STRUCTURED ABSTRACT
Background
Levels of B-type natriuretic peptide (BNP), a prognostic marker in patients with heart failure (HF), are lower among HF patients with obesity or preserved Left Ventricular Ejection Fraction (LVEF). We examined the distribution and prognostic value of BNP across BMI categories in acute decompensated heart failure (ADHF) patients with preserved vs. reduced LVEF.
Methods
We analyzed data from the Atherosclerosis Risk in Communities (ARIC) HF surveillance study which sampled and adjudicated ADHF hospitalizations in patients aged ≥ 55 years from 4 US communities (2005–2009). We examined 5 BMI categories: underweight (<18.5 kg/m2), normal weight (18.5–<25), overweight (25–<30), obese (30–<40) and morbidly obese (≥40) in HF with preserved LVEF (HFpEF) and reduced LVEF (HFrEF). The outcome was 1-year mortality from admission. We used ANCOVA to model log BNP and logistic regression for 1-year mortality, both adjusted for demographics and clinical characteristics.
Results
The cohort included 9820 weighted ADHF hospitalizations (58% HFrEF; 42% HFpEF). BNP levels were lower in HFpEF compared to HFrEF (p < 0.001) and decreased as BMI increased within the LVEF groups (p<0.001). After adjustment for covariates, log10 BNP independently predicted 1-year mortality (adjusted OR 1.62 (95% CI 1.17–2.24) with no significant interaction by BMI or LVEF groups.
Conclusions
BNP levels correlated inversely with BMI, and were higher in HFrEF compared to HFpEF. Obese patients with HFpEF and ADHF had a significant proportion with BNP levels below clinically accepted thresholds. Nevertheless, BNP was a predictor of mortality in ADHF across groups of BMI in HFpEF and HFrEF.
Keywords: B-type natriuretic peptide, heart failure, obesity, body mass index
INTRODUCTION
Obesity is a major public health problem in the United States, and is associated with a high incidence of cardiovascular risk factors including hypertension, diabetes, and dyslipidemia as well as with the development of heart failure (HF).1, 2 Furthermore, obesity is associated with an even higher incidence of HF with preserved ejection fraction (HFpEF), which constitutes approximately half of all HF patients in the community.3 Natriuretic peptides, such as B-type natriuretic peptide (BNP) and NT-pro BNP, are known to be useful in the diagnosis of HF, especially in patients with acute decompensated HF (ADHF), and in the prognostic assessment of HF.4, 5 However, natriuretic peptide levels vary by patient characteristics and are known to be lower in patients with obesity,4, 6, 7 and in those with HFpEF.8, 9
Although studies have evaluated the association of either BNP with BMI or BNP with left ventricular ejection fraction (LVEF), the association of BNP with HFpEF vs. HF with reduced LVEF (HFrEF) within BMI categories has not been examined in detail. Furthermore, the diagnostic and prognostic value of natriuretic peptides in obese patients with HFpEF is not well established. Accordingly, the objectives of our study were to examine the distribution of BNP levels by BMI categories in patients with acute decompensated HF (ADHF) with preserved vs. reduced ejection fraction, and to evaluate the prognostic value of BNP levels in obese vs. lean patients with ADHF and preserved vs. reduced ejection fraction. We divided our sample population into five BMI categories and two LVEF groups, which offered a unique design to study our objectives in many stratified sub-groups. We examined the study questions in a population-based sample of ADHF hospitalizations in 4 US communities in the Atherosclerosis Risk in Communities (ARIC) Heart Failure Community Surveillance Study.10, 11
METHODS
Study Population
The Atherosclerosis Risk in Communities (ARIC) Heart Failure Community Surveillance Study samples hospitalizations for ADHF in 4 US communities including Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota; and Washington County, Maryland, in individuals ≥ 55 years of age. Methods of event ascertainment and classification have been described previously.10, 11 Briefly, beginning in 2005, hospitalizations were randomly sampled within strata defined by targeted HF ICD-9-CM discharge diagnosis codes for HF or HF-related conditions in any position (398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 415.0, 416.9, 425.4, 428.x, 518.4, and 786.0x), age, sex, race, and community of residence.10, 11 Sampling probabilities could vary by strata and were selected to achieve similar standard errors for HF event rates across strata. (https://www2.cscc.unc.edu/aric/surveillance-manuals).
