Abstract
Background
Chronic pain patients increasingly seek treatment through mindfulness meditation.
Purpose
This study aims to synthesize evidence on efficacy and safety of mindfulness meditation interventions for the treatment of chronic pain in adults.
Method
We conducted a systematic review on randomized controlled trials (RCTs) with meta-analyses using the Hartung-Knapp-Sidik-Jonkman method for random-effects models. Quality of evidence was assessed using the GRADE approach. Outcomes included pain, depression, quality of life, and analgesic use.
Results
Thirty-eight RCTs met inclusion criteria; seven reported on safety. We found low-quality evidence that mindfulness meditation is associated with a small decrease in pain compared with all types of controls in 30 RCTs. Statistically significant effects were also found for depression symptoms and quality of life.
Conclusions
While mindfulness meditation improves pain and depression symptoms and quality of life, additional well-designed, rigorous, and large-scale RCTs are needed to decisively provide estimates of the efficacy of mindfulness meditation for chronic pain.
Electronic supplementary material
The online version of this article (doi:10.1007/s12160-016-9844-2) contains supplementary material, which is available to authorized users.
Keywords: Chronic pain, Mindfulness, Meditation, Systematic review
Introduction
Chronic pain, often defined as pain lasting longer than 3 months or past the normal time for tissue healing [1], can lead to significant medical, social, and economic consequences, relationship issues, lost productivity, and larger health care costs. The Institute of Medicine recognizes pain as a significant public health problem that costs our nation at least $560–635 billion annually, including costs of health care and lost productivity [2]. Further, chronic pain is frequently accompanied by psychiatric disorders such as pain medication addiction and depression that make treatment complicated [3]. The high prevalence and refractory nature of chronic pain, in conjunction with the negative consequences of pain medication dependence, has led to increased interest in treatment plans that include adjunctive therapy or alternatives to medication [4]. One such modality that pain patients are using is mindfulness meditation. Based on ancient Eastern meditation practices, mindfulness facilitates an attentional stance of detached observation. It is characterized by paying attention to the present moment with openness, curiosity, and acceptance [5, 6]. Mindfulness meditation is thought to work by refocusing the mind on the present and increasing awareness of one’s external surroundings and inner sensations, allowing the individual to step back and reframe experiences. Current research using neuroimaging to elucidate neurological mechanisms underlying effects of mindfulness has focused on brain structures such as the posterior cingulate cortex, which appear to be involved in self-referential processing [7, 8]. Clinical uses of mindfulness include applications in substance abuse [9], tobacco cessation [10], stress reduction [11], and treatment of chronic pain [12–14].
Early mindfulness studies in pain patients showed promising outcomes on pain symptoms, mood disturbance, anxiety, and depression, as well as pain-related drug utilization [5]. Numerous systematic reviews on the effects of mindfulness meditation have been published in recent years. Of those that report pain outcomes, several have focused on specific types of pain such as low back pain [13], fibromyalgia [15], or somatization disorder [16]. Others were not limited to RCTs [14, 17]. There have been several comprehensive reviews focused on controlled trials of mindfulness interventions for chronic pain including a review [4] that showed improvements in depressive symptoms and coping, another review [18] on mindfulness for chronic back pain, fibromyalgia, and musculoskeletal pain that showed small positive effects for pain, and the most recent review [19] on various pain conditions which found improvements in pain, pain acceptance, quality of life, and functional status. Authors of these reviews echoed concerns that there is limited evidence for efficacy of mindfulness-based interventions for patients with chronic pain because of methodological issues. They have concluded that additional high-quality research was needed before a recommendation for the use of mindfulness meditation for chronic pain symptoms could be made.
The purpose of this study was to conduct a systematic review and meta-analysis of the effects and safety of mindfulness meditation, as an adjunctive or monotherapy to treat individuals with chronic pain due to migraine, headache, back pain, osteoarthritis, or neuralgic pain compared with treatment as usual, waitlists, no treatment, or other active treatments. Pain was the primary outcome, and secondary outcomes included depression, quality of life, and analgesic use. The systematic review protocol is registered in an international registry for systematic reviews (PROSPERO 2015:CRD42015025052).
Methods
Search Strategy
We searched the electronic databases PubMed, Cumulative Index to Nursing and Allied Health Literature (CINAHL), PsycINFO, and Cochrane Central Register of Controlled Trials (CENTRAL) for English-language-randomized controlled trials from inception through June 2016. We combined pain conditions and design terms with the following mindfulness search terms: “Mindfulness” [Mesh]) or “Meditation” [Mesh] or mindfulness* or mindfulness-based or MBSR or MBCT or M-BCT or meditation or meditat* or Vipassana or satipaṭṭhāna or anapanasati or Zen or Pranayama or Sudarshan or Kriya or zazen or shambhala or buddhis*.” In addition to this search and the reference mining of all included studies identified through it, we reference mined prior systematic reviews and retrieved all studies included therein.
Eligibility Criteria
Parallel group, individual or cluster RCTs of adults who report chronic pain were included. Studies where the author defined chronic pain and studies in patients reporting pain for a minimum of 3 months were included. Studies were required to involve mindfulness meditation, either as an adjunctive or monotherapy; studies testing other meditation interventions such as yoga, tai chi, qigong, and transcendental meditation techniques without reference to mindfulness were excluded. Mindfulness interventions that did not require formal meditation, such as acceptance and commitment therapy (ACT) were also excluded. Only studies that reported pain measures or change in analgesic use were included. Dissertations and conference abstracts were excluded.
Procedures
Two independent reviewers screened titles and abstracts of retrieved citations—following a pilot session to ensure similar interpretation of the inclusion and exclusion criteria. Citations judged as potentially eligible by one or both reviewers were obtained as full text. The full text publications were then dually screened against the specified inclusion criteria. The flow of citations throughout this process was documented in an electronic database, and reasons for exclusion of full-text publications were recorded. Data abstraction was also conducted in dual. Risk of bias was assessed using the Cochrane Risk of Bias tool [20]. Other biases related to the US Preventive Services Task Force’s (USPSTF) criteria for internal validity of included studies were assessed [21, 22]. These criteria were used to rate the quality of evidence as good, fair, or poor for each included study.
Meta-analytic Techniques
When sufficient data were available and statistical heterogeneity was below agreed thresholds [20], we performed meta-analysis to pool efficacy results across included studies for the outcomes of interest and present a forest plot for the main meta-analysis. We used the Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis using unadjusted means and measures of dispersion [23–25]. For studies reporting multiple pain outcomes, we used specific pain measures, such as the McGill Pain Questionnaire (MPQ) for the main meta-analysis rather than the pain subscale of the SF-36, and average or general pain measures rather than situational measures such as pain at the time of assessment. Due to the small number of adverse events reported, quantitative analysis was not conducted. We conducted subgroup analyses and meta-regressions to address whether there were differences in effect sizes between different interventions types, populations, or when used as monotherapy versus an adjunctive therapy. The quality of the body of evidence was assessed using the GRADE approach [22, 26] by which a determination of high, moderate, low, or very low was made for each major outcome [27].
