Abstract
Wnt signaling is a critical component during embryonic development and also plays an important role in regulating adult tissue homeostasis. Abnormal activation of Wnt signaling has been implicated in many cancers, while reduced activity of Wnt signaling leads to poor wound healing and structural formations. Thus, extensive efforts have been focused on developing small molecules that have potential to either inhibit or activate the pathway, hoping these molecules can offer leads for novel approaches in treating different human diseases. Many small‐molecule inhibitors specifically target various elements, such as Frizzled, Disheveled, Porcupine, or Tankyrase, within the Wnt signaling pathways. These small molecules not only have the potential to be further developed as therapeutic reagents, but they may also be used as chemical probes to dissect the underlying mechanism of the Wnt signaling pathways. Therefore, their respective mechanisms and effective dosages are highly pertinent. Aiming to provide an overview of those molecules in a concise, easy‐to‐use manner, we summarize and organize the current research on them so that it may be helpful for utilization in different studies.
Keywords: Wnt signaling pathway, small‐molecule inhibitors, cancer, stem cells, Frizzled, Disheveled, GSK‐3β, Axin, β‐catenin, T‐cell factor/lymphoid enhancer‐binding factor, Porcupine, and Tankyrase
Abbreviations
- APC
adenomatous polyposis coli
- BIO
6‐bromoindirubin‐3′oxime
- CBP
cyclic AMP response‐element binding protein
- CRD
cysteine‐rich domain
- Dkk
Dickkopf
- Dvl
Dishevelled
- FRPs
Frizzled‐related proteins
- Fzd
Frizzled
- GSK‐3β
glycogen synthase kinase‐3
- HAT
histone acetyltransferase
- IWP
inhibitors of Wnt production
- LRP
lipoprotein‐receptor‐related proteins
- NMR
nuclear magnetic resonance
- MBOAT
membrane‐bound O‐acetyltransferase
- NSAID
non‐steroidal anti‐inflammatory drug
- Prcn
Porcupine
- TCF/LEF
T‐cell factor/lymphoid enhancer‐binding factor
- TNKS
Tankyrase.
Introduction
In the past thirty years, research into the Wnt signaling pathways has revealed their undeniable significance in human cell development, making it one of the important pathways to be studied in the current cancer and stem cells research community.1 Wnt signaling pervades all parts of the human body.2 Thus, abnormal Wnt signaling has been implicated in many different types of cancers, including, but not limited to: gastrointestinal cancers, leukemia, melanoma, and breast cancer.3 Extensive efforts have focused on inhibition at various points of the Wnt pathway in the hopes of stopping excess nuclear localization of β‐catenin and the subsequent overexpression of Wnt ligands that lead to cancer. On the other hand, because Wnt signaling plays an important role in regulating stem cells, studies of Wnt activation can also benefit medicine as well since reduced Wnt signaling is detrimental to injury repair.4 Moreover, the efforts of targeting the Wnt signaling pathways with small molecules have expanded greatly in concert with technological advancements of the scientific tools needed to examine it. Particularly, the creation of the Top‐FLASH assay, a luciferase‐based Wnt reporter, has allowed more convenient experiments to be conducted.5 Furthermore, with chemical libraries now approaching 10 million, docking screens have been utilized to find many new ligands that can bind efficiently to a target molecule.6
Here, we focus specifically on the canonical Wnt signaling pathway. In the canonical Wnt signaling pathway, Wnt proteins are secreted molecules that, after acylation by Porcupine (Prcn), bind the seven‐transmembrane receptor Frizzled (Fzd) and lipoprotein‐receptor‐related protein (LRP) 5/6, activating the Disheveled (Dvl) proteins.7 Dvl then binds to the C‐terminus of Fzd while also recruiting Axin from the β‐catenin destruction complex, which normally consists of Axin, glycogen synthase kinase‐3β (GSK‐3β), and adenomatous polyposis coli (APC). Without this Wnt signaling, β‐catenin is phosphorylated by the APC‐Axin‐GSK‐β‐catenin complex, and the phosphorylated β‐catenin can be removed through the ubiquitin proteasome system.8 With Wnt signaling, however, LRP5/6 protein is phosphorylated by casein kinase 1 (CK1γ) and GSK‐3β, which are recruited by the Dvl‐bound Axin. In addition, there are many Wnt signaling regulators, one of which is Tankyrase (TNKS). TNKS phosphorylates Axin and the phosphorylated Axin can be destroyed by the ubiquitin proteasome system.8 Without Axin, β‐catenin cannot be effectively phosphorylated by GSK‐3β, resulting in stabilization of β‐catenin. Accumulated β‐catenin is transported to the nucleus and initiates transcription of the Wnt target genes by binding to T‐cell factor/lymphoid enhancer‐binding factor (TCF/LEF) transcription reporters, Creb‐binding protein, or p300 protein. The canonical Wnt signaling pathway is visually represented in Figure 1.
Figure 1.
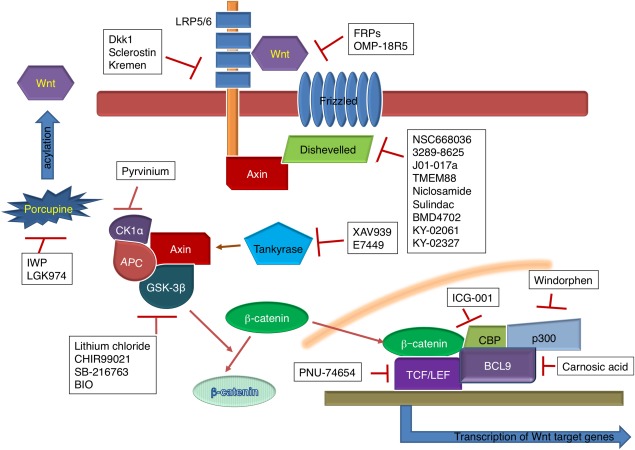
Overview of the Wnt signaling pathway and targets of small‐molecule inhibitors.
Most of the discovered Wnt signaling inhibitors and activators have focused on several specific protein‐protein interactions. Specific sites of inhibition include: the Fzd protein, the Dvl protein, the β‐catenin destruction complex, nuclear β‐catenin, and the enzymes Prcn and TNKS. Table 1 lists the small‐molecule inhibitors that are discussed in this paper. The most prominent Wnt signaling activators focus on inhibiting GSK‐3β, which normally disrupts the β‐catenin destruction complex, allowing transportation of β‐catenin into the nucleus to participate in gene transcription and expression; those molecules are listed in Table 2. Because understanding how different small molecules can interact with different parts of the pathway is crucial to future research, in this paper, we (1) review the most prominent molecules that interact with each of the above points in the Wnt signaling pathway, (2) explain the mechanism through which the molecules act, (3) indicate the effective dosages of the molecules discovered within the literature, and (4) make suggestions for utilization of those molecules to target Wnt signaling in biological systems.
