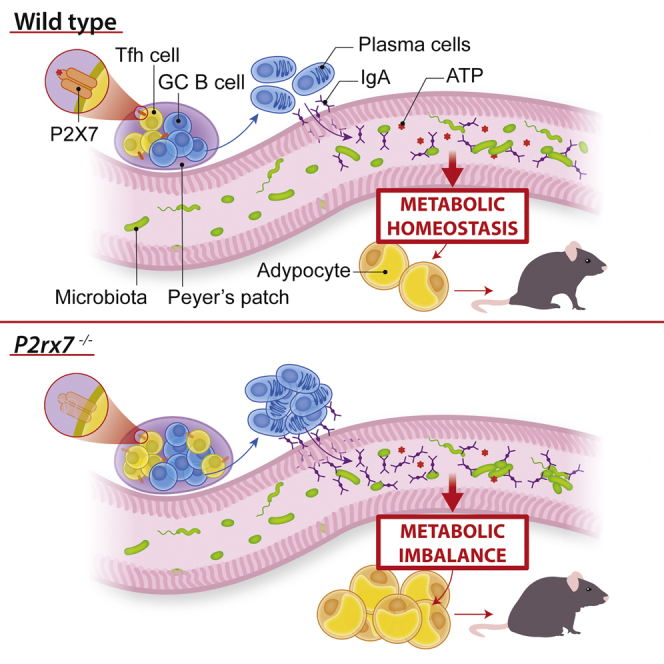
Summary
The ATP-gated ionotropic P2X7 receptor regulates T follicular helper (Tfh) cell abundance in the Peyer’s patches (PPs) of the small intestine; deletion of P2rx7, encoding for P2X7, in Tfh cells results in enhanced IgA secretion and binding to commensal bacteria. Here, we show that Tfh cell activity is important for generating a diverse bacterial community in the gut and that sensing of microbiota-derived extracellular ATP via P2X7 promotes the generation of a proficient gut ecosystem for metabolic homeostasis. The results of this study indicate that Tfh cells play a role in host-microbiota mutualism beyond protecting the intestinal mucosa by induction of affinity-matured IgA and suggest that extracellular ATP constitutes an inter-kingdom signaling molecule important for selecting a beneficial microbial community for the host via P2X7-mediated regulation of B cell help.
Keywords: T follicular helper cells, IgA, P2rx7, ATP, microbiota, metabolism
Graphical Abstract
Highlights
-
•
P2X7 receptor activity in Tfh cells is important for shaping the gut microbiota
-
•
Control of secretory IgA by Tfh cells promotes a healthy gut ecosystem
-
•
Lack of P2X7 in Tfh cells results in selection of an obesogenic microbiota
-
•
Sensing of extracellular ATP by P2X7 in Tfh cells promotes host metabolic balance
Gut commensals contribute to host metabolic homeostasis. ATP released by bacteria limits IgA secretion in the small intestine via the P2X7 receptor expressed in T follicular helper cells. Perruzza et al. show that this regulatory mechanism is important for shaping a diverse microbiota that promote host metabolic homeostasis.
Introduction
The gastrointestinal tract of mammals is colonized by bacteria at birth, and, thereafter, a mutualistic interaction with the evolving microbiota is established. The microbiota regulates the metabolic balance of the organism by generating bioactive molecules that are absorbed through the intestinal epithelium. A comparison of conventionally reared and germ-free (GF) mice showed that the gut microbiota regulates host fat storage. The transfer of cecal microbiota from conventional to GF mice results in a significant increase in body fat content and insulin resistance (Bäckhed et al., 2004). A primary role of intestinal commensals in the pathophysiology of metabolism was shown by reproduction of an obese phenotype in GF mice by transplantation of the microbiota isolated from obese animals (Turnbaugh et al., 2006).
Bacteria stimulate the development of gut-associated lymphoid tissue (GALT) (Macpherson and Harris, 2004). The intestinal tissue must integrate commensal bacteria and maintain their number and composition without inducing inflammation-mediated tissue damage (Littman and Pamer, 2011). Central in this homeostatic relationship is the production of immunoglobulin A (IgA). T follicular helper (Tfh) cells in the Peyer’s patches (PPs) of the small intestine promote germinal center (GC) reactions and affinity maturation of IgA responses that are critical for efficient mucosal defense by limiting the translocation of potentially invasive bacteria and microbial inflammatory compounds from the gut lumen into the organism (Fagarasan et al., 2002, Shroff et al., 1995, Wei et al., 2011). Importantly, regulation of high-affinity IgA responses by T follicular regulatory (Tfr) cells promotes the diversification and influences the composition of the microbiota in the gut (Kawamoto et al., 2014).
In mice deficient for the ATP-gated ionotropic receptor P2X7 (P2rx7−/−), Tfh cells are significantly increased in PPs because of resistance to cell death induced by extracellular ATP. The altered regulation of Tfh cells in these mice results in enhanced secretory IgA responses (Proietti et al., 2014). Here we show that lack of P2X7-mediated control of Tfh cells results in altered microbiota composition that is responsible for impaired glucose homeostasis and enhanced fat deposition.