Results
Description of Included Studies
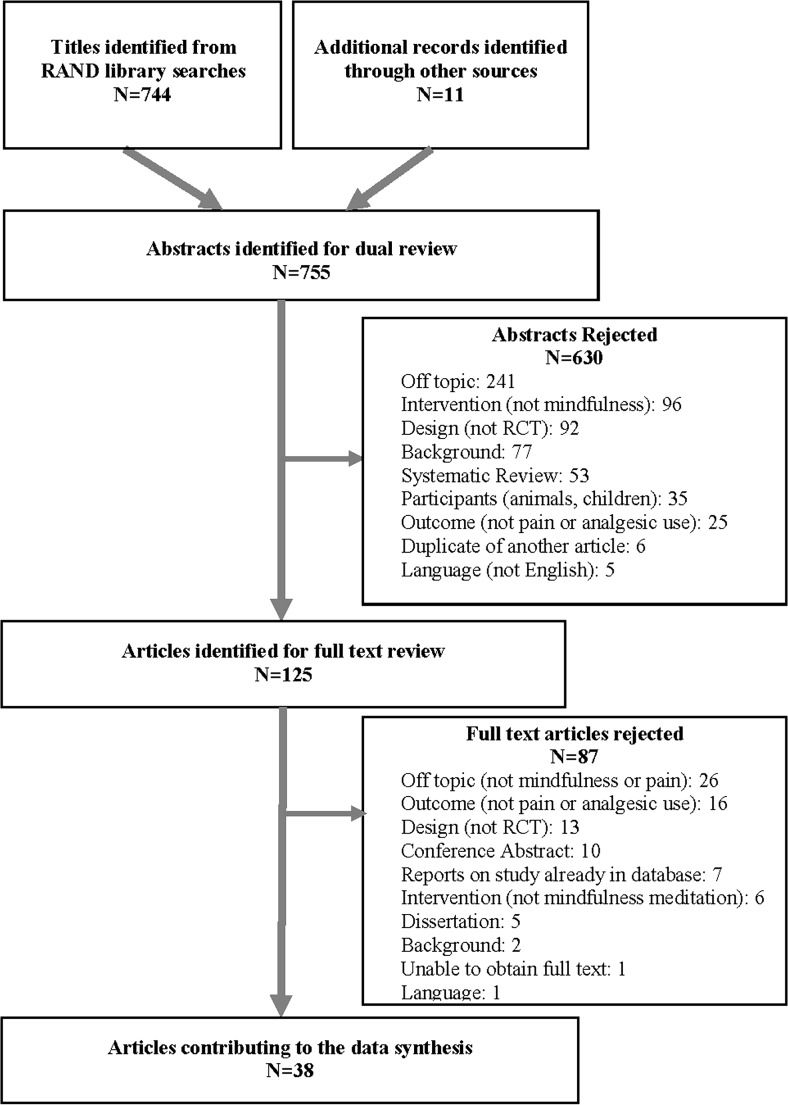
We identified 744 citations through searches of electronic databases and 11 additional records identified through other sources (see Fig. 1). Full texts were obtained for 125 citations identified as potentially eligible by two independent reviewers; 38 RCTs met inclusion criteria. Details of study characteristics are displayed in Table 1 and effects for individual studies are displayed in Table 2.
Fig. 1.
Literature flow diagram
Table 1.
Characteristics of included studies
| Study | Sample size | Location | Source of pain | % male | Age (M (SD)) | Intervention | Comparators | Quality rating |
|---|---|---|---|---|---|---|---|---|
| Astin et al. [53] | 128 | USA | Fibromyalgia | 0.7 | 48 (10.6) | MBSR and Qigong for 8 weeks | Education support group | Poor |
| Bakhshani et al. [61] | 40 | Middle East | Migraine, headache | 35.1 | Intervention, 30 (9.08); control, 31 (9.57) | MBSR for 8 weeks with TAU | TAU | Poor |
| Banth and Ardebil [60] | 88 | Middle East | Back pain | 0 | 40.3(8.2) | MBSR for 8 weeks with TAU | TAU | Poor |
| Brown and Jones [54] | 40 | Europe | Fibromyalgia, rheumatoid arthritis, other musculoskeletal | 26 | Intervention, 48 (10); control, 45 (12) | Mindfulness-based pain management program: breath awareness, body awareness, gentle movement, pain management, compassion training for 8 weeks | TAU | Poor |
| Cash et al. [39] | 91 | USA | Fibromyalgia | 0 | Not reported | MBSR for 8 weeks | Waitlist control group | Fair |
| Cathcart et al. [40] | 58 | Australia | Headache | 37 | Intervention, 46 (13.10); control, 45 (14.2) | Brief mindfulness-based therapy, based on MBSR and MBCT for 3 weeks | Waitlist control group | Fair |
| Cherkin et al. [38] | 342 | USA | Back pain | 34.3 | 49 (12.3) | MBSR for 8 weeks with TAU | CBT with TAU or TAU alone | Good |
| Davis and Zautra [42] | 79 | USA | Fibromyalgia | 2 | 46; range = 22–81 | Mindful social emotional regulation internet intervention in 12 modules for 6 weeks | Healthy lifestyle tips via internet | Fair |
| Day et al. [41] | 36 | USA | Migraine, headache | 11 | 42 (12.0) | MBCT for 8 weeks adapted for headache pain with TAU | Waitlist control group | Fair |
| Dowd et al. [43] | 124 | Europe | Headache, back pain, osteoarthritis, fibromyalgia, nerve pain, neuropathy | 10 | 45 (12.25) | MBCT computerized: included audio-recorded meditation, psychoeducation, mindfulness, and a cognitive and behavioral change for 6 weeks | Psychoeducation | Fair |
| Esmer et al. [55] | 40 | USA | Back pain, leg pain | 56 | Intervention, 55 (11.2); control, 58 (9.5) | MBSR for 8 weeks with TAU | TAU | Poor |
| Fjorback et al. [32] | 120 | Europe | Bodily distress syndrome | 20 | Intervention, 38 (9); control, 40 (8) | MBSR for 8 weeks with TAU | Enhanced TAU of 2-h specialist medical care and brief CBT | Good |
| Fogarty et al. [30] | 51 | New Zealand | Rheumatoid arthritis | 12 | Intervention, 52 (12); control, 55 (13) | MBSR for 8 weeks and TAU | TAU | Good |
| Garland et al. [44] | 115 | USA | Osteoarthritis, fibromyalgia | 32 | 48 (14) | MORE: multimodal intervention of mindfulness, CBT, positive psychology for 8 weeks with TAU | Support group with TAU | Fair |
| Gaylord et al. [45] | 75 | USA | Irritable bowel syndrome | 0 | Intervention, 45 (12.55); control, 41 (14.68) | Mindfulness training tailored for IBS population for 8 weeks with TAU | TAU and support group | Fair |
| Jay et al. [50] | 112 | Europe | Musculoskeletal pain | 0 | Intervention, 45.5 (9.0); control, 47.6 (8.2) | Mindfulness pain and stress workplace program for 10 weeks | TAU | Fair |
| Johns et al. [37] | 71 | USA | Cancer | 9.9 | Intervention, 56 (9.9); control, 56 (12.7) | MBSR for 8 weeks | Psychoeducation support group | Good |
| Kanter et al. [62] | 20 | USA | Interstitial cystitis, bladder pain syndrome | 0 | Intervention, 46 (15.2); control, 44 (13.9) | MBSR for 8 weeks with TAU | TAU | Poor |
| Kearney et al. [51] | 55 | USA | Gulf War illness | 85.5 | Intervention,51 (6.8); control, 48 (7.4) | MBSR for 8 weeks with TAU | TAU | Fair |
| la Cour and Petersen [46] | 109 | Europe | Varied, non-specific pain | 15 | Intervention, 47 (12.42); control, 49 (12.20) | MBSR: Standard program modified for chronic pain patients for 8 weeks with co-intervention TAU | Waitlist, TAU | Fair |
| Lengacher et al. [52] | 322 | USA | Cancer | 0 | 56.6 (9.7) | MBSR modified for breast cancer patients for 6 weeks with TAU | TAU | Fair |
| Ljotsson et al. [33] | 85 | Europe | Irritable bowel syndrome | 15 | 35 (9.4) | MBCT protocol via Internet for IBS group treatment for 10 weeks | Online discussion forum | Good |
| Ljotsson et al. [34] | 195 | Europe | Irritable bowel syndrome | 21 | 39 (11.1) | MBCT protocol via internet for IBS group treatment for 10 weeks | Online stress management program | Good |
| Meize-Grochowski et al. [56] | 31 | USA | Postherpetic neuralgia | 44 | Overall, 72 (9.6) | MBSR: 1 h instruction focusing breathing while seated comfortably, daily meditation using CD, phone call reminders, daily journal, for 6 weeks with TAU | TAU | Poor |
| Morone et al. [47] | 37 | USA | Back pain | 43 | Intervention, 74 (6.1); controls, 76 (5.0) | Modified MBSR: (1) the body scan; (2) sitting practice; (3) walking meditation for 8 weeks | Waitlist controls | Fair |
| Morone et al. [57] | 40 | USA | Back pain | 37 | Intervention, 78(7.1); control, 73(6.2) | Modified MBSR: (1) the body scan; (2) sitting practice; (3) walking meditation for 8 weeks; Over the counter and prescribed medications | Over the counter and prescribed medications | Poor |
| Morone et al. [36] | 282 | USA | Back pain | 33.7 | 74.5 (6.6) | MBSR for 8 weeks | Health education program | Good |
| Omidi and Zargar [58] | 66 | Middle East | Headache | 20 | Intervention, 35 (2.41); control, 32 (3.2) | MBSR for 8 weeks | TAU | Poor |
| Parra-Delgado and Latorre-Postigo [31] | 33 | Europe | Fibromyalgia | 0 | 53 (10.08) | MBCT for 12 weeks with TAU | TAU | Good |