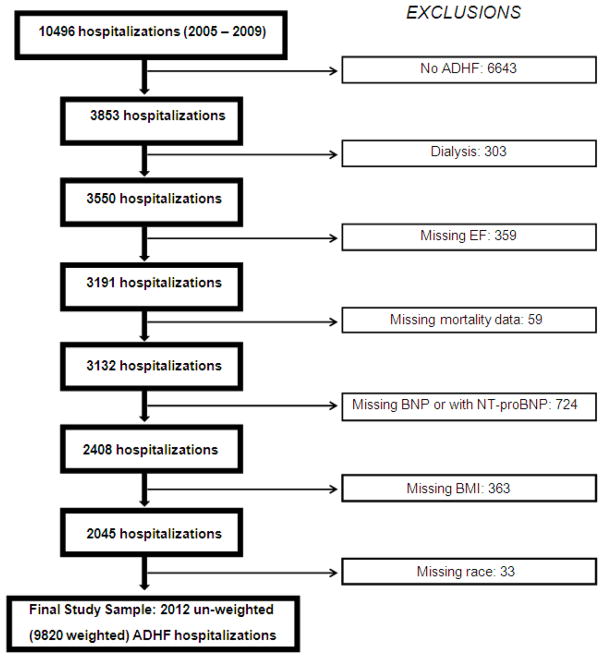
Sampled hospitalizations from 2005 to 2009 (unweighted n = 10, 496) were abstracted by trained abstractors if the medical record documented any evidence of decompensation or new onset of HF symptoms, or any mention by a physician that HF was the reason for hospitalization. Fully abstracted cases (unweighted n = 6,399) were independently classified by 2 physicians of the ARIC Mortality and Morbidity Classification Committee or a computerized algorithm into 1 of 5 categories: definite ADHF, possible ADHF, chronic stable HF, HF unlikely, or unclassifiable. Definite or possible ADHF required evidence from symptoms, signs, imaging, or treatment of an acute exacerbation, worsening or new onset of symptoms, or other decompensated circulatory state. For this study, definite and possible ADHF have been combined into a single category, I.E. ADHF. The ADHF events were further classified by HF type. Heart failure with reduced EF was defined as evidence in the medical record of LVEF < 50% within two years prior to the current hospitalization based on (in priority order) reviewer assessment, quantitative LVEF based on various imaging modalities (prioritized according to reliability), or qualitative LVEF based on echocardiography. If all LVEF ≥ 50% within two years then the case was classified as HFpEF. Otherwise, HF type was set to missing. There were a total of 359 ADHF hospitalizations with missing LVEF, which were excluded from the analysis (Figure 1). Detailed abstraction also included general medical history, physical examination, diagnostic and laboratory tests, and medications. Mortality within 1 year from the date of admission was determined by linkage with the National Death Index, with mortality data available for events with admission dates through 2009.
Figure 1.
Study cohort of acute decompensated heart failure hospitalizations
Figure 1 details the selection of our study sample from all un-weighted, ADHF hospitalizations. Since only a small proportion of hospitalizations had NT-proBNP levels available (unweighted N = 359) and because NT-proBNP cannot be interpreted on the same scale as BNP, we only included hospitalizations with BNP levels recorded. In addition, we excluded hospitalizations of patients on dialysis, because BNP levels in patients with end stage renal disease are higher, vary with timing of dialysis and type of dialysis membrane used, and may not correlate with estimated volume overload. 12–14
Data Analysis
We used measurements of weight, height, systolic blood pressure and heart rate at the time of admission. For laboratory values including BNP, serum sodium, hemoglobin, blood urea nitrogen (BUN) and creatinine, specific admission values were not available. The most abnormal values and discharge values were accessible; we used the most abnormal values recorded during the hospitalization for the analysis. Specifically for BNP, their highest levels are likely to be more accurate than the admission BNP levels, as some patients may have developed acute heart failure during their hospitalization stay. Glomerular filtration rate (GFR) was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.15 The presence of comorbidities and the medications at discharge were abstracted from the medical record. The patients were divided into five categories based on their body mass index (BMI): underweight (< 18.5 kg/m2), normal weight (18.5–24.99 kg/m2), overweight (25–29.99 kg/m2), obese (30–39.99 kg/m2) and morbidly obese (≥ 40 kg/m2).
All statistical analyses were conducted using survey procedures and were weighted using sampling weights, defined by the inverse of the sampling probability, to appropriately account for the stratified sampling design. Therefore, all results are presented in the manuscript (including Tables and Figures) as weighted number of hospitalizations. BNP levels were log transformed prior to analysis to account for their skewed distribution. Linear regression was used to model log10 BNP, and logistic regression was used for 1-year case fatality, adjusted for demographics, clinical characteristics and interactions between BMI, LVEF type and log BNP. Potential confounders were included in the model of 1-year case fatality; a forward selection procedure (entry criterion p-value < 0.20) was used to select covariates in the case fatality model. In secondary analyses, BMI was modeled as an ordinal and continuous predictor to assess trend. All analyses were completed using survey procedures in SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
The final analysis sample included 2012 un-weighted hospitalizations, corresponding to 9820 weighted ADHF hospitalizations. Overall, 42.4% of all hospitalizations were classified as HFpEF and 57.6% were classified as HFrEF. The majority of HF hospitalizations were in patients with BMI ≥ 25 kg/m2 (overweight 29.7%, obese 24.3%, and morbidly obese 9.3%), while 32.4% were normal weight, and a small proportion were underweight (4.2%). As shown in Table 1, hospitalizations for underweight patients had a lower proportion of HFpEF (35.7%) while hospitalizations for morbidly obese patients had a higher percentage of HFpEF (63%).
Table 1.