Results
Increased Body Weight and Impaired Glucose Metabolism in P2rx7−/− Mice
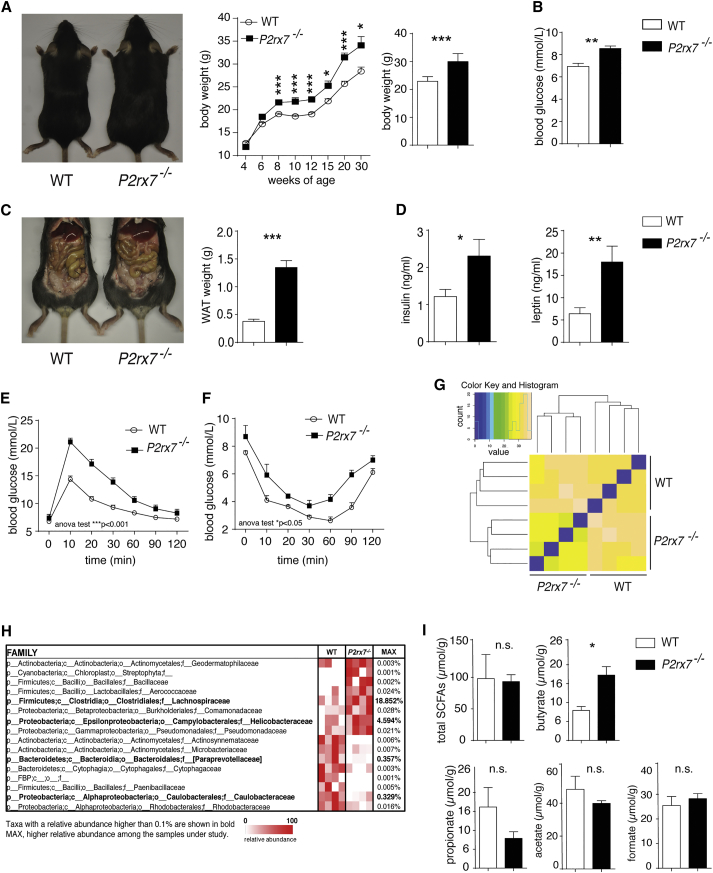
A characteristic trait of P2rx7−/− mice is the increase in body weight with respect to wild-type (WT) littermates (Beaucage et al., 2014; Figure 1A). P2rx7−/− animals at 8 weeks had a significant increase in blood glucose, white adipose tissue (WAT) mass, serum insulin, and leptin with respect to WT littermates (Figures 1B–1D). Moreover, P2rx7−/− mice showed significant glucose intolerance and decreased insulin sensitivity, as measured by glucose tolerance test (GTT) and insulin tolerance test (ITT) (Figures 1E and 1F). Food consumption was not different between the two strains of mice (data not shown), as were plasma total cholesterol (76.9 ± 23.1 mg/dL versus 66.5 ± 17.5 mg/dL in 9-week-old WT and P2rx7−/− animals, respectively; n = 5) and plasma triglyceride levels (104.2 ± 25.4 mg/dL versus 95.4 ± 14.9 mg/dL in WT and P2rx7−/− animals, respectively).
Figure 1.
Alterations of Metabolic Parameters and Microbiota Composition in P2rx7−/− Mice
(A–F) P2rx7−/− and WT littermates, weight gain in WT and P2rx7−/− mice, and body weight (n = 20) (A) and blood glucose concentration (n = 20) (B) at 9 weeks. Also shown are representative abdomens and statistics of WAT weights (n = 20) (C). Serum insulin and leptin concentrations (n = 20) are shown (D) as well as glucose homeostasis determined by GTT (E) and ITT (F) in WT and P2rx7−/− mice (n = 5).
(G) Similarity in mouse microbiota by Euclidean distances between cecal samples from WT and P2rx7−/− mice based on the taxonomic assignment at family rank. Dendrograms show the Euclidean distances between cecal samples, and the matrix colors are proportional to the observed distances.
(H) Heatmap of bacterial families in cecal microbiota that discriminate WT from P2rx7−/− mice. Families were selected according to p < 0.1 with two-tailed unpaired Student’s t test. Each line represents one family, and each column represents an individual mouse. Mean relative abundances of families detected in WT and P2rx7−/− mice and the p value for each family are shown. Operational taxonomic units (OTUs) with a relative abundance higher than 0.1% in at least one sample are shown in bold.
(I) SCFA quantification in cecum content of WT and P2rx7−/− mice (n = 5).
Means ± SEM are shown, and Mann-Whitney test (A–D, and I) and two-way ANOVA (E and F) were used. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; n.s., non-significant.
Macroscopic analysis at 9 weeks revealed liver enlargement (weights, WT: 1.057 ± 0.04 g; P2rx7−/−: 1.346 ± 0.038 g; n = 10; p = 0.0006) that was associated with significantly elevated glycogen in fasted P2rx7−/− compared with WT mice, which exhibited only minimal, scattered glycogen deposition (Figure S1A). Glucokinase (GCK) mRNA levels were significantly increased in P2rx7−/− mice, suggesting that hyperglycemia could result in increased glucose flux (Figure S1B). However, intracellular glucose does not appear to enter glycolysis because transcript levels of the key enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDH), which catabolizes the conversion of glucose 1,3 biphospate to 1,3-bisphosphoglyceric acid and fosters glycolysis, were reduced (Figure S1B). This implies that increased intracellular glucose could be accumulated as glycogen and, indeed, could explain the observed phenotype. Other metabolic pathways in the liver did not appear to be affected (Figure S1B). These results suggest that P2X7 activity is important in the regulation of glucose homeostasis. The altered metabolic control we observed in P2rx7−/− mice was worsened by a high-fat diet, which induced a significant increase in body and WAT weights as well as significantly impaired glucose tolerance and insulin sensitivity with respect to WT littermates (Figures S1C and S1D), suggesting that P2rx7−/− mice are more sensitive to increased caloric intake.