| Plews-Ogan et al. [59] | 30 | USA | Musculoskeletal pain | 23 | 47 (NR) | MBSR for 8 weeks | TAU (this group used in analysis) or massage | Poor |
| Rahmani and Talepasand [63] | 24 | Middle East | Cancer | 0 | Intervention, 43 (3.07); control, 45 (3.28) | MBSR and group conscious yoga for 8 weeks | No treatment | Poor |
| Schmidt et al. [48] | 177 | Europe | Fibromyalgia | 0 | 53 (9.6) | Modified MBSR: mindfulness, yoga, and social interaction topics for 8 weeks | Waitlist (this group used in analysis), relaxation and stretching support group | Fair |
| Teixeira [64] | 22 | USA | Diabetic peripheral neuropathy | 25 | 75 (10.8) | Modified MBSR: mindfulness meditation instruction and compact disk 5 days/week over a 4-week period for 4 weeks | Nutritional information and food diary | Poor |
| Wells et al. [49] | 19 | USA | Migraine | 11 | Intervention, 46 (17); control, 45 (12) | MBSR for 8 weeks | TAU | Fair |
| Wong [65] | 100 | Asia | Unspecified | NR | NR | MBSR for 8 weeks | Multidisciplinary education program | Poor |
| Wong [28] | 100 | Asia | Unspecified | NR | 48 (7.84) | MBSR for 8 weeks | Multidisciplinary pain intervention | Good |
| Zautra et al. [29] | 144 | USA | Rheumatoid arthritis | 32 | Men, 62 (NR); women, 51 (NR) | Mindfulness meditation based on MBSR and emotion regulation therapy offered in sessions and home practice for 8 weeks | Education group (this group used in analysis), cognitive behavioral therapy | Good |
| Zgierska et al. [35] | 35 | USA | Back pain | 20 | 51.8 (9.7) | MBCT-manualized program 6 days/week for 8 weeks with TAU | TAU and opioid therapy | Good |
Note. Age (M (SD)) age mean (standard deviation, range, or not reported), CBT cognitive behavioral therapy, IBS irritable bowel syndrome, MBCT mindfulness-based cognitive therapy, MBSR mindfulness-based stress reduction, MORE mindfulness-oriented recovery enhancement, NR not reported, TAU treatment as usual
Table 2.
Effects for individual studies
| Study | Outcome | Measure | % pain change Tx Grp | % pain change Ctrl Grp | SMD (95 % CI) | Follow-up (week) |
|---|---|---|---|---|---|---|
| Astin et al. [53] | Pain | SF-36 pain score | −31.58 % | −34.39 % | 0.02 (−0.47, 0.5) | 14 |
| −28.79 % | −35.03 % | −0.04 (−0.52, 0.45) | 24 | |||
| −23.22 % | −29.94 % | −0.05 (−0.54, 0.43) | 8 | |||
| Depression | BDI | – | – | 0.15 (−0.35, 0.64) | 24 | |
| Bakhshani et al. [61] | Pain | Significant improvement in pain, quality of life; sample size of groups at follow-up not reported | ||||
| Quality of life | ||||||
| Banth and Ardebil [60] | Pain | MPQ | −47.92 % | −11.62 % | 2.5 (1.94, 3.07) | 12 |
| Quality of life | SF-12 mental health | – | – | 1.11 (0.65, 1.56) | 8 | |
| – | – | 1.49 (1.01, 1.97) | 12 | |||
| SF-12 physical health | – | – | 1.34 (0.88, 1.81) | 8 | ||
| – | – | 1.86 (1.36, 2.37) | 12 | |||
| Brown and Jones [54] | Pain | Laser pain unpleasantness | 9.26 % | 6.78 % | 0.24 (−0.51, 0.98) | 24 |
| Quality of life, mental | SF-36 mental composite | – | – | 1.16 (0.36, 1.96) | 24 | |
| Quality of life, physical | SF-36 physical composite | – | – | −0.42 (−1.17, 0.33) | 24 | |
| Cash et al. [39] | Pain | VAS | −4.26 % | −5.92 % | 0 (−0.42, 0.41) | 16 |
| −11.31 % | −1.01 % | 0.32 (−0.1, 0.74) | 8 | |||
| Cathcart et al. [40] | Pain | Headache intensity | −4.42 % | −11.16 % | 0.08 (−0.52, 0.69) | 8 |
| Cherkin et al. [38] | Pain | Significant reductions in pain and disability; Reported only adjusted data | ||||
| Disability | ||||||
| Davis and Zautra [42] | Pain | Pain | −0.93 % | −1.52 % | −0.24 (−0.69, 0.2) | 6 |
| Day et al. [41] | Pain | BPI intensity | −11.98 % | −6.53 % | −0.01 (−0.66, 0.65) | 8 |
| Dowd et al. [43] | Pain | Average pain | 1.26 % | −11.77 % | 0 (−0.36, 0.35) | 6 |
| 7.18 % | 1.71 % | −0.19 (−0.54, 0.17) | 30 | |||
| Esmer et al. [55] | Pain | VAS | −29.74 % | −0.82 % | 0.3 (−0.5, 1.1) | 12 |
| Disability | Roland-Morris disability | – | – | 0.90 ( 0.06 , 1.74 ) | 12 | |
| Fjorback et al. [32] | Pain | SF-36 bodily pain | −31.25 % | −7.38 % | 0.15 (−0.23, 0.53) | 12 |
| −44.12 % | −12.08 % | 0.23 (−0.18, 0.63) | 36 | |||
| −34.93 % | −31.54 % | −0.1 (−0.51, 0.31) | 60 | |||
| Quality of life, mental | SF-36 mental composite | – | – | −0.04 (−0.42, 0.34) | 60 | |
| Quality of life, physical | SF-36 physical composite | – | – | 0.22 (−0.16, 0.61) | 60 | |
| Fogarty et al. [30] | Pain | Significant reduction reported; no usable data as only reported change within group | ||||
| Garland et al. [44] | Pain | BPI | −12.32 % | 11.11 % | 0.76 (0.38, 1.14) | 20 |
| −10.66 % | 4.01 % | 0.57 (0.19, 0.94) | 8 | |||
| Gaylord et al. [45] | Pain | Pain severity | −42.96 % | −14.73 % | 0.53 (0.06, 0.99) | 20 |
| −35.83 % | −5.36 % | 0.54 (0.08, 1) | 8 | |||
| Depression | BSI-18 depression | – | – | 0.03 (−0.42, 0.49) | 20 | |
| Quality of life, general | IBS quality of life | – | – | 0.25 (−0.21, 0.7) | 20 | |
| Jay et al. [50] | Pain | Significant reduction reported; no usable data as only reported differences | ||||
| Johns et al. [37] | Pain | BPI | −35.19 % | −30.90 % | −0.07 (−0.54, 0.41) | 24 |
| Kanter et al. [62] | Pain | VAS | −16.95 % | −19.30 % | −0.14 (−1.05, 0.77) | 8 |
| Quality of life | SF-12 MCS | 0.7 (−0.24, 1.64) | 8 | |||
| SF-12 PCS | −0.02 (−0.93, 0.89) | 8 | ||||
| Kearney et al. [51] | Pain | MPQ | −23.87 % | −5.67 % | 0.41 (−0.13, 0.94) | 24 |
| Depression | PHQ | – | – | 0.34 (−0.2, 0.87) | 8 | |
| – | – | 0.47 (−0.07, 1) | 24 | |||
| la Cour and Petersen [46] | Pain | BPI | −1.05 % | −6.77 % | −0.16 (−0.53, 0.22) | 8 |
| Depression | HADS | – | – | 0.37 (−0.01, 0.75) | 8 | |
| Quality of life, mental | SF-36 mental composite | – | – | 0.53 (0.15, 0.91) | 8 | |
| Quality of life, physical | SF-36 physical composite | – | – | 0.00 (−0.38, 0.38) | 8 | |
| Lengacher et al. [52] | Pain | BPI | −25.92 % | −10.63 % | 0.02 (−0.2, 0.25) | 12 |
| Depression | CES-D score | – | – | 0.12 (−0.11, 0.35) | 6 | |
| – | – | 0.04 (−0.18, 0.27) | 12 | |||
| Quality of life | QoL MOS SF-36 | – | – | −0.05 (−0.28, 0.17) | 6 | |
