Abstract
A number of findings suggested that general anesthetics induced neural cell death by apoptosis in various animal models. Although clinical evidence regarding the correlation between anesthetic exposures at young age and subsequent cognitive impairments remains unclear, repeated or consistent exposures to general anesthetics may be a potential harmful risk in developing human brains. The mechanisms underlying the anesthetic neurotoxicity have received extensive attention recently. We will attempt a brief review to summarize current understanding on the role of both apoptosis and autophagic cell death mediated by calcium dysregulation in anesthetic neurotoxicity.
Keywords: Anesthesia, Anesthetics, Autophagy, Calcium, Apoptosis, Neurodegeneration
The neurotoxic effects of general anesthetics (GAs) have been widely concerned ever since the report of halothane-related morphologic changes and functional deficits of the brain in rats (Uemura et al., 1985) and the striking finding of anesthetics mediated apoptosis in developing brains (Jevtovic-Todorovic et al., 2003). Accumulating evidence suggested that many anesthetics including midazolam (Young et al., 2005), ketamine (Fredriksson et al., 2004; Garcia et al., 2003; Hayashi et al., 2002; Slikker et al., 2007; Young et al., 2005; Zou et al., 2009a; Zou et al., 2009b), propofol (Cattano et al., 2008; Pesic et al., 2009; Vutskits et al., 2005), isoflurane (Istaphanous et al., 2011; Loepke et al., 2009; Ma et al., 2007; Nikizad et al., 2007; Palanisamy et al., 2011; Sanders et al., 2009), sevoflurane (Istaphanous et al., 2011; Satomoto et al., 2009; Zhang et al., 2008), and desflurane (Istaphanous et al., 2011), induced neural cell death by apoptosis in various tissue cultures and animal models. In particular, a number of animal studies revealed that excessive anesthesia exposures in the developing brains may impair neurodevelopment and cause long-term neurocognitive deficits (Hayashi et al., 2002; Zou et al., 2009a). Although clinical evidence regarding the correlation between anesthetic exposures at young age and subsequent cognitive impairments remains unclear, multiple or prolonged exposures to anesthetics may be a potential harmful risk in developing human brains (Flick et al., 2011; Sprung et al., 2012). Therefore, the mechanisms underlying the anesthetic neurotoxicity are of great importance to guide the use of general anesthesia safely in patients. In this brief review, we have focused on the summary of current knowledge on different types of cell death (Type 1 apoptosis vs. type 2 autophagic cell death) mediated by calcium dysregulation in anesthetic neurotoxicity.
Intracellular Ca2+ serves as a biological messenger that is involved in controlling almost all cell processes, including muscle contraction, exocytosis, proliferation, differentiation, protein synthesis, and gene expression, etc (Berridge et al., 2003). Therefore, maintenance of calcium homeostasis plays a crucial role for cell survival. Under physiological conditions, the concentration of cytosolic free calcium ([Ca2+]c) fluctuates at around 100 nM, much lower than that in endoplasmic reticulum (ER) (~300 to 500 µM) and mitochondrion (~0.5 mM), as well as extracellular calcium level (~1.2 mM) (Orrenius et al., 2003). The recent studies indicated that disruption of intracellular calcium homeostasis may be an emerging mechanism involved in anesthetic-induced neuroapoptosis (Joseph, J Donald et al., 2014; Wei, 2011; Zhao et al., 2013). Previous evidence demonstrated that inhalational anesthetics induced cell damage by causing abnormal calcium release from the ER via over-activation of Inositol trisphosphate receptors (InsP3Rs) and/or ryanodine receptors (RyRs) in a concentration- and duration-dependent manner (Inan and Wei, 2010; Joseph, J. D. et al., 2014; Orrenius et al., 2003; Stutzmann et al., 2007; Yang et al., 2008). The increase in the [Ca2+]c regulates two different self-destructive processes, Type 1 apoptosis and type 2 autophagic cell death (Medina et al., 2015; Orrenius et al., 2003), and whose interaction may determine cell fate (Maiuri et al., 2007) as shown in Fig. 1.
