Abstract
Articular cartilage defects at the knee joint are identified and treated with increasing frequency. Autologous chondrocytes may have the strongest potential to generate high-quality repair tissue within the defective region. Autologous chondrocyte implantation is not available in every country. We present a surgical technique where the surgeon can apply autologous chondrocytes in a one-step procedure to treat articular cartilage defects at the knee joint.
Traumatic or degenerative knee joint cartilage defects may lead to the development of premature osteoarthritis. An optimal and individualized treatment protocol has to be performed to return the patient to full activity and prevent general joint degeneration. According to current evidence, large-diameter and also possibly patellar retrosurface cartilage defects should be treated by the use of cell-based repair techniques such as autologous chondrocyte implantation (ACI).1 Because ACI is not always available to the surgeon due to several reasons, we here present a technique to apply autologous chondrocytes during a one-step procedure (particulate cartilage fragments) not only for the treatment of large-diameter cartilage defects but also for fresh purely chondral flakes that cannot be refixed. This technique had already been described in the early 1980s,2 it was further developed by different authors, and 2-year data were recently presented by Christensen et al.3
Surgical Technique
The technique described here can be applied at the patella, trochlea, and both femoral condyles. The presented surgical description is for the patellar undersurface, and identical for all locations. Coexisting pathology such as ligamentous instability, mechanical axis malalignment, or patellar instability has to be identified and treated accordingly to protect the cartilage repair. Such intervention can be performed simultaneously with the particulate cartilage repair procedure. Copathology is not the topic of this technical description and will not be further specified. Preoperative planning is mandatory and should include magnetic resonance imaging (MRI) of the index joint. Furthermore, conventional radiograph of the knee joint including long-standing hip-knee-ankle radiographs is recommended to complete the diagnostic radiological check-up. Varus and valgus deformities more than 3° to 5° and tibiofemoral cartilage defects at the overloaded compartment should be corrected. Severe patellar instability and/or malalignment has to be identified and addressed accordingly. Clinical examination of the index knee joint and examination under anesthesia are mandatory. Perioperative antibiotic prophylaxis is recommended, yet not without conflicting evidence. The patient position is supine. A tourniquet is recommended, using the limb occlusion pressure system to implant the cartilage under dry (bloodless) settings. Every indicated (comparable with ACI indication1) and planned minced cartilage procedure is initiated via arthroscopy of the index knee joint including possible cointerventions such as meniscus surgery (Video 1). The intended-to-treat cartilage defect is well inspected and final indication is given during arthroscopy. Smaller than expected cartilage defects at the distal femur might be approached by, for example, the use of the microfracture technique. After finishing the arthroscopic procedure the portals are closed. An average 8-cm-long, vertical and patella-central skin incision (variations apply) is performed at 30° of knee flexion and medial arthrotomy gives access to the joint. After macroscopic inspection of the joint, the patella (in this case) is everted at full extension and the defect inspected. The size of the defect is measured before debridement, and the extraction number of healthy cartilage cylinders is calculated. The knee joint is then flexed to approximately 80°, and using a standard ACI biopsy instrument (4-mm-diameter hollow punch; i.e., Cartilage Biopsy Extractor, Storz, Tuttlingen, Germany) on average 3 osteochondral cylinders (larger defects might require more cylinders) are taken from the low-weight-bearing intercondylar notch as described before.4 Cylinder quantity depends on defect size and can be adapted. Donor site morbidity is minimal to nonexistent.5 The cylinders are further prepared at the back table. If more particulate cartilage is required, one can extract more cylinders from the