Table 1.
Effective Dosages and CAS Numbers of Small‐Molecule Inhibitors Discussed in This Paper Grouped by Their Targets
| Target | Compounds | Effective dosage | CAS No. | References |
|---|---|---|---|---|
| Dvl protein | NSC668036 | 237 µM | 144678‐63‐7 | 19 |
| 3289‐8625 | 10.6 µM | 294891‐81‐9 | 20 | |
| J01‐017a | 1.5 ± 0.5 μM | 22 | ||
| TMEM88 | 2 ± 0.2 µM | 24 | ||
| KY‐02061 | 0.4–12 µM | 25 | ||
| KY‐02327 | 0.13–4 µM | 25 | ||
| BMD4702 | 38 µM | 335206‐54‐7 | 26 | |
| Niclosamide | 24 µM | 50‐65‐7 | 28 | |
| DK‐520 | 8.308 ± 0.8 µM | 29 | ||
| Sulindac | 0.186 µM | 38194‐50‐2 | 31 | |
| β‐catenin destruction complex | Pyrvinium | <10 nM | 7187‐62‐4 | 37 |
| Natural compounds | Derricin | 10 µM | 34211‐25‐1 | 40 |
| Derricidin | 50 µM | 38965‐74‐1 | 40 | |
| Carnosic acid | 5‐20 µM | 3650‐09‐7 | 41 | |
| TCF/LEF transcription reporter | ICG‐001 | 3 µM | 847591‐62‐2 | 43 |
| PNU‐74654 | 100 µM | 113906‐27‐7 | 45 | |
| Windorphen | 4.2 µM | 19881‐70‐0 | 47 | |
| Prcn | IWP‐L6 | 10 nM | 1427782‐89‐5 | 48 |
| Wnt‐C59 | >60 µM | 1243243‐89‐1 | 49 | |
| LGK974 | 0.3–1 nM | 1243244‐14‐5 | 51 | |
| ETC‐159 | 2.9 nM | 1638250‐96‐0 | 52 | |
| TNKS | XAV939 | 0.099 µM | 284028‐89‐3 | 55 |
| E7449 | 50‐120 nM | 1140964‐99‐3 | 57 |
Table 2.
Effective Dosages and CAS Numbers of GSK‐3β Inhibitors That Could Be Used to Activate the Wnt Pathway
Inhibitors Blocking Wnt Interactions With Fzd
Wnt signaling is initiated by the binding of a secreted Wnt molecule to its receptor, Fzd. A Fzd antibody, OMP‐18R5, appears to block canonical Wnt signaling and inhibit human tumor growth of multiple types, including lung, breast, colon, and pancreatic tumors.9 By binding to many binding sites designated as Fzd cysteine‐rich domains (CRD), OMP‐18R5 possibly blocks Wnt proteins from binding to Fzd through steric hindrance, resulting in accumulation of β‐catenin.9 This discovery ultimately suggests that Fzd CRDs could be promising targets for the development of new therapeutic cancer drugs.10 Additionally, Gurney et al. observed OMP‐18R5 interacting synergistically with several standard‐of‐care chemotherapeutic agents, especially taxol, making it a compelling candidate for further study in the field of cancer therapy.9
Secreted frizzled‐related proteins (SFRPs) are a group of molecules with an N‐terminal CRD similar to the CRDs of Fzd Wnt receptors.11 Using a T‐cell factor luciferase reporter to measure Wnt function, Bafico et al. showed that FRPs inhibit Wnt signaling by a paracrine model.11 Their studies revealed two possible models that SFRPs could use to inhibit Wnt signaling. The first possibility is that FRPs bind to Wnt molecule itself, altering the ability of the Wnt ligand to bind with its normal Fzd receptors. The alternative possibility is that FRPs form complexes with the Fzd receptor instead, changing its binding capabilities to Wnt protein. While further research is needed to determine which one is the exact mechanism of how FRPs antagonize Wnt binding, efforts have been made to generate recombinant SFRPs as Wnt inhibitors.12 Further studies on SFRPs have revealed that depending on cellular context and concentration, SFRPs have potential to both inhibit and activate the Wnt signaling pathway.12 Undoubtedly, determining the exact conditions that lead to SFRPs' seemingly contradictory role in different contexts will shed additional light on its utility in molecular biology.
In the canonical Wnt signaling pathway, LRP5/6 works with Fzd as a Wnt co‐receptor. Dickkopf1 (Dkk1) inhibits Wnt signaling by binding to LRP5/6 in the Wnt signaling pathway. Related to Dkk1 are Kremen1 and Kremen2, high‐affinity Dkk1 receptors that contribute to blocking Wnt/β‐catenin signaling through binding with Dkk1.13 Dkk1 and its Kremen receptor form a ternary complex with LRP6, leading to rapid endocytosis and disposal of the Wnt receptor from the plasma membrane, preventing any of its subsequent interactions and signal transmittances. However, after investigating Dkk1 molecules that were mutant at the aforementioned residue sites and finding their antagonistic ability unaffected, Ellwanger et al. suggested that Kremen proteins were not essential to Dkk1's ability to inhibit the Wnt signaling pathway, and that they may only be needed when cells express a high level of LRP5/6.14 Further study on the interaction of Kremen proteins and Dkk could yield more specific and useful insights on how to reach more targeted goals, such as in Liedert et al.'s study which, after determining the significance of Kremen2 in bone injury repair, suggested antagonizing Kremen2 to improve bone fracture healing under compromised circumstances.15
Sclerostin also inhibits the Wnt signaling pathway through interactions with LRP5/6. However, unlike Dkk1, which binds to the first and third propeller of domains of LRP5/6, sclerostin only binds the first or the first two propeller domains.16 By mutating the Asn‐93 residue in the PNAIG sequence of sclerostin, Holdsworth et al. destroyed sclerostin's ability to inhibit Wnt1 signaling and bind to LRP6, revealing the importance of that particular residue in the interaction between the two.17 Wnt3A and Wnt9B were not affected, though, suggesting that those Wnts interact with LRP6 in different ways.17 Further research on the differences between the structures of Dkk1 and sclerostin will help clarify the mechanisms of how these inhibitors work, potentially allowing researchers to better differentiate between their respective therapeutic values.