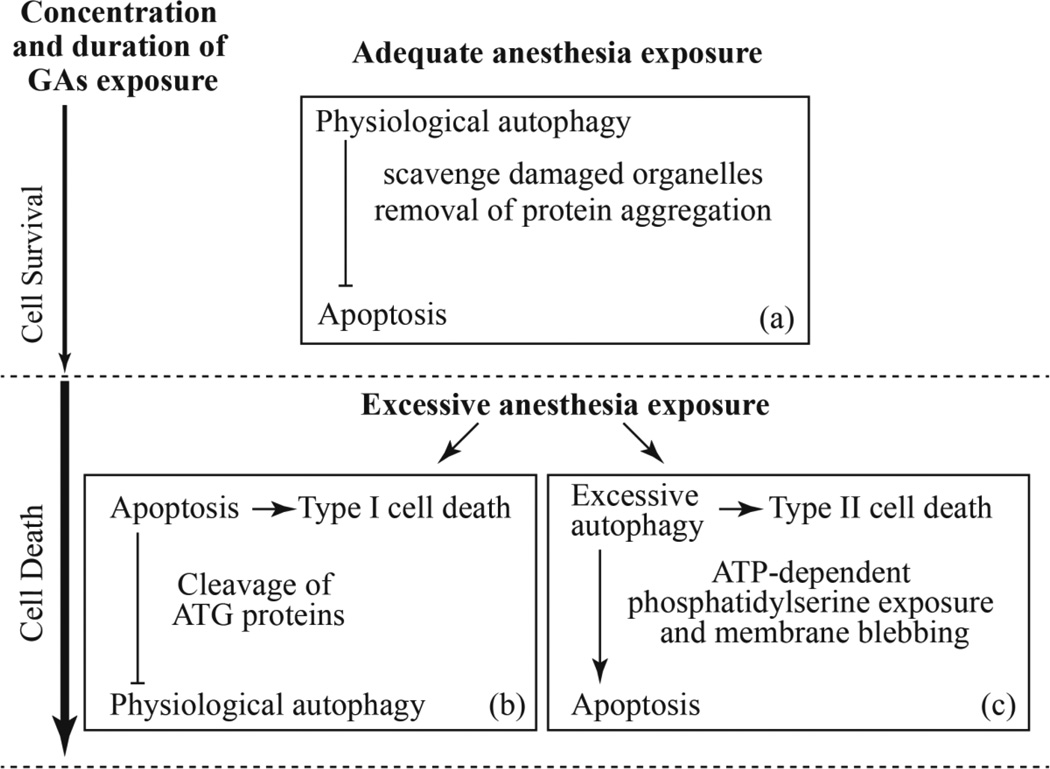
Fig. 1.
Interaction between autophagy and apoptosis induced by general anesthetics with various degrees of concentrations and durations. (a) Physiological autophagy plays a protective role for cell survival by inhibiting apoptosis induction after adequate general anesthetics (GAs) at a low concentration and short exposure. However, excessive GAs at a persistent and high concentration result in type I cell death by apoptosis and inhibit protective autophagy activity via cleavage of ATG protein required for autophagy (b), and over-activated autophagy that cause type II autophagic cell death and aggravation of apoptosis (c).
General anesthetics at low concentration for short exposure may activate autophagy to scavenge damaged organelles and remove protein aggregation, as a normal physiological function, and therefore elevating the threshold for apoptosis induction. Zhou et al. showed that sevoflurane induced ER stress and activated autophagy, which protected against sevoflurane-induced apoptosis in H4 cells (Zhou et al., 2016). It also been suggested that isoflurane-induced increase in cleaved caspase-3 in the neonatal rat brain can be ameliorated by preconditioning with a brief anesthetic exposure (Peng et al., 2014). Consistent with these findings, it was proposed that adequate promotion of autophagy may play a protective role for cell survival by preventing developmental neurotoxicity of general anesthetics (Li and Yu, 2014). Therefore, the moderate anesthesia that induces protectively autophagic responses may be beneficial for an “adequate” adaptation to potential neurotoxicity of general anesthetics, as referred to adequate anesthesia exposure in Fig.1 (a).
