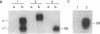Abstract
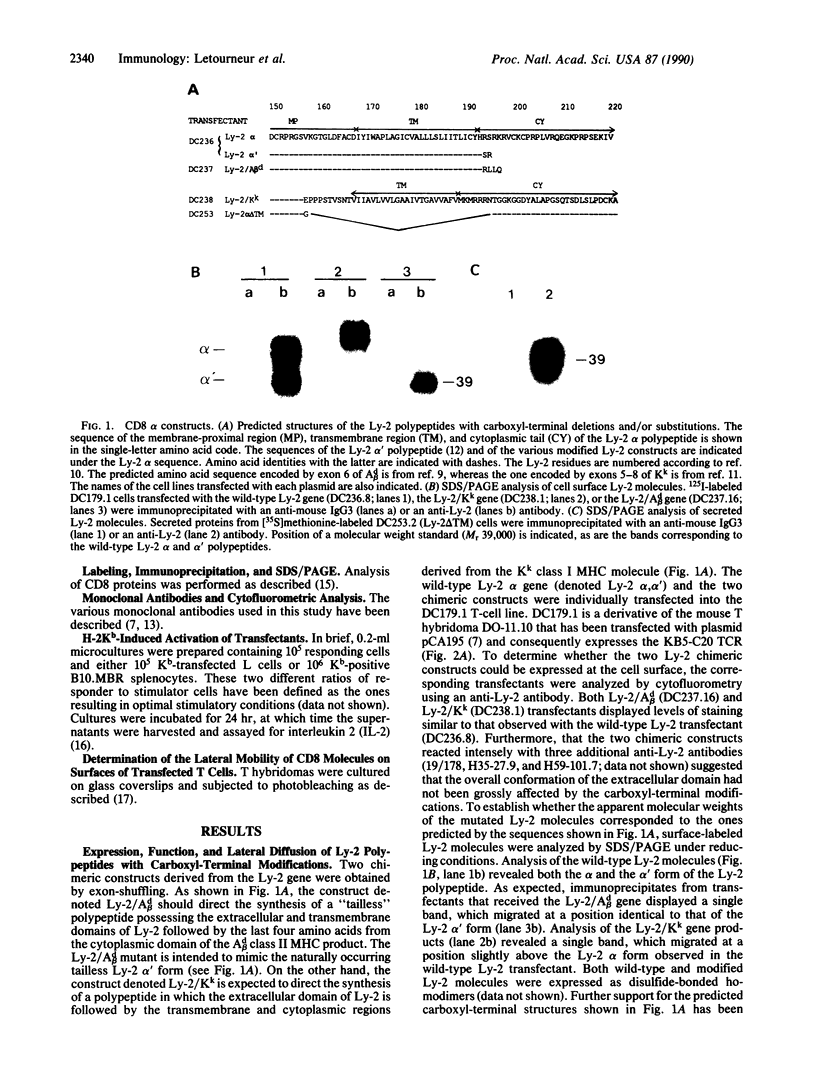
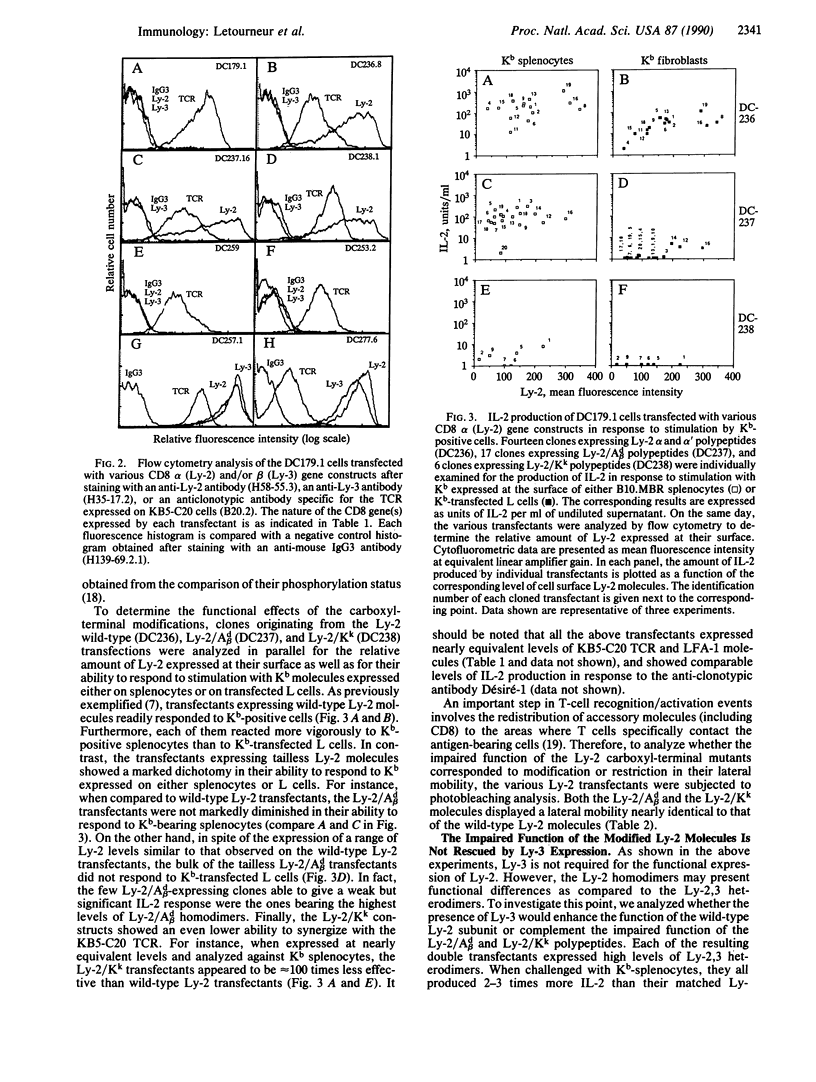
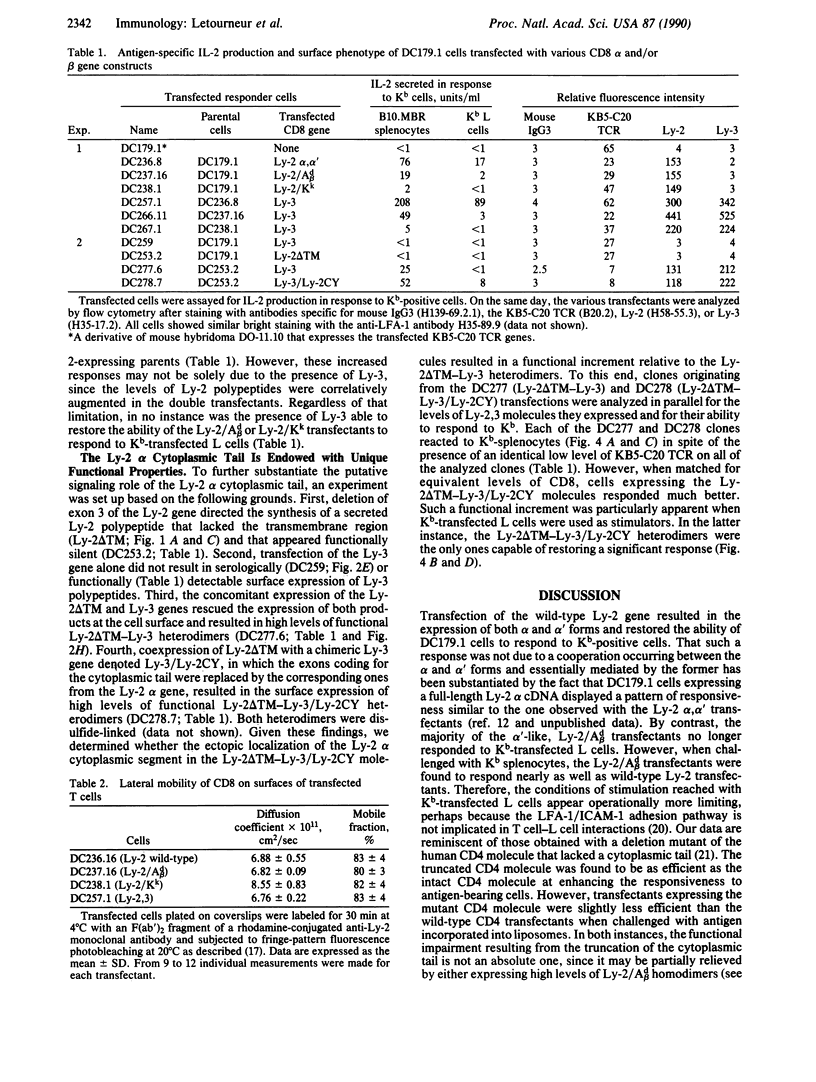
To test for the functional importance of the cytoplasmic segment of the CD8 molecule, a mouse T-cell hybridoma expressing a T-cell receptor specific for the class I major histocompatibility complex product H-2Kb was transfected with a set of CD8 alpha-chain (Ly-2) and/or beta-chain (Ly-3) genes encoding polypeptides with carboxyl-terminal truncations or substitutions. When challenged with Kb-positive splenocytes, transfectants expressing Ly-2 homodimers that lacked cytoplasmic tails responded nearly as effectively as wild-type Ly-2 transfectants. However in marked contrast to the wild-type Ly-2 transfectants, tailless Ly-2 transfectants were greatly impaired in their ability to respond to Kb-transfected L cells. Coexpression of the Ly-3 gene did not restore this impaired response. The unique functional property of the Ly-2 alpha cytoplasmic segment was further supported by the analysis of a chimeric Ly-3 subunit in which the cytoplasmic segment was replaced by the one from the Ly-2 alpha subunit. When associated with a soluble Ly-2 subunit lacking a transmembrane segment, the chimeric Ly-3 was indeed sufficient to restore the response to Kb-transfected L cells. Since the lateral mobility of the tailless Ly-2 molecules on the cell surface was nearly identical to that of the wild-type Ly-2 molecules, their partially impaired function may indicate that they have lost their cis-acting signaling properties but retained their ability to bind class I products of the major histocompatibility complex.