| – | – | 0.01 (−0.21, 0.24) | 12 | |||
| Ljotsson et al. [33] | Pain | Total pain | −46.15 % | 0.00 % | 0.64 (0.19, 1.08) | 10 |
| Depression | MADRS-S | – | – | 0.43 (−0.02, 0.87) | 10 | |
| Quality of life, general | IBS quality of life | – | – | 0.95 (0.49, 1.41) | 10 | |
| Disability | Sheehan disability scale | – | – | 0.47 ( 0.02, 0.91) | 10 | |
| Ljotsson et al. [34] | Pain | Significant reduction IBS pain/discomfort; no usable data as did not report pain measure | 24 | |||
| Depression | HADS | – | – | 0 (−0.28, 0.28) | 24 | |
| Quality of life, general | IBS quality of life | – | – | 0.51 (0.22, 0.8) | 24 | |
| Meize-Grochowski et al. [56] | Pain | SF MPQ—total pain | −8.57 % | −4.17 % | −0.48 (−1.25, 0.28) | 2 |
| SF MPQ—total pain | −25.71 % | −12.50 % | −0.31 (−1.07, 0.45) | 8 | ||
| Depression | CES-D | – | – | 0.32 (−1.08, 0.44) | 8 | |
| Quality of life, mental | Emotional well being | – | – | 0.07 (−0.69, 0.82) | 8 | |
| Quality of life, physical | Average physical subscales | – | – | −0.02 (−0.77, 0.74) | 8 | |
| Morone et al. [47] | Pain | SF MPQ | −11.61 % | 3.29 % | 0.23 (−0.42, 0.88) | 8 |
| Quality of life, mental | SF-36 mental composite | – | – | 0.22 (−0.43, 0.86) | 8 | |
| Quality of life, physical | SF-36 physical composite | – | – | 0.11 (−0.53, 0.76) | 8 | |
| Disability | Roland-Morris disability | – | – | 0.23 (−0.42, 0.87) | 8 | |
| Morone et al. [57] | Pain | SF MPQ—total pain | −22.44 % | −26.71 % | −0.04 (−0.7, 0.63) | 24 |
| SF MPQ—total pain | −26.28 % | −29.19 % | −0.01 (−0.68, 0.65) | 8 | ||
| Morone et al. [36] | Pain | Numeric pain rating—average | −13.64 % | 0.95 % | 0.22 (−0.01, 0.46) | 24 |
| Quality of life | SF-36 global health composite | – | – | 0.17 (−0.06, 0.41) | 8 | |
| – | – | 0.03 (−0.2, 0.26) | 24 | |||
| SF-36 physical health composite | – | – | 0.17 (−0.06, 0.4) | 8 | ||
| – | – | 0 (−0.23, 0.23) | 24 | |||
| Plews-Ogan et al. [59] | Pain | Pain unpleasantness vs. TAU | −7.46 % | −8.70 % | 0.02 (CI, −1.04, 1.07) | 12 |
| Pain unpleasantness vs. massage | −7.46 % | −25.35 % | −0.16 (−1.19, 0.88) | 12 | ||
| Pain unpleasantness vs. TAU | −16.42 % | −13.04 % | 0.07 (CI, −0.99, 1.13) | 4 | ||
| Pain unpleasantness vs. massage | −16.42 % | −12.68 % | −0.11 (CI, −0.92, 1.14) | 4 | ||
| Pain unpleasantness vs. TAU | −13.43 % | −1.45 % | 0.17 (CI, −0.89, 1.23) | 8 | ||
| Pain unpleasantness vs. massage | −13.43 % | −39.44 % | −0.3 (CI, −1.34, 0.74) | 8 | ||
| Quality of Life, mental | SF-12 mental composite | – | – | 0.67 (−0.42, 1.75) | 12 | |
| Rahmani and Talepasand [63] | Pain | Global quality symptoms—pain | −26.52 % | 11.11 % | 1.85 (0.89, 2.8) | 16 |
| −44.89 % | −1.85 % | 3.24 (2.02, 4.46) | 8 | |||
| Quality of life, general | Global quality total score | – | – | 1.18 (0.32, 2.05) | 16 | |
| Schmidt et al. [48] | Pain | Pain perception scale vs. waitlist | −13.19 % | −6.90 % | 0.17 (−0.2, 0.55) | 16 |
| Pain perception scale vs. active | −13.19 % | −7.40 % | 0.15 (−0.22, 0.53) | 16 | ||
| Pain Perception Scale vs. waitlist | −11.87 % | −8.00 % | 0.08 (−0.3, 0.45) | 8 | ||
| Pain perception scale vs. active | −11.87 % | −4.86 % | 0.22 (−0.16, 0.6) | 8 | ||
| Depression | CES-D | – | – | 0.1 (−0.27, 0.48) | 16 | |
| Quality of Life, General | QoL profile for chronically ill | – | – | 0.26 (−0.12, 0.63) | 16 | |
| Teixeira [64] | Pain | NeuroQoL pain | Missing baseline mean | 0.14 (−0.74, 1.01) | 4 | |
| Quality of life, general | NeuroQoL overall | – | – | 0.79 (−0.12, 1.7) | 4 | |
| Wells et al. [49] | Pain | Headache severity | −25.00 % | 0.00 % | 0.99 (0.04, 1.95) | 12 |
| Headache severity | −27.27 % | 8.33 % | 1.5 (0.48, 2.51) | 8 | ||
| Depression | PHQ | – | – | 0.59 (−0.33, 1.51) | 8 | |
| Quality of life, general | Migraine-specific QoL | – | – | −0.43 (−1.34, 0.48) | 8 | |
| Wong [65] | Pain | Significant pain decrease; no usable data | ||||
| Wong [28] | Pain | No significant effect; no usable data | ||||
| Zautra et al. [29] | Pain | Pain vs. education | −14.49 % | −17.70 % | 0.22 (−0.2, 0.63) | 8 |
| Pain vs. cognitive behavior therapy | −14.49 % | −14.34 % | 0.56 (0.16, 0.96) | 8 | ||
| Depression | Depressive symptoms | – | – | 0.28 (−0.13, 0.7) | 8 | |
| Zgierska et al. [35] | Pain | Significant pain decrease; no follow-up data available |
Note. BDI Beck depression inventory, BPI brief pain index; BS brief symptom inventory, CES-D The Center for Epidemiological Studies-Depression Scale, CI confidence interval, MADRS Montgomery-Asberg Depression Rating Scale, HADS Hospital Anxiety and Depression Scale; MPQ McGill Pain Questionnaire, NPS Neuropathic Pain Scale, NeuroQoL Quality of Life in Neurological Disorders; PHQ Patient Health Questionnaire, QoL quality of life, SF-36 Short-Form Health Survey 36, SF MPQ Short-Form McGill Pain Questionnaire, SMD standardized mean difference, TAU treatment as usual, VAS Visual Analog Scale
In total, studies assigned 3536 participants; sample sizes ranged from 19 to 342. Fifteen studies reported an a priori power calculation with targeted sample size achieved, ten studies did not report information about a power calculation, and three studies were unclear in the reporting of a power calculation. Ten studies noted there was insufficient power; the authors considered these pilot studies. The majority of the studies were conducted in North America or Europe. The mean age of participants ranged from 30 (SD, 9.08) to 78 years (SD, 7.1. Eight studies included only female participants.
Medical conditions reported included fibromyalgia in eight studies and back pain in eight studies. (Categories are not mutually exclusive; some studies included patients with different conditions.) Osteoarthritis was reported in two studies and rheumatoid arthritis in three. Migraine headache was reported in three studies and another type of headache in five studies. Three studies reported irritable bowel syndrome (IBS). Eight studies reported other causes of pain and three studies did not specify a medical condition or source of chronic pain.