Altered Microbiota Composition in P2rx7−/− Mice
Hierarchical clustering of mice for cecal microbiota showed that P2rx7−/− animals clustered together and separately with respect to WT littermates (Figure 1G). Among the most represented families, we detected the increase of Lachnospiraceae and Helicobacteraceae in P2rx7−/− mice. In contrast, Paraprevotellaceae and Caulobacteraceae were enriched in WT animals (Figure 1H). The increase of Lachnospiraceae within gut commensals has been associated with obesity (Cho et al., 2012). Many species belonging to this family have been shown to produce butyrate (Duncan et al., 2002, Meehan and Beiko, 2014), the abundance of which has been associated with obesity (Cho et al., 2012, Turnbaugh et al., 2006). Quantification of short chain fatty acids (SCFAs) in cecal content of WT and P2rx7−/− mice revealed a significant increase in butyrate in mutant mice (Figure 1I). These data suggest that variations in selected families of the microbiota might result from deletion of P2rx7 and contribute to the observed altered glucose metabolism.
Cell-Intrinsic Role of Tfh Cells in Glucose Homeostasis
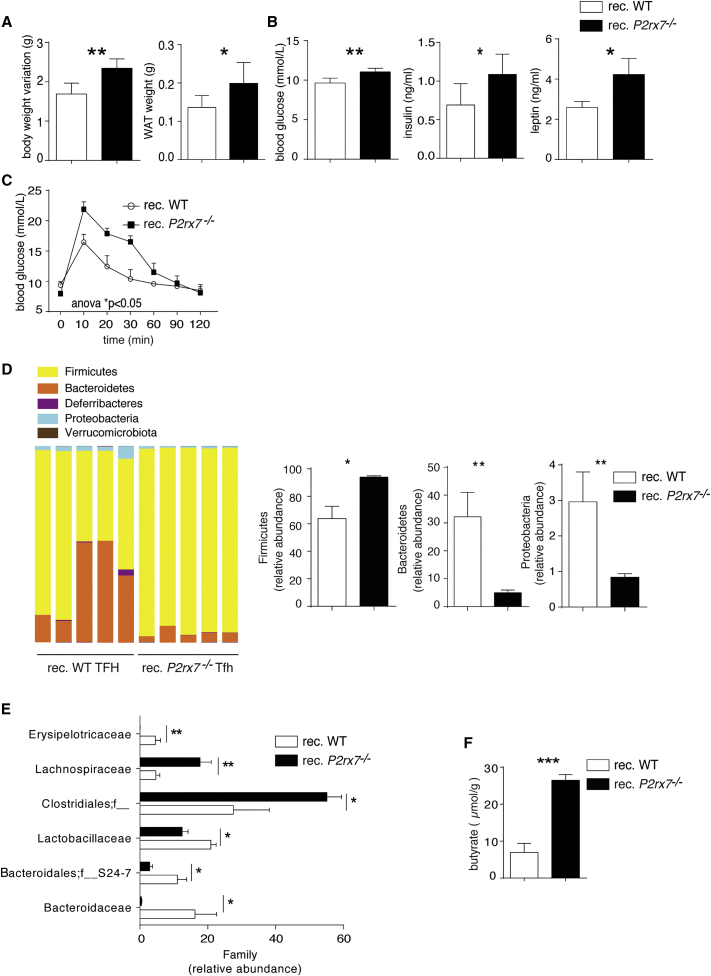
Adoptive transfer of P2rx7−/− Tfh cells into lymphopenic mice results in enhanced expansion in PPs with respect to WT cells (Proietti et al., 2014; Figure S2A). 1 month after transfer into Cd3e−/− mice, both WT and P2rx7−/− cells maintained the Tfh phenotype, characterized by CXCR5, Bcl6, PD1, and inducible T-cell costimulator (ICOS) expression, with few cells expressing Foxp3 (Figures S2B–S2D). Transfer of mutant cells resulted in significantly enhanced GC reactions (Figure S2E), increased body and WAT weights, and increased blood glucose, insulin, and leptin levels (Figures 2A and 2B). GTT also revealed impaired glucose sensitivity (Figure 2C). Firmicutes were significantly increased, with concomitant reduction of both Bacteroidetes and Proteobacteria (Figure 2D). The analysis of variations in family abundances showed a significant increase in Lachnospiraceae, a characteristic feature of P2rx7−/− mice (Figure 1H), and of an undetermined family belonging to the order Clostridiales in P2rx7−/− chimeric mice (Figure 2E). Moreover, quantification of SCFAs showed a significant increase in butyrate (Figure 2F). Therefore, P2rx7−/− Tfh cells appear to be sufficient for determining the modifications in metabolic parameters and microbiota composition that were observed in P2rx7−/− mice.
Figure 2.
Cell-Intrinsic Role of Tfh Cells in Regulating Glucose Metabolism
(A–C) Body weight variation and WAT weight (A); blood glucose, insulin, and leptin levels (B); and GTT (C) in Cd3e−/− mice reconstituted with WT or P2rx7−/− Tfh cells (n = 5).
(D and E) Phylum (D) and family (E) relative abundances in Cd3e−/− mice reconstituted with WT or P2rx7−/− Tfh cells (n = 5).
(F) Butyrate quantification in cecum content of Cd3e−/− mice reconstituted with WT or P2rx7−/− Tfh cells (n = 5).