notch. In parallel defect preparation is further realized. Using a No. 15 blade the defect is circumcised. By the use of a ringed curette, installation of a 360° healthy surrounding cartilage wall at 90° is established. Defect dimensions are measured again for the operative protocol and study purposes. It is recommended to prepare until clearly healthy remaining cartilage is reached. Cartilage chips that are retrieved at areas of marginally defective cartilage/into the healthy cartilage can also be brought to the back table to be included for the preparation of the notch fragments. For fragmentation at the back table, it is recommended to sit down and use a No. 10 blade. The blade should be renewed frequently. The manual mincing procedure should be performed within a drop of water for better handling of the cartilage pieces. All bone is removed from the notch cylinders. It can be used to fill up small cysts at the defect itself or put back to the notch donor site. Yet, bone marrow influx into the joint from the donor holes at the notch could be beneficial by ways of growth factor and cytokine influx. The fragments should be minced until dimensions are smaller than 1 × 1 × 1 mm. A paste-like appearance is optimal.6 The paste is finally stored and kept at the margin of a kidney basin within a drop of water. Defect preparation is finalized by the removal of the calcified layer with the aim of avoiding bleeding from the subchondral bone. It can be eased by the use of a motorized high-speed burr that should be used under water only. A matrix (e.g., aluminum) is taken for defect dimensions that can be recorded from that for the operative report as well. A Chondro-Gide (Geistlich, Wolhusen, Switzerland) membrane is cut slightly undersizing the match of the matrix. After humidification of the membrane for better handling, it does swell around 10% to 15% in size that has to be considered further when covering the defect with it. For the final step, all requirements have to be kept ready at once (Fig 1). After final seasoning of the defect, the cartilage paste is dried out as well by the use of a tissue wherein the water is sucked in passively and the paste becomes totally dry. Then, a thin layer of fibrin (Tisseel fibrin sealant, Baxter, Deerfield, IL) glue is applied to the defect button. Herewith, minimal unwanted bleeding can also be stopped. Thereafter the dry cartilage paste is applied to the defect to fill out the whole thickness. Care is taken not to harm the cartilage and foremost the chondrocytes within because these are very susceptible to mechanical force.7 Fragments are primarily fixed within the defect using a second fibrin glue application that mixes with the fragments and generates a stable fragment and/or fibrin clot as also described by Christensen et al.3 After this step of the procedure, the defect is already filled up to the level of the surrounding cartilage or slightly beneath so the membrane can be placed with ease. Finally and before drying out of the fibrin, the Chondro-Gide membrane is placed on top of the fibrin glue-coated chips (rough side facing the chips) and directly sutured to the surrounding healthy cartilage. Speedy application of the membrane generates a bond between the fibrin and the membrane. It is recommended to minimally irrigate the membrane for better handling during placement and fixation. Next, the membrane is fixed to the surrounding cartilage by using sutures as has already been established for the first- or second-generation ACI.8 A 6.0 monofil suture material is recommended with interrupted sutures and knot placement on top of the Chondro-Gide (Fig 2). Herewith, a stable fixation of the fragment and/or fibrin complex can be achieved that is important at the patellar retrosurface where high shearing forces may be present. A membrane-associated application is also recommended at the trochlea. Finally, the joint is rinsed multiple times. A drain is applied. The Chondro-Gide surface is dried and sealed using a final fibrin glue application to make it water tight and generate a “bioactive chamber.” Hereafter the patella is reverted and the joint closed in layers. A brace is placed while the patient is still under anesthesia. The patient is on bed rest for the first 24 postoperative hours. Then rehabilitation similar to patients with ACI starts as described elsewhere.9
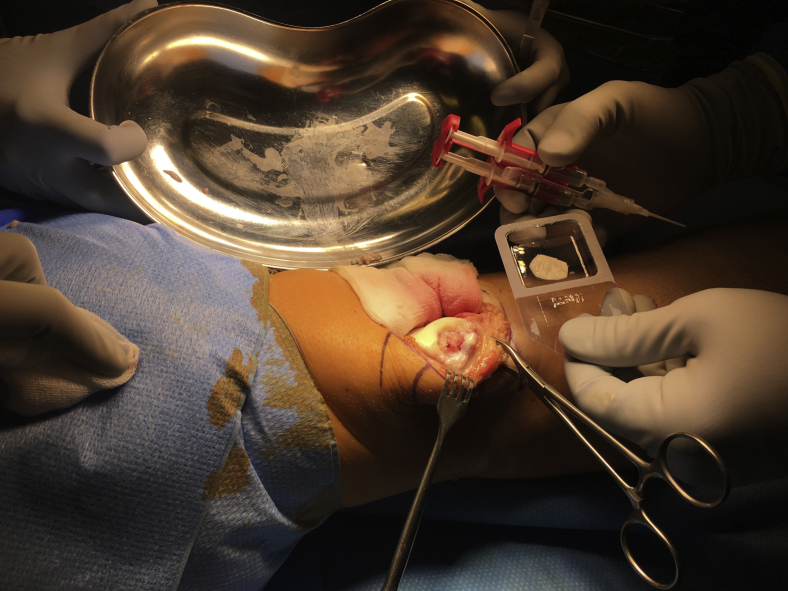
Fig 1.