However, contrast to the moderate anesthesia, a prolonged and high concentration exposure of general anesthetics can induce ER stress and subsequent apoptosis and/or excessive autophagy, which eventually results in type I or II cell death respectively. Loepke et al. showed that prolonged isoflurane exposure in neonatal mice led to increased immediate brain cell degeneration (Loepke et al., 2009). It was reported that ER stress pathway mediated isoflurane-induced neuroapoptosis and cognitive impairments in aged rats (Ge et al., 2015). These evidence implicate that aged and developing brain may be more vulnerable to long-term exposure to general anesthetics. It was also suggested that sevoflurane induced apoptosis, degeneration and decreased self-renewal capacity of neural stem cells in a time-dependent way (Nie et al., 2013; Qiu et al., 2015). The overmuch anesthesia that exceed the physiological adaptation of neural cells may be “excessive” and detrimental. As illustrated in Fig 1(b and c), this excessive anesthesia exposure may cause neurotoxicity by inducing apoptosis and/or excessive autophagy.
Excessive autophagy facilitates ATP-dependent events such as phospholipids (PS) exposure and membrane blebbing by producing more ATP, and promoting apoptosis. On the other hand, excessive autophagy may cause cell death directly in a “self-eating” mode (Fig. 1(c)). Apoptosis is a suicidal cell death program, accompanied by caspase activation and degradation of cell component. The caspases trigger the cleavage of multiple proteins including autophagy-related (ATG) proteins to weaken the apoptosis induction and accelerate cell demise (Fig. 1(b)).
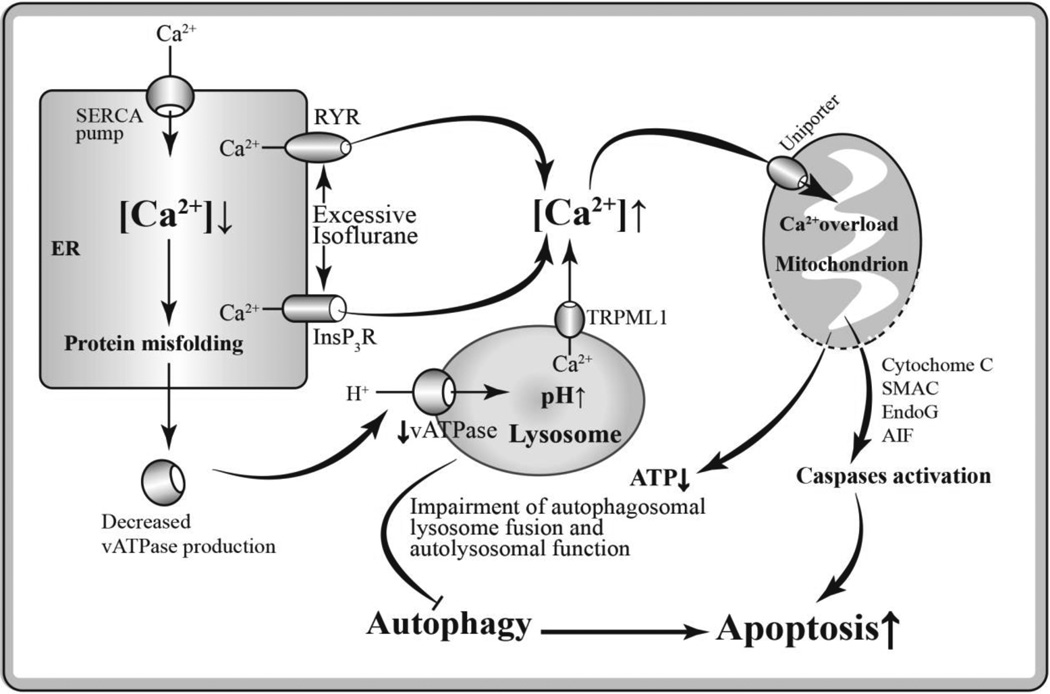
As shown in Figure 2, a prolonged exposure of general anesthetics such as isoflurane at high concentration causes Ca2+ release from the ER into the cytoplasm by over-activation of InsP3Rs and/or ryanodine receptor. This may elicit an abnormal rise of [Ca2+]c and a detrimental decrease of Ca2+ level in ER. As protein-folding reaction depends on high concentrations of Ca2+ in ER lumen (Görlach et al., 2006), the decreased Ca2+ level in ER will result in accumulation of misfolded protein including vATPase, a proton-pumping membrane protein in lysosome. Once vATPase maturation in ER is disturbed, vATPase will fail to drive protons into the lysosome lumen to maintain its proper pH value. That will lead to impaired lysosomal acidification which is essential for macromolecule degradation (Mindell, 2012). Additionally, elevated pH value in lysosome activates transient receptor potential cation channel mucolipin subfamily member 1 (TRPML1), a low H+-sensitive endolysosomal Ca2+ channel, causing Ca2+ efflux into cytoplasm (Lee et al., 2015), further aggravating the abnormal elevation of cytosolic Ca2+. In our previous research work, we have demonstrated that isoflurane has greater potency than sevoflurane or desflurane to cause calcium release from the ER and to induce neurotoxicity in different kinds of neurons (Yang et al., 2008). These findings may provide a valuable basis for the clinical use of different inhalational anesthetics in patients, especially for those patients vulnerable to anesthesia-mediated cell damage.