Inhibitors Targeting Dvl Protein
Wong et al. indicated that there is also an essential interaction between the C‐terminal sequence of the seventh transmembrane helix of Fzd and the PDZ domain of the Dvl protein that aids the transduction of downstream Wnt signals.18 Shan et al.'s discovery of NSC668036 and Grandy et al.'s discovery of 3289‐8625 as inhibitors of this Fzd‐PDZ interaction demonstrated that structure‐based virtual ligand screening, along with nuclear magnetic resonance (NMR) spectroscopy, are valid methods of identifying small‐molecule inhibitors using the structural features of a target protein.19, 20 Additionally, Shan and Zheng established the viability of in silico searching as another method of screening compounds for inhibitor potential as well.21 Shan et al. displayed J01‐017a as the most potent inhibitor of the Dvl‐PDZ domain that has been produced thus far.22 It is definitely a molecule of interest for researchers wishing to analyze the PDZ domain and how it affects Wnt signaling. Other molecules of interest include those that end with the tripeptides VVV and VWV, such as TMEM88, since these tripeptides seem to modulate Dvl‐PDZ protein interactions.23, 24 The structures of these molecules can be used as models to adjust in order to find the best antagonist for targeting interactions between Dvl and Fzd.
With NMR titration analysis, Kim et al. identified KY‐02061, along with an analog, KY‐02327, as potential candidates for the development of bone anabolic anti‐osteoporosis drugs.25 These molecules block the Dvl‐CXXC5 interaction that works as a negative feedback loop for the Wnt signaling pathway. Thus, blocking this interaction activates the Wnt signaling pathway, which may allow for enhanced rescue of bone loss in ovariectomized mice.25 KY‐02061 interacts with the β‐sheet complex within the Dvl‐PDZ domain in order to achieve inhibition of the Dvl‐CXXC5 interaction. The IC50 value of KY‐02061 was found to be 24 µM. However, because it displayed poor metabolic ability in vivo, the analog, KY‐02327, was created, and the improved molecule demonstrated a 7.7‐fold lower IC50 than the original.25
Using a structure‐based pharmacophore model of the Dvl‐PDZ domain, Choi et al. discovered BMD4702 as a highly potent compound that bound to the Dvl‐PDZ domain with an 11.2 µM affinity and 0.186 µM Kd value.26 Through structural‐kinetic relationship analyses and docking studies, they postulated that the ligand‐binding site of this molecule is composed of three hydrogen bonds, from neighboring carboxyl groups, and three hydrophobic features. The success of this molecule as an inhibitor makes it a good model to be studied for further optimization in drug development.
Niclosamide inhibits the Wnt signaling pathway by downregulating Dvl2 expression, resulting in decreased downstream β‐catenin signaling. A study by Osada et al. demonstrated that niclosamide has promise as a treatment for colorectal cancer since it inhibits Wnt signaling with no significant toxicity against non‐tumor cells.27 Oral administration seemed to be sufficient to distribute the drug to tumor tissue as niclosamide concentrations in tissue corresponded closely to those found in plasma. About 0.1‐0.2 μM of niclosamide was found in plasma from 0.5 h to 12 h after oral intake (200 mg/kg). Overall, the IC50 of niclosamide has been reviewed to range from 0.4 µM to 12 µM, depending on the type of tissue.28 In more recent studies, niclosamide has improved further as a potential drug through the identification of an acyl derivative, DK‐520. Once metabolized in vivo, this derivative increased both plasma concentration of the drug and duration of exposure while administered orally with an IC50 range of 0.13‐4 μM.29
Sulindac and its metabolites have appeared in recent studies as a desirable drug to target cancer cells due to good bioavailability and its ability to cross the blood‐brain barrier.30 It is part of a group of non‐steroidal anti‐inflammatory drugs (NSAIDs) that have been used in conjunction with other conventional therapies for cancer, chosen for their ability to selectively reduce tumor growth and induce apoptosis of cancer cells without harm to normal tissue.31 Sulindac appears to bind specifically to the PDZ domain of Dvl, a key molecule that relays Wnt signals from initial receptors to various downstream components.32 The specificity of binding is largely determined by the side chain on arginine residue 322.33 Lee et al. explained that sulindac competes with the C‐terminus of Dapper, another Wnt signaling antagonist, to bind to the PDZ domain of Dvl.34 The subsequent suppression of β‐catenin leads to further suppression of downstream components such as PDE5 and S100A4.31, 35 Sulindac sulfide has been reported to have an IC50 value of 38 μM; perhaps other analogs can be studied to find one with a lower nanomolar range.31 Like with any NSAID though, one has to carefully evaluate its safety. One of the safest NSAIDs of recent note is the compound, phospho‐sulindac.36 Considering the efficacy of phospho‐sulindac in inhibiting the Wnt signaling pathway would be a novel way to help progress the field. The structures of these Dvl inhibitors are shown in Figure 2.
Figure 2.
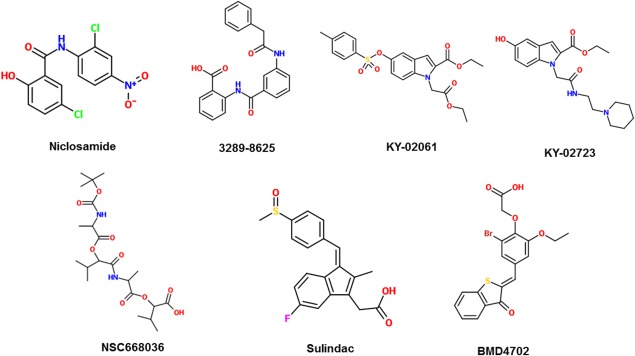
2‐D structures of small‐molecule inhibitors that target Disheveled protein.
Inhibitor Stabilizing the β‐Catenin Destruction Complex
Pyrvinium (Fig. 3) is one of the more potent inhibitors of the Wnt signaling pathway as it has been shown to inhibit proliferation of lung cancer cells in vitro at a dose below 10 nM.37 Saraswati et al. illustrated that pyrvinium binds to and activates casein kinase 1α (CK1α), which is part of the β‐catenin destruction complex.38 Therefore, activating it contributes to the destruction of β‐catenin, stopping further relays of the Wnt signal. The discovery of this mechanism revealed that CK1α may be a viable target for drugs in the future that have the aim of inhibiting Wnt signaling.39 However, further studies on decreasing pyrvinium's toxicity need to be conducted before it can progress as a therapeutic agent.
Figure 3.
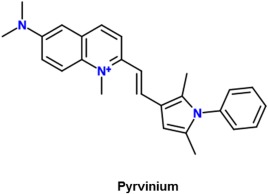
2‐D structure of pyrvinium, which interferes with the β‐catenin destruction complex.
Natural Compound Inhibitors
Chalcones such as derricin and derricidin are newly discovered inhibitors of the Wnt signaling pathway. Their inhibition is signified by the lowered amount of nuclear β‐catenin found in treated cells. Not much is yet understood about them but they offer the advantage of having a low risk of mutagenesis due to their minimal interaction with DNA.40 Derricin strongly inhibited Wnt signaling at a concentration of 10 µM, while derricidin was not as effective until it reached 50 μM.40 Further research can be performed on how the differences in these two structures influence their different mechanisms in inhibiting Wnt signaling as it is not yet known exactly which component of Wnt signaling pathway they interact with.