Means ± SEM are shown, and Mann-Whitney test (A, B, and D–F) and two-way ANOVA (C) were used. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Selective Role of Tfh Cells in Regulating Glucose Metabolism
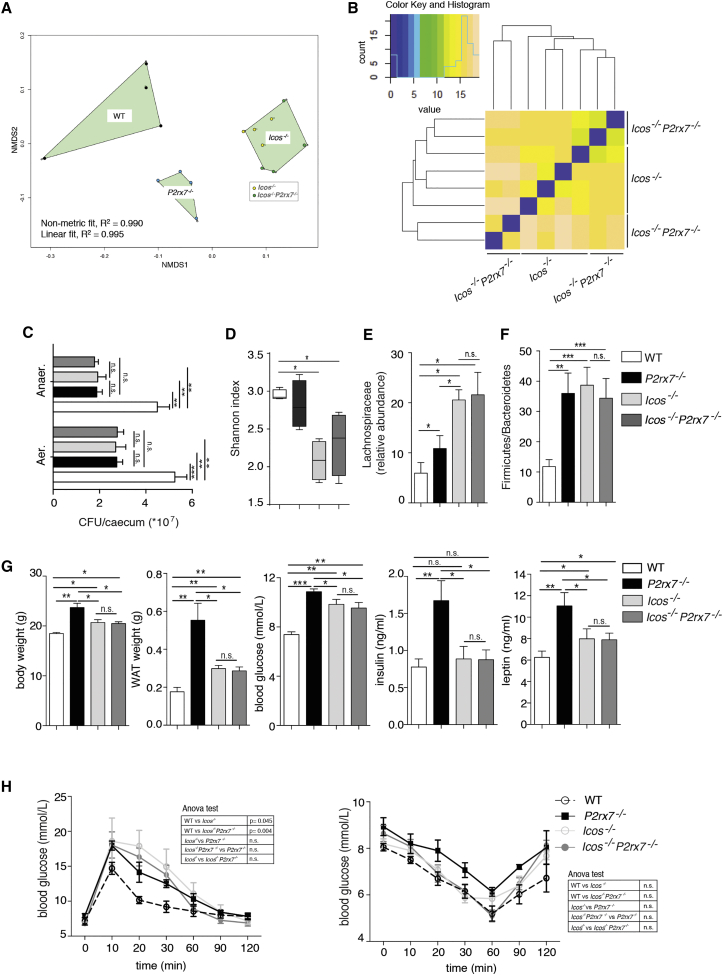
To understand whether Tfh cell activity in P2rx7−/− mice played a causative role in the development of metabolic syndrome by shaping the microbiota, we compared Icos−/− and Icos−/−P2rx7−/− double mutant mice, which are devoid of Tfh and GC B cells (Figures S3A and S3B), for microbiota composition and metabolic parameters. Icos−/− mice exhibit normal CD4 and CD8 cell populations in lymph nodes and spleen (Dong et al., 2001). However, effector/memory CD4 (CD44+CD62L−) as well as T regulatory and Th17 cells in PPs and mesenteric lymph nodes (MLNs) were significantly reduced (Figures S3C and S3D). Icos−/− mice were characterized by an increase in IgA-coated bacteria in stools with respect to WT mice (Figure S4A), a finding consistent with a predominant T-independent IgA response to commensals (Bunker et al., 2015) and modulation of the secretory IgA response by Tfh cells (Kawamoto et al., 2014). Concomitant deletion of P2rx7 did not affect IgA coating in ICOS-deficient mice, indicating that lack of P2X7 in ICOS− cells does not influence the enhanced IgA response (Figure S4A). Notably, culture of aerobic and anaerobic bacteria from the cecum revealed the significant reduction of colony-forming units (CFUs) in samples from ICOS-deficient and P2rx7−/− mice (Figure 3C), suggesting that deregulated IgA might impair the expansion of the cecal microbial community.
Figure 3.
Crucial Role of Tfh Cells in Shaping Commensal Microbiota Composition
(A) Similarity among cecal microbiota through non-metric multidimensional scaling (NMDS) based on an unweighted Unifrac dissimilarity matrix.
(B) Euclidean distances inferred on taxonomic assignment at family rank between cecal samples from Icos−/− and Icos−/−P2rx7−/− mice.
(C) Statistical analysis of CFUs of aerobic and anaerobic bacteria recovered from the ceca of WT, P2rx7−/− (n = 10), Icos−/−, and Icos−/−P2rx7−/− mice (n = 5).
(D) Box and whisker plots of the Shannon diversity index at the bacterial family level in WT, P2rx7−/−, Icos−/−, and Icos−/−P2rx7−/− mouse cecal samples (n = 4).
(E and F) Relative abundance of the Lachnospiraceae family (E) and Firmicutes/Bacteroidetes ratio (F) in the indicated mice (n = 4).
(G) Body and WAT weights and blood glucose, insulin, and leptin concentrations (n = 10).
(H) GTT (left) and ITT (right) in WT (white dots), P2rx7−/− (black squares), Icos−/− (light gray) and Icos−/−P2rx7−/− (dark gray) mice (n = 10).
Means ± SEM are shown, and Mann-Whitney test (C–G) and two-way ANOVA (H) were used. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
The overall microbiota compositions of Icos−/− mutants clustered together by β-diversity analysis and were well separated from WT- and P2rx7−/−-derived samples (Figure 3A). Icos−/− also co-clustered with Icos−/−P2rx7−/− mice for microbiota composition in a hierarchical clustering analysis (Figure 3B). These data indicate that Icos deletion (i.e., lack of Tfh cells) causes drastic changes in the gut microbial taxonomic structure. Moreover, α-diversity was significantly reduced in ICOS-deficient animals, supporting the importance of Tfh cell activity in generating a diverse microbiome (Kawamoto et al., 2014; Figure 3D). The reduction of microbial biodiversity in Icos−/−-associated microbiota was reflected by depletion of microbial functions through phylogenetic investigation of communities by reconstruction of unobserved states (PICRUSt) prediction of metabolic potential (Langille et al., 2013; Figure S4B). In Icos−/− and Icos−/−P2rx7−/− mice, we observed a significant increase in the Lachnospiraceae (Figure 3E) and Firmicutes to Bacteroidetes ratio (Figure 3F) together with significantly increased body and WAT weights, blood glucose, and leptin (Figure 3G). Moreover, glucose tolerance was impaired. Different from P2rx7−/− mice, serum insulin and insulin tolerance tests were not altered in both Icos−/− and Icos−/−P2rx7−/− mice with respect to WT mice (Figures 3G–3H). Notably, Icos−/− and Icos−/−P2rx7−/− mice were characterized by indistinguishable metabolic parameters, ruling out a contribution of P2X7 in ICOS− cells to these phenotypic traits. Altogether, these results underscore the importance of Tfh cells in selecting a proficient microbiota for host glucose homeostasis.