Baseline Characteristics by Body Mass Index (BMI) Groups
| Variable | Under-weight BMI < 18.5 kg/m2 (n = 415) | Normal Weight BMI 18.5–24.99 kg/m2 (n = 3206) | Overweight BMI 25– 29.99 kg/m2 (n = 2919) | Obese BMI 30–39.99 kg/m2 (n = 2428) | Morbidly Obese BMI > 40 kg/m2 (n = 916) |
|---|---|---|---|---|---|
| Age (years) | 79.8 ± 1.1 | 78.6 ± 0.4 | 76.6 ± 0.5 | 72.8 ± 0.5 | 68.5 ± 0.6 |
| Gender (male %) | 40 | 48 | 57 | 49 | 29 |
| Race (Caucasian %) | 76 | 77 | 75 | 69 | 60 |
| HFpEF (%) | 36 | 37 | 38 | 49 | 62 |
| Prior HF hospitalization (%) | 27 | 37 | 34 | 35 | 35 |
| MI (%) | 23 | 31 | 29 | 31 | 20 |
| Coronary Heart Disease (%) | 56 | 61 | 63 | 64 | 55 |
| Diabetes (%) | 25 | 27 | 42 | 61 | 71 |
| Hypertension (%) | 76 | 80 | 83 | 89 | 88 |
| Valvular Heart Disease (%) | 35 | 26 | 24 | 21 | 18 |
| Atrial Fibrillation (%) | 34 | 33 | 36 | 36 | 41 |
| PVD (%) | 12 | 14 | 10 | 15 | 11 |
| Stroke or TIA (%) | 21 | 19 | 19 | 20 | 21 |
| Pulmonary embolism (%) | 2 | 2 | 2 | 2 | 6 |
| Lung Disease (%) | 10 | 8 | 12 | 16 | 20 |
| Pulmonary hypertension (%) | 13 | 16 | 15 | 18 | 27 |
| Obstructive Sleep Apnea (%) | 4 | 2 | 6 | 15 | 31 |
| Current Smoker (%) | 21 | 15 | 13 | 14 | 14 |
| Connective Tissue Disease (%) | 3 | 5 | 5 | 6 | 7 |
| Depression (%) | 17 | 15 | 14 | 17 | 16 |
| ACEI/ARBs (%) | 38 | 55 | 54 | 60 | 60 |
| Beta-blockers (%) | 59 | 69 | 76 | 71 | 63 |
| Diuretics (%) | 82 | 85 | 85 | 86 | 89 |
| Heart Rate (beats/min) | 93.0 ± 3.4 | 91.1 ± 1.1 | 88.0 ± 1.2 | 87.2 ± 1.2 | 90.5 ± 2.1 |
| Systolic Blood Pressure (mmHg) | 137.8 ± 3.9 | 138.7 ± 1.5 | 141.5 ± 1.6 | 145.7 ± 1.5 | 143.0 ± 2.7 |
| Serum BUN (mg/dL) | 39.9 ± 2.4 | 37.5 ± 1.0 | 40.7 ± 1.2 | 38.7 ± 1.2 | 39.9 ± 1.8 |
| Estimated GFR (ml/min/1.73m2) | 44.2 ± 2.6 | 43.2 ± 0.9 | 39.7 ± 1.0 | 42.5 ± 1.4 | 42.4 ± 1.6 |
| Serum Hemoglobin (gm/dL) | 11.0 ± 0.2 | 10.6 ± 0.1 | 10.8 ± 0.1 | 11.1 ± 0.1 | 10.8 ± 0.2 |
| Serum Sodium (mEq/L) | 136.5 ± 0.4 | 135.6 ± 0.2 | 136.4 ± 0.2 | 136.6 ± 0.2 | 136.9 ± 0.3 |
For all above listed variables, the p-values for differences across five BMI categories were statistically significant (i.e. < 0.01); n = weighted number of hospitalizations in each BMI category.
Mean ± standard error is reported for continuous variables.
HFpEF=Heart Failure with preserved Ejection Fraction, MI=Myocardial Infarction, PVD=Peripheral Vascular Disease, TIA=Transient ischemic attack, ACEI=Angiotensin converting enzyme inhibitors, ARB=angiotensin receptor blocker, BUN=Blood Urea Nitrogen, GFR=Glomerular Filtration Rate.
Table 1 shows the baseline characteristics of the five BMI groups. With increasing BMI, the hospitalized HF patients tended to be younger, more often non-white, more likely to have history of diabetes, hypertension, lung disease, pulmonary hypertension, obstructive sleep apnea, and higher systolic blood pressure, but were less likely to have a history of valvular heart disease. Morbidly obese patients more frequently had a history of pulmonary embolism whereas a higher proportion of underweight patients were current smokers.