Macroscopic image. Right knee joint with the everted patella. Cartilage defect at medial facet already prepared, dry, and not bleeding. Particulate cartilage chips (dry) ready for implantation sitting at the edge of the kidney basin. Fibrin glue ready. A Chondro-Gide membrane slightly soaked with water for better handling is cut to defect dimensions.
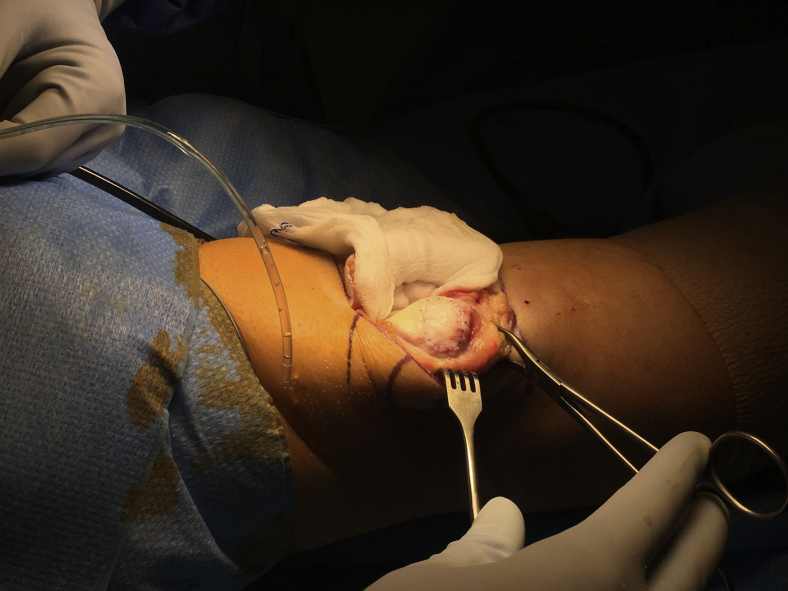
Fig 2.
Macroscopic image. Right knee joint with everted patella. Cartilage defect at medial facet now covered by particulate cartilage chips and a Chondro-Gide membrane on top. The membrane is secured to the surrounding cartilage by the use of 6-0 interrupted sutures.
Discussion
Chondral and osteochondral lesions at the knee joint surface represent a severe medical as well as socioeconomic dilemma. If not treated well, such lesions clearly predispose for early onset osteoarthritis.1 At present many different surgical techniques to repair defective cartilage are on offer for the managing physician. The goal of cartilage repair surgery must be (1) to generate tissue that will finally be as close to the surrounding native tissue as possible (i.e., hyaline cartilage), (2) to reverse clinical symptoms with return to previous activity, and (3) to provide with long-term durability. Tissue quality and clinical symptoms are most likely closely connected. Current evidence is supportive of the fact that autologous tissue, in such autologous chondrocytes, may result in the highest possible repair tissue quality within the defective region. Clinical outcome, return to sport, and long-term durability have been reported to be most satisfying after ACI when compared with other techniques.10 Defect size exceeding 2.5 to 3 cm2 should be approached by the use of cell-based techniques.1, 11 Furthermore, according to the existing evidence, retropatellar lesions have a high rate of failure when using either microfracture or osteochondral cylinder repair.12 At such locations cell-based techniques should be applied with an even lighter indication behavior. ACI is a precious and furthermore 2-step procedure. Naturally, it is not available in every country worldwide. Yet, there are many patients who show a good indication for ACI. Furthermore, there is a collective of patients who suffer from fresh, but irrefixable (purely chondral, comminuted) flake fractures or just lately separated osteochondritis dissecans lesions. This collective of subjects could also represent indicative for particulate or minced cartilage procedures as such technique is gaining rising interest.13 A recent case report provided an excellent clinical and MR outcome after a spontaneous minced cartilage procedure that was combined with subchondral drilling (a large localized bone marrow edema was present) to simultaneously relieve pressure and revitalize the subchondral bone and the particulate cartilage.14 The defect in that case report had a lesion diameter of 3.75 cm2. With calculation by the use of the AMADEUS (Area Measurement And DEpth and Underlying Structures) cartilage defect score15 it was coded as 3.75cBE with a total score of 40 points and thus grade 3. Already 6 months postoperatively the MOCART (magnetic resonance observation of cartilage repair tissue) score on MRI was 80 points that can be considered very satisfactory.16 MR data provided by Christensen et al.3 were satisfactory as well. The idea to apply particulate