Fig. 2.
Hypothetical pathways of anesthetic neurotoxicity via impaired autophagy secondary to intracellular Ca2+ dysregulation. A prolonged exposure of GAs such as isoflurane at high concentration induces excessive Ca2+ release from the ER into the cytoplasm by over-activation of InsP3Rs and/or ryanodine receptor (RYR). The decreased Ca2+ level in ER causes accumulation of misfolded protein including vATPase, resulting in elevated lysosomal pH value and impaired autolysosomal function. Abnormally increased cytosolic Ca2+ leads to mitochondrial Ca2+ overload, and resulting in uncoupling of oxidative phosphorylation and release of catabolic hydrolases such as cytochrome c, apoptosis inducing factor (AIF), endonuclease G (EndoG) and secondary mitochondria-derived activator of caspase (SMAC), and result in apoptosis. In addition, the mitochondrial Ca2+ overload may induce decreased ATP production and inhibited ATP-dependent autophagic cytoprotection, thus promoting apoptosis.
Since mitochondria locate close to ER, the bulk cytosolic free Ca2+ released from ER transports into mitochondria via uniporter rapidly, causing mitochondrial Ca2+ overload (Gunter and Sheu, 2009; Rizzuto et al., 2009; Szabadkai and Rizzuto, 2004). Abnormally increased Ca2+ in mitochondria induces high-conductance permeability transition pores opening in mitochondria (Pinton et al., 2008). Loss of mitochondrial membrane potential, uncoupling of oxidative phosphorylation and release of catabolic hydrolases such as cytochrome c and apoptosis inducing factor (AIF) may occur as a result of increased permeability of mitochondria (Lemasters et al., 2009). The disruption of mitochondrial respiratory chain will shut down ATP production, resulting impaired ATP-dependent autophagy activity such as autophagosomal lysosome fusion. On the other hand, the components leaked from mitochondria trigger intrinsic apoptotic process. It has been proved that cytochrome c initiates activation of caspase cascade and form apoptosome (McIlwain et al., 2013; Parrish et al., 2013). The impairment of autolysosome function weakens the autophagic cytoprotection and drives process of neuroapoptosis which eventually lead to cell death.
Besides ER calcium release sites, there are several proposed targets for inhaled anesthetics mediated neurotoxicity (Jackson et al., 2016; Wang et al., 2015). Recent studies have proposed the role of p75 growth factor activation on anesthetics mediated neurotoxicity (Pearn et al., 2012). Studies have suggested that general anesthetics may affect the activity of the brain cells via promoting g-aminobutyric acid (GABA) receptors and/or inhibiting N-methyl-D-aspartic acid (NMDA) receptors, since both of them control neurotransmitter transport (de Sousa et al., 2000; Nelson et al., 2002; Sato et al., 2005). Moreover, recent data showed that sevoflurane induced Tau phosphorylation, glycogen synthase kinase 3β activation, and cognitive impairment in the young mice (Tao et al., 2014). It was also suggested that sevoflurane anesthesia in pregnant mice may impair learning and memory in offspring mice by decreasing postsynaptic density-95 levels in the neurons (Zheng et al., 2013). The latest evidence showed isoflurane-induced cognitive deficits may stem from upregulation of hippocampal IL-1b, partially via activation of the canonical NF-κb pathway, in aged rats (Li et al., 2013). Hence, GAs induced neurotoxicity has been appreciated as multifaceted mechanism involving calcium dysregulation, functional alteration of receptors and/or ion channels within plasma membrane, transcription factor dysfunction and some other underlying mechanisms.