Carnosic acid, derived from rosemary, is another natural compound which has been shown to decrease transcriptional β‐catenin.41 De La Roche et al. identified it when they developed an ELISA‐based “plus‐minus” assay that screened for small molecules that disrupt the binding of β‐catenin to BCL9 without affecting its binding to TCF.41 They estimated the Kd range of carnosic acid to be between 5‐20 µM. NMR and analytical ultracentrifugation have demonstrated that attenuation of β‐catenin by carnosic acid relies on an intrinsically labile α‐helix (H1) amino‐terminally adjacent to the BCL9‐binding site in β‐catenin.41 The capabilities of carnosic acid to attenuate oncogenic β‐catenin in colorectal cells highlights that H1 could be a weakness with potential to be exploited in order to further degrade β‐catenin and prevent overexpression of Wnt/β‐catenin genes. Though this method has worked in vitro, in vivo tests have yet to be run in order to evaluate the viability of this site as a therapeutic target. The structures of derricin, derricidin, and carnosic acid are shown in Figure 4.
Figure 4.
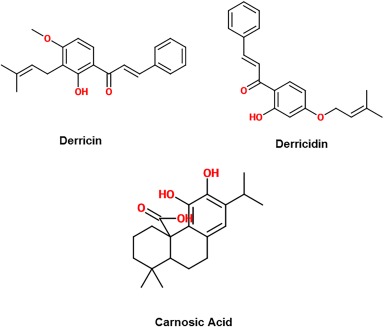
2‐D structure of natural compound inhibitors of the Wnt signaling pathway.
Inhibitors Affecting Nuclear β‐Catenin Activity
Cyclic AMP response‐element binding protein (CBP) forms a complex with T‐cell factor (TCF) to serve as a coactivator of several transcription factors of Wnt signaling. The structures of the following small‐molecule inhibitors that affect nuclear β‐catenin activity are shown in Figure 5. ICG‐001 is a small molecule that blocks the interaction between CBP and β‐catenin, thereby disrupting the signaling between β‐catenin and TCF as well.42 Kim and Kahn reported ICG‐001 to have an IC50 of 3 µM.43 A study done by Henderson et al. has noted that this can prevent pulmonary fibrosis or even reverse established fibrosis.44 In addition, research by Grigson et al. further supports that ICG‐001 administration resulted in inhibition of TCF reporter activity in multiple myeloma cell lines and a significant increase in cleavage of caspase 3.42 It is currently unknown if ICG‐001 may bind to other transcriptional factors as well as CBP; this could be a further area of study for this molecule.
Figure 5.
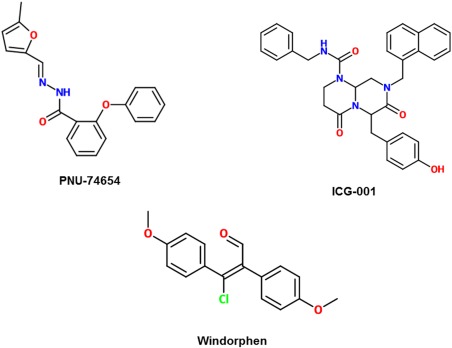
2‐D structures of inhibitors that target nuclear β‐catenin activity.
PNU‐74654 is a competitive inhibitor of β‐catenin by directly binding to the same site on TCF that β‐catenin binds to. With inhibition achieved at 100 µM, Leal et al.'s experiment tested this molecule with adrenocortical tumor cells in vitro, resulting in decreased viability and increased apoptosis of the treated cells.45
Windorphen, unlike ICG‐001, disrupts interactions between β‐catenin and p300 instead of CBP or the LEF/TCF reporter.46 It does so by targeting the C‐terminal transactivation domain of β‐catenin‐1. It also targets p300 histone acetyltransferase (HAT) activity with an IC50 of 4.2 µM.47 Because overexpression of p300 is associated with poor prognosis in human colon cancers, windorphen may offer an advantage in treating it.47
Inhibitors of Prcn Activity
Inhibitors of Wnt production (IWPs) are a group of small molecules (shown in Fig. 6) that target Prcn, a membrane‐bound O‐acyltransferase (MBOAT) family protein. Prcn is an enzyme that catalyzes the palmitoylation of Wnt protein, leading to its secretion and ultimate activation of cellular responses. In particular, IWP‐L6 appears to be the most potent inhibitor of Wnt signaling within this set of molecules. Doses of 10 nM significantly reduced morphogenesis of the tailfin of zebrafish, while doses of 50 nM and above blocked morphogenesis of the tailfin completely.48 Meanwhile, a 5 μM dose of IWP‐2, a widely used inhibitor, was required for the same results.48
Figure 6.
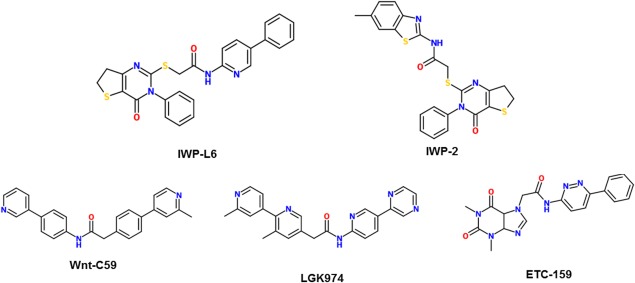
2‐D structures of inhibitors that target enzyme activity of Porcupine.
Another prcn inhibitor, Wnt‐C59, was also shown to effectively inhibit the Wnt signaling pathway, significantly reducing the effects of kidney fibrosis.48 By preventing the expression of Wnt target genes, it interrupts β‐catenin signaling, attenuating the signaling cascades of fibrotic disorders. Also, in mouse models, Wnt‐C59 suppressed the growth of nasopharyngeal tumors and arrested cancer stem cells in HNE1 and SUNE1 cells with IC50 values greater than 60 µM.49
LGK974 has demonstrated the ability to inhibit Wnt signaling both in vitro and in vivo by reducing LRP6 phosphorylation and Axin2 expression. Liu et al.'s study highlighted that the molecule is highly effective in both human and mouse tumor models, with IC50 values ranging between 0.3 nM to 1 nM, and is also well‐tolerated in normal tissue, making it a compelling potential drug for targeting cancers, once its toxicity is assessed.50
One more inhibitor, ETC‐159, discovered by Madan et al. has potential as a therapeutic drug because as a Prcn inhibitor, it does not produce intestinal or skin toxicity in mouse models. Its highly efficient treatment of models of genetically defined cancers makes it especially promising. It inhibited β‐catenin reporter activity in a dose‐dependent manner with an IC50 of 2.9 nM.51
Inhibitors of TNKS Enzyme
The enzyme TNKS antagonizes β‐catenin destruction complex activity by destroying a key protein of the complex, Axin.52 Thus, inhibiting TNKS stabilizes more of the β‐catenin destruction complexes, preventing β‐catenin's accumulation and excess signaling of Wnt. It was suggested, though, that TNKS inhibition may not be as valuable as other modes due to LEF1 and B9L shielding β‐catenin in the nucleus and protecting it from Axin‐mediated destruction.53 Two TNKS inhibitors have been developed (Fig. 7).
