Abstract
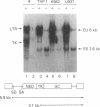
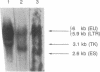
Retroviral gene transfer has been used successfully to correct the glucocerebrosidase (GCase) deficiency in primary hematopoietic cells from patients with Gaucher disease. For this model of somatic gene therapy, we developed a high-titer, amphotropic retroviral vector designated NTG in which the human GCase gene was driven by the mutant polyoma virus enhancer/herpesvirus thymidine kinase gene (tk) promoter (Py+/Htk). NTG normalized GCase activity in transduced Gaucher fibroblasts and efficiently infected human monocytic and erythroleukemic cell lines. RNA blot-hybridization (Northern blot) analysis of these hematopoietic cell lines showed unexpectedly high-level expression from the Moloney murine leukemia virus long terminal repeat (Mo-MLV LTR) and levels of Py+/Htk enhancer/promoter-initiated human GCase RNA that approximated endogenous GCase RNA levels. Furthermore, NTG efficiently infected human hematopoietic progenitor cells. Detection (by means of the polymerase chain reaction) of the provirus in approximately one-third of NTG-infected progenitor colonies that had not been selected in G418-containing medium indicates that relative resistance to G418 underestimated the actual gene transfer efficiency. Northern blot analysis of NTG-infected, progenitor-derived cells showed expression from both the Mo-MLV LTR and the Py+/Htk enhancer/promoter. NTG-transduced hematopoietic progenitor cells from patients with Gaucher disease generated progeny in which GCase activity had been normalized.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armentano D., Yu S. F., Kantoff P. W., von Ruden T., Anderson W. F., Gilboa E. Effect of internal viral sequences on the utility of retroviral vectors. J Virol. 1987 May;61(5):1647–1650. doi: 10.1128/jvi.61.5.1647-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADY R. O., KANFER J. N., SHAPIRO D. METABOLISM OF GLUCOCEREBROSIDES. II. EVIDENCE OF AN ENZYMATIC DEFICIENCY IN GAUCHER'S DISEASE. Biochem Biophys Res Commun. 1965 Jan 18;18:221–225. doi: 10.1016/0006-291x(65)90743-6. [DOI] [PubMed] [Google Scholar]
- Barton N. W., Furbish F. S., Murray G. J., Garfield M., Brady R. O. Therapeutic response to intravenous infusions of glucocerebrosidase in a patient with Gaucher disease. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1913–1916. doi: 10.1073/pnas.87.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender M. A., Miller A. D., Gelinas R. E. Expression of the human beta-globin gene after retroviral transfer into murine erythroleukemia cells and human BFU-E cells. Mol Cell Biol. 1988 Apr;8(4):1725–1735. doi: 10.1128/mcb.8.4.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine D. M., Karlsson S., Nienhuis A. W. Combination of interleukins 3 and 6 preserves stem cell function in culture and enhances retrovirus-mediated gene transfer into hematopoietic stem cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8897–8901. doi: 10.1073/pnas.86.22.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordignon C., Yu S. F., Smith C. A., Hantzopoulos P., Ungers G. E., Keever C. A., O'Reilly R. J., Gilboa E. Retroviral vector-mediated high-efficiency expression of adenosine deaminase (ADA) in hematopoietic long-term cultures of ADA-deficient marrow cells. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6748–6752. doi: 10.1073/pnas.86.17.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Correll P. H., Fink J. K., Brady R. O., Perry L. K., Karlsson S. Production of human glucocerebrosidase in mice after retroviral gene transfer into multipotential hematopoietic progenitor cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8912–8916. doi: 10.1073/pnas.86.22.8912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber H. E., Finley K. D., Hershberg R. M., Katzman S. S., Laikind P. K., Seegmiller J. E., Friedmann T., Yee J. K., Jolly D. J. Retroviral vector-mediated gene transfer into human hematopoietic progenitor cells. Science. 1985 Nov 29;230(4729):1057–1061. doi: 10.1126/science.3864246. [DOI] [PubMed] [Google Scholar]
- Hock R. A., Miller A. D. Retrovirus-mediated transfer and expression of drug resistance genes in human haematopoietic progenitor cells. Nature. 1986 Mar 20;320(6059):275–277. doi: 10.1038/320275a0. [DOI] [PubMed] [Google Scholar]
- Hogge D. E., Humphries R. K. Gene transfer to primary normal and malignant human hemopoietic progenitors using recombinant retroviruses. Blood. 1987 Feb;69(2):611–617. [PubMed] [Google Scholar]
- Hughes P. F., Eaves C. J., Hogge D. E., Humphries R. K. High-efficiency gene transfer to human hematopoietic cells maintained in long-term marrow culture. Blood. 1989 Nov 1;74(6):1915–1922. [PubMed] [Google Scholar]
- Karlsson S., Bodine D. M., Perry L., Papayannopoulou T., Nienhuis A. W. Expression of the human beta-globin gene following retroviral-mediated transfer into multipotential hematopoietic progenitors of mice. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6062–6066. doi: 10.1073/pnas.85.16.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson S., Humphries R. K., Gluzman Y., Nienhuis A. W. Transfer of genes into hematopoietic cells using recombinant DNA viruses. Proc Natl Acad Sci U S A. 1985 Jan;82(1):158–162. doi: 10.1073/pnas.82.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson S., Papayannopoulou T., Schweiger S. G., Stamatoyannopoulos G., Nienhuis A. W. Retroviral-mediated transfer of genomic globin genes leads to regulated production of RNA and protein. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2411–2415. doi: 10.1073/pnas.84.8.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laneuville P., Chang W., Kamel-Reid S., Fauser A. A., Dick J. E. High-efficiency gene transfer and expression in normal human hematopoietic cells with retrovirus vectors. Blood. 1988 Mar;71(3):811–814. [PubMed] [Google Scholar]
- Reiner O., Wilder S., Givol D., Horowitz M. Efficient in vitro and in vivo expression of human glucocerebrosidase cDNA. DNA. 1987 Apr;6(2):101–108. doi: 10.1089/dna.1987.6.101. [DOI] [PubMed] [Google Scholar]
- Starer F., Sargent J. D., Hobbs J. R. Regression of the radiological changes of Gaucher's disease following bone marrow transplantation. Br J Radiol. 1987 Dec;60(720):1189–1195. doi: 10.1259/0007-1285-60-720-1189. [DOI] [PubMed] [Google Scholar]
- Sutherland H. J., Eaves C. J., Eaves A. C., Dragowska W., Lansdorp P. M. Characterization and partial purification of human marrow cells capable of initiating long-term hematopoiesis in vitro. Blood. 1989 Oct;74(5):1563–1570. [PubMed] [Google Scholar]
- Szilvassy S. J., Fraser C. C., Eaves C. J., Lansdorp P. M., Eaves A. C., Humphries R. K. Retrovirus-mediated gene transfer to purified hemopoietic stem cells with long-term lympho-myelopoietic repopulating ability. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8798–8802. doi: 10.1073/pnas.86.22.8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]