Role of Commensal Microbiota in Altered Glucose Metabolism of P2rx7−/− Mice
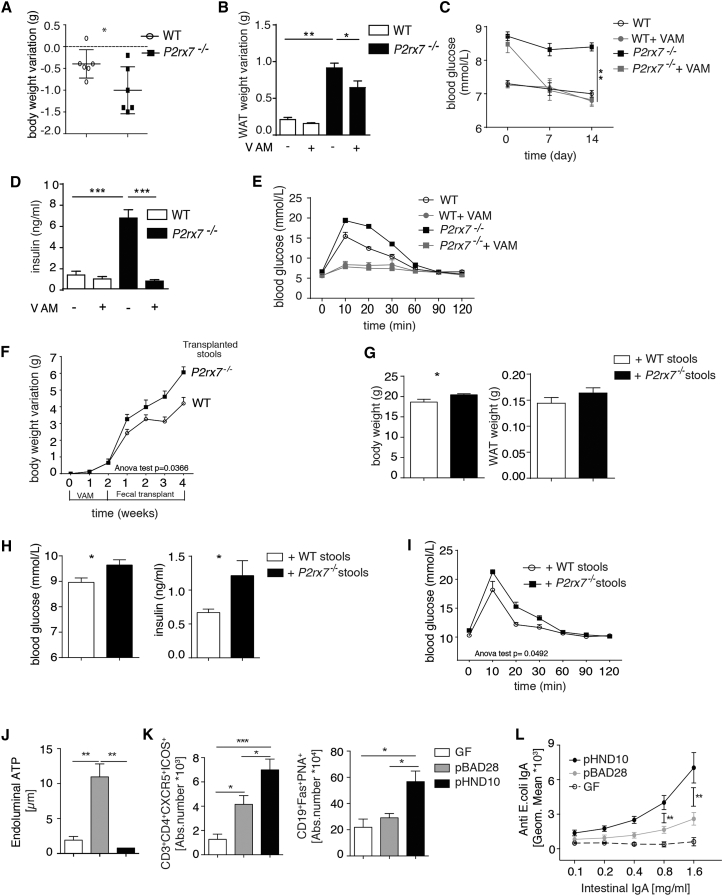
We treated WT and P2rx7−/− mice with vancomycin, ampicillin, and metronidazole (VAM) to deplete most bacterial species present in the gut. As expected, Tfh cells from P2rx7−/− mice were relatively resistant to cell death induced by massive release of bacterial ATP by VAM compared with WT cells (Figure S5A). Administration of VAM resulted in a significantly enhanced reduction in body and WAT weights as well as blood glucose in P2rx7−/− mice compared with WT littermates (Figures 4A–4C). In addition, serum concentrations of insulin and GTTs of VAM-treated P2rx7−/− mice became indistinguishable from their WT counterparts (Figures 4D and 4E). GF P2rx7−/− mice at 10 weeks showed analogous body and WAT weights to WT littermates and indistinguishable glucose serum levels as well as tolerance to glucose bolus. Some reduction of insulin serum concentration, albeit not reaching statistical significance, was detected in P2rx7−/− mice (Figures S5C and S5D). These data confirm the role of the microbiota in the metabolic phenotype of P2rx7−/− mice. To understand whether the changes in the gut microbiota characteristic of P2rx7−/− mice were the cause of the observed metabolic phenotype, we transplanted cecal content isolated from P2rx7−/− mice into VAM-treated WT animals. Administration of VAM with depletion of the microbiota resulted in significant diminution of Tfh cells; fecal transplant restored Tfh cell abundance. Microbiota isolated from P2rx7−/− mice induced a significant increase in Tfh cells compared with autochthonous microbiota (Figure S5B), suggesting that microbiota conditioned by P2rx7−/− Tfh cells can amplify the regulation of Tfh cells in a feedforward loop. Mice transplanted with microbiota from P2rx7−/− mice gained significantly more weight compared with mice transplanted with bacteria from WT mice (Figure 4F). Moreover, body and WAT weights and blood glucose and insulin concentrations 4 weeks after transplant were all increased in mice harboring bacteria isolated from P2rx7−/− mice (Figures 4G and 4H), and tolerance to glucose was significantly impaired (Figure 4I). These results suggest that the gut microbiota associated with P2rx7−/− mice has the transmissible capacity to promote the fat deposition and development of metabolic features characteristic of these mutant animals.
Figure 4.
Role of Microbiota of P2rx7−/− Mice in Altering Glucose Metabolism
(A–E) Body (A) and WAT (B) weight variation, blood glucose (C) and insulin levels (D), and GTT (E) in WT and P2rx7−/− mice after 14 days of VAM (n = 5).
(F–I) Weight gain (F), body and WAT weights (G), blood glucose and insulin (H), and GTT (I) in WT mice transplanted with WT or P2rx7−/− microbiota (n = 5).
(J) Concentrations of ATP in ilea from GF mice either non-colonized (GF) or colonized with pBAD28 or pHND10 bearing E. coli.