Figure 7.
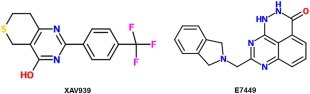
2‐D structures of inhibitors that target enzyme activity of Tankyrase.
XAV939 inhibits the TNKS enzymes that stabilize Axin, allowing Axin to play its proper role in the destruction of β‐catenin, with a reported Kd of 0.099 µM.54 In Tian et al.'s study, neuroblastoma cell lines were treated with the drug, resulting in downregulation of target proteins downstream of β‐catenin as well as destruction of β‐catenin itself.55 Additional in vivo tests could build further upon these studies.
Another more promising TNKS inhibitor, E7449, has also been recently discovered. Its mechanism is very similar to XAV939, but its IC50 values have ranged between 50 to 120 nM.56 Moreover, it appears to be lower in intestinal toxicity than most other TNKS inhibitors, which makes it even more enticing for use as a possible new drug.56
Activation of Wnt Signaling Through Inhibition of GSK‐3β
Just like with excess Wnt activation, excess inhibition can have negative health effects as well. For example, loss of Wnt signaling has been implicated in β‐amyloid dependent neurodegeneration found in the brains of Alzheimer's patients.57 Also, in a study by Ho et al., down‐regulation of Wnt/β‐catenin activity due to overexpression of GSK‐3β activity found in the β‐catenin destruction complex was implicated in the pathway of diabetes.58 Thus, activation of the Wnt pathway also has the capacity to be a treatment option for various health problems.
Currently, the most notable Wnt activators work by inhibiting the GSK‐3β enzyme found in the β‐catenin destruction complex. By inhibiting that enzyme, those molecules disrupt the ability of the complex to degrade β‐catenin, allowing β‐catenin to then accumulate in the nucleus and relay the Wnt signal further for transcription.
It is known that high concentration (∼ mM) lithium chloride (LiCl) inhibits GSK‐3β activity. Indeed, treatment of mesenchymal C2C12 cells with LiCl stimulated Wnt signaling and expression of Wnt markers, ultimately promoting osteoblast differentiation.59 Moreover, LiCl was also shown to improve bone mass in mice through LRP5‐independent activation of Wnt signaling at a serum level below the reported IC50 of 2 mM.60 Lithium has been used as an effective treatment for bipolar disorder, with little toxicity, for over half a century.61 Therefore, a new application of it for bone diseases such as osteoporosis and osteopenia will be easier to establish. Future studies utilizing LiCl could focus on whether or not it has an effect on other biological pathways as well.
Another GSK‐3β inhibitor, CHIR99021, has been used as a Wnt signaling activator in a variety of studies. Zhang et al. used CHIR99021 to highlight the significance of the Wnt signaling pathway in human lung development.62 This study exposed potential therapeutic targets in the treatment of lung disease and illuminated the possibility of using agonists to increase injury repair or regeneration. Also using CHIR99021, Li et al. discovered that there appears to be an undeniable link between the Wnt and Notch signaling pathways in non‐small cell lung cancer.63 Indeed, studying the Wnt signaling pathway with agonists have just as much promise as studying antagonists in unraveling key mysteries about the complexity of human biological pathways. Future studies using CHIR99021 or other agonists on different cell types and contexts would give much needed elucidation on how the Wnt signaling pathway may affect different parts of the bodies in different ways.
A useful application of Wnt activator studies is the elucidation of the mechanisms of various diseases in which Wnt genes are overexpressed. Research using SB‐216763, a highly selective cell‐permeable inhibitor of GSK‐3β, highlighted the contribution of overactive Wnt signaling to diseases such as dermal fibrosis and chronic lymphocytic leukemia.64, 65 Another activator, 6‐bromoindirubin‐3’‐oxime (BIO), was used in a study by Yang et al. to support that Wnt activation is what gives hepatocellular carcinoma cells an advantage over chemotherapy, explaining why chemotherapy may lead to remission, but rarely cures.66
Developing Wnt activators may also be helpful when stem cells are needed for therapy of some sort. SB‐216763 mimics the Wnt3a activity that leads to retinal stem cell proliferation.67 Because SB‐216763 is less expensive than Wnt3a protein, this discovery could reduce the cost of tissue engineering for regeneration of damaged retinal cells. BIO‐mediated Wnt activation has also proven to be applicable to stem cell engineering since it maintains undifferentiated phenotypes in both human and mouse embryonic stem cells. Notably, BIO‐mediated Wnt activation is reversible once BIO is withdrawn, opening up new possibilities for the use of human embryonic stem cells in regenerative medicine.68 In a study documenting the cytotoxicity and activation potential of various GSK3 inhibitors, CHIR99021 and SB‐216763 were found to have lower toxicity in mouse embryonic stem cells while BIO had a higher toxicity.69 Naujok et al. reported an IC50 value of 0.48 µM for BIO, 5.7 µM for SB‐216763, and 4.9 µM for CHIR99021. They ultimately concluded that CHIR99021 was the optimal molecule because it had both high potency and low toxicity.69 Each of the GSK‐3β inhibitors discussed here have their own advantages and disadvantages to be considered when designing experiments. Structures of GSK‐3β inhibitors are shown in Figure 8.
Figure 8.
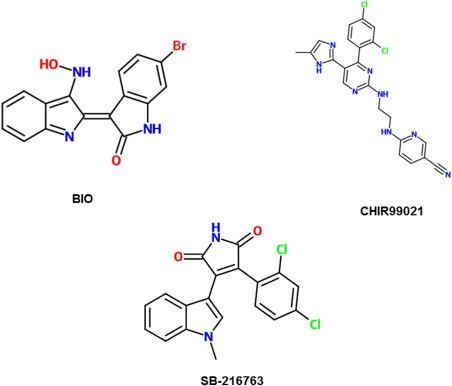
2‐D structures of GSK‐3β inhibitors, which result in activation of the Wnt pathway.
Conclusions and Perspectives
Current advances in Wnt signaling research have displayed these key points as promising sites of inhibition: the Fzd protein, the Dvl protein, the β‐catenin destruction complex, nuclear β‐catenin, and the enzymes, Prcn and TNKS. Each of these have several small‐molecule inhibitors associated with it that have been discovered over the years. Likewise, the most prominent activation site in Wnt signaling research, GSK‐3β, has several small molecules associated with it as well. Structure‐based screening, large‐scale screening and chemical genomics approaches have been critical to the discovery of small molecules that interact with Wnt signaling pathway.22, 26 Undoubtedly, these methods will continue to be of essential use to the future research on the biological effects of Wnt signaling.