In summary, general anesthetics can induce activation of autophagy and apoptosis by disrupting intracellular calcium homeostasis in a time- and concentration- dependent pattern. And the interplay between autophagy and apoptosis may ultimately determine the degree of neural cell injury. Understanding the mechanism of general anesthetics-mediated neurotoxicity will help to develop strategy minimizing the possible harmful effects of GAs in patients, especially in pediatric practice, such as minimizing the total exposure of GAs to patients.
Highlights.
GAs-induced neurotoxicity via disrupting intracellular calcium homeostasis.
Apoptosis vs. autophagic cell death are mediated by calcium dysregulation in anesthetic neurotoxicity.
Interplay between autophagy and apoptosis determines degree of neural cell injury.
Acknowledgments
We would like to thank Dr. Roderic G. Eckenhoff and Maryellen Eckenhoff from the Department of Anesthesiology and Critical Care, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, for their valuable discussion.
Funding
Supported by grants to HW from the NIH (K08-GM073224, R01GM084979, 3R01GM084979-02S1, 2R01GM084979-06A1), the March of Dimes Birth Defects Foundation (#12-FY08-167), and the bridging fund from the Department of Anaesthesiology, Perelman School of Medicine, University of Pennsylvania.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors report no conflict of interest.
References
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4(7):517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Cattano D, Young C, Straiko MM, Olney JW. Subanesthetic doses of propofol induce neuroapoptosis in the infant mouse brain. Anesth. Analg. 2008;106(6):1712–1714. doi: 10.1213/ane.0b013e318172ba0a. [DOI] [PubMed] [Google Scholar]
- de Sousa SL, Dickinson R, Lieb WR, Franks NP. Contrasting synaptic actions of the inhalational general anesthetics isoflurane and xenon. Anesthesiology. 2000;92(4):1055–1066. doi: 10.1097/00000542-200004000-00024. [DOI] [PubMed] [Google Scholar]
- Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, Sprung J, Weaver AL, Schroeder DR, Warner DO. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128(5):e1053–e1061. doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson A, Archer T, Alm H, Gordh T, Eriksson P. Neurofunctional deficits and potentiated apoptosis by neonatal NMDA antagonist administration. Behav. Brain Res. 2004;153(2):367–376. doi: 10.1016/j.bbr.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Görlach A, Klappa P, Kietzmann DT. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid. Redox Signal. 2006;8(9–10):1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Seidler FJ, Slotkin TA. Developmental neurotoxicity elicited by prenatal or postnatal chlorpyrifos exposure: effects on neurospecific proteins indicate changing vulnerabilities. Environ. Health Perspect. 2003;111(3):297. doi: 10.1289/ehp.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge HW, Hu WW, Ma LL, Kong FJ. Endoplasmic reticulum stress pathway mediates isoflurane-induced neuroapoptosis and cognitive impairments in aged rats. Physiol. Behav. 2015;151:16–23. doi: 10.1016/j.physbeh.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Gunter TE, Sheu SS. Characteristics and possible functions of mitochondrial Ca(2+) transport mechanisms. Biochim. Biophys. Acta. 2009;1787(11):1291–1308. doi: 10.1016/j.bbabio.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Dikkes P, Soriano SG. Repeated administration of ketamine may lead to neuronal degeneration in the developing rat brain. Paediatr. Anaesth. 2002;12(9):770–774. doi: 10.1046/j.1460-9592.2002.00883.x. [DOI] [PubMed] [Google Scholar]
