Abstract
Purpose
To evaluate the neutrophil to lymphocyte ratio (NLR) in patients with nonarteritic anterior ischemic optic neuropathy (NAION).
Methods
We investigated 112 subjects comprising 56 patients with NAION and 56 healthy controls at Süleyman Demirel University. Complete blood count, demographic, and clinic data from NAION patients were evaluated in this study. The NLR was calculated in all individuals and compared between the patient and control groups. Cut-off values were also determined. Then, the relationship between NLR and visual outcomes was investigated.
Results
The cut-off value for NLR was 1.64. NLR values were significantly higher in NAION patients than in healthy subjects (p < 0.001) and were directly correlated with erythrocyte sedimentation rate levels (r = 0.263, p = 0.006). Also, the NLR value was associated with visual outcomes. Receiver operator characteristic curve analysis revealed a 0.63 area under the curve (confidence interval, 53.7% to 74.1%), 85% sensitivity and 41% specificity at the cut-off NLR value.
Conclusions
The NLR may be a biomarker with good sensitivity that is quick, cost effective and easily detected in serum. It can be used in clinical practice to predict a NAION patient's prognosis in terms of visual outcomes.
Keywords: Neutrophil to lymphocyte ratio, Optic neuropathy, Prognosis, Useful marker, Vision
Nonarteritic anterior ischemic optic neuropathy (NAION) is the most common cause of acute optic neuropathy in adults above 50 years of age [1]. It is an idiopathic disease and most patients present with painless sudden visual loss, optic disc swelling and visual field defects. The patients generally have systemic vascular risk factors, such as hypertension, diabetes mellitus, hypercholesterolemia [2]. Other associations have been reported, including hyperhomocysteinemia, nocturnal hypotension, anemia, obstructive sleep apnea syndrome, and some coagulopathies [3]. The diagnosis of NAION in most patients is clinical, considering age, presence of systemic vascular risk factors, pattern of visual loss, and appearance of the swollen disc.
White blood cell count and its subtypes are well-known as classic inflammatory markers especially in cardiovascular diseases [4]. In recent years, the blood neutrophil to lymphocyte ratio (NLR) has been identified as a potentially useful marker of clinical outcome in inflammatory disease [5,6,7,8,9,10,11]. Also, neutrophil-mediated inflammation could play an important role in the pathogenesis of the ischemic disease of the optic nerve, and NLR may be used as a marker to diagnose and assess disease severity for NAION.
In the present study, we compared NLR values between NAION patients and healthy subjects. Also, we investigated the relationship between NLR and visual outcomes. To the best of our knowledge, this is the first study to evaluate the NLR in patients with NAION.
Materials and Methods
In this study, we included patients diagnosed with NAION at the Department of Ophthalmology at Süleyman Demirel University between January 2011 and June 2014. The study was conducted according to the Declaration of Helsinki and approved by the Süleyman Demirel University, Department of Medical Sciences ethics committee. Informed consent was obtained from each of the healthy subjects in the control group.
The study included 56 patients diagnosed with NAION and 56 healthy controls. The diagnosis of NAION was established according to the Ischemic Optic Neuropathy Decompression Trial criteria: sudden loss of vision within the previous 14 days, a relative afferent pupillary defect, optic disc edema, and visual field defects consistent with optic neuropathy. We differentiate NAION from ischemic optic neuropathy by the absence of complaints such as headache, weight loss, temporal artery tenderness, and jaw claudication. All patients were treated with intravenous corticosteroids (1 g/day methylprednisolone) for 3 days, followed by oral prednisone (1 mg per kilogram per day) for 11 days. Patients were excluded if they had active infection, chronic inflammatory disease or autoimmune disease, glaucoma, ophthalmic or neurological disease, any other retinal pathology, ocular trauma or if they had undergone previous eye surgery (other than uneventful cataract surgery). Ophthalmological examination included best-corrected visual acuity (BCVA [logarithm of the minimum angle of resolution]), slit-lamp examination, and visual field test (Zeiss Humphrey Systems, Dublin, CA, USA). BCVA was recorded at the initial onset of disease and after 6 months.