The total length of the interventions ranged from 3 to 12 weeks; the majority of interventions (29 studies) were 8 weeks in length. Twenty-one studies were conducted on mindfulness-based stress reduction (MBSR) and six on mindfulness-based cognitive therapy (MBCT). Eleven additional studies reported results on other types of mindfulness training. Thirteen RCTs provided the mindfulness intervention as monotherapy, and eighteen utilized a mindfulness intervention as adjunctive therapy, specifying that all participants received this in addition to other treatment such as medication. Seven of the studies were unclear as to whether the mindfulness intervention was monotherapy or adjunctive therapy. Nineteen RCTs used treatment as usual as comparators, thirteen used passive comparators, and ten used education/support groups as comparators. Beyond these common comparators, one study each used stress management, massage, a multidisciplinary pain intervention, relaxation/stretching, and nutritional information/food diaries as comparators; two studies used cognitive-behavioral therapy. Several studies had two comparison arms.
Study Quality and Risk of Bias
The study quality for each included study is displayed in Table 1. Eleven studies obtained a “good” quality rating [28–38]. Fourteen studies were judged to be of fair quality, primarily due to being unclear in some aspects of the methods [39–52]. Thirteen studies were judged to be poor; ten primarily due to issues with completeness of reporting outcome data such as inadequate or missing intention to treat (ITT) analysis and/or less than 80 % follow-up [53–62] and three due to unclear methods [63–65]. Details of the quality ratings and risk of bias for each included study is displayed in Electronic Supplementary Material 1.
Measures
Studies reported patient pain measures such as the Visual Analog Scale, the SF-36 pain subscale, and McGill Pain Questionnaire. Secondary outcome measures included depression symptoms (e.g., Beck Depression Inventory, Patient Health Questionnaire), physical and mental health-related quality of life (e.g., SF-36 mental and physical components), and functional impairment/disability (e.g., Roland-Morris Disability Questionnaire, Sheehan Disability Scale).
Chronic Pain Treatment Response
Thirty RCTs reported continuous outcome data on scales assessing chronic pain [29, 31–33, 36, 39–49, 51–60, 62–64, 66].
Eight studies met screening inclusion criteria but did not contribute to the meta-analysis because they did not report poolable data [28, 30, 34, 35, 38, 50, 61, 65]. Their study characteristics are displayed in Table 1, and study level effects along with the reasons they were not in pooled analyses are displayed in Table 2.
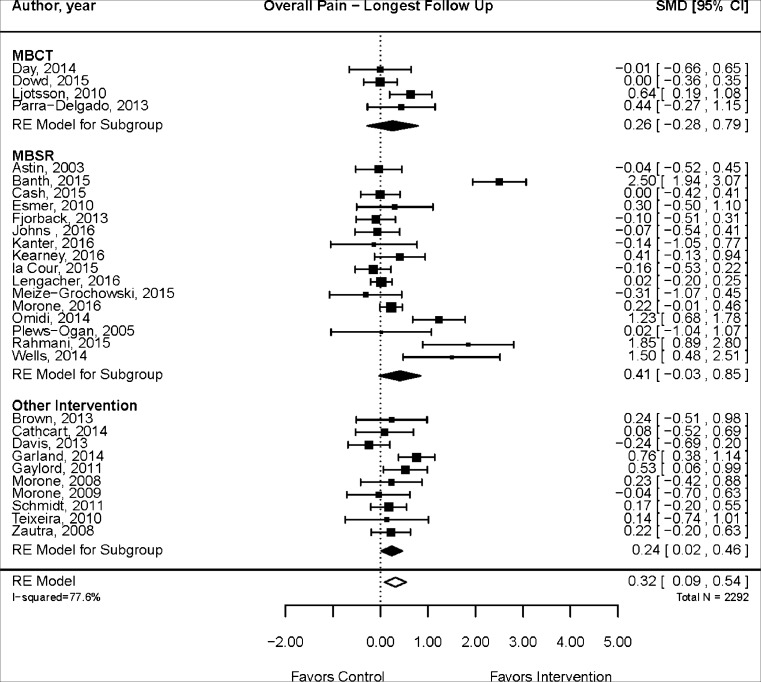
Pain scales and comparators varied from study to study. The median follow-up time was 12 weeks, with a range of 4 to 60 weeks. Figure 2 displays the results of meta-analysis using data at the longest follow-up for each study. The pooled analysis indicates a statistically significant effect of mindfulness meditation compared with treatment as usual, passive controls, and education/support groups (SMD, 0.32; 95 % CI, 0.09, 0.54; 30 RCTs). Substantial heterogeneity was detected (I 2 = 77.6 %). There was no evidence of publication bias (Begg’s p = 0.26; Egger’s test p = 0.09). To investigate whether the treatment estimate is robust when excluding poor-quality studies and to explore the possible source of the substantial heterogeneity, we conducted a sensitivity analysis including only fair or good quality studies. The improvement remained significant, the effect size was smaller (SMD, 0.19; 95 % CI, 0.03, 0.34; 19 RCTs), and there was less heterogeneity (I 2 = 50.5 %). Meta-regressions showed that changes in pain outcomes in good- (p = 0.42) and fair-quality (p = 0.13) studies were not significantly different from changes in poor-quality studies.
Fig. 2.
Mindfulness meditation effects on chronic pain
In subgroup analyses, the effect was not statistically significant at 12 weeks or less (SMD, 0.25; 95 % CI, −0.13, 0.63; 15 RCTs; I 2 = 82.6 %) but was significant for follow-up periods beyond 12 weeks (SMD, 0.31; 95 % CI, 0.04, 0.59; 14 RCTs, I 2 = 69.0 %). Begg’s test was not statistically significant (p = 0.16) but Egger’s test showed evidence of publication bias (p = 0.04). The quality of evidence that mindfulness meditation is associated with a decrease in chronic pain compared with control is low overall and for both short- and long-term follow-up due to inconsistency, heterogeneity, and possible publication bias. A detailed table displays the quality of evidence for findings for each major outcome in Electronic Supplementary Material 2.
In order to present clinically meaningful results, we calculated the percent change in pain symptoms from baseline to follow-up for mindfulness meditation and comparison groups for each study and displayed findings in Table 2. We then calculated the overall weighted mean percent change for mindfulness meditation groups versus comparison groups for effects of meditation for pain at longest follow-up. The mean percent change in pain for meditation groups was −0.19 % (SD, 0.91; min, −0.48; max, 0.10) while the mean percent change in pain for control groups was −0.08 % (SD, 0.74; min, −0.35; max, 0.11). The p value for the difference between groups was significant (p = 0.0031).
Depression
Depression outcomes were reported in 12 RCTs [29, 31, 33, 34, 45, 46, 48, 49, 51–53, 56]. Overall, meditation significantly lowered depression scores as compared with treatment as usual, support, education, stress management, and waitlist control groups (SMD, 0.15; 95 % CI, 0.03, 0.26; 12 RCTs; I 2 = 0 %). No heterogeneity was detected. The quality of evidence was rated as high due to lack of heterogeneity, consistent study results, and precision of effect (small confidence intervals).
Quality of Life
Sixteen studies reported mental health-related quality of life; the effect of mindfulness meditation was significant in the pooled analysis as compared with treatment as usual, support groups, education, stress management, and waitlist controls (SMD, 0.49; 95 % CI, 0.22, 0.76; I 2, 74.9 %). [32–34, 45–49, 52, 54, 56, 59, 60, 62–64]. Sixteen studies measured physical health-related quality of life [32–34, 36, 45–49, 52, 54, 56, 60, 62–64]. Pooled analyses showed a significant effect of mindfulness meditation as compared with treatment as usual, support groups, education, stress management, and waitlist controls (SMD, 0.34; 95 % CI, 0.03, 0.65; I 2, 79.2 %). Both quality-of-life analyses detected substantial heterogeneity, and the quality of evidence was rated as moderate for mental health (small confidence intervals, more consistent results) and low for physical health-related quality of life.