Because of Wnt signaling's implication in a variety of cancers, Wnt inhibitor studies have great value in determining how Wnt signaling is regulated and uncovering more clinically useful insights. Of course, this perspective can go both ways and so, investigating Wnt signaling activation can generate critical information as well, unearthing the secrets of better injury repair and other processes where Wnt signaling activation is required. Although research on small molecules affecting Wnt signaling has progressed significantly in recent years, several steps can still be taken to further close the gaps in current knowledge. First, analysis of binding affinities of these small molecules at different Wnt signaling sites and determination of their roles in other pathways need to be made so that it can be seen whether or not healthy cells will be affected by the introduction of these molecules into the pathway. Second, further elucidation on the three‐dimensional structures of key molecules and their mechanism of binding at the different Wnt signaling pathway sites will clarify the specificity of binding at these sites, allowing us to improve binding and discover other molecules that may bind well to those sites. Lastly, the efficacy and safety of these compounds need to be assessed.
Finding the best point of the Wnt pathway to influence and which molecule (or molecules combined) to affect such a point will surely be a complex road ahead. This review has attempted to address some of the above issues. The study of the Wnt signaling pathways continues to currently expand. Hopefully, this signifies that the near future will yield many definitive uses of small molecule Wnt signaling modulation in treating a variety of illnesses.
Acknowledgments
The authors thank Dr. Chi Zhang, Dr. Avery Sader, and Ms. Elizabeth Tannous for useful discussions.
References
- 1. Nusse R, Varmus H (2012) Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J 31:2670–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holland JD, Klaus A, Garratt AN, Birchmeier W (2013) Wnt signaling in stem and cancer stem cells. Curr Opin Cell Biol 25:254–264. [DOI] [PubMed] [Google Scholar]
- 3. Zhan T, Rindtorff N, Boutros M (2016) Wnt signaling in cancer. Oncogene [VOL:PAGE #S]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whyte JL, Smith AA, Helms JA (2012) Wnt signaling and injury repair. Cold Spring Harbor Perspect Biol 4:a008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H (1997) Constitutive transcriptional activation by a beta‐catenin‐Tcf complex in APC‐/‐ colon carcinoma. Science 275:1784–1787. [DOI] [PubMed] [Google Scholar]
- 6. Irwin JJ, Shoichet BK (2016) Docking screens for novel ligands conferring new biology. J Med Chem 59:4103–4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huelsken J, Behrens J (2002) The Wnt signalling pathway. J Cell Sci 115:3977–3978. [DOI] [PubMed] [Google Scholar]
- 8. Voronkov A, Krauss S (2013) Wnt/beta‐catenin signaling and small molecule inhibitors. Curr Pharm Des 19:634–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, Lam A, Lazetic S, Ma S, Mitra S, Park IK, Pickell K, Sato A, Satyal S, Stroud M, Tran H, Yen WC, Lewicki J, Hoey T (2012) Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci USA 109:11717–11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee HJ, Bao J, Miller A, Zhang C, Wu J, Baday YC, Guibao C, Li L, Wu D, Zheng JJ (2015) Structure‐based discovery of novel small molecule Wnt signaling inhibitors by targeting the cysteine‐rich domain of frizzled. J Biol Chem 290:30596–30606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bafico A, Gazit A, Pramila T, Finch PW, Yaniv A, Aaronson SA (1999) Interaction of frizzled related protein (FRP) with Wnt ligands and the frizzled receptor suggests alternative mechanisms for FRP inhibition of Wnt signaling. J Biol Chem 274:16180–16187. [DOI] [PubMed] [Google Scholar]
- 12. Xavier CP, Melikova M, Chuman Y, Üren A, Baljinnyam B, Rubin JS (2014) Secreted Frizzled‐related protein potentiation versus inhibition of Wnt3a/β‐catenin signaling. Cell Signal 26:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, Glinka A, Niehrs C (2002) Kremen proteins are Dickkopf receptors that regulate Wnt/beta‐catenin signalling. Nature 417:664–667. [DOI] [PubMed] [Google Scholar]
- 14. Ellwanger K, Saito H, Clement‐Lacroix P, Maltry N, Niedermeyer J, Lee WK, Baron R, Rawadi G, Westphal H, Niehrs C (2008) Targeted disruption of the Wnt regulator Kremen induces limb defects and high bone density. Mol Cell Biol 28:4875–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liedert A, Rontgen V, Schinke T, Benisch P, Ebert R, Jakob F, Klein‐Hitpass L, Lennerz JK, Amling M, Ignatius A (2014) Osteoblast‐specific Krm2 overexpression and Lrp5 deficiency have different effects on fracture healing in mice. PLoS One 9:e103250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boschert V, van Dinther M, Weidauer S, van Pee K, Muth EM, Ten Dijke P, Mueller TD (2013) Mutational analysis of sclerostin shows importance of the flexible loop and the cystine‐knot for Wnt‐signaling inhibition. PLoS One 8:e81710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holdsworth G, Slocombe P, Doyle C, Sweeney B, Veverka V, Le Riche K, Franklin RJ, Compson J, Brookings D, Turner J, Kennedy J, Garlish R, Shi J, Newnham L, McMillan D, Muzylak M, Carr MD, Henry AJ, Ceska T, Robinson MK (2012) Characterization of the interaction of sclerostin with the low density lipoprotein receptor‐related protein (LRP) family of Wnt co‐receptors. J Biol Chem 287:26464–26477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D, Mlodzik M, Shi DL, Zheng J (2003) Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C‐terminal region of Frizzled. Mol Cell 12:1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shan J, Shi DL, Wang J, Zheng J (2005) Identification of a specific inhibitor of the dishevelled PDZ domain. Biochemistry 44:15495–15503. [DOI] [PubMed] [Google Scholar]
