Scholar]
- 53.Lee LY, Ison MG. Diarrhea caused by viruses in transplant recipients. Transpl Infect Dis. 2014;16(3):347–358. doi: 10.1111/tid.12212. [DOI] [PubMed] [Google Scholar]
- 54.Bravo C, Gispert P, Borro JM, de la Torre M, Cifrian Martinez JM, Fernandez Rozas S, et al. Prevalence and management of gastrointestinal complications in lung transplant patients: MITOS study group. Transplant Proc. 2007;39(7):2409–2412. doi: 10.1016/j.transproceed.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 55.Lyu DM, Zamora MR. Medical complications of lung transplantation. Proc Am Thorac Soc. 2009;6(1):101–107. doi: 10.1513/pats.200808-077GO. [DOI] [PubMed] [Google Scholar]
- 56.Grimm JC, Lui C, Kilic A, Valero V, 3rd, Sciortino CM, Whitman GJ, et al. A risk score to predict acute renal failure in adult patients after lung transplantation. Ann Thorac Surg. 2015;99(1):251–257. doi: 10.1016/j.athoracsur.2014.07.073. [DOI] [PubMed] [Google Scholar]
- 57.George TJ, Arnaoutakis GJ, Beaty CA, Pipeling MR, Merlo CA, Conte JV, et al. Acute kidney injury increases mortality after lung transplantation. Ann Thorac Surg. 2012;94(1):185–192. doi: 10.1016/j.athoracsur.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacques F, El-Hamamsy I, Fortier A, Maltais S, Perrault LP, Liberman M, et al. Acute renal failure following lung transplantation: risk factors, mortality, and long-term consequences. Eur J Cardiothorac Surg. 2012;41(1):193–199. doi: 10.1016/j.ejcts.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Avery RK. Infections after lung transplantation. Semin Respir Crit Care Med. 2006;27(5):544–551. doi: 10.1055/s-2006-954612. [DOI] [PubMed] [Google Scholar]
- 60.Yun JH, Lee SO, Jo KW, Choi SH, Lee J, Chae EJ, et al. Infections after lung transplantation: time of occurrence, sites, and microbiologic etiologies. Korean J Intern Med. 2015;30(4):506–514. doi: 10.3904/kjim.2015.30.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weiss ES, Allen JG, Merlo CA, Conte JV, Shah AS. Factors indicative of long-term survival after lung transplantation: a review of 836 10-year survivors. J Heart Lung Transplant. 2010;29(3):240–246. doi: 10.1016/j.healun.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 62.Machuca TN, Schio SM, Camargo SM, Lobato V, Costa CDO, Felicetti JC, et al. Prognostic factors in lung transplantation: the Santa Casa de Porto Alegre experience. Transplantation. 2011;91:1297–1303. doi: 10.1097/TP.0b013e31821ab8e5. [DOI] [PubMed] [Google Scholar]
- 63.Feltracco P, Serra E, Barbieri S, Milevoj M, Furnari M, Rizzi S, et al. Noninvasive ventilation in postoperative care of lung transplant recipients. Transplantation proceedings. 2009;41:1339–1344. doi: 10.1016/j.transproceed.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 64.Torres A, Gatell JM, Aznar E, el-Ebiary M, Puig de la Bellacasa J, González J, et al. Re-intubation increases the risk of nosocomial pneumonia in patients needing mechanical ventilation. American journal of respiratory and critical care medicine. 1995;152:137–141. doi: 10.1164/ajrccm.152.1.7599812. [DOI] [PubMed] [Google Scholar]
- 65.Lum HD, Studenski Sa, Degenholtz HB, Hardy SE. Early hospital readmission is a predictor of one-year mortality in community-dwelling older Medicare beneficiaries. Journal of general internal medicine. 2012;27:1467–1474. doi: 10.1007/s11606-012-2116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Engelbert TL, Fernandes-Taylor S, Gupta PK, Kent KC, Matsumura J. Clinical characteristics associated with readmission among patients undergoing vascular surgery. Journal of vascular surgery. 2014;59:1349–1355. doi: 10.1016/j.jvs.2013.10.103. [DOI] [PubMed] [Google Scholar]
- 67.Li LT, Mills WL, White DL, Li A, Gutierrez AM, Berger DH, et al. Causes and prevalence of unplanned readmissions after colorectal surgery: a systematic review and meta-analysis. J Am Geriatr Soc. 2013;61(7):1175–1181. doi: 10.1111/jgs.12307. [DOI] [PubMed] [Google Scholar]
- 68.Serper M, Koppe S, Kang R, Changelian A, Ladner D, Levitsky J. Discharge destination, caregiver support, and clinical outcomes following adult liver transplantation. Hepatology. 2013;58:1036A–1037A. [Google Scholar]
- 69.Banga A, Sahoo D, Lane CR, Mehta AC, Akindipe O, Budev MM, et al. Characteristics and outcomes of patients with lung transplantation requiring admission to the medical ICU. Chest. 2014;146(3):590–599. doi: 10.1378/chest.14-0191. [DOI] [PubMed] [Google Scholar]
- 70.Ditewig JB, Blok H, Havers J, van Veenendaal H. Effectiveness of self-management interventions on mortality, hospital readmissions, chronic heart failure hospitalization rate and quality of life in patients with chronic heart failure: a systematic review. Patient Educ Couns. 2010;78(3):297–315. doi: 10.1016/j.pec.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 71.Burns ME, Galbraith AA, Ross-Degnan D, Balaban RB. Feasibility and evaluation of a pilot community health worker intervention to reduce hospital readmissions. Int J Qual Health Care. 2014;26(4):358–365. doi: 10.1093/intqhc/mzu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kirby SE, Dennis SM, Bazeley P, Harris MF. Activating patients with chronic disease for self-management: comparison of self-managing patients with those managing by frequent readmissions to hospital. Aust J Prim Health. 2013;19(3):198–206. doi: 10.1071/PY12030. [DOI] [PubMed] [Google Scholar]
- 73.Lorig KR, Sobel DS, Stewart AL, Brown BW, Jr, Bandura A, Ritter P, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Med Care. 1999;37(1):5–14. doi: 10.1097/00005650-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 74.Yam CH, Wong EL, Chan FW, Leung MC, Wong FY, Cheung AW, et al. Avoidable readmission in Hong Kong--system, clinician, patient or social factor? BMC Health Serv Res. 2010;10:311. doi: 10.1186/1472-6963-10-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Valapour M, Skeans MA, Heubner BM, Smith JM, Schnitzler MA, Hertz MI, et al. OPTN/SRTR 2012 Annual Data Report: lung. Am J Transplant. 2014;14(Suppl 1):139–165. doi: 10.1111/ajt.12584. [DOI] [PubMed] [Google Scholar]
- 76.Shankar N, Marotta P, Wall W, Albasheer M, Hernandez-Alejandro R, Chandok N. Defining readmission risk factors for liver transplantation recipients. Gastroenterology & hepatology. 2011;7:585–590. [PMC free article] [PubMed] [Google Scholar]
- 77.Kansagara D, Englander H, Salanitro A, Kagen D, Theobald C, Freeman M, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698. doi: 10.1001/jama.2011.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fidahussein SS, Croghan IT, Cha SS, Klocke DL. Posthospital follow-up visits and 30-day readmission rates in chronic obstructive pulmonary disease. Risk Manag Healthc Policy. 2014;7:105–112. doi: 10.2147/RMHP.S62815. [DOI] [PMC free article] [PubMed] [Google Scholar]