cartilage is not new and was, to our knowledge, initially described in 1982 by Albrecht.2 It was picked up again in the mid-2000s by Lu et al.17 and finally converted into a clinical product that is currently off market.18 Recently, Christensen et al.3 presented satisfactory 2-year data after an autologous particulate cartilage procedure using fibrin glue only. The principal biological functionality of chondrocyte outgrowth has been shown in vitro and in vivo.6 The intra-articular environment after surgery might be beneficial to act as an in situ bioreactor to promote outgrowth of the cells and initiate chondrogenesis.19 The complex and constantly changing cocktail of growth factors and cytokines is unique. Biomechanical stimulation via compression, shear, hydrostatic pressure, and fluid flow is inimitable. Such conditions cannot be copied under in vitro settings where current evidence has reported unsuccessful cellular outgrowth of cartilage pieces.20 Clinical studies using either autologous or allogenic minced cartilage pieces have proven to be successful in the past.21 The technique described here is very similar and inspired by the one previously published by Christensen et al.3 With regard to intraoperative considerations of using fibrin glue only that might be absorbed within a couple of days (outgrowth and matrix initiation have to be as fast), we decide to suture a Chondro-Gide on top of the construct. The chips are safely kept in place and are initially mechanically protected from the opposing joint surface via the smooth side of the Chondro-Gide. The rough side might even promote cell migration from the uppermost cartilage chips into the membrane. It should resorb while cell outgrowth and neomatrix formation is accomplished and gains enough mechanical stability (speculative). Such coverage might be of particular importance within weakly constrained joint compartments such as the patellofemoral joint. At the tibiofemoral compartment, it might be of less importance.14 By matrix coverage, the technique is coming closer to second-generation ACI. One can further modify by installation of multiple subchondral bone drillings using a 1.2 or 1.0 K-wire according to Pridie. Small-diameter K-wires are recommended because the damage to the subchondral bone plate is minimal while blood influx is still contingent.22 Herewith, the technique is applying aspects of the autogenous matrix-induced chondrogenesis approach when drilling and matrix are concerned. The advantage could be seen that the small fraction of stem cells that might be included within the super clot have the capability to activate the chondrocytes with regard to outgrowth and proliferation. Vice versa, the cartilage cells might promote the stem cells to differentiate via a chondrogenic lineage.23 Underlying bone marrow edema can be addressed simultaneously.
We present here an easy technique to safely apply autologous chondrocytes for coverage of large-diameter cartilage defects. Such a technique can be considered as rather novel besides previous publication effort and thus several pearls/advantages as well as pitfalls/disadvantages have to be considered (Table 1). Besides improved basic and animal experiment knowledge, prospective and comparative clinical long-term data among large patient cohorts including MRI have to be initiated to fully support general use of the technique described here.
Table 1.