Complete blood count data were obtained for all subjects. Blood sampling was performed at the time of diagnosis with NAION in the patient group. The hospital in the present study had been employing an automation system since 2008. Furthermore, age, sex, visual acuity, laboratory values including leukocytes, neutrophils, lymphocytes, NLR, mean platelet volume (MPV), red blood cell distribution width (RDW), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) parameters were recorded for these patients. In addition, we calculated NLR in all individuals, compared NLR between the patient and control groups, and determined cut-off NLR values. Then, we investigated the relationship between NLR and visual outcomes.
Complete blood and inflammatory-marker counts
Complete blood counts were performed by flow cytometry (Beckman Coulter LH 780 Analyzer; Beckman Coulter, Miami, FL, USA). Calibration of the analyzer was performed twice a day by the control blood samples with low and high parameters. Peripheral blood was obtained at the time of diagnosis. Venous blood samples were drawn into EDTA tubes for the evaluation of hematological parameters before treatment. Hematological parameters (leukocytes, neutrophils, lymphocytes, NLR, MPV, RDW, ESR, and CRP) were recorded. The NLR was calculated as follows: NLR = neutrophil count to lymphocyte count. CRP was measured by the turbidimetric method (Toshiba ACCUTE TBA-40FR; Toshiba Medical Systems, Tokyo, Japan). ESR was measured by spectrophotometric assay (Alifax test-1 THL, 950 nm; Alifax, Polverara, Italy).
Statistical analysis
All statistical analyses were performed using the SPSS for Windows ver. 20.0 (IBM Corp., Armonk, NY, USA). The Kolmogorov-Smirnov test was used to determine whether or not variables are normally distributed. Continuous variables were expressed as mean (standard deviation) or median according to distribution state. Categorical variables were expressed as numbers and percentage. The chi-square test was used to compare proportions in different groups. Student's t-test or Mann-Whitney U-test was used to compare the two independent groups according to distribution state. The Kruskal-Wallis test was used for comparing more than two independent groups for non-normal distributed variables. In cases where Kruskal-Wallis test yielded a statistical significance, post hoc analysis was performed to identify the groups that showed differences by Bonferroni-corrected Mann-Whitney U-test. Receiver-operating characteristic curves were used to determine the cut-off values of NLR. The correlation coefficients and their significance between non-normally distributed variables (NLR and other laboratory values: RDW, MPV, ESR, and CRP) were analyzed using the Spearman test. A p-value less than 0.05 was considered significant.
Results
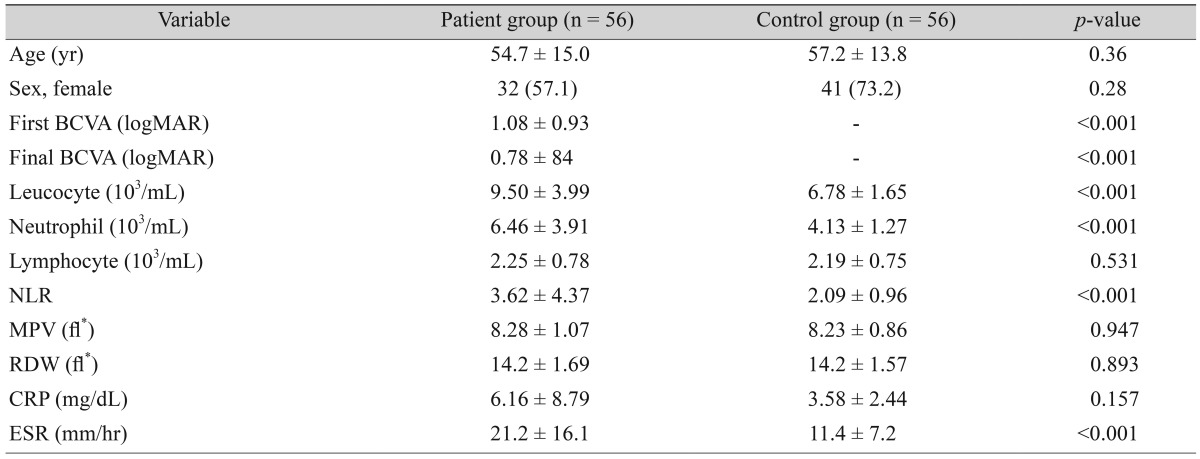
Fifty-six patients with NAION and 56 age- and sex-matched healthy subjects were included in this study. The mean age of patients with NAION and patients in the control group were 54.7 ± 15.0 and 57.2 ± 13.8 years, respectively (p = 0.36). The percentage of women was 57.1% (n = 32) for patients, and 73.2% (n = 41) for the controls (p = 0.28). Demographic data, clinical characteristics, and blood parameters are given in Table 1. The initial and final BCVA (logarithm of the minimum angle of resolution) were significantly lower in the patient group (p < 0.001).