Functional Impairment (Disability Measures)
Four studies reported poolable disability scores from the Roland-Morris Disability Questionnaire and the Sheehan Disability Scale [33, 36, 47, 55]. The difference between the mindfulness and comparison groups in follow-up was not statistically significant (SMD, 0.30; 95 % CI, −0.02, 0.62; I 2 = 1.7 %), although the results approached significance. No heterogeneity was detected. The quality of evidence was rated low due to imprecision and small total sample size.
Analgesic Use
Only four studies reported use of analgesics as an outcome. In a study of MBSR for treatment of chronic pain due to failed back surgery syndrome [55], at 12-week follow-up, the analgesic medication logs of the intervention group documented a decrease in analgesic use compared with those in the control group (−1.5 (SD = 1.8) vs. 0.4 (SD = 1.1), p = <0.001). A study of mindfulness meditation and cognitive-behavioral therapy vs. usual care for low back pain [35] reported that the mean morphine equivalent dose (mg/day) of opioids was not significantly different between groups at both 8 and 26 weeks. Likewise, a trial of MBSR for back pain [38] found no significant difference between groups in self-reported use of pain medication. Finally, a trial of mindfulness-oriented recovery enhancement (MORE) for chronic pain of various etiologies [44] found intervention participants significantly more likely to no longer meet criteria for opioid use disorder immediately following treatment (p = 0.05); however, these effects were not sustained at 3-month follow-up.
Adverse Events
Only 7 of the 38 included RCTs reported on adverse events. Four stated no adverse events occurred [36, 47, 50, 57]; one described that two participants experienced temporary strong feelings of anger toward their pain condition and two of the participants experienced greater anxiety [46]; two studies recorded mild side effects from yoga and progressive muscle relaxation [35, 38].
Study Characteristic Moderators
Meta-regressions were run to determine if changes in pain outcomes systematically differed by several subcategories. There was no difference in effect on pain between MBSR (16 studies) and MBCT (4 studies; p = 0.68) or other types of mindfulness interventions (10 studies; p = 0.68). When comparing MBSR (16 studies) to all other interventions (14 studies), there was also no difference in effect (p = 0.45). As stated in more detail above, medical conditions reported included fibromyalgia, back pain, arthritis, headache, and irritable bowel syndrome (IBS). Meta-regressions did not suggest differences between headache (six studies) and other conditions (p = 0.93), back pain (eight studies) and other conditions (p = 0.15), and fibromyalgia (eight studies) and other conditions (p = 0.29). Gender composition (% male) had no association with effect on pain (p = 0.26). The total length of the intervention program ranged from 3 to 12 weeks (mean was 8 weeks). Meta-regression did not suggest differences between high-frequency interventions and medium- (p = 0.16) or low-frequency (p = 0.44) interventions. No systematic difference in effect on pain between adjunctive therapy and monotherapy (p = 0.62) or between adjunctive therapy and interventions where this was unclear (p = 0.10) was found. Finally, there was no systematic difference in effect whether the comparator was treatment as usual, waitlist, or another intervention (p = 0.21).
Discussion
In sum, mindfulness meditation was associated with a small effect of improved pain symptoms compared with treatment as usual, passive controls, and education/support groups in a meta-analysis of 30 randomized controlled trials. However, there was evidence of substantial heterogeneity among studies and possible publication bias resulting in a low quality of evidence. The efficacy of mindfulness meditation on pain did not differ systematically by type of intervention, medical condition, or by length or frequency of intervention. Mindfulness meditation was associated with statistically significant improvement in depression, physical health-related quality of life, and mental health-related quality of life. Quality of evidence was high for depression, moderate for mental health-related quality of life, and low for physical health-related quality of life. Only four studies reported on change in analgesic use; results were mixed. Adverse events in the included RCTs were rare and not serious, but the vast majority of studies did not collect adverse events data.
This review has several methodological strengths: an a priori research design, duplicate study selection and data abstraction of study information, a comprehensive search of electronic databases, risk of bias assessments, and comprehensive quality of evidence assessments used to formulate review conclusions. One limitation is that we did not contact individual study authors; results reported in the review are based on published data. We excluded conference abstracts which do not contain enough data to evaluate study quality. In addition, we included only studies published in English.
The included studies had many limitations. Thirteen of the thirty-eight studies were rated as poor quality, primarily due to lack of ITT, poor follow-up, or poor reporting of methods for randomization and concealment of allocation. The authors of ten studies reported inadequate statistical power to detect differences in pain outcomes between mindfulness meditation and the comparator; the authors considered these pilot studies. Ten other studies did not report a power calculation. Sample sizes were small; 15 studies randomized fewer than 50 participants.
More well-designed, rigorous, and large RCTs are needed in order to develop an evidence base that can more decisively provide estimates of its effectiveness. Studies should enroll samples large enough to detect statistical differences in outcomes and should follow-up with participants for 6 to 12 months in order to assess the long-term effects of meditation. Adherence to mindfulness practice and simultaneous use of other therapies should be monitored frequently. Intervention characteristics, including the optimal dose, have also not yet conclusively been established. In order to detect intervention specific effects, studies need to have attention-matched controls. Smaller trials may be conducted to answer these questions. Other outcomes that were outside the scope of this review may be important to explore. As the impact of mindfulness may be related to the appraisal of the pain, it may be useful for future trials to focus primary outcomes on symptoms associated with pain such as quality of life, pain-related interference, pain tolerance, analgesic, and related issues such as opioid craving. Future publications on RCTs of mindfulness meditation should adhere to Consolidated Standards of Reporting Trials (CONSORT) standards.
Only three RCTs attributed minor adverse events to mindfulness meditation. However, only 7 of the 38 included RCTs mentioned whether adverse events were monitored and collected. Thus quality of evidence for adverse events reported in RCTs is inadequate for a comprehensive assessment. Given published reports of adverse events during meditation, including psychosis [67], future trials should actively collect adverse events data. In addition, a systematic review of observational studies and case reports would shed additional light on adverse events during mindfulness meditation.
Further research examining the effect of mindfulness meditation on chronic pain should also focus on better understanding whether there is a minimum frequency or duration of meditation practice for it to be effective. While recent studies have yielded similar positive effects of mindfulness for pain, these effects tend to be small to medium and based on a body of evidence that is, at best, of moderate quality. A potential way to advance research on chronic pain would be to improve intervention and control group descriptions, identify different effects of various components of complex interventions, and work toward a standard criterion for assessing therapeutic gain [68]. Head-to-head trials that compare mindfulness interventions of a similar category but with variations in components or dose may be helpful to tease out the most effective elements of these interventions [69].
Similar to previous reviews in this area, we conclude that while mindfulness meditation interventions showed significant improvements for chronic pain, depression, and quality of life, the weaknesses in the body of evidence prevent strong conclusions. The available evidence did not yield consistent effects for pain outcomes, and few studies were available for forms of mindfulness meditation other than MBSR. Quality of evidence for the efficacy of mindfulness interventions in reducing chronic pain is low. There was higher quality evidence of the efficacy of mindfulness meditation on depression and mental health-related quality-of-life outcomes. This review is consistent with previous reviews concluding that more well-designed, rigorous, and large RCTs are needed in order to develop an evidence base that can more decisively provide estimates of the efficacy of mindfulness meditation for chronic pain. In the meantime, chronic pain continues to pose a tremendous burden on society and individuals. A novel therapeutic approach for chronic pain management such as mindfulness meditation would likely be welcomed by patients suffering from pain.
Electronic Supplementary Material
(DOCX 33 kb)
(DOCX 26 kb)
Compliance with Ethical Standards
Funding and Disclaimer
The systematic review was sponsored by the Department of Defense Centers of Excellence for Psychological Health and Traumatic Brain Injury (contract number 14-539.2). The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the views of the Department of Defense Centers of Excellence for Psychological Health and Traumatic Brain Injury.
Authors Statement of Conflict of Interest and Adherence to Ethical Standards Authors
Authors Hilton, Hempel, Ewing, Apaydin, Xenakis, Newberry, Colaiaco, Maher, Shanman, Sorbero, and Maglione declare that they have no conflict of interest. All procedures, including the informed consent process, were conducted in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
References
- 1.Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health pathways to prevention workshop. Annals of Internal Medicine. 2015;162:276–286. doi: 10.7326/M14-2559. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine: Relieving pain in America: A blueprint for transforming prevention, care, education, and research (report brief). www.iom.edu/relievingpain. 2011. [PubMed]
- 3.Department of Veterans Affairs Department of Defense: VA/DoD clinical practice guideline for management of opioid therapy for chronic pain. May 2010.