Full text
PDF
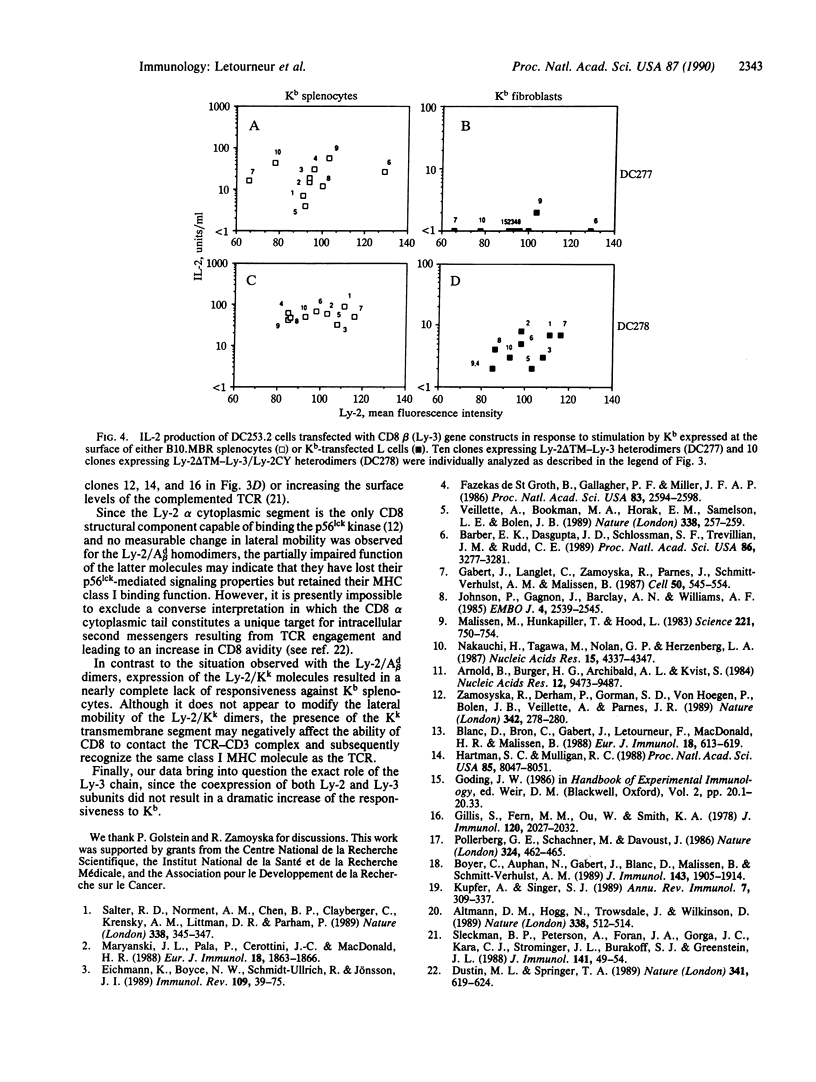
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann D. M., Hogg N., Trowsdale J., Wilkinson D. Cotransfection of ICAM-1 and HLA-DR reconstitutes human antigen-presenting cell function in mouse L cells. Nature. 1989 Apr 6;338(6215):512–514. doi: 10.1038/338512a0. [DOI] [PubMed] [Google Scholar]
- Arnold B., Burgert H. G., Archibald A. L., Kvist S. Complete nucleotide sequence of the murine H-2Kk gene. Comparison of three H-2K locus alleles. Nucleic Acids Res. 1984 Dec 21;12(24):9473–9487. doi: 10.1093/nar/12.24.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber E. K., Dasgupta J. D., Schlossman S. F., Trevillyan J. M., Rudd C. E. The CD4 and CD8 antigens are coupled to a protein-tyrosine kinase (p56lck) that phosphorylates the CD3 complex. Proc Natl Acad Sci U S A. 1989 May;86(9):3277–3281. doi: 10.1073/pnas.86.9.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc D., Bron C., Gabert J., Letourneur F., MacDonald H. R., Malissen B. Gene transfer of the Ly-3 chain gene of the mouse CD8 molecular complex: co-transfer with the Ly-2 polypeptide gene results in detectable cell surface expression of the Ly-3 antigenic determinants. Eur J Immunol. 1988 Apr;18(4):613–619. doi: 10.1002/eji.1830180419. [DOI] [PubMed] [Google Scholar]
- Boyer C., Auphan N., Gabert J., Blanc D., Malissen B., Schmitt-Verhulst A. M. Comparison of phosphorylation and internalization of the antigen receptor/CD3 complex, CD8, and class I MHC-encoded proteins on T cells. Role of intracytoplasmic domains analyzed with hybrid CD8/class I molecules. J Immunol. 1989 Sep 15;143(6):1905–1914. [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989 Oct 19;341(6243):619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Eichmann K., Boyce N. W., Schmidt-Ullrich R., Jönsson J. I. Distinct functions of CD8(CD4) are utilized at different stages of T-lymphocyte differentiation. Immunol Rev. 1989 Jun;109:39–75. doi: 10.1111/j.1600-065x.1989.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Fazekas de St Groth B., Gallagher P. F., Miller J. F. Involvement of Lyt-2 and L3T4 in activation of hapten-specific Lyt-2+ L3T4+ T-cell clones. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2594–2598. doi: 10.1073/pnas.83.8.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabert J., Langlet C., Zamoyska R., Parnes J. R., Schmitt-Verhulst A. M., Malissen B. Reconstitution of MHC class I specificity by transfer of the T cell receptor and Lyt-2 genes. Cell. 1987 Aug 14;50(4):545–554. doi: 10.1016/0092-8674(87)90027-4. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Hartman S. C., Mulligan R. C. Two dominant-acting selectable markers for gene transfer studies in mammalian cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8047–8051. doi: 10.1073/pnas.85.21.8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P., Gagnon J., Barclay A. N., Williams A. F. Purification, chain separation and sequence of the MRC OX-8 antigen, a marker of rat cytotoxic T lymphocytes. EMBO J. 1985 Oct;4(10):2539–2545. doi: 10.1002/j.1460-2075.1985.tb03968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Singer S. J. Cell biology of cytotoxic and helper T cell functions: immunofluorescence microscopic studies of single cells and cell couples. Annu Rev Immunol. 1989;7:309–337. doi: 10.1146/annurev.iy.07.040189.001521. [DOI] [PubMed] [Google Scholar]
- Malissen M., Hunkapiller T., Hood L. Nucleotide sequence of a light chain gene of the mouse I-A subregion: A beta d. Science. 1983 Aug 19;221(4612):750–754. doi: 10.1126/science.6410508. [DOI] [PubMed] [Google Scholar]
- Maryanski J. L., Pala P., Cerottini J. C., MacDonald H. R. Antigen recognition by H-2-restricted cytolytic T lymphocytes: inhibition of cytolysis by anti-CD8 monoclonal antibodies depends upon both concentration and primary sequence of peptide antigen. Eur J Immunol. 1988 Nov;18(11):1863–1866. doi: 10.1002/eji.1830181135. [DOI] [PubMed] [Google Scholar]
- Nakauchi H., Tagawa M., Nolan G. P., Herzenberg L. A. Isolation and characterization of the gene for the murine T cell differentiation antigen and immunoglobulin-related molecule, Lyt-2. Nucleic Acids Res. 1987 May 26;15(10):4337–4347. doi: 10.1093/nar/15.10.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollerberg G. E., Schachner M., Davoust J. Differentiation state-dependent surface mobilities of two forms of the neural cell adhesion molecule. Nature. 1986 Dec 4;324(6096):462–465. doi: 10.1038/324462a0. [DOI] [PubMed] [Google Scholar]
- Salter R. D., Norment A. M., Chen B. P., Clayberger C., Krensky A. M., Littman D. R., Parham P. Polymorphism in the alpha 3 domain of HLA-A molecules affects binding to CD8. Nature. 1989 Mar 23;338(6213):345–347. doi: 10.1038/338345a0. [DOI] [PubMed] [Google Scholar]
- Sleckman B. P., Peterson A., Foran J. A., Gorga J. C., Kara C. J., Strominger J. L., Burakoff S. J., Greenstein J. L. Functional analysis of a cytoplasmic domain-deleted mutant of the CD4 molecule. J Immunol. 1988 Jul 1;141(1):49–54. [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Samelson L. E., Bolen J. B. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck. Nature. 1989 Mar 16;338(6212):257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- Zamoyska R., Derham P., Gorman S. D., von Hoegen P., Bolen J. B., Veillette A., Parnes J. R. Inability of CD8 alpha' polypeptides to associate with p56lck correlates with impaired function in vitro and lack of expression in vivo. Nature. 1989 Nov 16;342(6247):278–281. doi: 10.1038/342278a0. [DOI] [PubMed] [Google Scholar]