Table 1. Demographic data, clinical characteristics, and blood parameters of the study groups.
Values are presented as mean ± standard deviation or number (%).
BCVA = best-corrected visual acuity; logMAR = logarithm of the minimum angle of resolution; NLR = neutrophil to lymphocyte ratio; MPV = mean platelet volume; RDW = red blood cell distribution width; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate.
*fl = (plateletcrit [%] / platelet count [×109/l]) × 105.
The patients with NAION had significantly higher neutrophil count (p < 0.001), NLR (p = 0.006) and ESR (p < 0.001) than control group (Table 1). No difference was detected in the levels of lymphocyte, RDW, MPV, and CRP between groups (p = 0.531, p = 0.893, p = 0.947, and p = 0.157, respectively). The NLR, and both the initial and final BCVA were negatively correlated (p = 0.014 and p = 0.04, respectively).
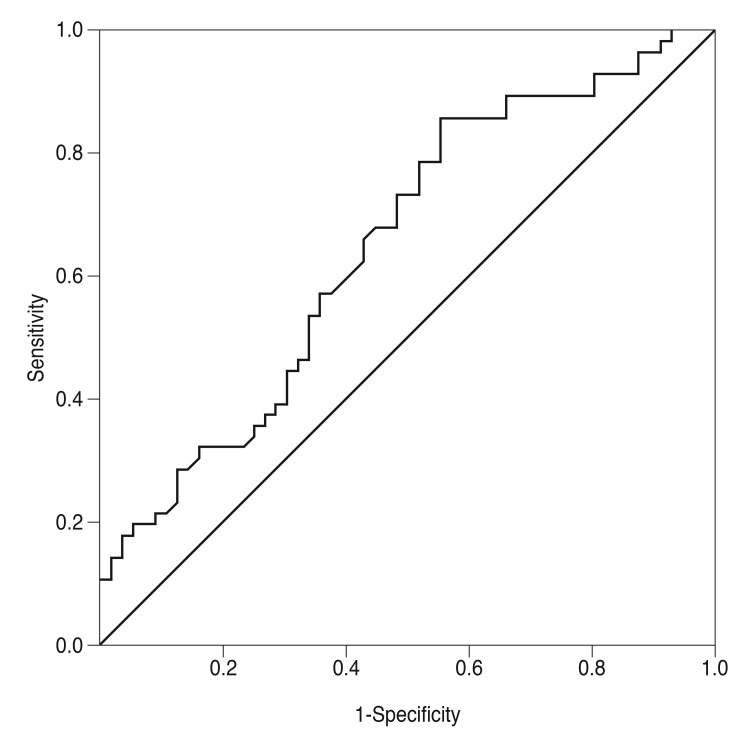
The cut-off value of NLR for predicting a NAION diagnosis was 1.64. Receiver operator characteristic analysis revealed a 0.63 area under the curve (confidence interval, 53.7% to 74.1%), 85% sensitivity and 41% specificity with the cut-off of NLR (Fig. 1). The positive predictive value and negative predictive value for NLR were 60% and 71%, respectively.
Fig. 1. Receiver operator characteristic curve showing specificity and sensitivity percentages of neutrophil to lymphocyte ratio in patients with nonarteritic anterior ischemic optic neuropathy. Area under the curve 0.63, neutrophil to lymphocyte ratio cut-off value 1.64, sensitivity 85%, specificity 41%.