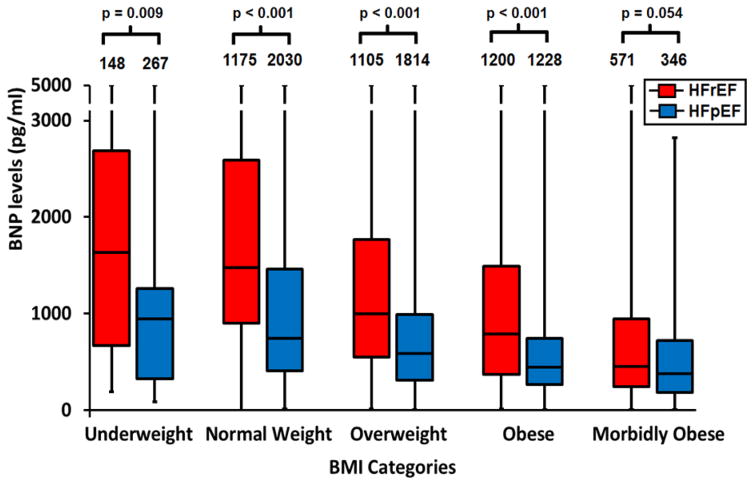
Figure 2 shows the distribution of BNP levels across the BMI and EF groups. In both EF groups, BNP decreased with increasing BMI (p trend <0.0001). Also, within each BMI category, BNP was significantly higher in the HFrEF groups compared to HFpEF groups (all p < 0.001) with the exception of the morbidly obese patients where the difference was not statistically significant (p = 0.054). Since the prevalence of diabetes increased markedly with higher BMI (Table 1), and given possible differences in prognosis for BMI categories based on the presence or absence of diabetes, 16 we also examined the distribution of BNP levels in patients with and without diabetes. The same trend of lower BNP levels with higher BMI and with HFpEF compared to HFrEF was noted, irrespective of diabetic status (data not shown).
Figure 2.
Box Plot of BNP levels (median, interquartile range) across BMI categories in HFrEF and HFpEF. P-values for trend across BMI categories < 0.0001 for both HFpEF and HFrEF. P-values are based on a linear model of log10 BNP adjusted for age, sex, race, BUN, systolic blood pressure at admission, and medications and comorbidities shown in Table 1.
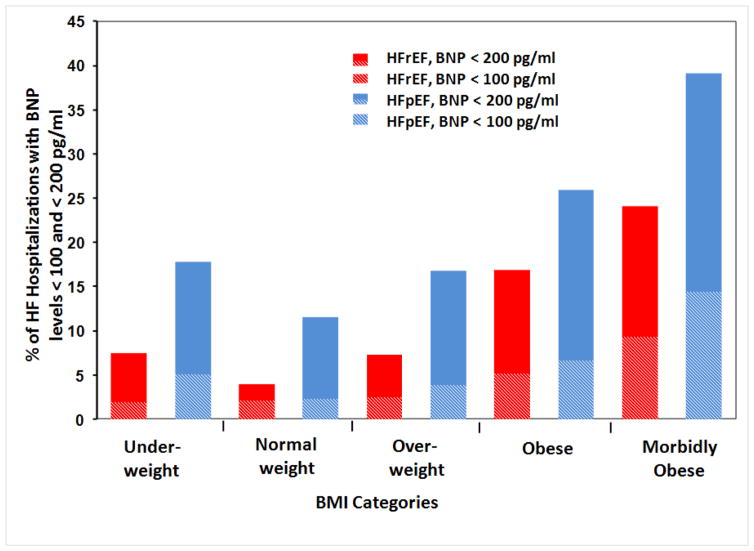
We also examined the prevalence of BNP levels < 100 pg/ml within the EF and BMI categories (Figure 3). Overall, the BNP levels were < 100 pg/ml in 4.3% of all ADHF hospitalizations with 5.7% in HFpEF and 3.3% in HFrEF hospitalizations. As BMI increased from normal weight to morbidly obese group, the percentage hospitalizations with BNP < 100 pg/mL also increased. BNP levels lower than 100 pg/ml were noted in 12.5% of all morbidly obese hospitalizations, with 14.4% in the morbidly obese HFpEF group. We further evaluated the prevalence of BNP levels < 200 pg/ml within BMI and LVEF categories. Overall, 14.2% of all ADHF hospitalizations had BNP < 200 pg/mL at admission. Within the HFpEF and HFrEF groups, 21.1% and 9.2% of patients had BNP < 200 pg/mL, respectively. As BMI increased from normal weight to morbidly obese group, the percent of hospitalizations with BNP < 200 pg/mL also increased; being present in 21.3% of obese and 33.5% of morbidly obese group hospitalizations, and specifically in 25.9% of obese and 39.1% of morbidly obese group hospitalizations with HFpEF (Figure 3).
Figure 3.
Percentage of acute decompensated heart failure hospitalizations with BNP levels less than 100 pg/ml and less than 200 pg/ml in BMI categories by HFrEF vs. HFpEF. The height of the bars represent hospitalizations with BNP levels less than 200 pg/ml and the height of the shaded areas of the bars represent hospitalizations with BNP levels less than 100 pg/ml.