- Inan S, Wei H. The cytoprotective effects of dantrolene: a ryanodine receptor antagonist. Anesth. Analg. 2010;111(6):1400–1410. doi: 10.1213/ANE.0b013e3181f7181c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Istaphanous GK, Howard J, Nan X, Hughes EA, McCann JC, McAuliffe JJ, Danzer SC, Loepke AW. Comparison of the neuroapoptotic properties of equipotent anesthetic concentrations of desflurane, isoflurane, or sevoflurane in neonatal mice. Anesthesiology. 2011;114(3):578–587. doi: 10.1097/ALN.0b013e3182084a70. [DOI] [PubMed] [Google Scholar]
- Jackson WM, Gray CD, Jiang D, Schaefer ML, Connor C, Mintz CD. Molecular Mechanisms of Anesthetic Neurotoxicity: A Review of the Current Literature. J Neurosurg Anesthesiol. 2016 doi: 10.1097/ANA.0000000000000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J. Neurosci. 2003;23(3):876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JD, Peng Y, Mak D-OD, Cheung K-H, Vais H, Foskett JK, Wei H. General anesthetic isoflurane modulates inositol 1, 4, 5-trisphosphate receptor calcium channel opening. Anesthesiology. 2014;121(3):528–537. doi: 10.1097/ALN.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph JD, Peng Y, Mak DO, Cheung KH, Vais H, Foskett JK, Wei H. General anesthetic isoflurane modulates inositol 1,4,5-trisphosphate receptor calcium channel opening. Anesthesiology. 2014;121(3):528–537. doi: 10.1097/ALN.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, McBrayer MK, Wolfe DM, Haslett LJ, Kumar A, Sato Y, Lie PP, Mohan P, Coffey EE, Kompella U, Mitchell CH, Lloyd-Evans E, Nixon RA. Presenilin 1 Maintains Lysosomal Ca(2+) Homeostasis via TRPML1 by Regulating vATPase-Mediated Lysosome Acidification. Cell Rep. 2015;12(9):1430–1444. doi: 10.1016/j.celrep.2015.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim. Biophys. Acta. 2009;1787(11):1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Yu B. Elevation of protective autophagy as a potential way for preventing developmental neurotoxicity of general anesthetics. Med. Hypotheses. 2014;82(2):177–180. doi: 10.1016/j.mehy.2013.11.032. [DOI] [PubMed] [Google Scholar]
- Li ZQ, Rong XY, Liu YJ, Ni C, Tian XS, Mo N, Chui DH, Guo XY. Activation of the canonical nuclear factor-kappaB pathway is involved in isoflurane-induced hippocampal interleukin-1beta elevation and the resultant cognitive deficits in aged rats. Biochem. Biophys. Res. Commun. 2013;438(4):628–634. doi: 10.1016/j.bbrc.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Loepke AW, Istaphanous GK, McAuliffe JJ, 3rd, Miles L, Hughes EA, McCann JC, Harlow KE, Kurth CD, Williams MT, Vorhees CV, Danzer SC. The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth. Analg. 2009;108(1):90–104. doi: 10.1213/ane.0b013e31818cdb29. [DOI] [PubMed] [Google Scholar]
- Ma D, Williamson P, Januszewski A, Nogaro M-C, Hossain M, Ong LP, Shu Y, Franks NP, Maze M. Xenon mitigates isoflurane-induced neuronal apoptosis in the developing rodent brain. Anesthesiology. 2007;106(4):746–753. doi: 10.1097/01.anes.0000264762.48920.80. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007;8(9):741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013;5(4):a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina DL, Di PS, Peluso I, Armani A, De SD, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso C, Forrester A, Settembre C, Wang W, Gao Q, Xu H, Sandri M, Rizzuto R, De Matteis MA, Ballabio A. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Rev. Mol. Cell Biol. 2015;17(3):288–299. doi: 10.1038/ncb3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindell JA. Lysosomal acidification mechanisms. Annu Rev Physiol. 2012;74:69–86. doi: 10.1146/annurev-physiol-012110-142317. [DOI] [PubMed] [Google Scholar]