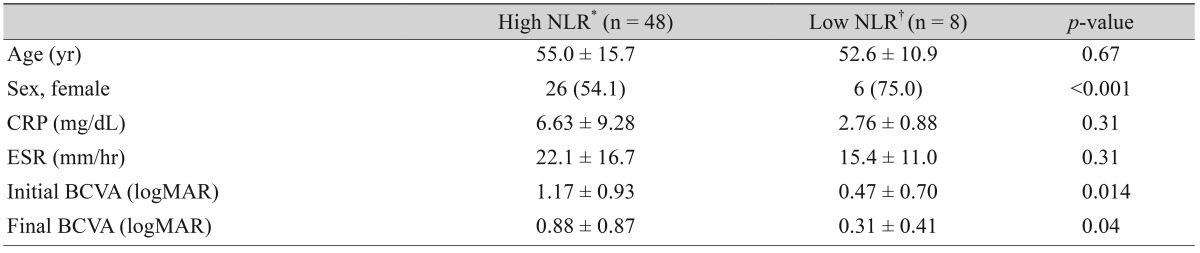
The study group was divided into two groups according to NLR (high NLR [≥1.64] and low NLR [<1.64]). The initial and final visual acuity were significantly worse in patients with high NLR than in patients with low NLR (Table 2). In addition, the majority of patients with low NLR were female (75.0%).
Table 2. The relationship between NLR, initial visual acuity, and final visual acuity in patients with NAION.
Values are presented as mean ± standard deviation or number (%).
NLR = neutrophil to lymphocyte ratio; NAION = nonarteritic anterior ischemic optic neuropathy; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; BCVA = best-corrected visual acuity; logMAR = logarithm of the minimum angle of resolution.
*NLR value of 1.64 or higher; †NLR value less than 1.64.
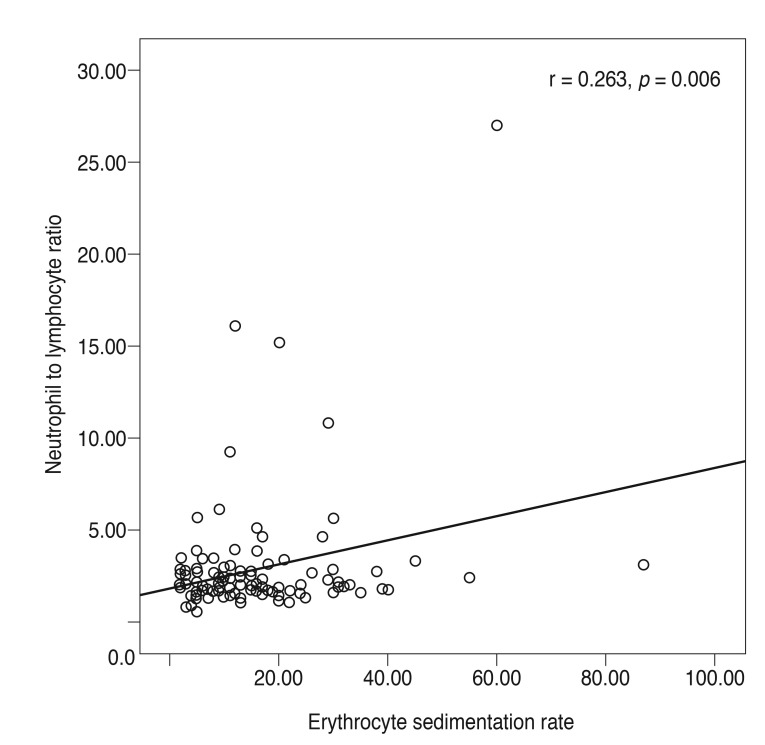
There was a statistically significant positive correlation between ESR and NLR (r = 0.263, p = 0.006) (Fig. 2). There was no correlation between NLR and MPV, RDW, and CRP (r = 0.061, p = 0.657; r = 0.061, p = 0.657; r = 0.199, p = 0.166, respectively).
Fig. 2. Correlations between neutrophil to lymphocyte ratio and erythrocyte sedimentation rate (mm/hr) level. r = Spearman correlation coefficient; p < 0.05 was statistically significant.