- 4.Chiesa A, Serretti A. Mindfulness-based interventions for chronic pain: a systematic review of the evidence. Journal of Alternative and Complementary Medicine. 2011;17:83–93. doi: 10.1089/acm.2009.0546. [DOI] [PubMed] [Google Scholar]
- 5.Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. Journal of Behavioral Medicine. 1985;8:163–190. doi: 10.1007/BF00845519. [DOI] [PubMed] [Google Scholar]
- 6.MARC: UCLA Mindfulness Awareness Research Center. Accessed May 29, 2015. http://marc.ucla.edu/default.cfm
- 7.Brewer JA, Garrison KA. The posterior cingulate cortex as a plausible mechanistic target of meditation: findings from neuroimaging. Ann NY Acad Sci. 2014;1307:19–27. doi: 10.1111/nyas.12246. [DOI] [PubMed] [Google Scholar]
- 8.Boccia M, Piccardi L, Guariglia P: The meditative mind: a comprehensive meta-analysis of MRI studies. Biomed Res Int 2015, Article ID 419808:1–11. [DOI] [PMC free article] [PubMed]
- 9.Chiesa A, Serretti A. Are mindfulness-based interventions effective for substance use disorders? A systematic review of the evidence. Substance Use and Misuse. 2014;49:492–512. doi: 10.3109/10826084.2013.770027. [DOI] [PubMed] [Google Scholar]
- 10.de Souza IC, de Barros VV, Gomide HP, et al. Mindfulness-based interventions for the treatment of smoking: a systematic literature review. Journal of Alternative and Complementary Medicine. 2015;21:129–140. doi: 10.1089/acm.2013.0471. [DOI] [PubMed] [Google Scholar]
- 11.Goyal M, Singh S, Sibinga EM, et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med. 2014;174:357–368. doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozasa EH, Tanaka LH, Monson C, et al. The effects of meditation-based interventions on the treatment of fibromyalgia. Curr Pain Headache Rep. 2012;16:383–387. doi: 10.1007/s11916-012-0285-8. [DOI] [PubMed] [Google Scholar]
- 13.Cramer H, Haller H, Lauche R, Dobos G. Mindfulness-based stress reduction for low back pain. A systematic review. BMC Complementary and Alternative Medicine. 2012;12:162. doi: 10.1186/1472-6882-12-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reiner K, Tibi L, Lipsitz JD. Do mindfulness-based interventions reduce pain intensity? A critical review of the literature. Pain Medicine. 2013;14:230–242. doi: 10.1111/pme.12006. [DOI] [PubMed] [Google Scholar]
- 15.Lauche R, Cramer H, Dobos G, Langhorst J, Schmidt S. A systematic review and meta-analysis of mindfulness-based stress reduction for the fibromyalgia syndrome. Journal of Psychosomatic Research. 2013;75:500–510. doi: 10.1016/j.jpsychores.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Lakhan SE, Schofield KL. Mindfulness-based therapies in the treatment of somatization disorders: a systematic review and meta-analysis. PloS One. 2013;8:e71834. doi: 10.1371/journal.pone.0071834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merkes M. Mindfulness-based stress reduction for people with chronic diseases. Aust J Prim Health. 2010;16:200–210. doi: 10.1071/PY09063. [DOI] [PubMed] [Google Scholar]
- 18.Lee C, Crawford C, Hickey A. Mind-body therapies for the self-management of chronic pain symptoms. Pain Medicine. 2014;15(Suppl 1):S21–39. doi: 10.1111/pme.12383. [DOI] [PubMed] [Google Scholar]
- 19.Bawa FL, Mercer SW, Atherton RJ, et al. Does mindfulness improve outcomes in patients with chronic pain? Systematic review and meta-analysis. British Journal of General Practice. 2015;65:e387–400. doi: 10.3399/bjgp15X685297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins J, Green S: Cochrane handbook for systematic reviews of interventions, version 5.1.0; 2011.
- 21.US Preventive Services Task Force: US Preventive Services Task Force Procedure Manual. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
- 22.The Lewin Group and ECRI Institute: Management of dyslipidemia: Evidence synthesis report. Clinical practice guideline. 2014.
- 23.Hartung J. An alternative method for meta-analysis. Biometrical Journal. 1999;41:901–916. doi: 10.1002/(SICI)1521-4036(199912)41:8<901::AID-BIMJ901>3.0.CO;2-W. [DOI] [Google Scholar]
- 24.Hartung J, Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Statistics in Medicine. 2001;20:3875–3889. doi: 10.1002/sim.1009. [DOI] [PubMed] [Google Scholar]
- 25.Sidik K, Jonkman JN. Robust variance estimation for random effects meta-analysis. Computational Statistics & Data Analysis. 2006;50:3681–3701. doi: 10.1016/j.csda.2005.07.019. [DOI] [Google Scholar]
- 26.Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong SY, Chan FW, Wong RL, et al. Comparing the effectiveness of mindfulness-based stress reduction and multidisciplinary intervention programs for chronic pain: a randomized comparative trial. Clinical Journal of Pain. 2011;27:724–734. doi: 10.1097/AJP.0b013e3182183c6e. [DOI] [PubMed] [Google Scholar]
- 29.Zautra AJ, Davis MC, Reich JW, et al. Comparison of cognitive behavioral and mindfulness meditation interventions on adaptation to rheumatoid arthritis for patients with and without history of recurrent depression. Journal of Consulting and Clinical Psychology. 2008;76:408–421. doi: 10.1037/0022-006X.76.3.408. [DOI] [PubMed] [Google Scholar]
- 30.Fogarty FA, Booth RJ, Gamble GD, Dalbeth N, Consedine NS. The effect of mindfulness-based stress reduction on disease activity in people with rheumatoid arthritis: a randomised controlled trial. Annals of the Rheumatic Diseases. 2015;74:472–474. doi: 10.1136/annrheumdis-2014-205946. [DOI] [PubMed] [Google Scholar]
- 31.Parra-Delgado M, Latorre-Postigo JM. Effectiveness of mindfulness-based cognitive therapy in the treatment of fibromyalgia: a randomised trial. Cognitive Therapy and Research. 2013;37:1015–1026. doi: 10.1007/s10608-013-9538-z. [DOI] [Google Scholar]
- 32.Fjorback LO, Arendt M, Ornbol E, et al. Mindfulness therapy for somatization disorder and functional somatic syndromes: randomized trial with one-year follow-up. Journal of Psychosomatic Research. 2013;74:31–40. doi: 10.1016/j.jpsychores.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Ljotsson B, Falk L, Vesterlund AW, et al. Internet-delivered exposure and mindfulness based therapy for irritable bowel syndrome--a randomized controlled trial. Behaviour Research and Therapy. 2010;48:531–539. doi: 10.1016/j.brat.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Ljotsson B, Hedman E, Andersson E, et al. Internet-delivered exposure-based treatment vs. stress management for irritable bowel syndrome: a randomized trial. American Journal of Gastroenterology. 2011;106:1481–1491. doi: 10.1038/ajg.2011.139. [DOI] [PubMed] [Google Scholar]
- 35.Zgierska AE, Burzinski CA, Cox J, et al. 2016 Mindfulness meditation and cognitive behavioral therapy intervention reduces pain severity and sensitivity in opioid-treated chronic low back pain: pilot findings from a randomized controlled trial. Pain Medicine [DOI] [PMC free article] [PubMed]
- 36.Morone NE, Greco CM, Moore CG, et al. A mind-body program for older adults with chronic low back pain: a randomized clinical trial. JAMA Intern Med. 2016;176:329–337. doi: 10.1001/jamainternmed.2015.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johns SA, Brown LF, Beck-Coon K, et al. 2016 Randomized controlled pilot trial of mindfulness-based stress reduction compared to psychoeducational support for persistently fatigued breast and colorectal cancer survivors. Supportive Care in Cancer [DOI] [PMC free article] [PubMed]
- 38.Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315:1240–1249. doi: 10.1001/jama.2016.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cash E, Salmon P, Weissbecker I, et al. Mindfulness meditation alleviates fibromyalgia symptoms in women: results of a randomized clinical trial. Annals of Behavioral Medicine. 2015;49:319–330. doi: 10.1007/s12160-014-9665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cathcart S, Galatis N, Immink M, Proeve M, Petkov J. Brief mindfulness-based therapy for chronic tension-type headache: a randomized controlled pilot study. Behavioural and Cognitive Psychotherapy. 2014;42:1–15. doi: 10.1017/S1352465813000234. [DOI] [PubMed] [Google Scholar]