(K) Quantification of Tfh and GC B cells in non-colonized or monocolonized animals as indicated.
(L) Intestinal anti-E. coli IgA quantification at fluorescence-activated cell sorting (FACS) (see Experimental Procedures) in GF mice or mice monocolonized with the indicated E. coli transformants (n = 5).
Means ± SEM are shown, and Mann-Whitney test (A–D, G, H, and J–L) and two-way ANOVA (E, F, and I) were used. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
ATP Released by Commensals Limits the Secretory IgA Response in the Small Intestine
We directly addressed whether bacterially derived ATP could influence T cell-dependent IgA responses via P2X7. The IgA response to E. coli is dependent on Tfh cells in PPs (Lécuyer et al., 2014) and is significantly more effective in P2rx7−/− mice (Proietti et al., 2014), suggesting that P2X7 activity can affect the T cell-dependent IgA response. We used a recombinant E. coli K-12 strain carrying the pHND10 plasmid, which encodes phoN2::HA fusion of Shigella flexneri, a periplasmic ATP-diphosphohydrolase (apyrase) (Santapaola et al., 2006, Scribano et al., 2014). Extracellular ATP released concomitantly with E. coli growth (Hironaka et al., 2013) was undetectable in cultures of these transformants, indicating that apyrase efficiently abrogated ATP secretion (data not shown). Accordingly, GF mice monocolonized with these bacteria showed significantly reduced ATP in the intestine compared with mice monocolonized with bacteria carrying an empty vector (Figure 4J). Consistent with a role of endoluminal ATP in regulating Tfh cell number and GC reaction in the PPs of the small intestine (Proietti et al., 2014), both Tfh and GC B cells were increased in animals colonized with apyrase-expressing bacteria (Figure 4K), as was E. coli-specific IgA in the intestinal fluid (Figure 4L). These data indicate that extracellular ATP released by commensals limits the secretory IgA response in the small intestine.
Discussion
The gut microbiota can affect many aspects of host metabolism, including energy harvesting from nutrients, hepatic lipogenesis, and adipose tissue development (Bäckhed et al., 2004, Cox and Blaser, 2013, Turnbaugh et al., 2006). Signaling pathways involved in reciprocal regulation of the adaptive immune system and microbiota to ensure the generation and maintenance of a healthy microbial community are poorly defined. We have shown that regulation of Tfh cell activity by the ATP-gated ionotropic P2X7 receptor contributes to the selection of a beneficial microbiota for glucose metabolism and fat deposition. Interestingly, hypo-functioning P2X7 variants were recently associated with impaired glucose homeostasis in both mice and humans (Todd et al., 2015). We observed that changes in the microbiota by enhanced Tfh cell activity were responsible for metabolic abnormalities and obesity. Previous microbial taxonomy of stool and mucus from P2rx7−/− mice did not reveal significant modifications with respect to the WT counterpart (Proietti et al., 2014). Apart from dissimilarities in accurately assigning amplicons to different taxa by the different techniques used for microbiota profiling (Kang et al., 2014), this apparent discrepancy could be due to selective variation in microbiota composition in the ceca of P2rx7−/− mice, where diversity of the bacterial ecosystem might be more sensitive to enhanced IgA coating. Bacterial coating by IgA restricts bacterial access to the epithelium and can promote the survival of specific bacteria that affect the intestinal as well as general metabolism (Mantis et al., 2011). Moreover, the diversified microbiota resulting from T-dependent secretory IgA pressure has been suggested to play a pivotal role in promoting the ecological adaptability and speciation potential of mammals (Sutherland et al., 2016).
Release of ATP from commensals limits Tfh cell-dependent helper activity, and abrogation of this release (Figure 4J) or signaling via P2X7 in Tfh cells (Proietti et al., 2014) results in enhanced taxon-specific secretory IgA responses. The results obtained with mice adoptively transferred with Tfh cells indicate that sensing of extracellular ATP by Tfh cells via P2X7 is exclusively responsible for the metabolic alterations observed in P2rx7−/− mice. Obesity was associated with an increased ratio of Firmicutes to Bacteroidetes (Cho et al., 2012, Ley et al., 2005, Turnbaugh et al., 2006), which was, in turn, associated with increased concentrations of butyrate in the intestine of obese versus lean mice (Cho et al., 2012, Turnbaugh et al., 2006). This interrelation with possible metabolic relevance might be sensitive to manipulation of the ATP/P2X7 axis in Tfh cells.
Tfh cells are regulated in the PPs by Tfr cells. IgA generated and selected in the presence of Tfr cells coated a larger diversity of bacterial taxa than in their absence. This coating was hypothesized to directly influence the diversity and phylogenetic structure of the intestinal bacterial ecosystem by contributing to the maintenance, rather than elimination, of indigenous bacteria (Kawamoto et al., 2014). We have previously shown that lack of P2X7 results in an increase in Tfh, but not Tfr, cell abundance in the PPs (Proietti et al., 2014). The deregulated activation of Tfh cells in the absence of the concomitant modulation by Tfr cells might result in loss of the controlled diversification of stimulatory bacteria that were hypothesized to promote a self-regulatory loop important for host-bacterial mutualism (Kawamoto et al., 2014). Defective Tfr activity as well as lack of Tfh cells (Icos−/− mice) resulted in a higher Firmicutes-to-Bacteroidetes ratio and expansion of bacteria belonging to Lachnospiraceae analogous to mice with P2X7-deficient Tfh cells. These results indicate that regulated GC reaction profoundly affects host metabolism by shaping a proficient microbiota. The lack of sensitivity of PP Tfh cells to endoluminal ATP compromises the host metabolism, thereby suggesting that ATP in the gut acts as an inter-kingdom signaling molecule ensuring the establishment of a healthy relationship between microbiota, the adaptive immune response, and systemic homeostasis.
