Abstract
This report describes a case of angiographically documented foveal avascular zone (FAZ) enlargement after a single intravitreal injection of bevacizumab for macular edema secondary to central retinal vein occlusion (CRVO). A 71-year-old female was treated with an intravitreal bevacizumab injection for macular edema following CRVO. Despite successfully decreased edema one month after injection, the postinjection best-corrected visual acuity immediately decreased from 20/40 to 20/1000 (Snellen equivalent). The FAZ area increased from 0.37 mm2 to 3.11 mm2 (8.4-fold increase). While intravitreal anti-vascular endothelial growth factor is effective and should be considered as a first-line treatment for macular edema secondary to CRVO, it may aggravate macular ischemia.
Keywords: Macular edema, central retinal vein, fovea centralis, bevacizumab, fluorescein angiography
INTRODUCTION
We previously reported the case of a diabetic patient with angiographically documented foveal avascular zone (FAZ) enlargement after intravitreal bevacizumab following vitrectomy and intravitreal bevacizumab (Avastin; Genentech, San Francisco, CA, USA).1 We also reported that macular ischemia may have a negative effect on visual outcomes after intravitreal bevacizumab injection, both in macular edema secondary to branch retinal vein occlusion and in diabetic macular edema.2,3 Intravitreal bevacizumab may be safe for choroidal neovascularization secondary to age-related macular degeneration or myopia, because of normal retinal vascular circulation. However, intravitreal bevacizumab may develop or worsen pre-existing retinal ischemia when retinal vascular disorders exist. The present report describes a case of angiographically documented FAZ enlargement following a single intravitreal injection of bevacizumab for macular edema secondary to central retinal vein occlusion (CRVO).
CASE REPORT
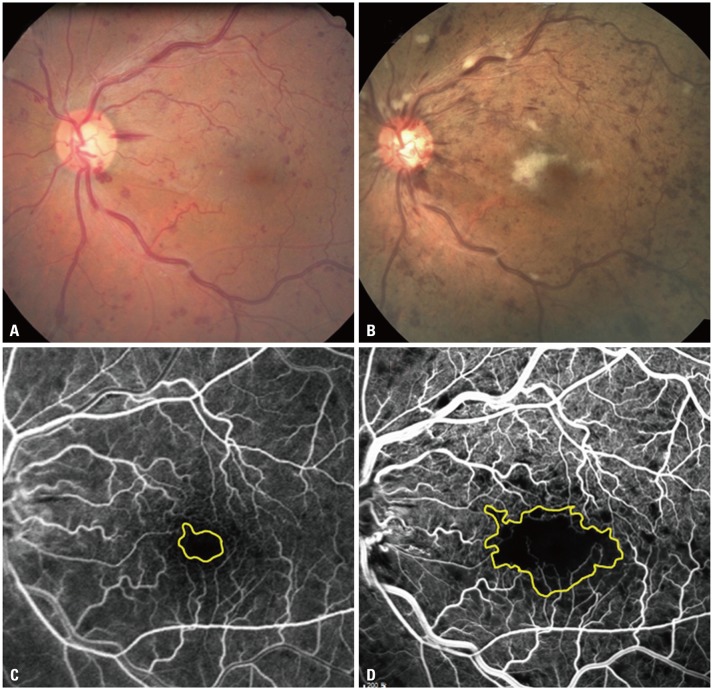
Informed consent was obtained from the patient after explanation of the nature and possible consequences of the study; the study protocol followed the tenets of the Declaration of Helsinki. A 71-year-old female who had typical fundus and angiographical change of CRVO with macular edema was treated with a single intravitreal injection of bevacizumab (Fig. 1A and C). There were no complications related to intravitreal injections, such as intraocular pressure elevation or infection, and no abrupt changes on fundus examination at immediate follow up and 1 day and 7 days after injection. One month later, despite successful resolution of macular edema confirmed by optical coherence tomography, the patient complained of an acute decrease in vision that developed over a few days after the injection. Her best-corrected visual acuity was 20/40 at the time of injection and 20/50 at 1 week after injection. However, it had decreased to 20/1000 (Snellen equivalent) by one month after injection. Postinjection fluorescein angiography images were taken to evaluate the retinal perfusion status. The FAZ area, which was calculated by manual outlining using the Heidelberg Eye Explorer program (Spectralis optical coherence tomography, version 1.5.12.0; Heidelberg Engineering, Heidelberg, Germany), revealed an increase in FAZ area from 0.37 mm2 to 3.11 mm2 (8.4-fold increase) (Fig. 1B and D). We put Avastin on hold and followed up on the patient without any treatment. After 1 month, the macular ischemia and cotton wool spots seemed to have been resolved.
Fig. 1. Fundus photography and fluorescein angiography, before (A and C) and one month after intravitreal bevacizumab injection (B and D). The macular edema resolved after a single intravitreal injection of 1.25 mg of bevacizumab. However, the foveal avascular zone area increased from 0.37 mm2 to 3.11 mm2, and best-corrected visual acuity decreased from 20/40 to 20/1000 (Snellen equivalent).