- Nelson L, Guo T, Lu J, Saper C, Franks N, Maze M. The sedative component of anesthesia is mediated by GABAA receptors in an endogenous sleep pathway. Nat. Neurosci. 2002;5(10):979–984. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- Nie H, Peng Z, Lao N, Dong H, Xiong L. Effects of sevoflurane on self-renewal capacity and differentiation of cultured neural stem cells. Neurochem. Res. 2013;38(8):1758–1767. doi: 10.1007/s11064-013-1074-4. [DOI] [PubMed] [Google Scholar]
- Nikizad H, YON JH, Carter L, Jevtovic-Todorovic V. Early exposure to general anesthesia causes significant neuronal deletion in the developing rat brain. Ann. N. Y. Acad. Sci. 2007;1122(1):69–82. doi: 10.1196/annals.1403.005. [DOI] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 2003;4(7):552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- Palanisamy A, Baxter MG, Keel PK, Xie Z, Crosby G, Culley DJ. Rats exposed to isoflurane in utero during early gestation are behaviorally abnormal as adults. Anesthesiology. 2011;114(3):521–528. doi: 10.1097/ALN.0b013e318209aa71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish AB, Freel CD, Kornbluth S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb. Perspect. Biol. 2013;5(6):a008672. doi: 10.1101/cshperspect.a008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearn ML, Hu Y, Niesman IR, Patel HH, Drummond JC, Roth DM, Akassoglou K, Patel PM, Head BP. Propofol neurotoxicity is mediated by p75 neurotrophin receptor activation. Anesthesiology. 2012;116(2):352–361. doi: 10.1097/ALN.0b013e318242a48c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Drobish JK, Liang G, Wu Z, Liu C, Joseph DJ, Abdou H, Eckenhoff MF, Wei H. Anesthetic preconditioning inhibits isoflurane-mediated apoptosis in the developing rat brain. Anesth. Analg. 2014;119(4):939–946. doi: 10.1213/ANE.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesic V, Milanovic D, Tanic N, Popic J, Kanazir S, Jevtovic-Todorovic V, Ruzdijic S. Potential mechanism of cell death in the developing rat brain induced by propofol anesthesia. Int. J. Dev. Neurosci. 2009;27(3):279–287. doi: 10.1016/j.ijdevneu.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27(50):6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Shi P, Mao W, Zhao Y, Liu W, Wang Y. Effect of apoptosis in neural stem cells treated with sevoflurane. BMC Anesthesiol. 2015;15:25. doi: 10.1186/s12871-015-0018-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rizzuto R, Marchi S, Bonora M, Aguiari P, Bononi A, De Stefani D, Giorgi C, Leo S, Rimessi A, Siviero R, Zecchini E, Pinton P. Ca(2+) transfer from the ER to mitochondria: when, how and why. Biochim. Biophys. Acta. 2009;1787(11):1342–1351. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders RD, Xu J, Shu Y, Januszewski A, Halder S, Fidalgo A, Sun P, Hossain M, Ma D, Maze M. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009;110(5):1077–1085. doi: 10.1097/ALN.0b013e31819daedd. [DOI] [PubMed] [Google Scholar]
- Sato Y, Kobayashi E, Murayama T, Mishina M, Seo N. Effect of N-methyl-d-aspartate Receptor ε1Subunit Gene Disruption of the Action of General Anesthetic Drugs in Mice. Anesthesiology. 2005;102(3):557–561. doi: 10.1097/00000542-200503000-00013. [DOI] [PubMed] [Google Scholar]
- Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110(3):628–637. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- Slikker W, Zou X, Hotchkiss CE, Divine RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC, Patterson TA, Hanig JP. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol. Sci. 2007;98(1):145–158. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- Sprung J, Flick RP, Katusic SK, Colligan RC, Barbaresi WJ, Bojanic K, Welch TL, Olson MD, Hanson AC, Schroeder DR, Wilder RT, Warner DO. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin. Proc. 2012;87(2):120–129. doi: 10.1016/j.mayocp.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann GE, Smith I, Caccamo A, Oddo S, Parker I, Laferla F. Enhanced ryanodine-mediated calcium release in mutant PS1-expressing Alzheimer's mouse models. Ann. N. Y. Acad. Sci. 2007;1097:265–277. doi: 10.1196/annals.1379.025. [DOI] [PubMed] [Google Scholar]