Discussion
NLR is a simple, cost-effective and reliable indicator of inflammation. To our knowledge, this is the first study of NLR in NAION patients and the first report of a relationship between NAION and NLR, and a significant correlation between NLR and visual outcomes in NAION.
NAION is one of the most common causes of vision loss worldwide [12]. So far, there is no generally accepted, well-proven, effective treatment for NAION. The primary medications used in the treatment of NAION are corticosteroids, but the mechanism by which steroids act in this disease is not known. Corticosteroid therapy has been suggested to be potentially beneficial in acute phase NAION through improvement of visual acuity and visual field [13].
NAION is known to be a consequence of acute ischemia of the optic nerve head, but the precise mechanism of the disease is unclear and presumably is multifactorial, including the development of a compartment syndrome [14], embolism, and vascular dysregulation [15]. Recently, early inf lammation components were determined in clinical NAION and its models [16,17,18]. NAION results in early cytokine mediated changes [19], and then sequential inflammatory cellular activation and infiltration are observed [17].
As a new inflammation marker, NLR represents the balance between neutrophil and lymphocyte levels, which are indicators of systemic inflammation [5,6,7,8,9,10,11]. Unlike other inflammatory markers, NLR is simple to calculate, easily applicable in daily practice, and inexpensive. In addition, a high NLR has been found to be correlated with severity and poor prognosis of several diseases [5,6,7,8,9,10,11]. Also, the NLR has been assessed in several studies on eye disease [20,21]. Ilhan et al. [20] found that patients with age-related macular degeneration have higher NLR compared with healthy subjects. Their study also showed that NLR correlates with disease severity. Karaca et al. [21] recently reported that the NLR is associated with progression of keratoconus. In our study, we found that patients with NAION have higher NLR and this value was correlated with visual results. According to our study, NLR may be helpful for diagnosing NAION and predicting the visual prognosis of NAION subjects.
In the previous study, a NLR result of 1.64 predicted the presence of NAION with 85% sensitivity and 41% specificity. The difference in initial and final visual acuity between patients with high and low NLR was significant (p = 0.014 and p = 0.04, respectively) (Table 2). According to our results, a high NLR value is associated with worse visual results.
Dirican et al. [7] reported that there was a correlation between NLR and levels of ESR in patients with sarcoidosis and the value of ESR increased significantly when the stage increased. In our study, we found higher level of ESR in NAION patients than in the control group, and there was a positive correlation between NLR and ESR values. But, we did not find any difference between NAION patients and the control group for the CRP value. According to these results, it is thought that the severity of disease may be more strongly associated with ESR than with CRP.
Our study had several limitations. This study had a retrospective design and evaluated a small patient group due to the rarity of the disease. In addition, usage of a single blood sample cannot be used to predict the persistence of NLR over time.
In conclusion, this study showed that NLR was significantly increased in patients with NAION compared with controls. We suggest that NLR can be used in clinical practice to predict prognosis for NAION patients in terms of visual outcomes.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Hattenhauer MG, Leavitt JA, Hodge DO, et al. Incidence of nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1997;123:103–107. doi: 10.1016/s0002-9394(14)70999-7. [DOI] [PubMed] [Google Scholar]
- 2.McCulley TJ, Lam BL, Feuer WJ. A comparison of risk factors for postoperative and spontaneous nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2005;25:22–24. doi: 10.1097/00041327-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Worrall BB, Moazami G, Odel JG, Behrens MM. Anterior ischemic optic neuropathy and activated protein C resistance: a case report and review of the literature. J Neuroophthalmol. 1997;17:162–165. [PubMed] [Google Scholar]