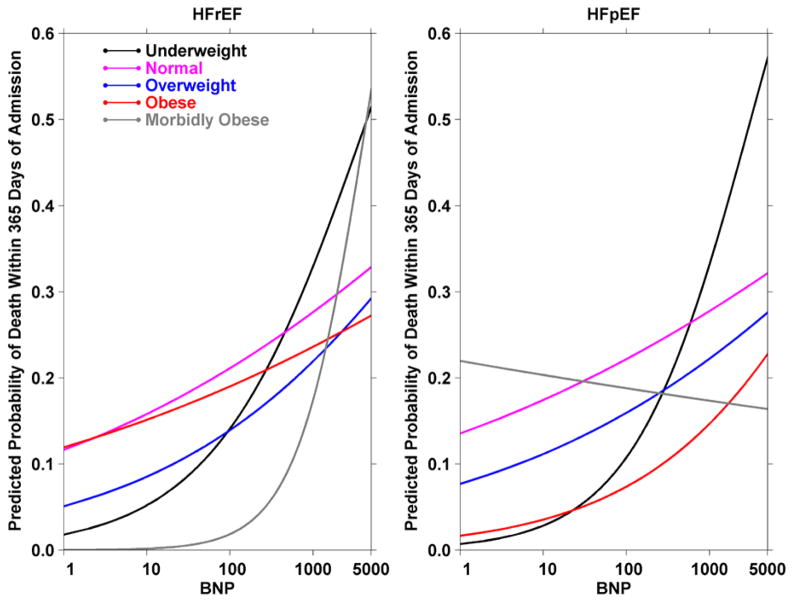
One-year case fatality was 31.9% for HFrEF and 29.6% for HFpEF admissions. After adjustment for covariates, log10 BNP remained an independent predictor of one-year all-cause mortality. In the overall cohort, the adjusted odds ratio of mortality per unit increase in log10 BNP, i.e. 10 fold increase in BNP, was 1.62 (95% CI 1.17 – 2.24). Adjusted odds ratios by BMI and heart failure type subgroups are reported in Table 2. The trend of higher odds ratios of mortality with increase in BNP was consistent across most subgroups of HF type or BMI category (Table 2), though it reached statistical significance only in morbidly obese subgroup with HFrEF. The interaction between BNP, BMI category and LVEF category was not statistically significant in the overall models (p=0.09). The adjusted probabilities of one-year mortality predicted from these models are plotted in Figure 4.
Table 2.
Odds Ratios for Mortality with increasing BNP Levels within Subgroups by BMI Category and Heart Failure Type
| BMI Category | HFrEF | HFpEF | ||
|---|---|---|---|---|
| 1-Yr mortality (%) | OR (95% CI) | 1-Yr mortality (%) | OR (95% CI) | |
| Underweight | 50.8 | 2.99 (0.44–20.42) | 48.3 | 4.12 (0.44–38.62) |
| Normal Weight | 37.0 | 1.43 (0.71–2.89) | 40.9 | 1.35 (0.62–2.95) |
| Overweight | 28.7 | 1.74 (0.77–3.89) | 30.4 | 1.51 (0.49–4.60) |
| Obese | 27.2 | 1.32 (0.54–3.22) | 18.0 | 2.16 (0.76–6.13) |
| Morbidly Obese | 20.4 | 11.21 (2.64–47.58) | 23.7 | 0.91 (0.34–2.38) |
P-value for interaction between BMI, LVEF categories and log BNP = 0.094
The adjusted Odd’s Ratios (ORs) and 95% Confidence Intervals (CI) are for mortality per unit increase in log10 BNP.
The models are adjusted for age, gender, race, systolic blood pressure at admission, highest BUN, prior history of heart failure hospitalization, beta-blockers at discharge, statins at discharge, ACE inhibitors or angiotensin II receptor blockers at discharge, intravenous diuretics during hospitalization, history of pulmonary hypertension, pulmonary embolism, connective tissue disease, stroke/TIA and the interactions terms for BMI, LVEF type and log BNP. The overall OR and 95% CI was 1.62 (1.17 – 2.24). The overall one-year mortality was 30.9%.
Figure 4.
Predicted probability of one-year mortality with increasing BNP in subgroups by BMI category and heart failure type. The models are adjusted for age, gender, race, systolic blood pressure at admission, highest BUN, beta-blockers at discharge, statins at discharge, ACE inhibitors or angiotensin II receptor blockers at discharge, intravenous diuretics during hospitalization, history of pulmonary hypertension, pulmonary embolism, connective tissue disease and stroke. Predicted probabilities were calculated for each subgroup by setting continuous covariates to mean values, and the binary variables were set 0 if the weighted mean was < 0.5 and 1 if the weighted mean ≥ 0.5 including race as white and gender as female.
As explained above in the methods section, our overall study group included hospitalizations with both definite and possible ADHF. We also performed a sensitivity analysis in order to evaluate hospitalizations with only definite ADHF, after excluding all hospitalizations with possible HF. Compared to 9820 ADHF hospitalizations in the overall group, there were 7974 weighted hospitalizations with definite HF in this sensitivity analysis. The adjusted odds ratio of mortality per unit increase in log10 BNP for this sub-group remained significant, equaling 1.96 (95% CI 1.32 – 2.91). Like the overall model, the trend of higher odds of mortality with increasing BNP remained consistent across the same sub-groups based on HF type or BMI category (data not shown). The interaction between BNP, BMI category and LVEF category also remained statistically non-significant in the sensitivity analysis (p=0.41).