- 41.Day MA, Thorn BE, Ward LC, et al. Mindfulness-based cognitive therapy for the treatment of headache pain: a pilot study. Clinical Journal of Pain. 2014;30:152–161. doi: 10.1097/AJP.0b013e318287a1dc. [DOI] [PubMed] [Google Scholar]
- 42.Davis MC, Zautra AJ. An online mindfulness intervention targeting socioemotional regulation in fibromyalgia: results of a randomized controlled trial. Annals of Behavioral Medicine. 2013;46:273–284. doi: 10.1007/s12160-013-9513-7. [DOI] [PubMed] [Google Scholar]
- 43.Dowd H, Hogan MJ, McGuire BE, et al. Comparison of an online mindfulness-based cognitive therapy intervention with online pain management psychoeducation: a randomized controlled study. Clinical Journal of Pain. 2015;31:517–527. doi: 10.1097/AJP.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 44.Garland EL, Manusov EG, Froeliger B, et al. Mindfulness-oriented recovery enhancement for chronic pain and prescription opioid misuse: results from an early-stage randomized controlled trial. Journal of Consulting and Clinical Psychology. 2014;82:448–459. doi: 10.1037/a0035798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaylord SA, Palsson OS, Garland EL, et al. Mindfulness training reduces the severity of irritable bowel syndrome in women: results of a randomized controlled trial. American Journal of Gastroenterology. 2011;106:1678–1688. doi: 10.1038/ajg.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.la Cour P, Petersen M. Effects of mindfulness meditation on chronic pain: a randomized controlled trial. Pain Medicine. 2015;16:641–652. doi: 10.1111/pme.12605. [DOI] [PubMed] [Google Scholar]
- 47.Morone NE, Greco CM, Weiner DK. Mindfulness meditation for the treatment of chronic low back pain in older adults: a randomized controlled pilot study. Pain. 2008;134:310–319. doi: 10.1016/j.pain.2007.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmidt S, Grossman P, Schwarzer B, et al. Treating fibromyalgia with mindfulness-based stress reduction: results from a 3-armed randomized controlled trial. Pain. 2011;152:361–369. doi: 10.1016/j.pain.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 49.Wells RE, Burch R, Paulsen RH, et al. Meditation for migraines: a pilot randomized controlled trial. Headache. 2014;54:1484–1495. doi: 10.1111/head.12420. [DOI] [PubMed] [Google Scholar]
- 50.Jay K, Brandt M, Hansen K, et al. Effect of individually tailored biopsychosocial workplace interventions on chronic musculoskeletal pain and stress among laboratory technicians: randomized controlled trial. Pain Physician. 2015;18:459–471. [PubMed] [Google Scholar]
- 51.Kearney DJ, Simpson TL, Malte CA, et al. Mindfulness-based stress reduction in addition to usual care is associated with improvements in pain, fatigue, and cognitive failures among veterans with gulf war illness. American Journal of Medicine. 2016;129:204–214. doi: 10.1016/j.amjmed.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 52.Lengacher CA, Reich RR, Paterson CL, et al. (2016) Examination of broad symptom improvement resulting from mindfulness-based stress reduction in breast cancer survivors: A randomized controlled trial. Journal of Clinical Oncology [DOI] [PMC free article] [PubMed]
- 53.Astin JA, Berman BM, Bausell B, et al. The efficacy of mindfulness meditation plus qigong movement therapy in the treatment of fibromyalgia: a randomized controlled trial. Journal of Rheumatology. 2003;30:2257–2262. [PubMed] [Google Scholar]
- 54.Brown CA, Jones AK. Psychobiological correlates of improved mental health in patients with musculoskeletal pain after a mindfulness-based pain management program. Clinical Journal of Pain. 2013;29:233–244. doi: 10.1097/AJP.0b013e31824c5d9f. [DOI] [PubMed] [Google Scholar]
- 55.Esmer G, Blum J, Rulf J, Pier J. Mindfulness-based stress reduction for failed back surgery syndrome: a randomized controlled trial. Journal of the American Osteopathic Association. 2010;110:646–652. [PubMed] [Google Scholar]
- 56.Meize-Grochowski R, Shuster G, Boursaw B, et al. Mindfulness meditation in older adults with postherpetic neuralgia: a randomized controlled pilot study. Geriatric Nursing (New York, N.Y.) 2015;36:154–160. doi: 10.1016/j.gerinurse.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morone NE, Rollman BL, Moore CG, Li Q, Weiner DK. A mind-body program for older adults with chronic low back pain: results of a pilot study. Pain Medicine. 2009;10:1395–1407. doi: 10.1111/j.1526-4637.2009.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Omidi A, Zargar F. Effect of mindfulness-based stress reduction on pain severity and mindful awareness in patients with tension headache: a randomized controlled clinical trial. Nursing and Midwifery. Studies. 2014;3:e21136. doi: 10.17795/nmsjournal21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Plews-Ogan M, Owens JE, Goodman M, Wolfe P, Schorling J. A pilot study evaluating mindfulness-based stress reduction and massage for the management of chronic pain. Journal of General Internal Medicine. 2005;20:1136–1138. doi: 10.1111/j.1525-1497.2005.0247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Banth S, Ardebil MD. Effectiveness of mindfulness meditation on pain and quality of life of patients with chronic low back pain. Int J Yoga. 2015;8:128–133. doi: 10.4103/0973-6131.158476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bakhshani NM, Amirani A, Amirifard H, Shahrakipoor M. The effectiveness of mindfulness-based stress reduction on perceived pain intensity and quality of life in patients with chronic headache. Glob J Health Sci. 2016;8:47326. doi: 10.5539/gjhs.v8n4p142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanter G, Komesu YM, Qaedan F, et al.: Mindfulness-based stress reduction as a novel treatment for interstitial cystitis/bladder pain syndrome: A randomized controlled trial. Int Urogynecol J. 2016. [DOI] [PMC free article] [PubMed]
- 63.Rahmani S, Talepasand S. The effect of group mindfulness—based stress reduction program and conscious yoga on the fatigue severity and global and specific life quality in women with breast cancer. Medical Journal of the Islamic Republic of Iran. 2015;29:175. [PMC free article] [PubMed] [Google Scholar]
- 64.Teixeira E. The effect of mindfulness meditation on painful diabetic peripheral neuropathy in adults older than 50 years. Holistic Nursing Practice. 2010;24:277–283. doi: 10.1097/HNP.0b013e3181f1add2. [DOI] [PubMed] [Google Scholar]
- 65.Wong SY. Effect of mindfulness-based stress reduction programme on pain and quality of life in chronic pain patients: a randomised controlled clinical trial. Hong Kong Medical Journal. Xianggang Yi Xue Za Zhi. 2009;15(Suppl 6):13–14. [PubMed] [Google Scholar]
- 66.Fjorback LO, Arendt M, Ornbol E, Fink P, Walach H. Mindfulness-based stress reduction and mindfulness-based cognitive therapy: a systematic review of randomized controlled trials. Acta Psychiatrica Scandinavica. 2011;124:102–119. doi: 10.1111/j.1600-0447.2011.01704.x. [DOI] [PubMed] [Google Scholar]
- 67.Kuijpers HJ, van der Heijden FM, Tuinier S, Verhoeven WM. Meditation-induced psychosis. Psychopathology. 2007;40:461–464. doi: 10.1159/000108125. [DOI] [PubMed] [Google Scholar]
- 68.Morley S, Williams A. New developments in the psychological management of chronic pain. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatri. 2015;60:168–175. doi: 10.1177/070674371506000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kerns RD, Burns JW, Shulman M, et al. Can we improve cognitive-behavioral therapy for chronic back pain treatment engagement and adherence? A controlled trial of tailored versus standard therapy. Health Psychology. 2014;33:938–947. doi: 10.1037/a0034406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 33 kb)
(DOCX 26 kb)