- Szabadkai G, Rizzuto R. Participation of endoplasmic reticulum and mitochondrial calcium handling in apoptosis: more than just neighborhood? FEBS Lett. 2004;567(1):111–115. doi: 10.1016/j.febslet.2004.04.059. [DOI] [PubMed] [Google Scholar]
- Tao G, Zhang J, Zhang L, Dong Y, Yu B, Crosby G, Culley DJ, Zhang Y, Xie Z. Sevoflurane induces tau phosphorylation and glycogen synthase kinase 3beta activation in young mice. Anesthesiology. 2014;121(3):510–527. doi: 10.1097/ALN.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura E, Ireland W, Levin E, Bowman R. Effects of halothane on the development of rat brain: a golgi study of dendritic growth. Exp. Neurol. 1985;89(3):503–519. doi: 10.1016/0014-4886(85)90002-0. [DOI] [PubMed] [Google Scholar]
- Vutskits L, Gascon E, Tassonyi E, Kiss JZ. Clinically relevant concentrations of propofol but not midazolam alter in vitro dendritic development of isolated γ-aminobutyric acid-positive interneurons. Anesthesiology. 2005;102(5):970–976. doi: 10.1097/00000542-200505000-00016. [DOI] [PubMed] [Google Scholar]
- Wang C, Liu F, Patterson TA, Paule MG, Slikker W. Anesthetic Drug-Induced Neurotoxicity and Compromised Neural Stem Cell Proliferation. J Drug Alcohol Res. 2015;(4):a1–a8. [Google Scholar]
- Wei H. The role of calcium dysregulation in anesthetic-mediated neurotoxicity. Anesth. Analg. 2011;113(5):972–974. doi: 10.1213/ANE.0b013e3182323261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei H. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology. 2008;109(2):243–250. doi: 10.1097/ALN.0b013e31817f5c47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C, Jevtovic-Todorovic V, Qin YQ, Tenkova T, Wang H, Labruyere J, Olney JW. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br. J. Pharmacol. 2005;146(2):189–197. doi: 10.1038/sj.bjp.0706301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Xue Z, Sun A. Subclinical concentration of sevoflurane potentiates neuronal apoptosis in the developing C57BL/6 mouse brain. Neurosci. Lett. 2008;447(2):109–114. doi: 10.1016/j.neulet.2008.09.083. [DOI] [PubMed] [Google Scholar]
- Zhao X, Yang Z, Liang G, Wu Z, Peng Y, Joseph DJ, Inan S, Wei H. Dual effects of isoflurane on proliferation, differentiation, and survival in human neuroprogenitor cells. Anesthesiology. 2013;118(3):537–549. doi: 10.1097/ALN.0b013e3182833fae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Dong Y, Xu Z, Crosby G, Culley DJ, Zhang Y, Xie Z. Sevoflurane anesthesia in pregnant mice induces neurotoxicity in fetal and offspring mice. Anesthesiology. 2013;118(3):516–526. doi: 10.1097/ALN.0b013e3182834d5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YF, Wang QX, Zhou HY, Chen G. Autophagy activation prevents sevoflurane-induced neurotoxicity in H4 human neuroglioma cells. Acta Pharmacol. Sin. 2016;37(5):580–588. doi: 10.1038/aps.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Patterson TA, Divine RL, Sadovova N, Zhang X, Hanig JP, Paule MG, Slikker W, Wang C. Prolonged exposure to ketamine increases neurodegeneration in the developing monkey brain. Int. J. Dev. Neurosci. 2009a;27(7):727–731. doi: 10.1016/j.ijdevneu.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Zou X, Patterson TA, Sadovova N, Twaddle NC, Doerge DR, Zhang X, Fu X, Hanig JP, Paule MG, Slikker W. Potential neurotoxicity of ketamine in the developing rat brain. Toxicol. Sci. 2009b;108(1):149–158. doi: 10.1093/toxsci/kfn270. [DOI] [PMC free article] [PubMed] [Google Scholar]