DISCUSSION
Among ADHF hospitalizations occurring in 4 US communities, the majority of patients were overweight, obese or morbidly obese, in both HFpEF and HFrEF groups. The BNP levels were lower in HFpEF, and decreased with increasing BMI. For example, compared to 4.3% in all ADHF hospitalizations, BNP levels were lower than 100 pg/mL in 12.5% of the morbidly obese group, and 14.4% of morbidly obese with HFpEF group. Nevertheless, our results suggest that increasing levels of BNP remain a predictor of mortality in ADHF across the spectrum of BMI groups with HFpEF and HFrEF.
Natriuretic peptides have been well studied as diagnostic and prognostic markers in HF. However, their levels are independently influenced by several factors which clinicians must take into consideration when interpreting BNP levels in HF patients.17 Our study underscores the importance of this issue in obese and morbidly obese patients, especially those with HFpEF. These patients represent a group of patients in which the clinical diagnosis of HF is often difficult and lower BNP levels may not be specific in ruling out the diagnosis. Levels of BNP lower than 100 pg/ml have been suggested as a threshold to rule out ADHF based on previous studies, particularly the Breathing-Not-Properly (BNP) Study.18 As noted above, about 1 in 8 morbidly obese patients with ADHF, and 1 in 7 morbidly obese patients with HFpEF may have levels below this threshold. In addition to LVEF and BMI, several other factors are also known to influence the levels of BNP, including age, renal function, and hemoglobin levels.17 Clinicians must keep these factors in mind when using BNP levels to aid in the diagnosis of ADHF, to use BNP levels to guide therapy or to use cutoffs to target higher risk patients at discharge. Similarly, when designing intervention trials using elevated BNP levels as screening or enrollment criteria, the effect of these factors need to be considered. For example, one of the inclusion criterion for the Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Heart Failure with Preserved Ejection Fraction (RELAX) trial19 was BNP levels > 200 pg/ml in a chronic HFpEF population. Based on our results, 26% of obese and 39% of the morbidly obese patients with HFpEF had BNP levels below this cutoff even in an ADHF setting, where levels are expected to be higher than those in the chronic HF population. This could lead to a disproportionate exclusion of obese patients from clinical trials of HFpEF, a fact that is even more pertinent given the fact that obese individuals have a higher burden of HFpEF.
The inverse relationship between BMI and natriuretic peptides has been demonstrated for BNP and NT-proBNP.7,20,21 In the present study, the inverse relationship between BMI and BNP was observed in both HFpEF and HFrEF groups. Several mechanisms have been suggested to explain the inverse correlation between BNP and obesity. Increased expression of natriuretic peptide clearance receptors (NPR-C) in obese individuals may facilitate removal of BNP from the circulation.7 This concept is strengthened by the fact that increased NPR-C gene expression has been shown in adipose tissue in humans.22 However, NT-proBNP which has also been inversely associated with obesity, does not show increased clearance through NPR-C receptors, suggesting that there may be other mechanisms involved.20 An alternative hypothesis has been the impaired synthesis or secretion of BNP from the myocardium in obesity, i.e. a BNP-deficient state.20 Furthermore, low BNP levels in obese patients may also reflect a less advanced staged of HF compared to lean patients.7 Finally, “cardiac cachexia” in advanced HF, characterized by greater neurohumoral activation may contribute to the higher BNP levels in lean patients compared to obese patients.23,24
We also investigated the diagnostic and prognostic utility of BNP with respect to LVEF. BNP levels were significantly higher in HFrEF compared to HFpEF within each BMI category, except for morbid obesity where we found the difference was not significant although the range in HFrEF was slightly larger. Generally, LV systolic dysfunction is associated with a larger ventricular chamber radius and greater wall stress. This leads to increased synthesis and release of BNP, and may explain why BNP levels are higher in patients with HFrEF.8 In addition, a complex interplay of neurohormonal factors may explain lower levels of BNP in HFpEF even with similar apparent severity of the HF state. 25
Although the prognostic value of BNP in HF had been demonstrated before,26 there is only limited data to suggest that higher BNP levels may predict mortality irrespective of LVEF.9 Our study builds further on these results and evaluated prognosis in 5 subgroups of BMI within HFpEF and HFrEF. Our findings suggest that higher levels of BNP were predictors of mortality in ADHF. The trend of higher odds of mortality with increase in BNP was consistent across most subgroups of HF types or BMI categories (Figure 4), although in the morbidly obese patients with HFpEF there was a suggestion that BNP may not be as helpful in predicting mortality. However, it should be noted that the overall statistical interaction for mortality predicted by BNP by EF and BMI groups was not statistically significant; therefore the finding of a lack of association of BNP with mortality in morbidly obese patients with HFpEF will need confirmation in future studies. It is possible that the overall lower BNP levels with a smaller range in this patient group may make BNP a less useful prognostic marker in this group of morbidly obese patients with HFpEF.