Experimental Procedures
Mice
All animal experiments were performed in accordance with the Swiss Federal Veterinary Office guidelines and as authorized by the Cantonal Veterinary Office. C57BL/6J, P2rx7−/− (B6.129P2-P2rx7tm1Gab/J), Icos−/−, and Cd3e−/− mice were bred in the specific pathogen-free (spf) facility at the Institute for Research in Biomedicine Switzerland.
Adoptive Transfer of Tfh Cells
CD4+CD8−CXCR5+ICOS+ cells from pooled PPs of WT or P2rx7−/− mice were sorted on a FACSAria. Eight-week-old Cd3e−/− mice were injected intravenously (i.v.) with 1 × 105 Tfh cells. Recipient mice were sacrificed 4 weeks after reconstitution.
Microbiota Transplantation
Eight-week-old C57BL/6J mice were gavaged for 2 weeks with vancomycin (1.25 mg), ampicillin (2.5 mg), and metronidazole (2.5 mg) in 200 μL PBS and then gavaged for 3 days with fresh cecal content (200 μL) collected from donor mice and resuspended in PBS (0.01 g/mL).
GTT, ITT, Serum Insulin, and Leptin Quantification
Animals were fasted for 12 hr (GTT) or 6 hr (ITT) and then received an intraperitoneal (i.p.) injection of glucose (2 g/kg of body weight) or insulin (0.6 U/kg). Blood glucose was monitored for 120 min using a glucometer on samples collected from the tip of the tail vein. Insulin and leptin were quantified by ELISA.
Taxonomic Analysis of Microbiota
For the evaluation of intestinal microbiota, the bacterial microbiota of cecal samples from WT, P2rx7−/−, Icos−/−, and Icos−/−P2rx7−/− mice has been investigated by sequencing the V5–V6 hypervariable regions of the 16S rDNA gene by using the Illumina MiSeq platform. The prokaryotic composition of the tested samples has been assessed by bioinformatic analysis of metagenomic amplicons (BioMaS) on the paired end (PE) reads generated by Illumina MiSeq sequencing. In experiments with adoptive transfer of Tfh cells, microbial V5 and V6 regions were sequenced on the Ion Torrent PGM (personal genome machine) system using a 316v2 chip and analyzed using QIIME V1.8.0. Operational taxonomic units (OTU) were generated using uclust and a 97% identity threshold. Taxonomy assignment was performed by blasting representative OTU sequences against the latest Greengenes database.
Statistical Analysis
The displayed data are representative of at least three independent experiments. Results were analyzed using the nonparametric Mann Whitney test, Student’s unpaired t test, and two-way ANOVA with Bonferroni post-test analysis. Results are presented as mean ± SEM. Values of p < 0.05 were considered statistically significant. For statistical analyses of microbiota, R statistic software (version 3.1.2) was used. Differences between the effects on microbiota composition were evaluated by analyzing the data with non-parametric Wilcoxon Mann-Whitney test with Benjamini-Hochberg correction, using paired data when possible, with which we could decide whether the population distributions were identical without assuming them to follow the normal distribution.
Author Contributions
F.G., L.P., and M.P. designed the experiments. L.P. performed most experiments. M.P., C.E.F., and T.R.J. performed experiments. A.M.D., C.M., and M.G. performed 16S metagenomic sequencing. L.M. and D.C. performed gas chromatography for SCFA quantification, G. Pellegrini performed the histological analysis. A.M. and G.D.N. performed the liver gene expression analysis. G.G., B.F., G. Pesole, M.B.G., and S.G. contributed bioinformatic analyses. M.G. and K.D.M. generated GF mice. D.S. and M.N. contributed E. coli transformants. F.G. and L.P. analyzed data and wrote the paper with contributions from G.G. and S.G.
Acknowledgments
We thank David Jarrossay (Institute for Research in Biomedicine) for cell sorting and Teresa De Filippis, Claudia Lionetti (University of Bari), and Caterina Manzari (Institute of Biomembranes and Bioenergetics) for contributing to 16S metagenomic sequencing. The work was supported by grant 310030_159491 and Sinergia CRSII2_127547 of the Swiss National Science Foundation (SNSF), grant 12A09 of the Novartis Stiftung für Medizinisch-Biologische Forschung, Nano-Tera Project 20NA21_128841, Fondazione Ticinese per la Ricerca sul Cancro, and Fondazione per la Ricerca sulla Trasfusione e sui Trapianti and Converge Biotech (to F.G.). The Ph.D. fellowship of L.P. was supported by Signora Alessandra. C.M. was supported by MD-PhD-Programm SNF323530_158124. K.D.M. is supported by a grant from the SNSF (SNSF310030_134902) and European Research Council (FP/2007-2013) agreement no. 281785.
Published: March 14, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and five figures and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2017.02.061.
Accession Numbers
The accession numbers for the sequences reported in this paper are SRA: SRP099819 and ENA: PRJEB19531 (for adoptive transfer of Tfh cells).