- 4.Arruda-Olson AM, Reeder GS, Bell MR, et al. Neutrophilia predicts death and heart failure after myocardial infarction: a community-based study. Circ Cardiovasc Qual Outcomes. 2009;2:656–662. doi: 10.1161/CIRCOUTCOMES.108.831024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azab B, Chainani V, Shah N, McGinn JT. Neutrophil-lymphocyte ratio as a predictor of major adverse cardiac events among diabetic population: a 4-year follow-up study. Angiology. 2013;64:456–465. doi: 10.1177/0003319712455216. [DOI] [PubMed] [Google Scholar]
- 6.Ertas G, Sonmez O, Turfan M, et al. Neutrophil/lymphocyte ratio is associated with thromboembolic stroke in patients with non-valvular atrial fibrillation. J Neurol Sci. 2013;324:49–52. doi: 10.1016/j.jns.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 7.Dirican N, Anar C, Kaya S, et al. The clinical significance of hematologic parameters in patients with sarcoidosis. Clin Respir J. 2016;10:32–39. doi: 10.1111/crj.12178. [DOI] [PubMed] [Google Scholar]
- 8.Ahsen A, Ulu MS, Yuksel S, et al. As a new inflammatory marker for familial Mediterranean fever: neutrophil-to-lymphocyte ratio. Inflammation. 2013;36:1357–1362. doi: 10.1007/s10753-013-9675-2. [DOI] [PubMed] [Google Scholar]
- 9.Dirican A, Kucukzeybek BB, Alacacioglu A, et al. Do the derived neutrophil to lymphocyte ratio and the neutrophil to lymphocyte ratio predict prognosis in breast cancer? Int J Clin Oncol. 2015;20:70–81. doi: 10.1007/s10147-014-0672-8. [DOI] [PubMed] [Google Scholar]
- 10.Kao SC, Pavlakis N, Harvie R, et al. High blood neutrophil-to-lymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res. 2010;16:5805–5813. doi: 10.1158/1078-0432.CCR-10-2245. [DOI] [PubMed] [Google Scholar]
- 11.Farah R, Khamisy-Farah R. Association of neutrophil to lymphocyte ratio with presence and severity of gastritis due to Helicobacter pylori infection. J Clin Lab Anal. 2014;28:219–223. doi: 10.1002/jcla.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayreh SS, Zimmerman MB. Nonarteritic anterior ischemic optic neuropathy: natural history of visual outcome. Ophthalmology. 2008;115:298–305. doi: 10.1016/j.ophtha.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayreh SS. Role of steroid therapy in nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2010;30:388–389. doi: 10.1097/WNO.0b013e3181fab01b. [DOI] [PubMed] [Google Scholar]
- 14.Tesser RA, Niendorf ER, Levin LA. The morphology of an infarct in nonarteritic anterior ischemic optic neuropathy. Ophthalmology. 2003;110:2031–2035. doi: 10.1016/S0161-6420(03)00804-2. [DOI] [PubMed] [Google Scholar]
- 15.Arnold AC. Pathogenesis of nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2003;23:157–163. doi: 10.1097/00041327-200306000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Zhang C, Guo Y, Miller NR, Bernstein SL. Optic nerve infarction and post-ischemic inf lammation in the rodent model of anterior ischemic optic neuropathy (rAION) Brain Res. 2009;1264:67–75. doi: 10.1016/j.brainres.2008.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salgado C, Vilson F, Miller NR, Bernstein SL. Cellular inflammation in nonarteritic anterior ischemic optic neuropathy and its primate model. Arch Ophthalmol. 2011;129:1583–1591. doi: 10.1001/archophthalmol.2011.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slater BJ, Vilson FL, Guo Y, et al. Optic nerve inflammation and demyelination in a rodent model of nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2013;54:7952–7961. doi: 10.1167/iovs.13-12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldenberg-Cohen N, Kramer M, Bahar I, et al. Elevated plasma levels of interleukin 8 in patients with acute anterior ischaemic optic neuropathy. Br J Ophthalmol. 2004;88:1538–1540. doi: 10.1136/bjo.2004.046524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilhan N, Daglioglu MC, Ilhan O, et al. Assessment of neutrophil/lymphocyte ratio in patients with age-related macular degeneration. Ocul Immunol Inflamm. 2014:1–4. doi: 10.3109/09273948.2014.921715. [DOI] [PubMed] [Google Scholar]
- 21.Karaca EE, Ozmen MC, Ekici F, et al. Neutrophil-to-lymphocyte ratio may predict progression in patients with keratoconus. Cornea. 2014;33:1168–1173. doi: 10.1097/ICO.0000000000000260. [DOI] [PubMed] [Google Scholar]