Advantages and Disadvantages of Second-Generation Minced Cartilage Technique
| Pearls/Advantages | Pitfalls/Disadvantages |
|---|---|
| Pure autologous material | Transplant overgrowth |
| Steep learning curve | Frequent application |
| One-step procedure | Long rehabilitation |
| Inexpensive | Frequent application |
| Application of chondrocytes | Generation of fibrous tissue |
| Application of chondrocytes | Incomplete defect filling/delamination |
| Pure autologous material | No long-term data available |
| Arthroscopy and arthrotomy required | Invasiveness |
| Usage of autologous cartilage | Potential donor site morbidity |
| Off-the-shelf application | Potential age limitation |
| Stable transplantation technique | Collagen membrane application required |
Footnotes
The authors report that they have no conflicts of interest in the authorship and publication of this article.
Supplementary Data
The second-generation particulate cartilage chips procedure is shown and explained step by step. After initial arthroscopy for joint inspection and finally defect exploration, final indication for the procedure is given. A medial arthrotomy is performed. The patella (in this case with patella cartilage defect) is everted. The defect is inspected with regard to dimension. In 90° of knee joint flexion, 3 osteochondral cylinders are taken from the notch. Those are prepared at the back table. There, bone is removed from the cylinders and the cartilage is minced into small pieces. Meanwhile the defect is debrided to form a stable base and border. Then the particulate cartilage pieces are brought into the defective region onto a fibrin base layer. Hereafter, a second fibrin application does fix the minced cartilage within the defect. Finally, a Chondro-Gide membrane is sutured on top and the construct is sealed with fibrin glue again. The joint is closed in layers.
References
- 1.Niemeyer P., Albrecht D., Andereya S. Autologous chondrocyte implantation (ACI) for cartilage defects of the knee: A guideline by the working group “Clinical Tissue Regeneration” of the German Society of Orthopaedics and Trauma (DGOU) Knee. 2016;23:426–435. doi: 10.1016/j.knee.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht F.H. [Closure of joint cartilage defects using cartilage fragments and fibrin glue] Fortschr Med. 1983;101:1650–1652. [in German] [PubMed] [Google Scholar]
- 3.Christensen B.B., Foldager C.B., Jensen J., Lind M. Autologous dual-tissue transplantation for osteochondral repair: Early clinical and radiological results. Cartilage. 2015;6:166–173. doi: 10.1177/1947603515580983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pestka J.M., Salzmann G.M., Sudkamp N.P., Niemeyer P. [Cartilage biopsy for autologous chondrocyte implantation (ACI)] Z Orthop Unfall. 2013;151:278–283. doi: 10.1055/s-0032-1328494. [in German] [DOI] [PubMed] [Google Scholar]
- 5.McCarthy H.S., Richardson J.B., Parker J.C., Roberts S. Evaluating joint morbidity after chondral harvest for autologous chondrocyte implantation (ACI): A study of ACI-treated ankles and hips with a knee chondral harvest. Cartilage. 2016;7:7–15. doi: 10.1177/1947603515607963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonasia D.E., Marmotti A., Mattia S. The degree of chondral fragmentation affects extracellular matrix production in cartilage autograft implantation: An in vitro study. Arthroscopy. 2015;31:2335–2341. doi: 10.1016/j.arthro.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 7.Hindle P., Hall A.C., Biant L.C. Viability of chondrocytes seeded onto a collagen I/III membrane for matrix-induced autologous chondrocyte implantation. J Orthop Res. 2014;32:1495–1502. doi: 10.1002/jor.22701. [DOI] [PubMed] [Google Scholar]
- 8.Jones D.G., Peterson L. Autologous chondrocyte implantation. J Bone Joint Surg Am. 2006;88:2502–2520. doi: 10.2106/00004623-200611000-00025. [DOI] [PubMed] [Google Scholar]
- 9.Hirschmuller A., Baur H., Braun S., Kreuz P.C., Sudkamp N.P., Niemeyer P. Rehabilitation after autologous chondrocyte implantation for isolated cartilage defects of the knee. Am J Sports Med. 2011;39:2686–2696. doi: 10.1177/0363546511404204. [DOI] [PubMed] [Google Scholar]