- 20. Grandy D, Shan J, Zhang X, Rao S, Akunuru S, Li H, Zhang Y, Alpatov I, Zhang XA, Lang RA, Shi DL, Zheng JJ (2009) Discovery and characterization of a small molecule inhibitor of the PDZ domain of dishevelled. J Biol Chem 284:16256–16263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shan J, Zheng JJ (2009) Optimizing Dvl PDZ domain inhibitor by exploring chemical space. J Comput Aided Mol Des 23:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shan J, Zhang X, Bao J, Cassell R, Zheng JJ (2012) Synthesis of potent dishevelled PDZ domain inhibitors guided by virtual screening and NMR studies. Chem Biol Drug Des 79:376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee HJ, Wang NX, Shao Y, Zheng JJ (2009) Identification of tripeptides recognized by the PDZ domain of Dishevelled. Bioorg Med Chem 17:1701–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee HJ, Finkelstein D, Li X, Wu D, Shi DL, Zheng JJ (2010) Identification of transmembrane protein 88 (TMEM88) as a dishevelled‐binding protein. J Biol Chem 285:41549–41556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim HY, Choi S, Yoon JH, Lim HJ, Lee H, Choi J, Ro EJ, Heo JN, Lee W, No KT, Choi KY (2016) Small molecule inhibitors of the Dishevelled‐CXXC5 interaction are new drug candidates for bone anabolic osteoporosis therapy. EMB Mol Med 8:375–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Choi J, Ma S, Kim HY, Yun JH, Heo JN, Lee W, Choi KY, No KT (2016) Identification of small‐molecule compounds targeting the dishevelled PDZ domain by virtual screening and binding studies. Bioorg Med Chem 24:3259–3266. [DOI] [PubMed] [Google Scholar]
- 27. Osada T, Chen M, Yang XY, Spasojevic I, Vandeusen JB, Hsu D, Clary BM, Clay TM, Chen W, Morse MA, Lyerly HK (2011) Antihelminth compound niclosamide downregulates Wnt signaling and elicits antitumor responses in tumors with activating APC mutations. Cancer Res 71:4172–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moskaleva EY, Perevozchikova VG, Zhirnik AS, Severin SE (2015) Molecular mechanisms of niclosamide antitumor activity. Biomed Khim 61:680–693. [DOI] [PubMed] [Google Scholar]
- 29. Mook RA, Jr , Wang J, Ren XR, Chen M, Spasojevic I, Barak LS, Lyerly HK, Chen W (2015) Structure‐activity studies of Wnt/beta‐catenin inhibition in the Niclosamide chemotype: identification of derivatives with improved drug exposure. Bioorg Med Chem 23:5829–5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. White MC, Johnson GG, Zhang W, Hobrath JV, Piazza GA, Grimaldi M (2013) Sulindac sulfide inhibits sarcoendoplasmic reticulum Ca2+ ATPase, induces endoplasmic reticulum stress response, and exerts toxicity in glioma cells: relevant similarities to and important differences from celecoxib. J Neurosci Res 91:393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li N, Xi Y, Tinsley HN, Gurpinar E, Gary BD, Zhu B, Li Y, Chen X, Keeton AB, Abadi AH, Moyer MP, Grizzle WE, Chang WC, Clapper ML, Piazza GA (2013) Sulindac selectively inhibits colon tumor cell growth by activating the cGMP/PKG pathway to suppress Wnt/beta‐catenin signaling. Mol Cancer Ther 12:1848–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang K, Song H, Yang P, Dai X, Li Y, Wang L, Du J, Pan K, Zhang T (2015) Silencing dishevelled‐1 sensitizes paclitaxel‐resistant human ovarian cancer cells via AKT/GSK‐3beta/beta‐catenin signalling. Cell Prolif 48:249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Simard JR, Rauh D (2009) Chemical and structural biology to direct the repurposing of sulindac. ChemMedChem 4:1793–1795. [DOI] [PubMed] [Google Scholar]
- 34. Lee HJ, Wang NX, Shi DL, Zheng JJ (2009) Sulindac inhibits canonical Wnt signaling by blocking the PDZ domain of the protein Dishevelled. Angew Chem Int Ed Engl 48:6448–6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stein U, Arlt F, Smith J, Sack U, Herrmann P, Walther W, Lemm M, Fichtner I, Shoemaker RH, Schlag PM (2011) Intervening in β‐catenin signaling by sulindac inhibits S100A4‐dependent colon cancer metastasis. Neoplasia 13:131. IN138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsioulias GJ, Go MF, Rigas B (2015) NSAIDs and colorectal cancer control: promise and challenges. Curr Pharmacol Rep 1:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang X, Lou Y, Zheng X, Wang H, Sun J, Dong Q, Han B (2015) Wnt blockers inhibit the proliferation of lung cancer stem cells. Drug Des Devel Ther 9:2399–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saraswati S, Alfaro MP, Thorne CA, Atkinson J, Lee E, Young PP (2010) Pyrvinium, a potent small molecule Wnt inhibitor, promotes wound repair and post‐MI cardiac remodeling. PLoS One 5:e15521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, Jernigan KK, Meyers KC, Hang BI, Waterson AG, Kim K, Melancon B, Ghidu VP, Sulikowski GA, LaFleur B, Salic A, Lee LA, Miller DM III, Lee E (2010) Small‐molecule inhibition of Wnt signaling through activation of casein kinase 1alpha. Nat Chem Biol 6:829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fonseca BF, Predes D, Cerqueira DM, Reis AH, Amado NG, Cayres MC, Kuster RM, Oliveira FL, Mendes FA, Abreu JG (2015) Derricin and derricidin inhibit Wnt/beta‐catenin signaling and suppress colon cancer cell growth in vitro. PLoS One 10:e0120919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de la Roche M, Rutherford TJ, Gupta D, Veprintsev DB, Saxty B, Freund SM, Bienz M (2012) An intrinsically labile alpha‐helix abutting the BCL9‐binding site of beta‐catenin is required for its inhibition by carnosic acid. Nat Commun 3:680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grigson ER, Ozerova M, Pisklakova A, Liu H, Sullivan DM, Nefedova Y (2015) Canonical Wnt pathway inhibitor ICG‐001 induces cytotoxicity of multiple myeloma cells in Wnt‐independent manner. PLoS One 10:e0117693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim YM, Kahn M (2014) The role of the Wnt signaling pathway in cancer stem cells: prospects for drug development. Res Rep Biochem 4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Henderson WR, Jr , Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, Borok Z, Knight DA, Kahn M (2010) Inhibition of Wnt/beta‐catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci USA 107:14309–14314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Leal LF, Bueno AC, Gomes DC, Abduch R, de Castro M, Antonini SR (2015) Inhibition of the Tcf/beta‐catenin complex increases apoptosis and impairs adrenocortical tumor cell proliferation and adrenal steroidogenesis. Oncotarget 6:43016–43032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, Moon RT, Teo JL, Kim HY, Moon SH, Ha JR, Kahn M (2004) A small molecule inhibitor of beta‐catenin/CREB‐binding protein transcription. Proc Natl Acad Sci USA 101:12682–12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ishihama K, Yamakawa M, Semba S, Takeda H, Kawata S, Kimura S, Kimura W (2007) Expression of HDAC1 and CBP/p300 in human colorectal carcinomas. J Clin Pathol 60:1205–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang X, Moon J, Dodge ME, Pan X, Zhang L, Hanson JM, Tuladhar R, Ma Z, Shi H, Williams NS, Amatruda JF, Carroll TJ, Lum L, Chen C (2013) The development of highly potent inhibitors for porcupine. J Med Chem 56:2700–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Madan B, Patel MB, Zhang J, Bunte RM, Rudemiller NP, Griffiths R, Virshup DM, Crowley SD (2016) Experimental inhibition of porcupine‐mediated Wnt O‐acylation attenuates kidney fibrosis. Kidney Int 89:1062–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cheng Y, Phoon YP, Jin X, Chong SY, Ip JC, Wong BW, Lung ML (2015) Wnt‐C59 arrests stemness and suppresses growth of nasopharyngeal carcinoma in mice by inhibiting the Wnt pathway in the tumor microenvironment. Oncotarget 6:14428–14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T, Kasibhatla S, Schuller AG, Li AG, Cheng D, Li J, Tompkins C, Pferdekamper A, Steffy A, Cheng Kowal C, Phung V, Guo G, Wang Y, Graham MP, Flynn S, Brenner JC, Li C, Villarroel MC, Schultz PG, Wu X, McNamara P, Sellers WR, Petruzzelli L, Boral AL, Seidel HM, McLaughlin ME, Che J, Carey TE, Vanasse G, Harris JL (2013) Targeting Wnt‐driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci USA 110:20224–20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Madan B, Ke Z, Harmston N, Ho SY, Frois AO, Alam J, Jeyaraj DA, Pendharkar V, Ghosh K, Virshup IH, Manoharan V, Ong EH, Sangthongpitag K, Hill J, Petretto E, Keller TH, Lee MA, Matter A, Virshup DM (2016) Wnt addiction of genetically defined cancers reversed by PORCN inhibition. Oncogene 35:2197–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Croy HE, Fuller CN, Giannotti J, Robinson P, Foley AV, Yamulla RJ, Cosgriff S, Greaves BD, von Kleeck RA, An HH, Powers CM, Tran JK, Tocker AM, Jacob KD, Davis BK, Roberts DM (2016) The poly(ADP‐ribose) polymerase enzyme tankyrase antagonizes activity of the beta‐catenin destruction complex through ADP‐ribosylation of axin and APC2. J Biol Chem 291:12747–12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Callow MG, Tran H, Phu L, Lau T, Lee J, Sandoval WN, Liu PS, Bheddah S, Tao J, Lill JR, Hongo JA, Davis D, Kirkpatrick DS, Polakis P, Costa M (2011) Ubiquitin ligase RNF146 regulates tankyrase and Axin to promote Wnt signaling. PLoS One 6:e22595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huang SM, Mishina YM, Liu S, Cheung A, Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner S, Hild M, Shi X, Wilson CJ, Mickanin C, Myer V, Fazal A, Tomlinson R, Serluca F, Shao W, Cheng H, Shultz M, Rau C, Schirle M, Schlegl J, Ghidelli S, Fawell S, Lu C, Curtis D, Kirschner MW, Lengauer C, Finan PM, Tallarico JA, Bouwmeester T, Porter JA, Bauer A, Cong F (2009) Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461:614–620. [DOI] [PubMed] [Google Scholar]
- 56. Tian XH, Hou WJ, Fang Y, Fan J, Tong H, Bai SL, Chen Q, Xu H, Li Y (2013) XAV939, a tankyrase 1 inhibitior, promotes cell apoptosis in neuroblastoma cell lines by inhibiting Wnt/beta‐catenin signaling pathway. J Exp Clin Cancer Res 32:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. McGonigle S, Chen Z, Wu J, Chang P, Kolber‐Simonds D, Ackermann K, Twine NC, Shie JL, Miu JT, Huang KC, Moniz GA, Nomoto K (2015) E7449: a dual inhibitor of PARP1/2 and tankyrase1/2 inhibits growth of DNA repair deficient tumors and antagonizes Wnt signaling. Oncotarget 6:41307–41323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Inestrosa NC, Toledo EM (2008) The role of Wnt signaling in neuronal dysfunction in Alzheimer's Disease. Mol Neurogener 3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ho C, Lee PH, Hsu YC, Wang FS, Huang YT, Lin CL (2012) Sustained Wnt/beta‐catenin signaling rescues high glucose induction of transforming growth factor‐beta1‐mediated renal fibrosis. Am J Med Sci 344:374–382. [DOI] [PubMed] [Google Scholar]
- 60. Galli C, Piemontese M, Lumetti S, Manfredi E, Macaluso GM, Passeri G (2013) GSK3b‐inhibitor lithium chloride enhances activation of Wnt canonical signaling and osteoblast differentiation on hydrophilic titanium surfaces. Clin Oral Implants Res 24:921–927. [DOI] [PubMed] [Google Scholar]
- 61. Clement‐Lacroix P, Ai M, Morvan F, Roman‐Roman S, Vayssiere B, Belleville C, Estrera K, Warman ML, Baron R, Rawadi G (2005) Lrp5‐independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci USA 102:17406–17411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schou M (2001) Lithium treatment at 52. J Affect Disord 67:21–32. [DOI] [PubMed] [Google Scholar]
- 63. Zhang M, Shi J, Huang Y, Lai L (2012) Expression of canonical WNT/beta‐CATENIN signaling components in the developing human lung. BMC Dev Biol 12:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li C, Zhang S, Lu Y, Zhang Y, Wang E, Cui Z (2013) The roles of Notch3 on the cell proliferation and apoptosis induced by CHIR99021 in NSCLC cell lines: a functional link between Wnt and Notch signaling pathways. PLoS One 8:e84659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lu D, Zhao Y, Tawatao R, Cottam HB, Sen M, Leoni LM, Kipps TJ, Corr M, Carson DA (2004) Activation of the Wnt signaling pathway in chronic lymphocytic leukemia. Proc Natl Acad Sci USA 101:3118–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bergmann C, Akhmetshina A, Dees C, Palumbo K, Zerr P, Beyer C, Zwerina J, Distler O, Schett G, Distler JH (2011) Inhibition of glycogen synthase kinase 3beta induces dermal fibrosis by activation of the canonical Wnt pathway. Ann Rheum Dis 70:2191–2198. [DOI] [PubMed] [Google Scholar]
- 67. Yang W, Yan HX, Chen L, Liu Q, He YQ, Yu LX, Zhang SH, Huang DD, Tang L, Kong XN, Chen C, Liu SQ, Wu MC, Wang HY (2008) Wnt/beta‐catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res 68:4287–4295. [DOI] [PubMed] [Google Scholar]
- 68. Inoue T, Kagawa T, Fukushima M, Shimizu T, Yoshinaga Y, Takada S, Tanihara H, Taga T (2006) Activation of canonical Wnt pathway promotes proliferation of retinal stem cells derived from adult mouse ciliary margin. Stem Cells 24:95–104. [DOI] [PubMed] [Google Scholar]
- 69. Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH (2004) Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK‐3‐specific inhibitor. Nat Med 10:55–63. [DOI] [PubMed] [Google Scholar]
- 70. Naujok O, Lentes J, Diekmann U, Davenport C, Lenzen S (2014) Cytotoxicity and activation of the Wnt/beta‐catenin pathway in mouse embryonic stem cells treated with four GSK3 inhibitors. BMC Res Notes 7:273. [DOI] [PMC free article] [PubMed] [Google Scholar]