Limitations
This is a retrospective observational study with limitations inherent to study design. The diagnosis of ADHF was made by review of hospitalization records, and because HF is a clinical diagnosis, an ideal gold standard does not exist. However, in this study, data were abstracted by trained data abstractors only when the initial screening suggested acute HF decompensation. Two physicians of the ARIC Mortality and Morbidity Classification Committee reviewed the detailed clinical, diagnostic and treatment data, and were responsible for confirming the diagnosis of definite or possible ADHF based on evidence from symptoms, signs, imaging, or treatment of an acute exacerbation, worsening or new onset of symptoms, or other decompensated circulatory state. In spite of this, misdiagnosis of HF remains possible, especially in obese patients where signs and symptoms of HF may not be specific. It should be noted that the results remained similar when a sensitivity analysis using only the subset of patients classified as definite ADHF were examined. Levels of BNP were available when the diagnosis of HF was confirmed by the reviewers; however, this would likely only underestimate the diagnosis of ADHF in patients with low BNPs. In addition, because admission BMI values were used given that the majority of patients had weights recorded at admission (compared to a lower proportion at discharge), there may be some inherent inaccuracy due to volume overload at the time of admission. Lastly, we used BMI as a measure of obesity, as has been used in the majority of studies that have examined obesity and HF. We did not have measures of central obesity such as waist circumference or waist-hip ratio, which some, although not all studies have suggested may be more associated with heart failure than BMI. 27–29
Conclusions
Our results demonstrate that most patients with ADHF hospitalizations across 4 US communities were overweight, obese or morbidly obese. BNP levels correlated inversely with respect to BMI, and were higher in HFrEF compared to HFpEF. Obese patients with HFpEF and ADHF have a significant proportion with BNP levels below the clinically accepted thresholds. Nevertheless, BNP was found to be an independent predictor of mortality in ADHF in most groups of BMI in HFpEF and HFrEF. The results are relevant in making diagnostic and prognostic decisions in patients with ADHF.
Acknowledgments
Grant support: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
The authors thank the staff and participants of the ARIC study for their important contributions.
LIST OF ABBREVIATIONS
- BNP
B-type Natriuretic Peptide
- BMI
Body Mass Index
- HF
Heart Failure
- LVEF
Left Ventricular Ejection Fraction
- ARIC
Atherosclerosis Risk in Communities
- ADHF
Acute Decompensated Heart Failure
- HFpEF
Heart Failure with Preserved Left Ventricular Ejection Fraction
- HFrEF
Heart Failure with Reduced Left Ventricular Ejection Fraction
Footnotes
Conflict(s) Of Interest/Disclosure(s): The authors report no relationships that could be construed as a conflict of interest
CONFLICTS OF INTEREST
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allison DB, Fontaine KR, Manson JE, Stevens J, VanItallie TB. Annual deaths attributable to obesity in the United States. JAMA. 1999 Oct 27;282(16):1530–8. doi: 10.1001/jama.282.16.1530. [DOI] [PubMed] [Google Scholar]
- 2.Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, et al. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2004 Nov 2;110(18):2952–67. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 3.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004 Feb 4;43(3):317–27. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 4.Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002 Jul 18;347(3):161–7. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 5.Tang WH, Francis GS, Morrow DA, Newby LK, Cannon CP, Jesse RL, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical utilization of cardiac biomarker testing in heart failure. Clin Biochem. 2008 Mar;41(4–5):210–21. doi: 10.1016/j.clinbiochem.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 6.van KR, van DF, Bakker J, Nijhuis J, Crijns H, Buurman W, et al. Is brain natriuretic peptide production decreased in obese subjects? J Am Coll Cardiol. 2006 Feb 21;47(4):886–7. doi: 10.1016/j.jacc.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Horwich TB, Hamilton MA, Fonarow GC. B-type natriuretic peptide levels in obese patients with advanced heart failure. J Am Coll Cardiol. 2006 Jan 3;47(1):85–90. doi: 10.1016/j.jacc.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 8.O’Donoghue M, Chen A, Baggish AL, Anwaruddin S, Krauser DG, Tung R, et al. The effects of ejection fraction on N-terminal ProBNP and BNP levels in patients with acute CHF: analysis from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) study. J Card Fail. 2005 Jun;11(5 Suppl):S9–14. doi: 10.1016/j.cardfail.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 9.van Veldhuisen DJ, Linssen GC, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JG, et al. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol. 2013 Apr 9;61(14):1498–506. doi: 10.1016/j.jacc.