DISCUSSION
Anti-vascular endothelial growth factor (VEGF) agents are safe and effective treatments for patients with macular edema secondary to CRVO,4 and have become the first-line treatment for many other retinal diseases. Campochiaro, et al.5 reported that treatment with ranibizumab (Lucentis; Genentech, South San Francisco, CA, USA) did not worsen retinal nonperfusion in patients with retinal vein occlusion, but rather reduced its occurrence, and that timely, aggressive blockade of VEGF prevented the worsening of retinal nonperfusion, by promoting reperfusion. In addition, a previous study using fluorescein angiography reported no evidence of increasing retinal ischemia after intravitreal bevacizumab injections.6
Nevertheless, the systemic use of bevacizumab in cancer chemotherapy has been found to be related to an increased risk of cardiovascular ischemia, and the use of bevacizumab is known to increase the risk of cerebrovascular events, including central nervous system (CNS) ischemic events and CNS hemorrhages.7 Thromboembolic events have also been reported in metastatic carcinoma patients treated with bevacizumab.8
Intravitreal bevacizumab may develop or even worsen pre-existing retinal ischemia, by blocking physiological VEGF, which is essential for maintaining retinal circulation when retinal vascular disorders exist. A nonselective anti-VEGF antibody, such as bevacizumab, not only blocks pathological VEGF, but also physiological VEGF, which is essential for maintaining foveal circulation. VEGF promotes endothelial cell proliferation and stimulates endothelial cell survival.9 Moreover, VEGF enhances endothelial nitric oxide (NO) synthase activity and upregulates the message and protein levels of the VEGF receptor in human endothelial cells. Thus, inhibition of VEGF could weaken the regenerative capacity of endothelial cells, leading to thrombosis or hemorrhage.10 In addition, NO inhibition and VEGF blockade can result in several common complications, such as endothelial dysfunction or vascular occlusive events. Endothelial dysfunction disturbs homeostatic balance, thereby prompting vasoconstriction of vessel walls, platelet activation, leukocyte adherence, thrombosis, impaired coagulation, vascular inflammation, and atherosclerosis.10 Leukocyte recruitment and adherence may also cause blockage of capillaries, resulting in capillary occlusion, which may account for bevacizumab-induced retinal ischemia when it is administered intravitreously.
While intravitreal anti-VEGF is effective and should be considered as a first-line treatment for macular edema secondary to CRVO, it may lead to or aggravate macular ischemia. One should be aware of these possible complications during intravitreal anti-VEGF administration. Further studies using serial optical coherence tomography angiography should prove helpful in determining whether anti-VEGF exacerbates macular ischemia in retinal vessel disorders.
ACKNOWLEDGEMENTS
Hyoung Jun Koh was a consultant/advisor for Allergan, Bayer, and Novartis Pharmaceuticals Corporation.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Lee SJ, Koh HJ. Enlargement of the foveal avascular zone in diabetic retinopathy after adjunctive intravitreal bevacizumab (avastin) with pars plana vitrectomy. J Ocul Pharmacol Ther. 2009;25:173–174. doi: 10.1089/jop.2008.0092. [DOI] [PubMed] [Google Scholar]
- 2.Chung EJ, Hong YT, Lee SC, Kwon OW, Koh HJ. Prognostic factors for visual outcome after intravitreal bevacizumab for macular edema due to branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2008;246:1241–1247. doi: 10.1007/s00417-008-0866-8. [DOI] [PubMed] [Google Scholar]
- 3.Chung EJ, Roh MI, Kwon OW, Koh HJ. Effects of macular ischemia on the outcome of intravitreal bevacizumab therapy for diabetic macular edema. Retina. 2008;28:957–963. doi: 10.1097/IAE.0b013e3181754209. [DOI] [PubMed] [Google Scholar]
- 4.Yeh S, Kim SJ, Ho AC, Schoenberger SD, Bakri SJ, Ehlers JP, et al. Therapies for macular edema associated with central retinal vein occlusion: a report by the American Academy of Ophthalmology. Ophthalmology. 2015;122:769–778. doi: 10.1016/j.ophtha.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Campochiaro PA, Bhisitkul RB, Shapiro H, Rubio RG. Vascular endothelial growth factor promotes progressive retinal nonperfusion in patients with retinal vein occlusion. Ophthalmology. 2013;120:795–802. doi: 10.1016/j.ophtha.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 6.Neubauer AS, Kook D, Haritoglou C, Priglinger SG, Kampik A, Ulbig MW, et al. Bevacizumab and retinal ischemia. Ophthalmology. 2007;114:2096. doi: 10.1016/j.ophtha.2007.05.057. [DOI] [PubMed] [Google Scholar]
- 7.Zuo PY, Chen XL, Liu YW, Xiao CL, Liu CY. Increased risk of cerebrovascular events in patients with cancer treated with bevacizumab: a meta-analysis. PLoS One. 2014;9:e102484. doi: 10.1371/journal.pone.0102484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scappaticci FA, Skillings JR, Holden SN, Gerber HP, Miller K, Kabbinavar F, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–1239. doi: 10.1093/jnci/djm086. [DOI] [PubMed] [Google Scholar]
- 9.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mourad JJ, des Guetz G, Debbabi H, Levy BI. Blood pressure rise following angiogenesis inhibition by bevacizumab. A crucial role for microcirculation. Ann Oncol. 2008;19:927–934. doi: 10.1093/annonc/mdm550. [DOI] [PubMed] [Google Scholar]