- 10.Campbell A.B., Pineda M., Harris J.D., Flanigan D.C. Return to sport after articular cartilage repair in athletes' knees: A systematic review. Arthroscopy. 2016;32:651–668.e1. doi: 10.1016/j.arthro.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 11.Lacy K.W., Cracchiolo A., Yu S., Goitz H. Medial femoral condyle cartilage defect biomechanics: Effect of obesity, defect size, and cartilage thickness. Am J Sports Med. 2016;44:409–416. doi: 10.1177/0363546515613517. [DOI] [PubMed] [Google Scholar]
- 12.Arendt E.A., Berruto M., Filardo G. Early osteoarthritis of the patellofemoral joint. Knee Surg Sports Traumatol Arthrosc. 2016;24:1836–1844. doi: 10.1007/s00167-016-4103-4. [DOI] [PubMed] [Google Scholar]
- 13.Bonasia D.E., Marmotti A., Rosso F., Collo G., Rossi R. Use of chondral fragments for one stage cartilage repair: A systematic review. World J Orthop. 2015;6:1006–1011. doi: 10.5312/wjo.v6.i11.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salzmann G.M., Baumann G.A., Preiss S. Spontaneous minced cartilage procedure for unexpectedly large femoral condyle surface defect. Case Rep Orthop. 2016;2016:1498135. doi: 10.1155/2016/1498135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jungmann PM, Welsch GH, Brittberg M, et al. Magnetic resonance imaging score and classification system (AMADEUS) for assessment of preoperative cartilage defect severity [published online August 25, 2016]. Cartilage. doi:10.1177/1947603516665444. [DOI] [PMC free article] [PubMed]
- 16.Marlovits S., Singer P., Zeller P., Mandl I., Haller J., Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: Determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57:16–23. doi: 10.1016/j.ejrad.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 17.Lu Y., Dhanaraj S., Wang Z. Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair. J Orthop Res. 2006;24:1261–1270. doi: 10.1002/jor.20135. [DOI] [PubMed] [Google Scholar]
- 18.Cole B.J., Farr J., Winalski C.S. Outcomes after a single-stage procedure for cell-based cartilage repair: A prospective clinical safety trial with 2-year follow-up. Am J Sports Med. 2011;39:1170–1179. doi: 10.1177/0363546511399382. [DOI] [PubMed] [Google Scholar]
- 19.Wang N., Grad S., Stoddart M.J. Particulate cartilage under bioreactor-induced compression and shear. Int Orthop. 2014;38:1105–1111. doi: 10.1007/s00264-013-2194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zingler C., Carl H.D., Swoboda B., Krinner S., Hennig F., Gelse K. Limited evidence of chondrocyte outgrowth from adult human articular cartilage. Osteoarthritis Cartilage. 2016;24:124–128. doi: 10.1016/j.joca.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Farr J., Cole B.J., Sherman S., Karas V. Particulated articular cartilage: CAIS and DeNovo NT. J Knee Surg. 2012;25:23–29. doi: 10.1055/s-0031-1299652. [DOI] [PubMed] [Google Scholar]
- 22.Eldracher M., Orth P., Cucchiarini M., Pape D., Madry H. Small subchondral drill holes improve marrow stimulation of articular cartilage defects. Am J Sports Med. 2014;42:2741–2750. doi: 10.1177/0363546514547029. [DOI] [PubMed] [Google Scholar]
- 23.de Windt T.S., Vonk L.A., Slaper-Cortenbach I.C. Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-stage cartilage repair in humans upon mixture with recycled autologous chondrons. Stem Cells. 2017;35:256–264. doi: 10.1002/stem.2475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The second-generation particulate cartilage chips procedure is shown and explained step by step. After initial arthroscopy for joint inspection and finally defect exploration, final indication for the procedure is given. A medial arthrotomy is performed. The patella (in this case with patella cartilage defect) is everted. The defect is inspected with regard to dimension. In 90° of knee joint flexion, 3 osteochondral cylinders are taken from the notch. Those are prepared at the back table. There, bone is removed from the cylinders and the cartilage is minced into small pieces. Meanwhile the defect is debrided to form a stable base and border. Then the particulate cartilage pieces are brought into the defective region onto a fibrin base layer. Hereafter, a second fibrin application does fix the minced cartilage within the defect. Finally, a Chondro-Gide membrane is sutured on top and the construct is sealed with fibrin glue again. The joint is closed in layers.