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 10.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012 Mar 1;5(2):152–9. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang PP, Chambless LE, Shahar E, Bertoni AG, Russell SD, Ni H, et al. Incidence and survival of hospitalized acute decompensated heart failure in four US communities (from the Atherosclerosis Risk in Communities Study) Am J Cardiol. 2014 Feb 1;113(3):504–10. doi: 10.1016/j.amjcard.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madsen LH, Ladefoged S, Corell P, Schou M, Hildebrandt PR, Atar D. N-terminal pro brain natriuretic peptide predicts mortality in patients with end-stage renal disease in hemodialysis. Kidney Int. 2007 Mar;71(6):548–54. doi: 10.1038/sj.ki.5002087. [DOI] [PubMed] [Google Scholar]
- 13.Sheen V, Bhalla V, Tulua-Tata A, Bhalla MA, Weiss D, Chiu A, et al. The use of B-type natriuretic peptide to assess volume status in patients with end-stage renal disease. Am Heart J. 2007 Feb;153(2):244–5. doi: 10.1016/j.ahj.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 14.Wahl HG, Graf S, Renz H, Fassbinder W. Elimination of the cardiac natriuretic peptides B-type natriuretic peptide (BNP) and N-terminal proBNP by hemodialysis. Clin Chem. 2004 Jun;50(6):1071–4. doi: 10.1373/clinchem.2003.030692. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zamora E, Lupón J, Enjuanes C, Pascual-Figal D, de Antonio M, Domingo M, Comín-Colet J, Vila J, Peñafiel J, Farré N, Alonso N, Santesmases J, Troya M, Bayés-Genís A. No benefit from the obesity paradox for diabetic patients with heart failure. Eur J Heart Fail. 2016 Jul;18(7):851–8. doi: 10.1002/ejhf.576. [DOI] [PubMed] [Google Scholar]
- 17.Januzzi JL., Jr Natriuretic peptides, ejection fraction, and prognosis: parsing the phenotypes of heart failure. J Am Coll Cardiol. 2013 Apr 9;61(14):1507–9. doi: 10.1016/j.jacc.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 18.Maisel AS, Clopton P, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, et al. Impact of age, race, and sex on the ability of B-type natriuretic peptide to aid in the emergency diagnosis of heart failure: results from the Breathing Not Properly (BNP) multinational study. Am Heart J. 2004 Jun;147(6):1078–84. doi: 10.1016/j.ahj.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013 Mar 27;309(12):1268–77. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das SR, Drazner MH, Dries DL, Vega GL, Stanek HG, Abdullah SM, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation. 2005 Oct 4;112(14):2163–8. doi: 10.1161/CIRCULATIONAHA.105.555573. [DOI] [PubMed] [Google Scholar]
- 21.Bayes-Genis A, Lloyd-Jones DM, van Kimmenade RR, Lainchbury JG, Richards AM, Ordonez-Llanos J, et al. Effect of body mass index on diagnostic and prognostic usefulness of amino-terminal pro-brain natriuretic peptide in patients with acute dyspnea. Arch Intern Med. 2007 Feb 26;167(4):400–7. doi: 10.1001/archinte.167.4.400. [DOI] [PubMed] [Google Scholar]
- 22.Pivovarova O, Gogebakan O, Kloting N, Sparwasser A, Weickert MO, Haddad I, et al. Insulin up-regulates natriuretic peptide clearance receptor expression in the subcutaneous fat depot in obese subjects: a missing link between CVD risk and obesity? J Clin Endocrinol Metab. 2012 May;97(5):E731–E739. doi: 10.1210/jc.2011-2839. [DOI] [PubMed] [Google Scholar]
- 23.Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997 Apr 12;349(9058):1050–3. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 24.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990 Jul 26;323(4):236–41. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 25.Bishu K, Deswal A, Chen HH, LeWinter MM, Lewis GD, Semigran MJ, et al. Biomarkers in acutely decompensated heart failure with preserved or reduced ejection fraction. Am Heart J. 2012 Nov;164(5):763–70. doi: 10.1016/j.ahj.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cleland JG, Taylor J, Freemantle N, Goode KM, Rigby AS, Tendera M. Relationship between plasma concentrations of N-terminal pro brain natriuretic peptide and the characteristics and outcome of patients with a clinical diagnosis of diastolic heart failure: a report from the PEP-CHF study. Eur J Heart Fail. 2012 May;14(5):487–94. doi: 10.1093/eurjhf/hfs049. [DOI] [PubMed] [Google Scholar]
- 27.Russo C, Sera F, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Abdominal adiposity, general obesity, and subclinical systolic dysfunction in the elderly: A population-based cohort study. Eur J Heart Fail. 2016 May;18(5):537–44. doi: 10.1002/ejhf.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark AL, Fonarow GC, Horwich TB. Waist circumference, body mass index, and survival in systolic heart failure: the obesity paradox revisited. J Card Fail. 2011 May;17(5):374–8. doi: 10.1016/j.cardfail.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Loehr LR, Rosamond WD, Poole C, McNeill AM, Chang PP, Folsom AR, Chambless LE, Heiss G. Association of multiple anthropometrics of overweight and obesity with incident heart failure: the Atherosclerosis Risk in Communities study. Circ Heart Fail. 2009 Jan;2(1):18–24. doi: 10.1161/CIRCHEARTFAILURE.108.813782. [DOI] [PMC free article] [PubMed] [Google Scholar]