Supplemental Information
References
- Bäckhed F., Ding H., Wang T., Hooper L.V., Koh G.Y., Nagy A., Semenkovich C.F., Gordon J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaucage K.L., Xiao A., Pollmann S.I., Grol M.W., Beach R.J., Holdsworth D.W., Sims S.M., Darling M.R., Dixon S.J. Loss of P2X7 nucleotide receptor function leads to abnormal fat distribution in mice. Purinergic Signal. 2014;10:291–304. doi: 10.1007/s11302-013-9388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunker J.J., Flynn T.M., Koval J.C., Shaw D.G., Meisel M., McDonald B.D., Ishizuka I.E., Dent A.L., Wilson P.C., Jabri B. Innate and adaptive humoral responses coat distinct commensal bacteria with immunoglobulin A. Immunity. 2015;43:541–553. doi: 10.1016/j.immuni.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho I., Yamanishi S., Cox L., Methé B.A., Zavadil J., Li K., Gao Z., Mahana D., Raju K., Teitler I. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature. 2012;488:621–626. doi: 10.1038/nature11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox L.M., Blaser M.J. Pathways in microbe-induced obesity. Cell Metab. 2013;17:883–894. doi: 10.1016/j.cmet.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C., Juedes A.E., Temann U.A., Shresta S., Allison J.P., Ruddle N.H., Flavell R.A. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- Duncan S.H., Barcenilla A., Stewart C.S., Pryde S.E., Flint H.J. Acetate utilization and butyryl coenzyme A (CoA):acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl. Environ. Microbiol. 2002;68:5186–5190. doi: 10.1128/AEM.68.10.5186-5190.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagarasan S., Muramatsu M., Suzuki K., Nagaoka H., Hiai H., Honjo T. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298:1424–1427. doi: 10.1126/science.1077336. [DOI] [PubMed] [Google Scholar]
- Hironaka I., Iwase T., Sugimoto S., Okuda K., Tajima A., Yanaga K., Mizunoe Y. Glucose triggers ATP secretion from bacteria in a growth-phase-dependent manner. Appl. Environ. Microbiol. 2013;79:2328–2335. doi: 10.1128/AEM.03871-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.S., Jeraldo P.R., Kurti A., Miller M.E., Cook M.D., Whitlock K., Goldenfeld N., Woods J.A., White B.A., Chia N., Fryer J.D. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol. Neurodegener. 2014;9:36. doi: 10.1186/1750-1326-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S., Maruya M., Kato L.M., Suda W., Atarashi K., Doi Y., Tsutsui Y., Qin H., Honda K., Okada T. Foxp3(+) T cells regulate immunoglobulin a selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity. 2014;41:152–165. doi: 10.1016/j.immuni.2014.05.016. [DOI] [PubMed] [Google Scholar]
- Langille M.G., Zaneveld J., Caporaso J.G., McDonald D., Knights D., Reyes J.A., Clemente J.C., Burkepile D.E., Vega Thurber R.L., Knight R. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lécuyer E., Rakotobe S., Lengliné-Garnier H., Lebreton C., Picard M., Juste C., Fritzen R., Eberl G., McCoy K.D., Macpherson A.J. Segmented filamentous bacterium uses secondary and tertiary lymphoid tissues to induce gut IgA and specific T helper 17 cell responses. Immunity. 2014;40:608–620. doi: 10.1016/j.immuni.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Ley R.E., Bäckhed F., Turnbaugh P., Lozupone C.A., Knight R.D., Gordon J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman D.R., Pamer E.G. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe. 2011;10:311–323. doi: 10.1016/j.chom.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson A.J., Harris N.L. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- Mantis N.J., Rol N., Corthésy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4:603–611. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan C.J., Beiko R.G. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol. Evol. 2014;6:703–713. doi: 10.1093/gbe/evu050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietti M., Cornacchione V., Rezzonico Jost T., Romagnani A., Faliti C.E., Perruzza L., Rigoni R., Radaelli E., Caprioli F., Preziuso S. ATP-gated ionotropic P2X7 receptor controls follicular T helper cell numbers in Peyer’s patches to promote host-microbiota mutualism. Immunity. 2014;41:789–801. doi: 10.1016/j.immuni.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Santapaola D., Del Chierico F., Petrucca A., Uzzau S., Casalino M., Colonna B., Sessa R., Berlutti F., Nicoletti M. Apyrase, the product of the virulence plasmid-encoded phoN2 (apy) gene of Shigella flexneri, is necessary for proper unipolar IcsA localization and for efficient intercellular spread. J. Bacteriol. 2006;188:1620–1627. doi: 10.1128/JB.188.4.1620-1627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scribano D., Petrucca A., Pompili M., Ambrosi C., Bruni E., Zagaglia C., Prosseda G., Nencioni L., Casalino M., Polticelli F., Nicoletti M. Polar localization of PhoN2, a periplasmic virulence-associated factor of Shigella flexneri, is required for proper IcsA exposition at the old bacterial pole. PLoS ONE. 2014;9:e90230. doi: 10.1371/journal.pone.0090230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff K.E., Meslin K., Cebra J.J. Commensal enteric bacteria engender a self-limiting humoral mucosal immune response while permanently colonizing the gut. Infect. Immun. 1995;63:3904–3913. doi: 10.1128/iai.63.10.3904-3913.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland D.B., Suzuki K., Fagarasan S. Fostering of advanced mutualism with gut microbiota by immunoglobulin A. Immunol. Rev. 2016;270:20–31. doi: 10.1111/imr.12384. [DOI] [PubMed] [Google Scholar]
- Todd J.N., Poon W., Lyssenko V., Groop L., Nichols B., Wilmot M., Robson S., Enjyoji K., Herman M.A., Hu C. Variation in glucose homeostasis traits associated with P2RX7 polymorphisms in mice and humans. J. Clin. Endocrinol. Metab. 2015;100:E688–E696. doi: 10.1210/jc.2014-4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Wei M., Shinkura R., Doi Y., Maruya M., Fagarasan S., Honjo T. Mice carrying a knock-in mutation of Aicda resulting in a defect in somatic hypermutation have impaired gut homeostasis and compromised mucosal defense. Nat. Immunol. 2011;12:264–270. doi: 10.1038/ni.1991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.